Introduction
Cachexia is a significant cause of morbidity and
mortality in patients with advanced cancer, occurring in up to 80%
of cases (1,2). Weight loss, anorexia, inflammation,
insulin resistance and increased muscle protein breakdown are
frequently associated with cachexia (3). Although the precise mechanism of
cancer cachexia has not been fully elucidated, it is now clear that
the persistent inflammatory response of the host, in conjunction
with inappropriate production and release of cytokines, such as
tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, are
central to the pathogenesis of this disease (4,5).
There is increasing evidence that eicosanoids, such as
prostaglandins have an important function in the development of
cachexia (6,7). Non-steroidal anti-inflammatory drugs
(NSAIDs) have been demonstrated to block the protein-catabolizing
effects that occur in cachectic mice and prevent muscle protein
breakdown in tumor-bearing rats (8,9).
Cyclooxygenases (COXs) are key rate-limiting enzymes
in the conversion of arachidonic acid to prostaglandins. There are
at least two isoforms of cyclooxygenase, including COX-1 and COX-2
(10). COX-1 is constitutively
expressed in numerous types of cell and is important in
homeostasis, whereas COX-2 is usually absent but inducible by
various cytokines, growth factors and mitogens. Overexpression of
COX-2 has been demonstrated in tissues from several types of tumor
tissue and in various cell lines (11,12).
Selective COX-2 inhibitors exhibit marked antineoplastic activity
in a number of model tumor systems (13–15).
It has also been observed that acute treatment of severely
cachectic tumor-bearing animals with the COX-2 inhibitor celecoxib
maintained or reduced the serum calcium level and produced a rapid
and significant weight gain, compared with tumor-matched
vehicle-treated animals (16). The
studies suggested that COX-2 inhibitors may be a potential
treatment approach to cachexia; however, little is known about the
underlying mechanisms.
In the present study, the colon 26 carcinoma mouse
model of cachexia, a well-studied model of cancer cachexia, was
used with the aim to investigate the possible effects of celecoxib
on cancer cachexia progression and to elucidate the complex
involvement of cytokines. The results demonstrated that the
reduction of serum vascular endothelial growth factor (VEGF)
concentration was associated with COX-2 inhibition. These findings
suggest a novel mechanism by which a COX-2 inhibitor ameliorates
cancer cachexia.
Materials and methods
Cell culture
Colon 26 cells were maintained in Dulbecco’s
modified Eagle’s medium supplemented with 10% fetal bovine serum,
sodium pyruvate, non-essential amino acids and L-glutamine
(Gibco-BRL, Paisley, UK) in a 37°C, 5% CO2 humidified
incubator. Colon 26 cells were harvested from subconfluent cultures
with 0.25% trypsin (Hyclone, Logan, UT, USA) and resuspended in
phosphate-buffered saline (PBS; Sigma, Santa Clara, CA, USA) at
107 cells/ml for injection, prior to use.
Animals
Seven-week-old male BALB/c mice were purchased from
the Center for New Drug Evaluation (Shandong University, Jinan,
China) and housed in a barrier system with controlled light
(12/12-h light/dark cycle) and temperature (21–22°C). All animal
experiments were conducted in accordance with the guidelines for
animal experimentation of the Shandong Provincial Animal Board
(Jinan, China).
Mouse model of cancer cachexia
Tumor cells were subcutaneously implanted at a
density of ~1×106 subcutaneously in the backs of the
BALB/c mice following anesthesia, which was induced and maintained
by inhalation of halothane (Sigma). All experimental animals were
observed to have a palpable tumor within five days of the start of
the experiment. Treatment was initiated when tumors reached a mean
volume of 0.1 ml, according to a previous study (; the selective
COX-2 inhibitor celecoxib (250 mg/kg/day; Pfixer, New York, NY,
USA) was administered in the diet. This treatment was maintained
for the duration of the experiment. The VEGF-neutralizing antibody
bevacizumab (5 mg/kg; Roche Diagnostics, Basel, Switzerland) was
intraperitoneally injected twice per week, with a total of three
injections administered to each mouse. Body weight was measured
three times a week. Each animal group was administered 150 g food,
the following day the remaining food was weighed and the reduction
in weight was divided by the number of mice to determine the
average food intake per animal. Tumor growth was measured every
three days and tumor volume was calculated as follows: Tumor volume
= length × (width)2 × 0.52.
Tissue and organ collection
Tumor-bearing and control mice were sacrificed at
different time points. At the time of sacrifice, animals were
anesthesized with halothane and exsanguinated via closed cardiac
puncture, blood was allowed to clot for 2 h at room temperature
then centrifuged for 20 min at 2,000 × g. Serum was removed and
stored at −80°C until required. Whole blood (100 μl) was prepared
with EDTA (Sigma) as an anticoagulant to determine hematological
parameters. Primary tumors were excised, the liver, kidneys and
spleen were resected and weighed, and were then immediately fixed
with 10% paraformaldehyde overnight, followed by washing with PBS.
A fraction of the tissues and organs was embedded with
paraffin.
Determination of circulating
cytokines
Cytokine levels in serum samples were assessed using
the Quantikine ELISA kits (R&D Systems, Inc., Minneapolis, MN,
USA).
Histological studies
Malignant and non-malignant paraffin-embedded
tissues were cut to a thickness of 4 μm for hematoxylin-eosin
(H&E) staining. Slides were examined under a light microscope
(DM500; Leica, Mannheim, Germany) and images were captured at a
magnification ×200.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Analyses of different treatment groups were conducted
using Student’s t-test in the SPSS statistical software package,
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. A
Kaplan-Meier survival curve was generated using Statistica, version
5.0 (Statsoft, Inc., Tulsa, OK, USA).
Results
Celecoxib reduces tumor growth and
increases survival rate in mice with cancer cachexia
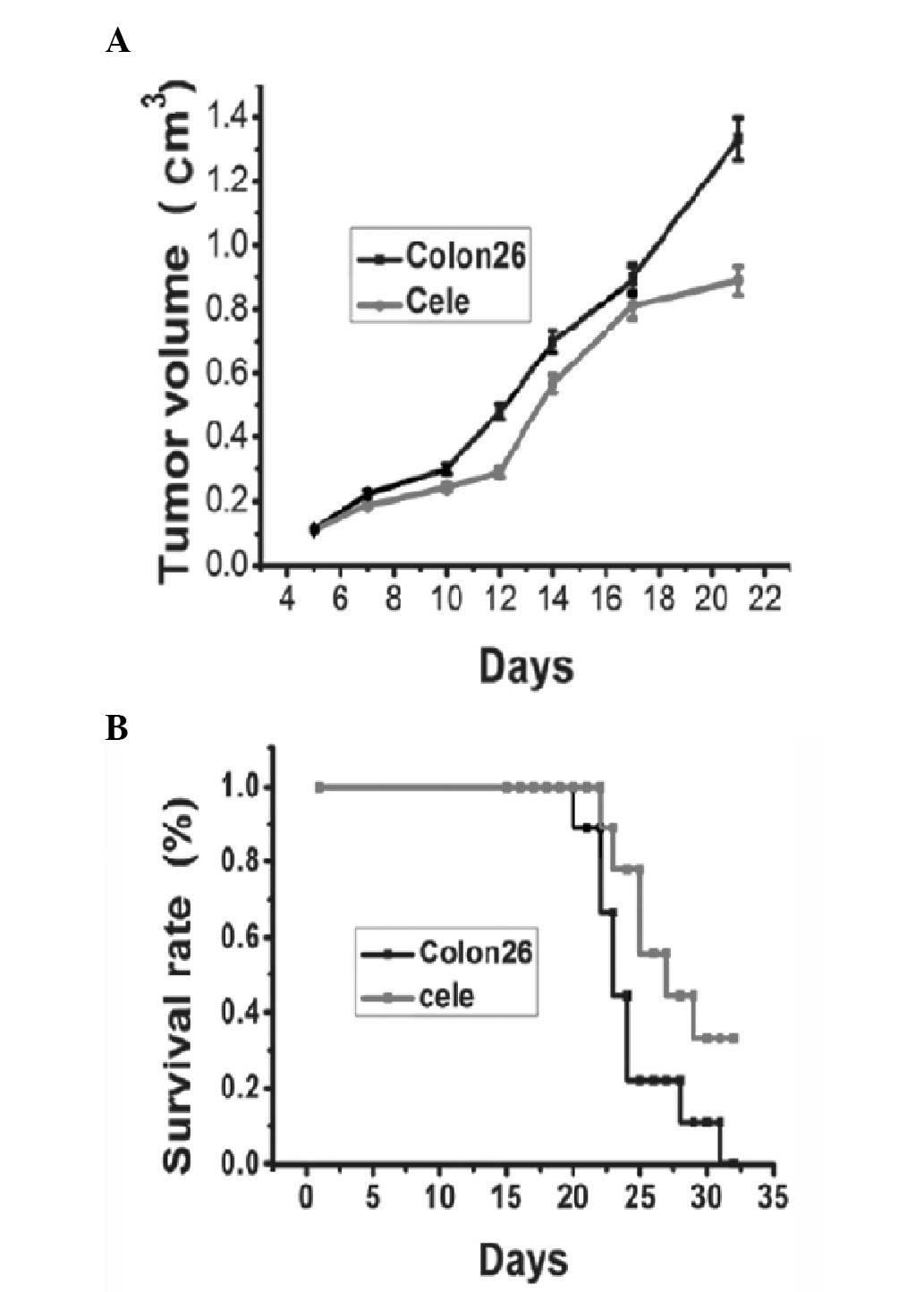
Tumor appearances were observed on day five, and the
volumes of the tumors increased until the day on which the mice
were sacrificed. Fig. 1A shows a
representative tumor growth curve of the colon 26 tumor group
compared with the celecoxib treatment group. The celecoxib-treated
group presented delayed tumor growth compared with the control
group. There was a significant reduction in tumor volume between
the treated and non-treated groupon day 21 (Fig. 1A, P<0.05). Mortality occurred in
mice injected with colon 26 tumors ~21 days following tumor
injection, and the survival rate reduced to 0 on day 32. Between
days 23 and 32 post-tumor inoculation, treatment of tumor bearing
mice with celecoxib led to a significant increase in survival rate
compared with that of the control tumor-bearing mice (Fig. 1B).
Celecoxib reduces weight loss and
increases food intake in mice with cancer cachexia
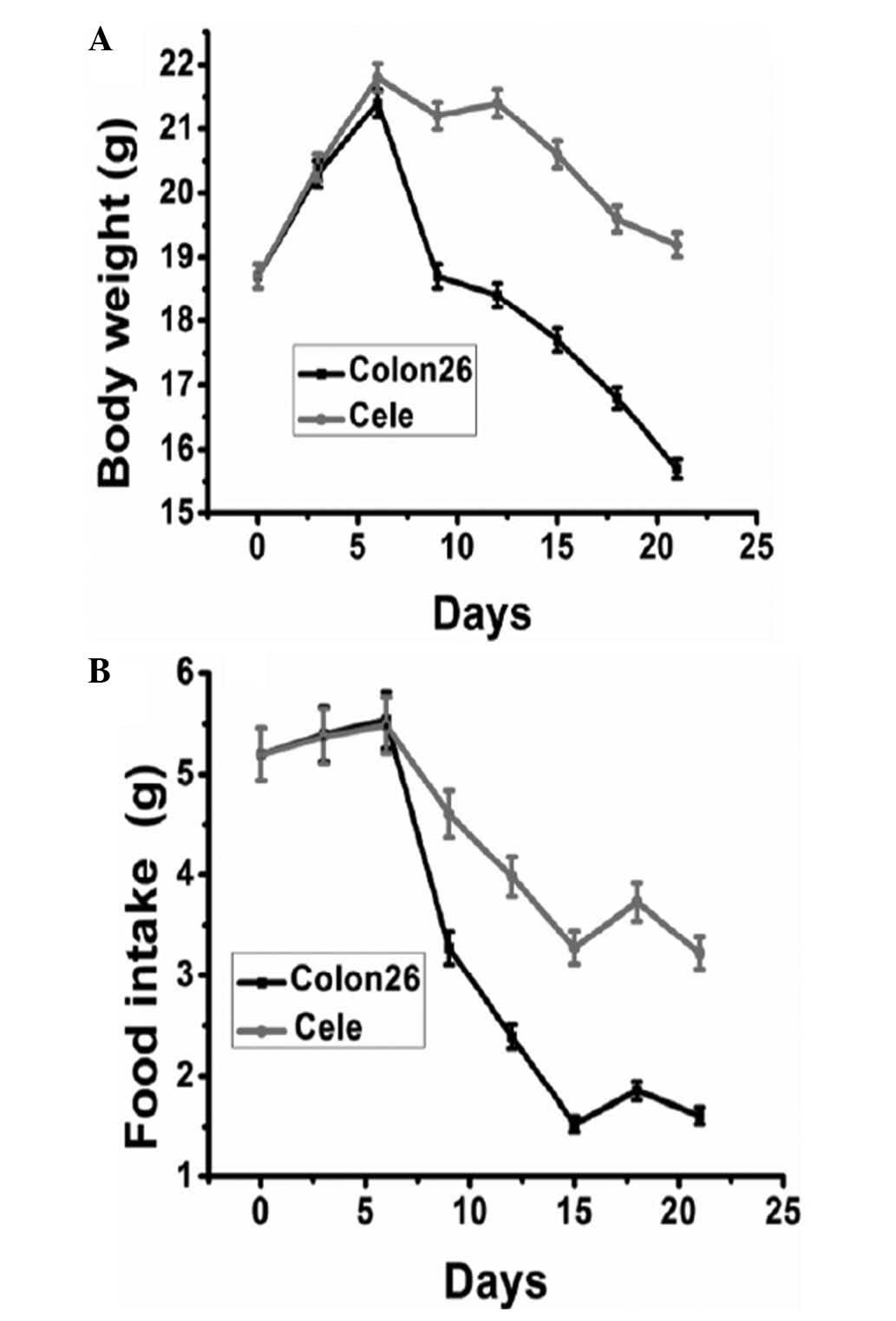
Body weight and food intake are considered to be
indicators of cancer cachexia in animal models. No significant
difference was identified among groups in initial body weight and
food intake. The body weight of mice carrying colon 26 tumors
started to reduce around day nine post-inoculation. From day 12 to
21 post-inoculation, prior to the occurrence of cachexia-mediated
mortality of tumor-bearing mice, the body weights of mice receiving
celecoxib treatment were identified to be significantly greater
than those of tumor-bearing control mice (Fig. 2A). The average body weight of mice
at the time of tumor inoculation was 18.7 g, this then reduced to
14.6 g in tumor-bearing control mice on day 21, while it reduced to
17.8 g in the mice receiving daily celecoxib treatment. Food intake
was similar in the two groups prior to day seven (Fig. 2B). However, a significant reduction
in food intake was observed from day eight in the control group,
which was significantly ameliorated by celecoxib treatment.
Celecoxib ameliorates anemia in mice with
cancer cachexia
Hematological analysis of peripheral blood indicated
a significant reduction in hematocrit at day 21 post colon 26 tumor
implantation. The level of hemoglobin and the number of
erythrocytes in peripheral blood were significantly reduced. These
results demonstrate that colon 26 tumor-bearing mice suffered from
severe anemia. Erythrocyte count and hemoglobin concentration in
the celecoxib-treated group were significantly higher than those of
the tumor-bearing control mice on day 21 post-inoculation (Table I).
 | Table IEffect of celecoxib on hemograms of
colon 26-adenocarcinoma model mice. |
Table I
Effect of celecoxib on hemograms of
colon 26-adenocarcinoma model mice.
| Group | Control mice | Celecoxib treated
mice |
|---|
| Erythrocyte
(×1012/l) | 5.4±0.7 | 7.6±0.4b |
| Hemoglobin (g/l) | 11.4±1.6 | 13.7±2.1a |
| Platelets
(×109/l) | 727±23.1 | 769.5±37.2 |
| White blood cells (×
109/l) | 5.7±0.4 | 5.9±0.7 |
Celecoxib reduces host inflammatory
response in mice with cancer cachexia
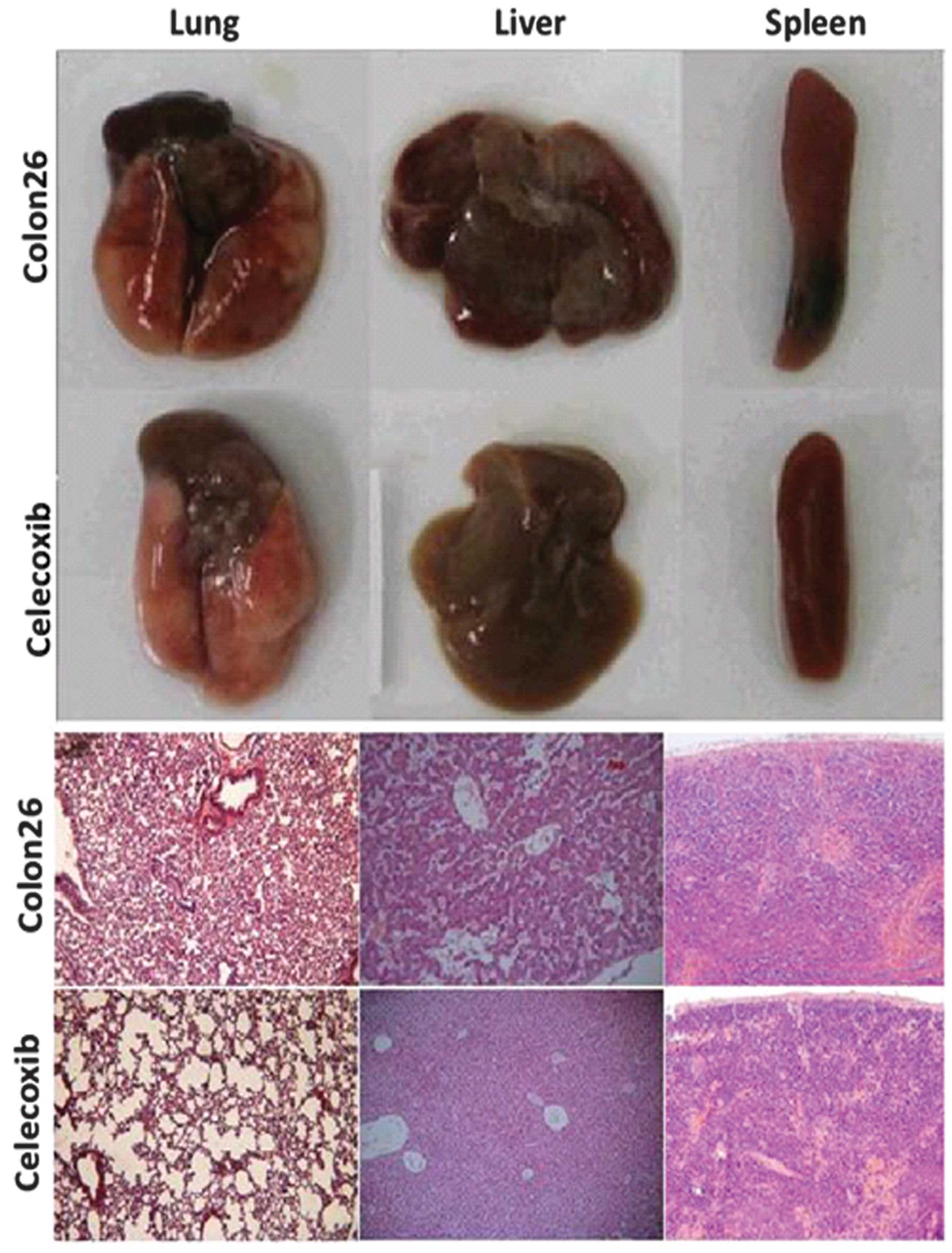
The injection of colon 26 cells into mice resulted
in increases in the volumes of liver, lung and spleen. These
increases were reduced by oral administration of celecoxib.
Histological examination demonstrated inflammatory changes in the
organs of colon 26 tumor-bearing mice. Following treatment with
celecoxib, H&E staining indicated a marked reduction in
inflammatory cell infiltration in the lung, liver and spleen
(Fig. 3).
Celecoxib alters the expression of
inflammatory cytokines in mice with cancer cachexia
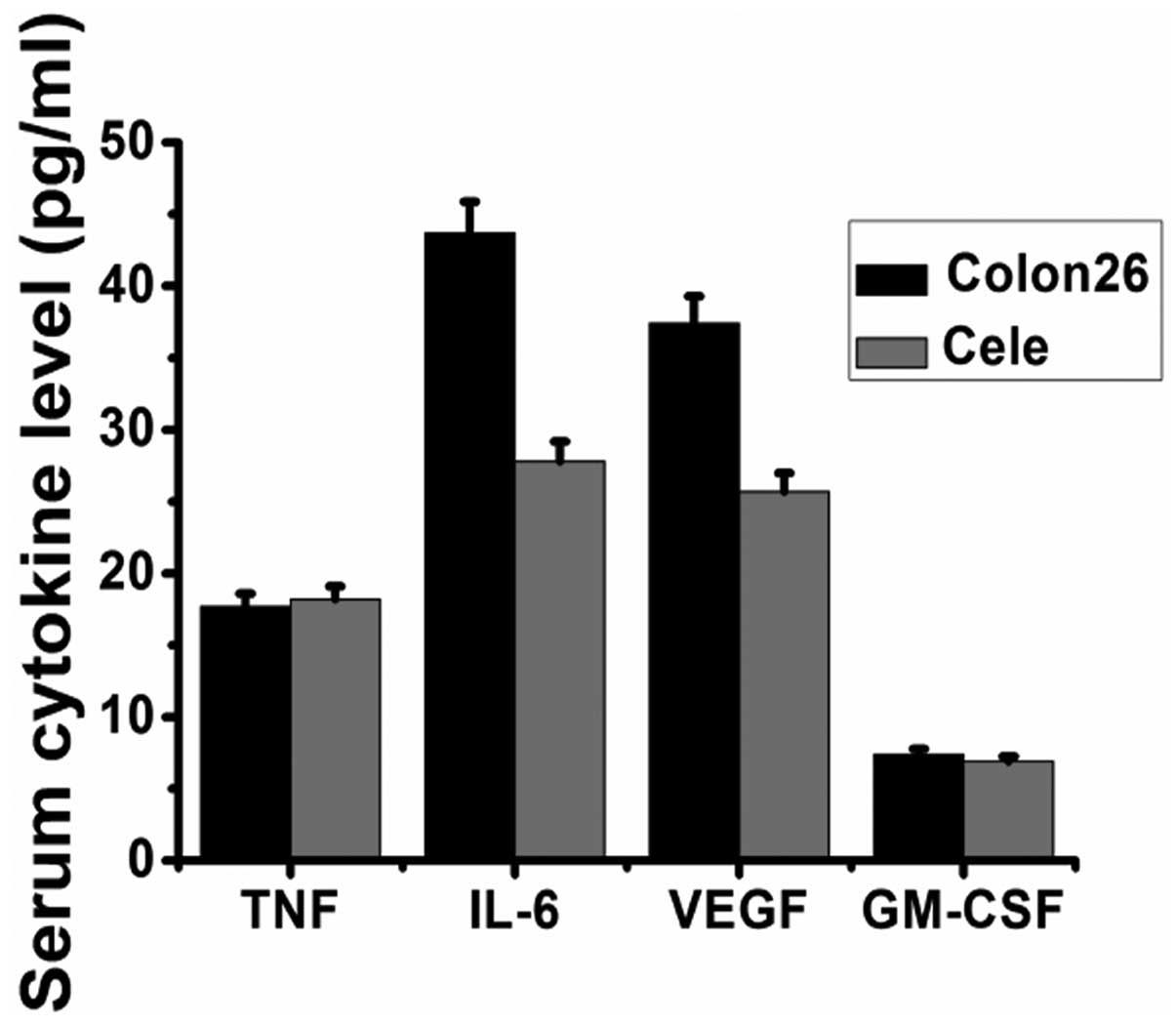
Serum concentrations of VEGF, granulocyte-macrophage
colony-stimulating factor (GM-CSF), IL-6 and TNF-α were measured by
ELISA in the sera of the controls and celecoxib-treated tumor mice.
As presented in Fig. 4, the levels
of IL-6 and VEGF were reduced significantly by oral administration
of celecoxib, while levels of TNF-α and GM-CSF were not affected by
the administration of celecoxib.
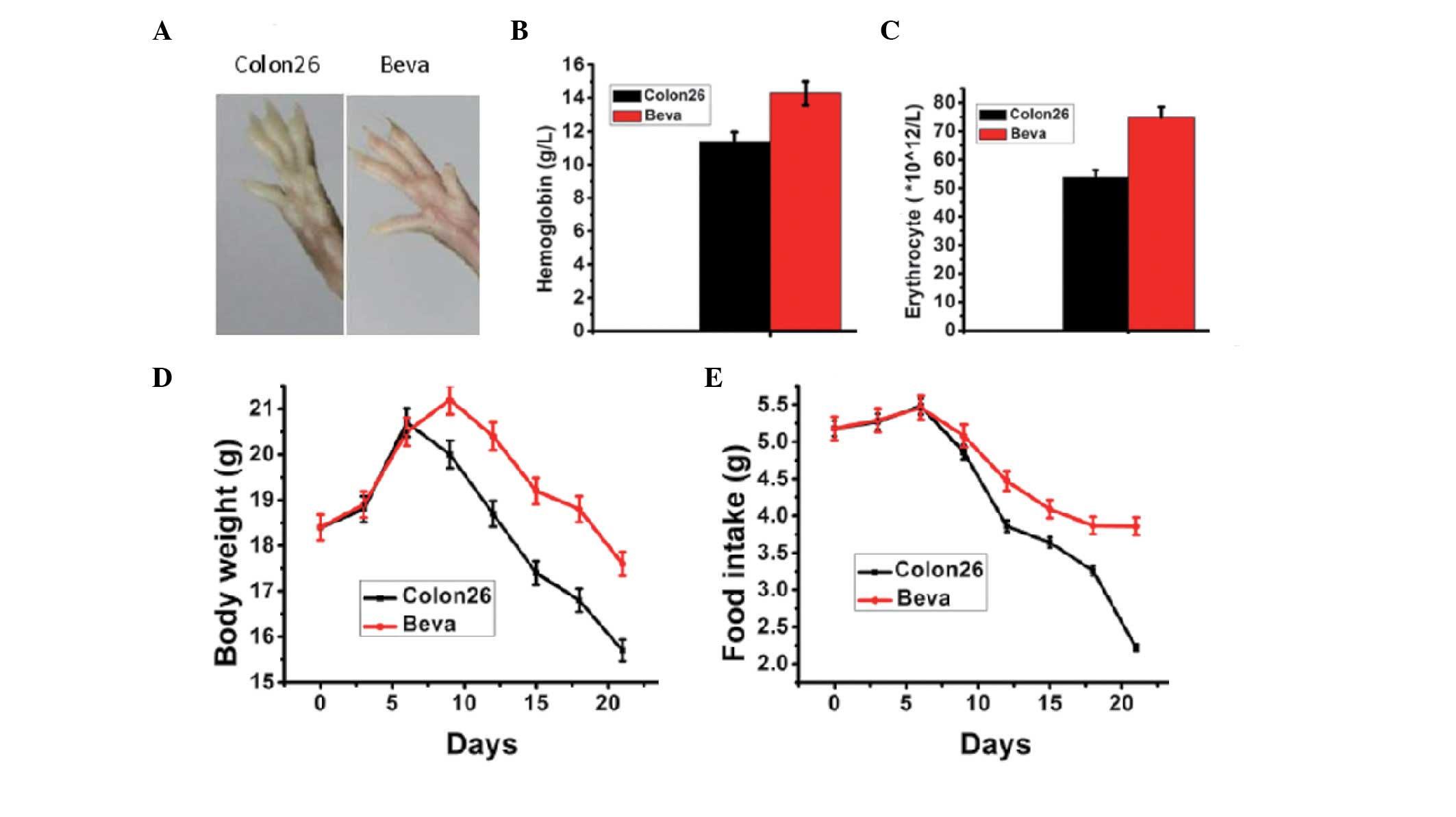
Anti-VEGF ameliorates cachexia events in
mice with cancer cachexia
In order to investigate whether an anti-VEGF agent
has the ability to reverse cachexia in mice injected by colon 26
tumor cells, a neutralizing anti-mouse VEGF antibody (bevacizumab)
was administered to the tumor-bearing mice for 10 days. The tumor
induced an anemic phenotype, including paleness of the paws, which
was reverted by anti-VEGF treatment (Fig. 5A). The erythrocyte count and
hemoglobin concentration in the bevacizumab group were
significantly higher than in untreated tumor-bearing mice on day 21
post-inoculation (Fig. 5B and C).
The food intake and body weight of the mice receiving bevacizumab
were significantly increased compared with the untreated
tumor-bearing mice (Fig. 5D and
E).
Discussion
Cancer cachexia occurs in a large percentage of
cases of advanced cancer, and reduces the strength of the patient,
the effectiveness of cancer treatments and the quality of life
(1,2). In the current study, treatment with a
selective COX-2 inhibitor, celecoxib, was demonstrated to attenuate
symptoms of cachexia induced by colon 26 tumor injection, including
severe anemia, loss of body weight and a reduction in food intake.
Increasing evidence denotes that eicosanoids, such as
prostaglandins are involved in the development of cachexia
(3,15,16,18),
and other studies have suggested that the selective COX-2 inhibitor
celecoxib is able to rapidly reverse this action (19–21).
The observations of the present study, that celecoxib ameliorates
cancer cachexia in colon 26 tumor model mice, is consistent with
the results of the previous studies.
Although the precise mechanism underlying cancer
cachexia remains to be fully elucidated, a current emerging
hypothesis is that cachexia is caused predominantly by cytokines,
either produced by cancer cells or released by the immune system in
response to the presence of the cancer (22,23).
High serum levels of TNF-α, IL-1 and IL-6 have been observed in
cancer patients, and the levels of these cytokines appear to
correlate with the incidence of cancer cachexia (24,25).
Of these cytokines, IL-6 has been demonstrated to enhance the
reduction in fat and muscle tissue of cachectic animals (26). In the current study, administration
of a COX-2 inhibitor significantly reduced the serum concentration
of IL-6 in tumor-bearing mice. Therefore, the COX-2 inhibitor
exerts an anticachectic effect, potentially through the inhibition
of IL-6 production in colon 26 tumor-bearing mice.
In the present study, administration of celecoxib
also significantly reduced the serum concentration of VEGF in
tumor-bearing mice. In order to further investigate the mechanisms
underlying the anticachectic effect of the COX-2 inhibitor,
bevacizumab, a VEGF antibody was used to treat the cachexia in
mice, and the results indicated that anti-VEGF treatment was able
to improve anemia, and increase food intake and body weight. VEGF
is one of the most potent angiogenic factors (27) and it has been implicated in
pathological angiogenesis associated with tumors and intraocular
neovascular disorders (28).
Another study suggested that tumor-derived VEGF is able to induce
symptoms that resemble cancer cachexia and paraneoplastic syndromes
(29). Kemik et al
(30) demonstrated that
tumor-derived VEGF induces cancer-associated systemic syndrome
(CASS), a cancer-associated systemic syndrome with features similar
to cachexia, by damaging the structure and function of multiple
tissues and organs, and an anti-VEGF antibody reversed VEGF-induced
CASS in tumor-bearing mice. COX-2 expression in cancer cells
stimulates the production of VEGF, and the expression of VEGF by
cancer cells can be inhibited using NSAIDs (31,32).
In the present study, it was demonstrated that COX-2 inhibitor
treatment reduces the level of VEGF in serum, and anti-VEGF
treatment attenuates cachectic events in tumor-bearing mice. These
results provide a novel mechanistic insight into the role of the
COX-2 inhibitor in the treatment of cachexia.
In conclusion, the present study demonstrated that
the COX-2 inhibitor celecoxib exerts an anticachectic effect in
colon 26 tumor-bearing mice through downregulation of VEGF. The
present findings may provide an alternative mechanism by which
COX-2 inhibitors can enhance the quality of life for patients
suffering from cancer cachexia.
Acknowledgements
The current work was supported by the National
Natural Science Foundation of China (grant no. 81272351) and the
Natural Science Foundation of Shandong Province (grant nos.
2012G0021826 and 2R2012HM020).
References
|
1
|
Fearon K, Strasser F, Anker SD, et al:
Definition and classification of cancer cachexia: an international
consensus. Lancet Oncol. 12:489–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar NB, Kazi A, Smith T, et al: Cancer
cachexia: traditional therapies and novel molecular mechanism-based
approaches to treatment. Curr Treat Options Oncol. 11:107–117.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donohoe CL, Ryan AM and Reynolds JV:
Cancer cachexia: mechanisms and clinical implications.
Gastroenterol Res Pract. 2011:6014342011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Argilés JM, Busquets S, Toledo M and
López-Soriano FJ: The role of cytokines in cancer cachexia. Curr
Opin Support Palliat Care. 3:263–268. 2009.
|
|
5
|
Gupta SC, Kim JH, Kannappan R, et al: Role
of nuclear factor κB-mediated inflammatory pathways in
cancer-related symptoms and their regulation by nutritional agents.
Exp Biol Med (Maywood). 236:658–671. 2011.
|
|
6
|
Clària J: Publication of a special issue:
resolution of acute inflammation and the role of lipid mediators.
Scientific World Journal. 10:1553–1555. 2010.PubMed/NCBI
|
|
7
|
Tan BH, Ross JA, Kaasa S, et al: European
Palliative Care Research Collaborative: Identification of possible
genetic polymorphisms involved in cancer cachexia: a systematic
review. J Genet. 90:165–177. 2011. View Article : Google Scholar
|
|
8
|
Argilés JM, Busquets S and López-Soriano
FJ: Anti-inflammatory therapies in cancer cachexia. Eur J
Pharmacol. 668(Suppl 1): S81–S86. 2011.
|
|
9
|
Batista ML Jr, Peres SB, McDonald ME, et
al: Adipose tissue inflammation and cancer cachexia: possible role
of nuclear transcription factors. Cytokine. 57:9–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiel A, Mrena J and Ristimäki A:
Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev.
30:387–395. 2011. View Article : Google Scholar
|
|
11
|
Turk HM, Camci C, Sevinc A, et al:
Cyclooxygenase-2 expression is not a marker of poor survival in
lung cancer. Asian Pac J Cancer Prev. 13:315–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morita Y, Hata K, Nakanishi M, et al:
Cyclooxygenase-2 promotes tumor lymphangiogenesis and lymph node
metastasis in oral squamous cell carcinoma. Int J Oncol.
41:885–892. 2012.PubMed/NCBI
|
|
14
|
Xin X, Majumder M, Girish GV, et al:
Targeting COX-2 and EP4 to control tumor growth, angiogenesis,
lymphangiogenesis and metastasis to the lungs and lymph nodes in a
breast cancer model. Lab Invest. 92:1115–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tisdale MJ: Cachexia in cancer patients.
Nat Rev Cancer. 2:862–871. 2002. View
Article : Google Scholar
|
|
16
|
Davis TW, Zweifel BS, O’Neal JM, et al:
Inhibition of cyclooxygenase-2 by celecoxib reverses tumor-induced
wasting. J Pharmacol Exp Ther. 308:929–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SY, Yu H, Krygier JE, Wooley PH and
Mott MP: High VEGF with rapid growth and early metastasis in mouse
osteosarcoma model. Sarcoma. 2007:956282007.PubMed/NCBI
|
|
18
|
Evans WJ, Morley JE, Argilés J, et al:
Cachexia: a new definition. Clin Nutr. 27:793–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serhan CN, Krishnamoorthy S, Recchiuti A
and Chiang N: Novel anti-inflammatory - pro-resolving mediators and
their receptors. Curr Top Med Chem. 11:629–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao QT, Yue SQ, Cui Z, et al: Potential
involvement of the cyclooxygenase-2 pathway in hepatocellular
carcinoma-associated angiogenesis. Life Sci. 80:484–492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seelaender M, Batista M Jr, Lira F,
Silverio R and Rossi-Fanelli F: Inflammation in cancer cachexia: to
resolve or not to resolve (is that the question?). Clin Nutr.
31:562–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Argilés JM, López-Soriano FJ and Busquets
S: Mechanisms to explain wasting of muscle and fat in cancer
cachexia. Curr Opin Support Palliat Care. 1:293–298.
2007.PubMed/NCBI
|
|
23
|
Lawrence T and Fong C: The resolution of
inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem
Cell Biol. 42:519–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patra SK and Arora S: Integrative role of
neuropeptides and cytokines in cancer anorexia-cachexia syndrome.
Clin Chim Acta. 413:1025–1034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laviano A, Molfino A, Seelaender M, et al:
Carnitine administration reduces cytokine levels, improves food
intake, and ameliorates body composition in tumor-bearing rats.
Cancer Invest. 29:696–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weidle UH, Klostermann S, Eggle D and
Krüger A: Interleukin 6/interleukin 6 receptor interaction and its
role as a therapeutic target for treatment of cachexia and cancer.
Cancer Genomics Proteomics. 7:287–302. 2010.PubMed/NCBI
|
|
27
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shojaei F and Ferrara N: Antiangiogenesis
to treat cancer and intraocular neovascular disorders. Lab Invest.
87:227–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krzystek-Korpacka M, Matusiewicz M,
Diakowska D, et al: Acute-phase response proteins are related to
cachexia and accelerated angiogenesis in gastroesophageal cancers.
Clin Chem Lab Med. 46:359–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kemik O, Sumer A, Kemik AS, et al: The
relationship among acute-phase response proteins, cytokines and
hormones in cachectic patients with colon cancer. World J Surg
Oncol. 8:852010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valcárcel M, Mendoza L, Hernández JJ, et
al: Vascular endothelial growth factor regulates melanoma cell
adhesion and growth in the bone marrow microenvironment via tumor
cyclooxygenase-2. J Transl Med. 9:1422011.PubMed/NCBI
|
|
32
|
Kim YY, Lee EJ, Kim YK, et al: Anti-cancer
effects of celecoxib in head and neck carcinoma. Mol Cells.
29:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|