Introduction
Nesprin-1 protein is a nuclear membrane protein
expressed in numerous tissues. In particular, the expression of
nesprin-1 expression levels are higher in skeletal muscles and
cardiac and vascular smooth muscles than those in other tissues
(1). It has been reported that
nesprin-1 not only has a key role in cell mitosis (2), RNA copy of transport and controlling
the stability of the nuclear membrane, but may also have an
important role in mediating cell differentiation (3,4).
Deficiencies in nesprin-1 or nesprin-2 protein may lead to
muscle-associated diseases, including dilated cardiomyopathy and
emery-dreifuss muscular dystrophy (5). Deficiencies may also cause
cytoskeletal reorganization and disorder of dynamic balance, which
results in a loss of cytoskeletal rigidity. The study of nesprin-1
may therefore aid the evaluation of potential therapeutic measures
for certain genetic diseases, including syndromes characterized by
a deficiency of the muscular or cardiovascular systems.
Myocardial ischemia may result in significant
myocardial dysfunction or even heart failure. Though
pharmacological and surgical treatments available may reduce tissue
ischemia, the damaged myocardial cells cannot be replaced (6). Mesenchymal stem cells (MSCs) are bone
marrow-derived cells that retain the capability to differentiate
into various types of tissue cells and contribute to the
regeneration of a variety of mesenchymal tissues, including bone,
cartilage, muscle and adipose tissue (5–7).
MSCs, transplanted into ischemic myocardial tissue, are able to
secrete a variety of factors including vascular endothelial growth
factor and therefore improve cardiac performance (8). The cardio-protective effects of MSCs
are known to be mediated by their differentiation into vascular
cells and cardiomyocyte-like cells and additionally by their
ability to supply large quantities of angiogenic, anti-apoptotic
and mitogenic factors (9–10). These results suggested that MSCs
have therapeutic potential for the treatment of heart failure.
Recently, it was reported that MSCs were able to be
differentiated into cardiomyocyte-like cells in vivo and
in vitro under specific conditions (7); however, the mechanisms underlying the
differentiation process has remained to be elucidated. The present
study aimed to investigate the expression of nesprin-1 protein and
its effects on the differentiation of rat bone-marrow MSCs in
vitro and in vivo. MSC differentiation was induced by
5-azacytidine treatment in vitro and the expression of
various structural proteins and nesprin-1 was analyzed. MSCs were
subsequently transplanted into an animal model of myocardial
infarction and the expression of structural genes and proteins was
further analyzed in vivo.
Materials and methods
Animals
Sprague-Dawley (SD) rats, weighing 250–300 g, were
obtained from the Medicine Animal Experimental Center of Shanghai
Jiao Tong University School (Shanghai, China). They were in normal
circadian, 26–27°C conditions and fed with normal feed. Production
license: scxk(hu)2004-0001, use license number: syxk(hu)2003–2009.
The experimental protocol was reviewed and approved by the
University of Shanghai Institutional Animal Care and Use Committee
(Shanghai, China). Procedures were performed in accordance with the
guidelines for animal experimentation of Shanghai Jiaotong
University, and were approved by the Shanghai Jiaotong University
Ethics Committee (Shanghai, China).
Reagents
Except where otherwise specified, all reagents were
purchased from Sigma-Aldrich (St. Louis, MO, USA) and Gibco-BRL
(Invitrogen Life Technologies, Carlsbad, CA, USA). Cell culture
mediums, low-glucose Dulbecco’s modified Eagle’s medium (DMEM) and
fetal bovine serum (FBS), were purchased from Gibco-BRL. The
cardiac-specific antibodies (cTnI, α-sarcomeric actin, actinin and
desmin), fluorescein isothiocyanate (FITC)-conjugated goat anti-rat
antibodies (CD45), phycoerythrin (PE)-conjugated rabbit anti-rat
antibodies (CD90), allophycocyanin (APC)-conjugated rabbit anti-rat
antibodies (CD29) and FITC-conjugated rabbit anti-rat nesprin-1
antibodies were purchased from Abcam (Cambridge, UK). The reagents
and instruments for immunohistochemistry, immunofluorescence and
western blot analysis were purchased from the Gibco-BRL, Abcam,
Qiagen (Santa Clara, CA, USA) and Roche Diagnostics (Mannheim,
Germany).
Cell culture
Eight-week-old SD rats (250–300 g) were used as
donors. Under general anesthesia with ether, ~100 μl bone marrow
was aspirated from the tibia and femur with a 20-gauge needle
attached to a 10-ml syringe containing 0.5 ml DMEM and 40 U/ml
heparin. Following aspiration, a cell suspension was obtained by
passing the aspirate through syringe needles of decreasing
sizes.
The concentration of cells in suspension was
adjusted to 5×105 mononuclear cells/ml DMEM,
supplemented with 20% FBS, penicillin (100 U/ml) and streptomycin
(100 μg/ml) at 37°C in humid air with 5% CO2. The cells
were subsequently seeded on culture plates, without removal of red
blood cells. BMSCs initially grow in colonies and do not reach
confluence over the entire culture dish; therefore, the cells were
first passed seven days following seeding, when half the colonies
had reached 70–80% confluence. The subsequent passages were
performed weekly, when the cells reached confluence. For
subcultures, adherent BMSCs were harvested using 0.125% trypsin and
plated at a ratio of 1:3.
For flow cytometry experiments, cells were detached
using accutase, for enhanced preservation of the cell surface
molecules.
Twenty-four hours following seeding of freshly
isolated BMSCs, 10 μmol/l 5-azacytidine was added to the culture
medium. Following incubation for a further 24 h, the BMSCs were
washed and further cultured in fresh medium that was changed every
48 h. The 5-azacytidine treatment was repeated two or three times,
depending on the specific experiment. In the control group, BMSCs
were cultured under the same conditions except that 5-azacytidine
exposure was omitted.
Labeling of MSCs
Following passage, two batches of cells became
almost confluent. Sterile DAPI solution was added to the culture
medium on the day of implantation at a final concentration of 50
mg/ml for 30 min (11). The MSCs
were subsequently rinsed six times in PBS to remove excess, unbound
DAPI. The MSCs were harvested (~1×106 cells for each
implantation) and resuspended in 50 μl serum-free DMEM.
Myocardial infarction model and stem cell
transplantation
SD rats were intubated under general anesthesia
using 4% chloral hydrate (4 mg/kg, administered intraperitoneally)
and ventilated with room air using a small animal ventilator
(Zhejiang University Apparatus, Hangzhou, China). Myocardial
infarction was induced by ligation of the left anterior descending
coronary artery 2–3 mm from the tip of the left auricle with a 7-0
silk suture (12). Successful
coronary occlusion was verified by blanching of the myocardium
distal to the coronary ligation. The sham-operation group received
the same thoracotomy procedure without coronary ligation. The rats
were divided randomly into three groups (8 rats per group):
Sham-operation group; MSC group where rats received an MSC
transplant two weeks following myocardial infarction by injection
of 1×106 cells in 50 μl DMEM directly into the infarct
border zone; and the DMEM group, where rats received an injection
of an identical volume of DMEM as the transplant subjects omitting
the MSCs.
Flow cytometric analysis
Flow cytometry was performed using a FACSAria
(Beckton-Dickinson, BD Biosciences, San Jose, CA, USA) flow
cytometer/cell sorter. Following accutase treatment, cells were
resuspended at a density of 105 cells/200 μl PBS with 2%
fetal calf serum (PBS-FCS) and incubations were performed on ice.
For each antibody used, 1×105 cells were stained. Cells
were incubated with the FITC-conjugated CD45 monoclonal antibody,
PE-conjugated CD90 monoclonal antibody or APC-conjugated CD29
monoclonal antibody (at the concentrations indicated by the
manufacturer) for 30 min at 4°C in the dark and subsequently washed
in PBS-FCS. Following washing, cells were analyzed using the flow
cytometer. A minimum of 5,000 events were analyzed for each sample.
Negative controls, used to detect unspecific binding, included an
irrelevant antibody or PBS-FCS alone. Data were analyzed using
Summit™ 5.2 software (Cytomation, Inc., Fort Collins, CO, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (0.5 μg) was isolated using the
guanidinium method (13) and was
reverse-transcribed in a 21-μl reaction mixture that contained 75
mmol/l KCl, 50 mmol/l Tris-HCl (pH 8.3), 3 mmol/l MgCl2,
0.5 mmol/l each of deoxyadenosine triphosphate (dATP),
deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate
(dGTP) and deoxythymidine triphosphate (dTTP), 600 ng random
hexamer primers, 10 mmol/l dithiothreitol, 2 U RNAse inhibitor and
10 U Superscript RNase H (Invitrogen Life Technologies) according
to the manufacturer’s instructions. 3-μl aliquots of total cDNA
were amplified (Mastercycler; Eppendorf, Hamburg, Germany) in a
25-μl reaction mixture containing 50 mmol/l KCl, 10 mmol/l Tris-HCl
(pH 8.3), 1.5 mmol/l MgCl2, 0.2 mmol/l each of dATP,
dCTP, dGTP and dTTP, 25 pmol each forward and reverse primer and
1.25 U of Taq polymerase (Applied Sangon Biotech Co., Ltd,
Shanghai, China). The same single-stranded cDNA product was used to
analyze the expression of all genes described. To assure that
amplification was in the exponential range, PCR progress was
determined by amplifying identical reaction mixtures for ascending
numbers of cycles. Following the cited number of PCR cycles, the
amplification rate was sufficient without reaching saturation for
any of the amplicons. PCR products were resolved using 2% agarose
gel electrophoresis and stained with ethidium bromide. Bands imaged
by a CCD camera (Biostep GmbH, Jahnsdorf, Germany) were analyzed
via optical densitometry with Phoretix Grabber 3.01 and Phoretix
Totallab 1.00 image processing and analying software (Biostep GmbH,
Jahnsdorf, Germany). The primers (Table I) were designed by Sangon Biotech
Co., Ltd (Shanghai, China) and the experiment was run three times.
As a control, the 530-bp band corresponding to human β-actin
transcript was amplified. α-actinin, desmin and cTnI mRNA
expression levels were calculated as the ratio of the intensity of
the corresponding band to the β-actin band by densitometry.
 | Table IPrimers used for reverse
transcription quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription quantitative polymerase chain reaction.
| Name | Primer
sequence | Length (bp) |
|---|
| α-actinin | F:
5′-TGGTCTTGGTTTCTGTGCCTTG-3′
R: 5′-CTGCTGTTTCCGCCTTCTGG-3′ | 251 |
| Desmin | F:
5′-AATGACCGCTTCGCCAACTAC-3′
R: 5′-TATCAGGTTGTCACGCTCCACG-3′ | 207 |
| Cardiac troponin
I | F:
5′-AAGCAGGAGATGGAGCGTGAG-3′
R: 5′-TCCTCCTTCTTCACCTGCTTG-3′ | 368 |
Immunohistochemical analysis
The MSCs and the cardiomyocyte-like cells that
differentiated from the MSCs adherent to chamber slides were fixed
for 10 min with methanol at −20°C. Following washing three times
with PBS, the cells were incubated at 4°C overnight with the
primary antibodies directed against cardiac-specific proteins,
including cTnI, desmin and α-sarcomeric actin. The cells were
subsequently incubated with the secondary antibodies at 37°C for 30
min, prior to incubation with diaminobenzidine (DAB) reagent for
5–10 min. Finally, the cells were mounted for microscopic
examination with neutral gum and cells exhibiting a brown granular
DAB/ H2O2 reaction product in the cytoplasm
were considered positive for the protein in question.
Immunofluorescence microscopy
BMSCs grown on glass coverslips were fixed by 20-min
incubation in 4% formaldehyde (freshly prepared from
paraformaldehyde), rinsed in PBS and stored in 70% ethanol at
−20°C. The fixed cells were blocked for 30 min in blocking solution
(PBS supplemented with 2% goat serum, 1% BSA, 0.1% gelatin, 0.1%
Triton X-100, and 0.05% Tween 10) and incubated overnight with the
primary antibody (at the dilution indicated by the manufacturer) at
4°C. Following washing, the cells were incubated with the secondary
antibody (FITC-conjugated anti-rat immunoglobulin G (IgG) for cTnI
and desmin; α-sarcomeric actin, and FITC-conjugated anti-rat IgG
for nesprin-1, respectively) for 30 min. Finally, the coverslips
were washed, mounted in glycerol and examined under an
epifluorescence microscope (Olympus Corp., Tokyo, Japan).
The subsets of animals were killed three weeks
following MSC transplantation (n=8). Following removal of the
heart, the free wall of the left ventricle, including the infarcted
and peri-infarcted regions, was embedded in tissue-frozen medium
(Thermo Fisher Scientific, Waltham, MA, USA). Frozen tissues were
sectioned onto 8-μm slides and stained with hematoxylin and eosin
(HE) (14). Survival of engrafted
cells was confirmed by identification of DAPI-positive spots under
fluorescent microscopy. Potential transdifferentiation of
myocardial-like cells from implanted MSCs was verified by antibody
immunostaining for rat cTnI and α-sarcomeric actin. Nesprin-1
protein expression was also verified by antibody immunostaining.
Briefly, frozen tissue sections were fixed in acetone at 4°C for 10
min and incubated separately with goat anti-rat cTnI, rabbit
anti-rat α-sarcomeric actin and rabbit anti-rat nesprin-1 (Abcam)
for 60 min at room temperature. Following one wash with PBS
solution, sections were incubated with secondary antibodies
(PE-conjugated IgG) for cTnI, α-sarcomeric actin and nesprin-1 and
examined under an epifluorescence microscope (Olympus BX61; Olympus
Corp.).
Identification of DAPI-labeled MSCs in
vivo following transplantation
The DAPI-labeled MSCs displayed clear nuclear and
faint cytoplasmic blue fluorescence when viewed under an
epifluorescence microscope. The labeling efficiency of cultured
MSCs with DAPI approached 100%. DAPI-labeled cells were identified
in all specimens three weeks following transplantation (eight rats
with transplanted MSCs at random). Three weeks following
engraftment, numerous scattered DAPI-labeled cells were identified
in the specimen.
Western blot analysis
Following washing with PBS, BMSCs were removed from
the culture dish and transferred to centrifuge tubes. The
cardiomyocytes of the myocardial ischemia model rats were collected
three weeks following MSC transplant (sham-operation group, DMEM
group and MSC group). Following centrifugation at 700 xg for 10 min
at 4°C, the pellets were lysed in hot Laemmli loading buffer [62.5
mmol/l Tris-HCl (pH6.8), 2% SDS, 10% glycerol, 0.05%
β-mercaptoethanol and 0.05% bromophenol blue]. Equal amounts of
protein extracts (20 μg/lane) were subjected to SDS-PAGE on a 5%
stacking gel and 10% separating gel, followed by transfer of
proteins onto a nitrocellulose membrane (20 min at 10 V). Following
blocking in PBS containing 0.05% Triton X-100 and 5% FCS for 1 h,
the blots were incubated overnight with rabbit anti-rat nesprin-1
at 4°C. Following washing, the membranes were incubated with the
secondary antibody (horseradish peroxidase-conjugated goat
anti-rabbit IgG) for 1 h and the bound antibodies were detected by
enhanced chemiluminescence (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). β-actin was used as a control.
Statistical analysis
Image programmer 5.1 software was used to analyze
images. Values are expressed as the mean ± standard deviation.
Statistical analyses were performed by paired Student’s t-tests
when applicable. Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 Demo software
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
Characterization of MSCs and
differentiated cardiomyomyte-like cells in vitro
Following discarding the non-adherent cells by the
first medium change and by washing with PBS three times at 24 h of
primary culture, ~80% MSCs had attached to culture dishes. The
medium was subsequently changed to remove the suspended
hematopoietic stem cells. Following three days of primary culture,
MSCs adhered to the plastic surface and presented a small
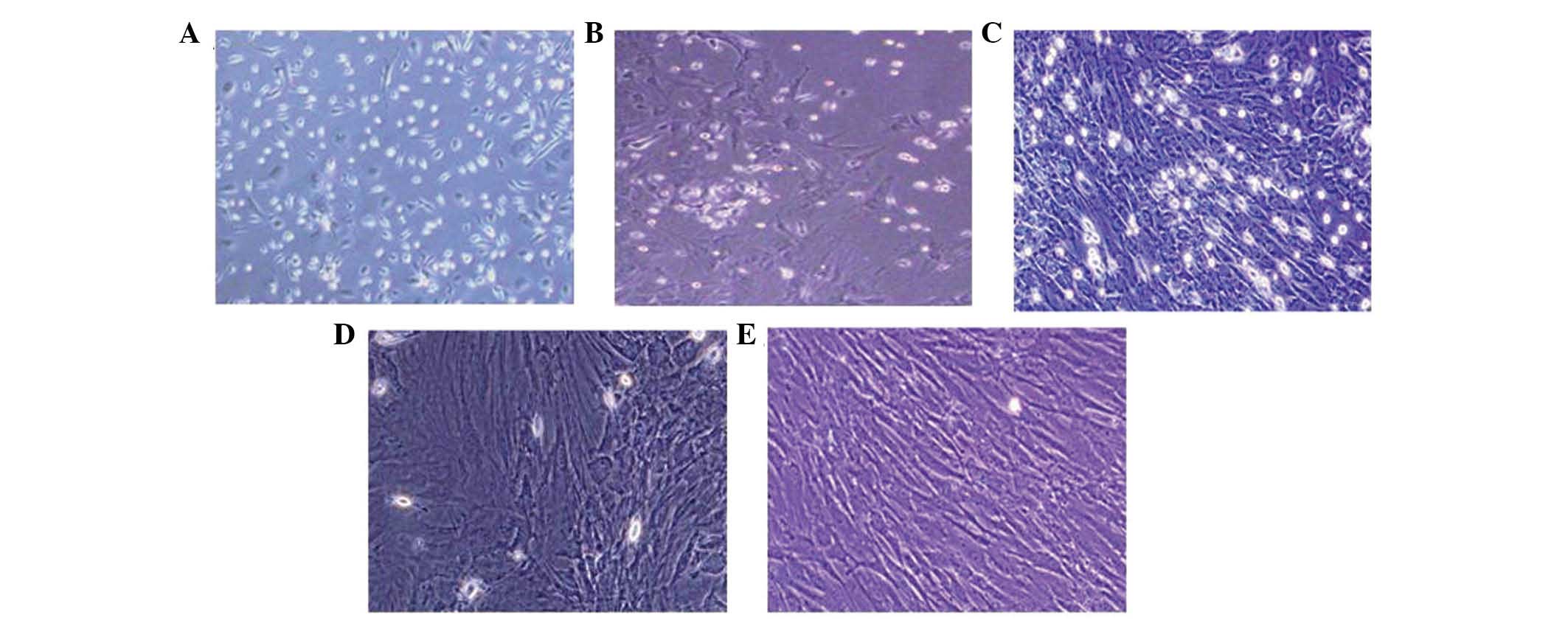
population of single cells. The cells were spindle shaped with one
nucleus (Fig. 1A). Seven to ten
days following initial plating, the cells developed into long
spindle-shaped fibroblastic cells and began to form colonies
(Fig. 1B and C). Following
replating, 100% of the cells had attached to the culture dishes and
were polygonal or spindle-shaped, with long processes.
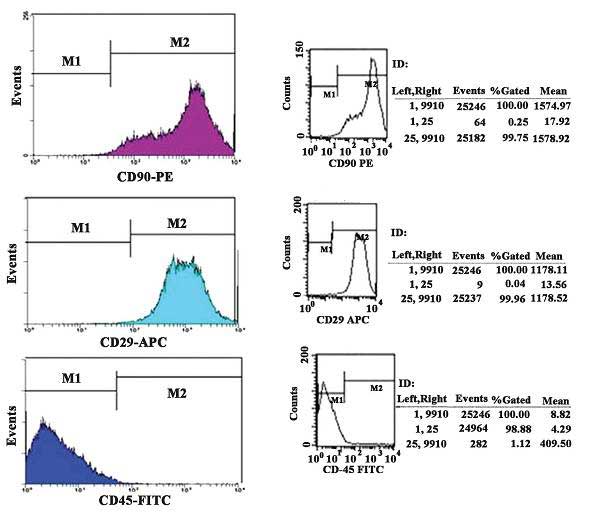
Rat MSC surface antigen profiles obtained by flow
cytometry (Fig. 2) were positive
for CD90 and CD29 and negative for CD45. The percentages of CD90-
and CD29-positive cells were 99.96 and 99.75%, respectively,
whereas the percentage of CD45-positive cells was 1.12%.
The morphological differentiation from MSCs to
cardiomyomytes-like cells developed gradually following
5-azacytidine induction. During exposure to 5-azacytidine, certain
adherent cells died and the surviving cells began to proliferate
and differentiate. One week later, ~30% of the remaining adherent
cells had enlarged and assumed ball-like or stick-like
morphologies. Two weeks later, the cells were observed to be
connected with adjoining cells (Fig.
1D and E).
Identification of myocardial infarction
and engrafted MSCs
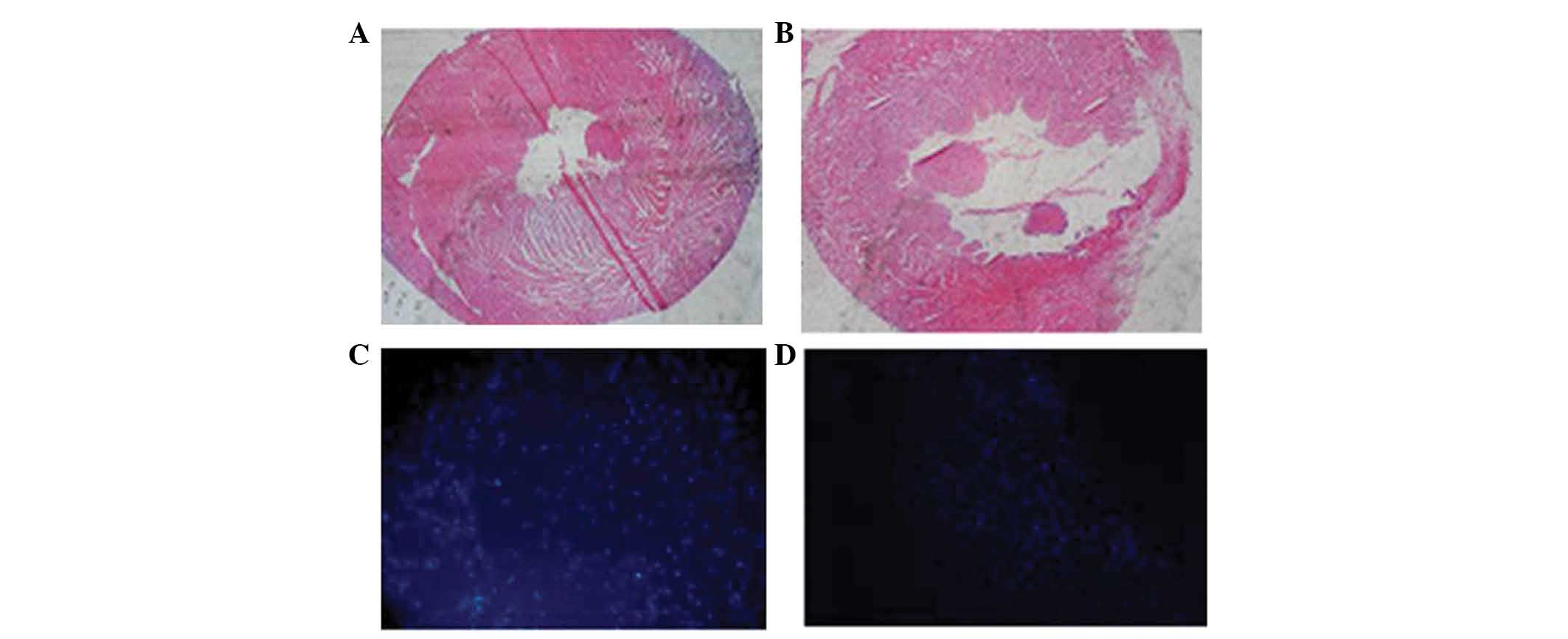
HE staining of cardiac tissue obtained three weeks
following myocardial infarction revealed fibrosis of the infarct
region in comparison to normal cardiac tissue (Fig. 3A and B). A greater number of
DAPI-positive cells were detected among the MSCs prior to
transplantation (Fig. 3C) than
that in the transplantation groups three weeks following
transplantation (Fig. 3D), which
may be due to fluorescence decay. Fig.
3D indicates engrafted cells in the ischemic myocardium, which
demonstrated that implanted cells were able to survive in the
peri-infarct region for three weeks post-transplantation.
5-azacytidine induces expression of
cardiac structural proteins and mRNA in MSCs
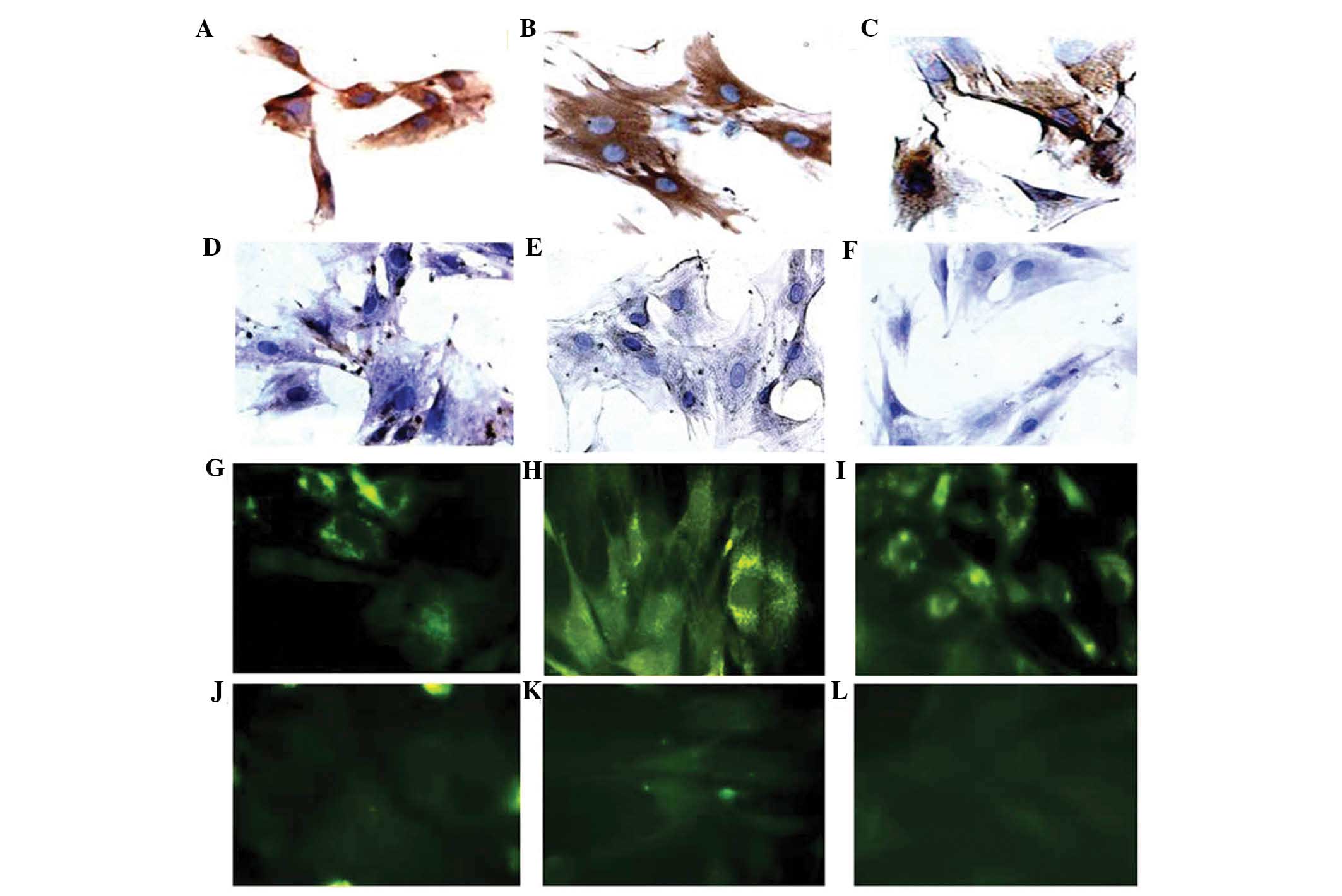
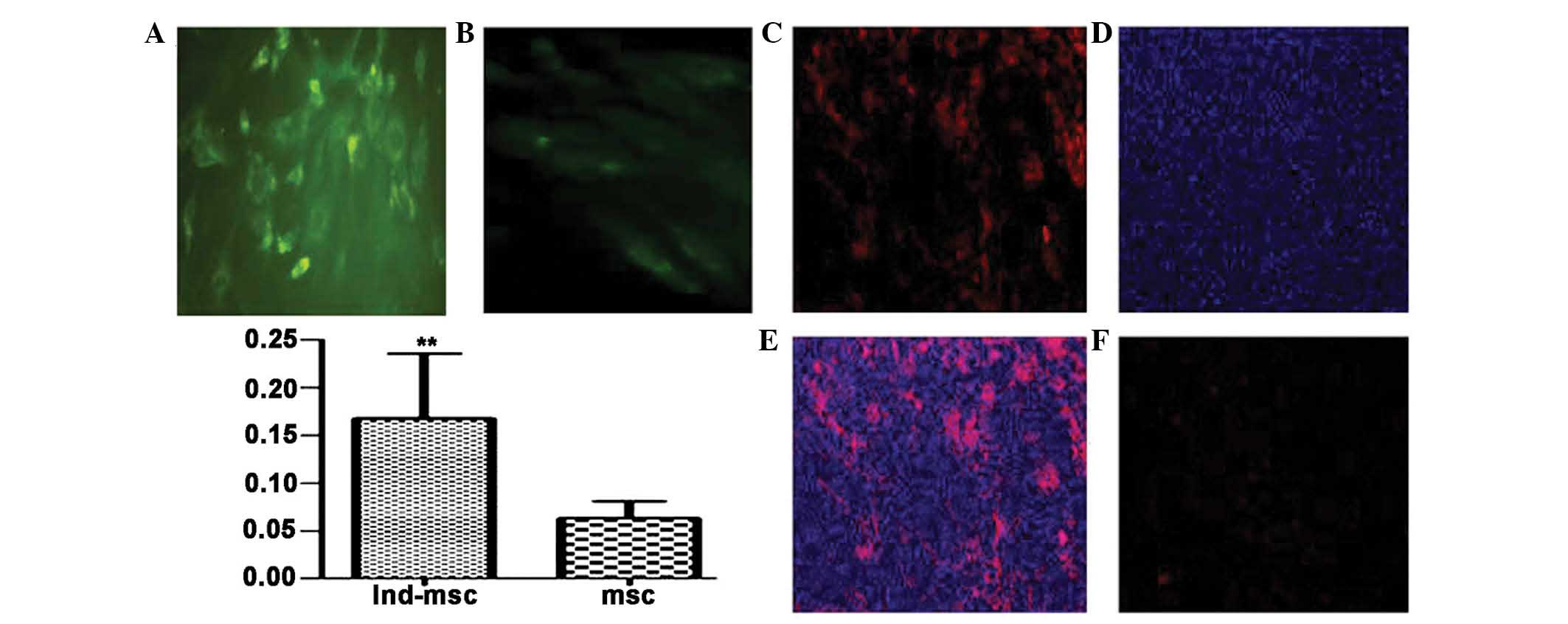
Immunohistochemistry and immunofluorescence assays
for cTnI, desmin and α-sarcomeric actin were performed four weeks
following MSC exposure to 5-azacytidine in vitro (Fig. 4). Untreated controls were also
analyzed to confirm that there were no changes in the expression of
markers of myogenic or cardiac differentiation, including the three
structural proteins. Treatment of MSCs for four weeks with 10
μmol/l 5-azacytidine induced differentiation into
cardiomyocyte-like cells as indicated by the expression of cTnI,
actinin and desmin genes (Fig. 5).
In the untreated control cells no expression of desmin, cTnI or the
cardiac isoform of actinin encoded by ACTN-2 was detected
(Fig. 5).
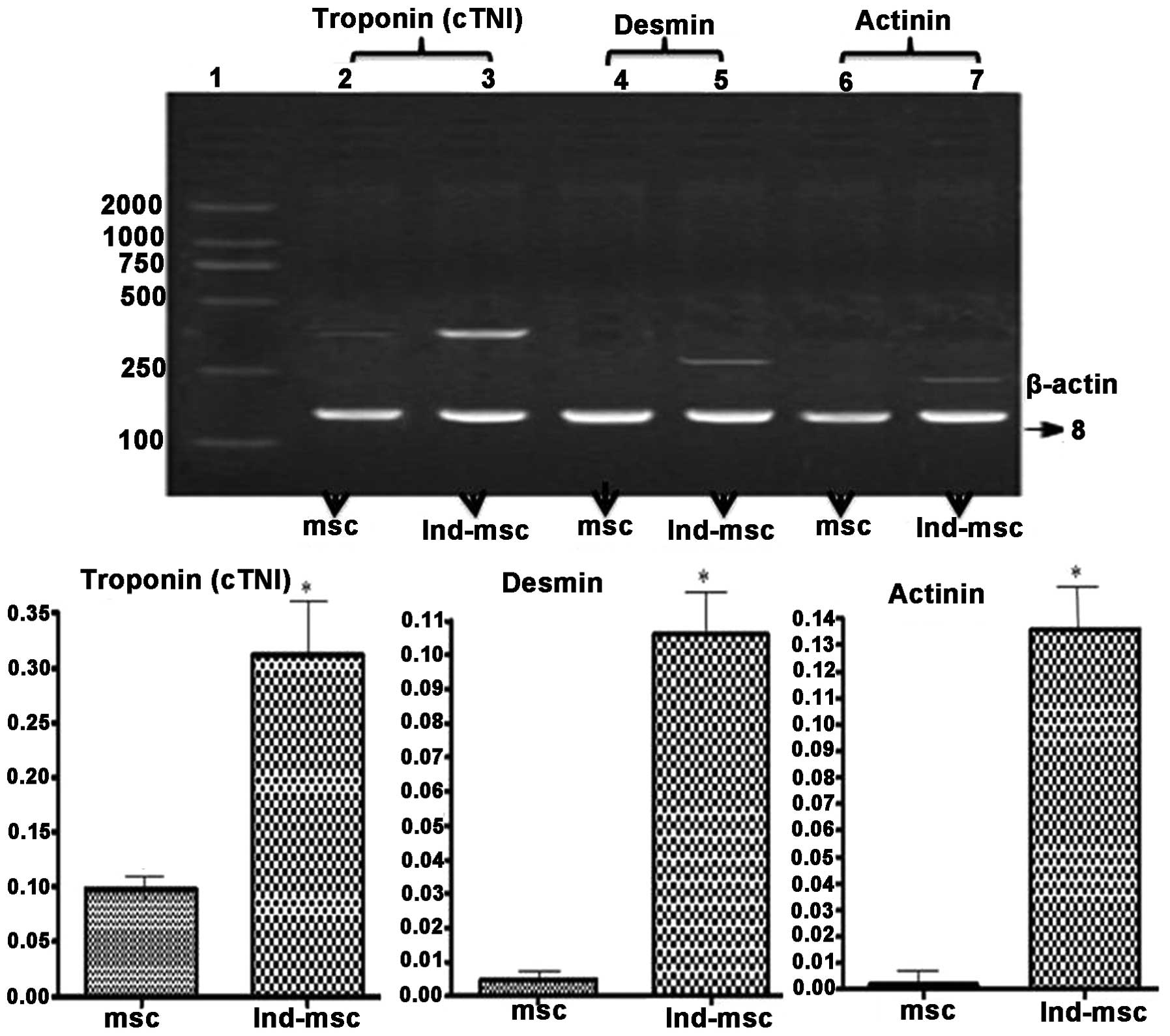 | Figure 5Effects of 5-azacytidine on the
expression of cardiac structural protein gene expression in MSCs.
Lanes: 1, marker; 2, 4 and 6, cTnI, desmin and actinin in untreated
MSCs, respectively; 3, 5 and 7, cTnI, desmin and actinin of MSCs
treated with 5-azacytidine, respectively. The number 8 indicates
the β-actin band. MSCs induced by 5-azacytidine were positive for
the expression of cTnI, desmin and actinin genes. The expression
levels of cardiac structural protein genes were significantly
higher in 5-azacytidine induced MSCs than those in untreated MSCs.
P=0.018 (cTnI), P=0.009 (desmin), P=0.007 (actinin) vs. untreated
MSCs; *P<0.05 vs. the untreated MSC group. MSCs,
mesenchymal stem cells; cTnI, cardiac troponin I; Ind-msc,
5-azacytidine-treated MSCs. |
MSC transplantation increases the
expression levels of cTnI and α-sarcomeric actin proteins in an
ischemic environment
The expression of cTnI and α-sarcomeric actin
proteins were examined in the myocardial infarction zone in
vivo, three weeks following MSC transplant. The expression
levels of cTnI and α-sarcomeric actin proteins were markedly higher
in the MSC group compared with those of the DMEM group (Fig. 6).
5-azacytidine increases nesprin-1
expression levels in MSCs in vitro and MSC transplantation
increases nesprin-1 expression levels in vivo
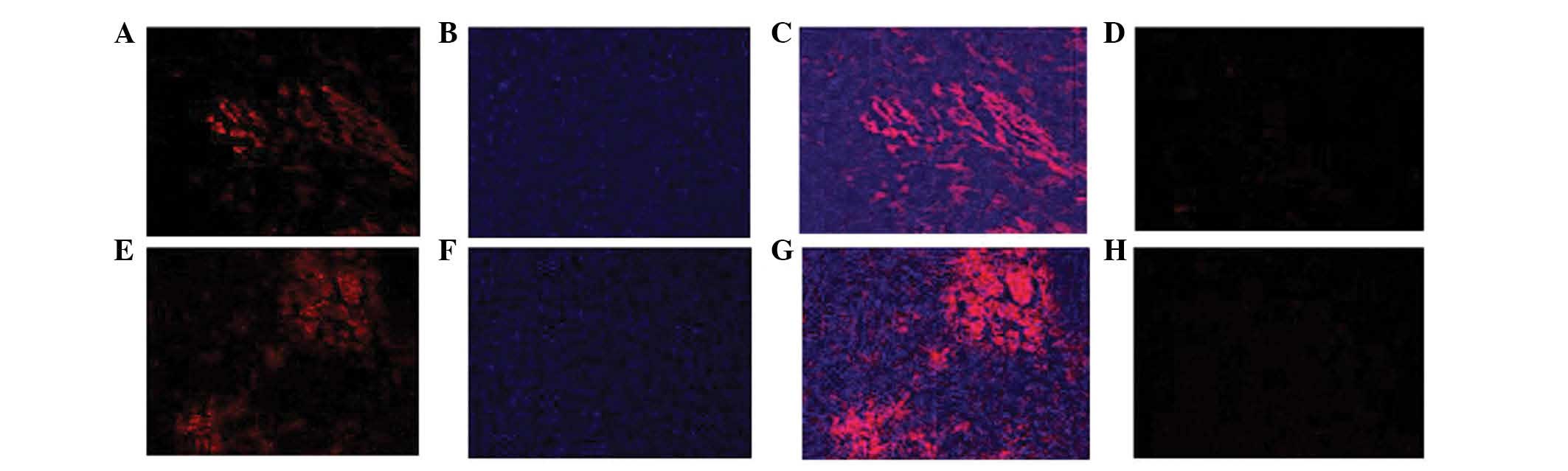
Immunofluorescent staining for nesprin-1 protein
expression verified the presence of the transplanted rat MSCs
(Fig. 7A and B). Nesprin-1 protein
expression levels were significantly higher in the MSCs treated
with 10 μmol/l 5-azacytidine in vitro for four weeks than
those in the untreated MSCs.
The results displayed in Fig. 7C and E indicated that nesprin-1
protein expression levels were markedly higher in the MSC group in
comparison with those in the control group. The expression of
nesprin-1 protein in the myocardial infarction zone was detected by
immunofluorescence three weeks following MSC transplantation.
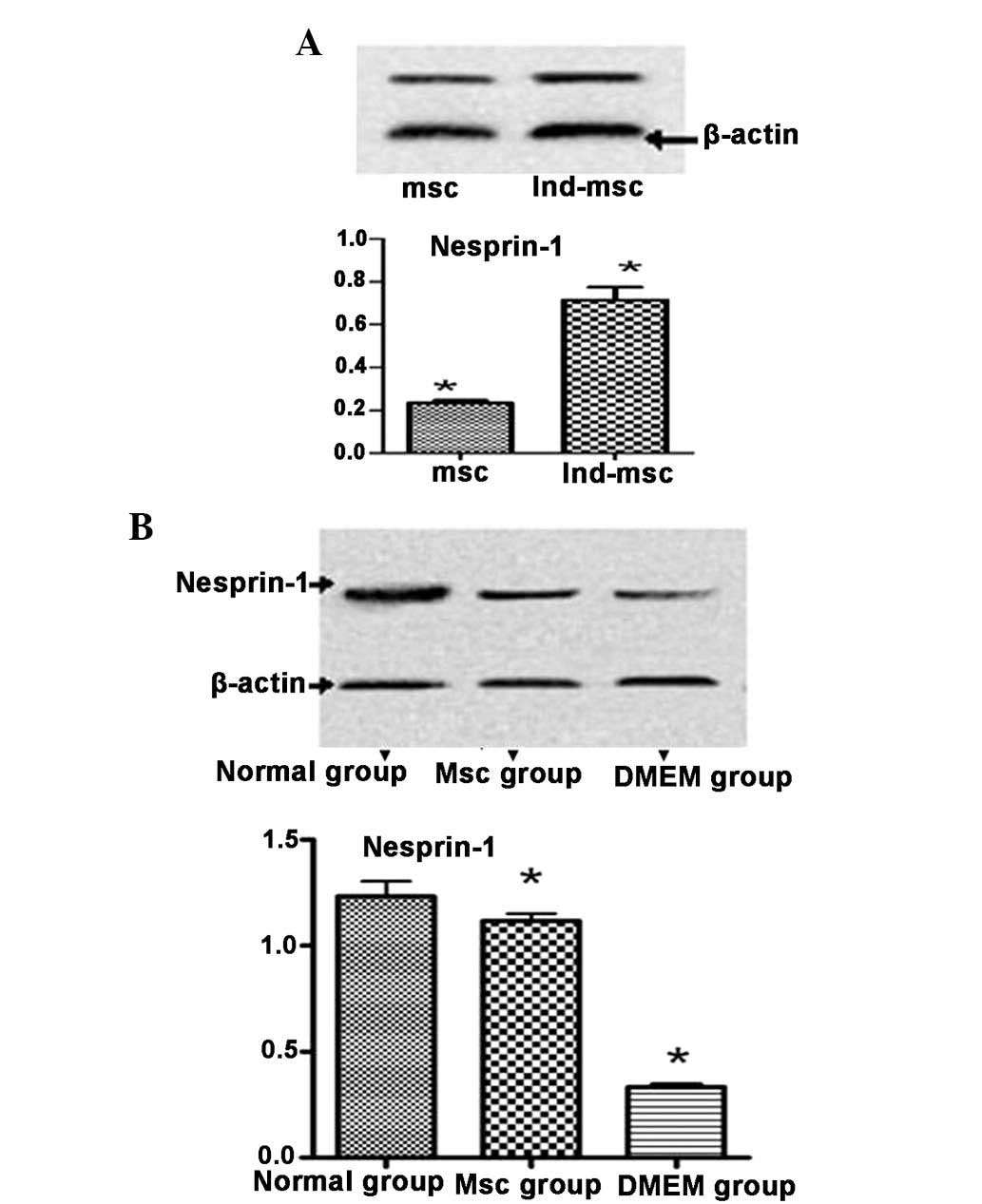
Nesprin-1 protein expression indicates
MSC differentiation
Nesprin-1 protein expression levels were higher in
the MSC group than those in the DMEM control group, but lower than
those in the normal group (Fig.
8B). Treatment of MSCs for four weeks with 10 μmol/l
5-azacytidine induced their differentiation into cardiomyocyte-like
cells, confirmed by the significantly higher expression levels of
nesprin-1 protein in the 5-azacytidine-treated MSCs compared with
those in the untreated MSCs.
Discussion
MSCs were first described in 1968 by Friedenstein
et al (15). These cells
can be expanded ex vivo and induced, either in vitro
or in vivo, to terminally differentiate into osteoblasts,
chondrocytes, adipocytes, tenocytes, myotubes, neural cells and
hematopoietic cells with strong self-renewal ability and genetic
stability in vitro (6).
Several studies reported that MSCs were able to proliferate and
potentially differentiate in vitro (16–18).
However, the ratio of MSCs in bone-marrow is only ~0.001–0.01%.
Hence, the separation and amplification of MSCs is of vital
importance. Wakitani et al (19) described a method to isolate MSCs
from rat bone marrow using Ficoll density gradient separation and
adherent culture. The International Society for Cellular Therapy
proposed three minimal criteria to identify MSCs: i) MSCs
must be plastic-adherent if maintained in standard culture
conditions; ii) MSCs must express CD105, CD73 and CD90, but
lack haematopoietic markers, including CD45, CD34, CD14 or CD11b,
and iii) MSCs must be capable of differentiating into
fibroblasts, osteoblasts, adipocytes and chondroblasts under the
corresponding lineage, particularly under in vitro
conditions (20). The MSC surface
antigens CD90 and CD29 were detected by flow cytometry; the
percentage of CD90 and CD29 detected was ~98%, whereas the
percentage of CD45 was ~1%.
Xu et al (21) reported that the ability of human
MSCs to proliferate remained strong between passages two and six.
Therefore, second-passage rat MSCs were selected for the present
study, to investigate whether these cells were able to
differentiate into cardiomyocyte-like cells in vitro
following 5-azacytidine treatment. Makino et al (22) and Toma et al (23) reported that following 5-azacytidine
treatment, rat MSCs differentiated into cardiomyocyte lineages
in vivo and in vitro. These MSCs developed into
cardiomyocyte-like cells, which expressed the cardiac myocyte
markers, myosin heavy chain and troponin T in cardiomyocyte medium
subjected to hypoxia re-oxygenation and treatment with hepatocyte
growth factor, bone morphogenetic protein 2 and fibroblast growth
factor 4 (24–26). Li et al (27) reported a localization of cardiac
troponin T (cTnT) in DAPI-labeled B-cell lymphoma-2-transduced MSCs
in a rat model of irreversible ligation of the left anterior
descending coronary artery, indicating differentiation towards
cardiomyocyte-like cells. In the present study, 10 μmol/l
5-azacytidine was used to induce rat MSCs to differentiate into
cardiomyocyte-like cells in vitro, which led to the adherent
cells enlarging and assuming ball-or stick-like morphologies. Four
weeks later, the expression of cTnI, actinin and desmin genes was
detected in cardiomyocyte-like cells by RT-qPCR. Expression of the
proteins cTnI, α-sarcomeric actin and desmin was also identified by
immunofluorescent staining.
Hu et al (28) reported that implanted MSCs were
able to survive in the peri-infarct region for ≥four weeks
post-transplantation when the MSCs were traced using DAPI. The
greatest number of DAPI-positive cells was detected in the
myocardium and the greatest functional benefit was observed when
MSCs were transplanted one week following myocardial infarction. In
the present study, DAPI was applied to label and trace MSCs which
were engrafted two weeks following myocardial infarction. Three
weeks following MSC transplant into the site of myocardial
infarction, expression of cTnI and α-sarcomeric actin was
detected.
Although positive results have been obtained in
cell-based therapies to treat myocardial infarction, the underlying
mechanisms have remained to be elucidated. Therefore, in the
present study the expression of nesprin-1 protein prior to and
following MSC differentiation was also examined. The results
revealed that nesprin-1 protein expression was higher in
cardiomyocyte-like cells than that in undifferentiated MSCs.
Nesprins are a family of proteins that bind to the
nuclear envelope (NE) and interact with emerin and lamin A/C
(2,4,29,30).
The structure of nesprin isoforms suggests that they form a protein
scaffold linking the NE to the nucleus, cytoplasmic organelles and
cell membrane via the actin cytoskeleton (29,30).
These studies suggested a role for nesprin in the structural
organization of the sarcomere and signaling between the
extracellular environment and nucleus (31,32).
Gough et al (33) concluded
that the Syne-1 (nesprin-1) gene was expressed in a variety of
forms that are multifunctional and capable of functioning at the
Golgi and the NE, including linkage of the two organelles during
muscle differentiation.
High expression levels of nesprin-1 were observed in
skeletal, cardiac and vascular smooth muscle. Zhang et al
(2) suggested that nesprin-1 may
have specific functions in muscle cell differentiation; however,
high expression levels of nesprin-1 were detected in peripheral
blood leukocytes and the spleen. Nesprin-1 is highly expressed in
muscle tissue and has muscle-specific isoforms (2,4,30).
During in vitro differentiation of C2C12 myoblasts into
myotubes, nesprin-1 localization shifts from the nuclei/NE to the
cytoplasm/sarcomere, indicating a specific role in muscle
differentiation (2,4). In the sarcomere of skeletal muscle
cells, various nesprin-1 epitopes are associated with the Z-line,
A/I junction, sarcoplasmic reticulum and mitochondrial membrane,
indicating that nesprin-1 may contribute to sarcomeric structure
maintenance (4,34). Furthermore, sarcomeric proteins
have been identified as potential interaction partners for
nesprin-1 and −2, including the ryanodine receptor and
muscle-specific A-kinase anchoring protein (mAKAP). mAKAP is
targeted to the NE by nesprin-1 and they interact through their
closely associated sarcoplasmic reticulums. Nesprins may
potentially be involved in maintaining and targeting protein
complexes common to the NE and the sarcoplasmic reticulum (4,35). A
study demonstrated that cardiomyocyte nuclei were elongated with
reduced heterochromatin in Delta/DeltaKASH mouse hearts (36). These findings reflected the results
of previous studies on lamin A/C gene mutations and therefore
reinforced the importance of an intact nuclear membrane complex for
a regularly functioning heart (36). During the development of immature
to mature muscle fibers in vivo, nesprin-2 was partially
replaced by nesprin-1 at the NE and short nesprin isoforms became
dominant. In emerin-negative skin fibroblasts, nesprin-2-giant was
relocated from the NE to the cytoplasm, while nesprin-1 remained at
the NE (37).
Nesprin-1 may therefore have a key function in
addition to its characterized roles in cell mitosis, RNA copy of
transport and the stability of the nuclear membrane. Nesprin-1 may
also have a significant role in cell differentiation. In the
present study, it was revealed that the expression levels of
nesprin-1 protein were higher in the infarcted myocardium implanted
with MSCs than those of the control group. In conclusion, it was
hypothesized that nesprin-1 had an important role in mediating the
differentiation of MSCs into cardiomyocyte-like cells. These
results may provide an experimental base from which to improve
cell-based therapies for the treatment of myocardial ischemia.
Acknowledgements
This study was supported by a grant from the Fund of
the School Medicine, Shanghai Jiao Tong University, Shanghai, China
(no. 13XJ10014).
References
|
1
|
Zhang Q, Skepper JN, Yang F, et al:
Nesprins: a novel family of spectrin-repeat-containing proteins
that localize to the nuclear membrane in multiple tissues. J Cell
Sci. 114:4485–4498. 2001.PubMed/NCBI
|
|
2
|
Apel ED, Lewis RM, Grady RM and Sanes JR:
Syne-1, a dystrophin- and Klarsicht-related protein associated with
synaptic nuclei at the neuromuscular junction. J Biol Chem.
275:31986–31995. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lammerding J, Schulze PC, Takahashi T, et
al: Lamin A/C deficiency causes defective nuclear mechanics and
mechanotransduction. J Clin Invest. 113:370–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Ragnauth CD, Skepper JN, et al:
Nesprin-2 is a multi-isomeric protein that binds lamin and emerin
at the nuclear envelope and forms a subcellular network in skeletal
muscle. J Cell Sci. 118(Pt 4): 673–687. 2005. View Article : Google Scholar
|
|
5
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520.
2001.PubMed/NCBI
|
|
7
|
Devine SM: Mesenchymal stem cells: will
they have a role in the clinic? J Cell Biochem Suppl. 38:73–79.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagaya N, Fujii T, Iwase T, et al:
Intravenous administration of mesenchymal stem cells improves
cardiac function in rats with acute myocardial infarction through
angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol.
287:H2670–H2676. 2004. View Article : Google Scholar
|
|
9
|
Miyahara Y, Nagaya N, Kataoka M, et al:
Monolayered mesenchymal stem cells repair scarred myocardium after
myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chedrawy EG, Wang JS, Nguyen DM, et al:
Incorporation and integration of implanted myogenic and stem cells
into native myocardial fibers: anatomic basis for functional
improvements. J Thorac Cardiovasc Surg. 124:584–590. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min JY, Sandmann S, Meissner A, et al:
Differential effects of mibefradil, verapamil, and amlodipine on
myocardial function and intracellular Ca(2+) handling in rats with
chronic myocardial infarction. J Pharmacol Exp Ther. 291:1038–1044.
1999.PubMed/NCBI
|
|
13
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martina JD1, Simmons C and Jukic DM:
High-definition hematoxylin and eosin staining in a transition to
digital pathology. J Pathol Inform. 2:452011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI, et al: Heterotopic transplants of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dennis JE and Charbord P: Origin and
differentiation of human and murine stroma. Stem Cells. 20:205–214.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campagnoli C, Roberts IA, Kumar S, et al:
Identification of mesenchymal stem/progenitor cells in human
first-trimester fetal blood, liver, and bone marrow. Blood.
98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin DR, Cox NR, Hathcock TL, et al:
Isolation and characterization of multipotential mesenchymal stem
cells from feline bone marrow. Exp Hematol. 30:879–886. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
21
|
Xu W, Zhang X, Qian H, et al: Mesenchymal
stem cells from adult human bone marrow differentiate into a
cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood).
229:623–631. 2004.PubMed/NCBI
|
|
22
|
Makino S, Fukuda K, Miyoshi S, et al:
Cardiomyocytes can be generated from marrow stromal cells in vitro.
J Clin Invest. 103:697–705. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toma C, Pittenger MF, Cahill KS, et al:
Human mesenchymal stem cells differentiate to a cardiomyocyte
phenotype in the adult murine heart. Circulation. 105:93–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon J, Min BG, Kim YH, et al:
Differentiation, engraftment and functional effects of pre-treated
mesenchymal stem cells in a rat myocardial infarct model. Acta
Cardiol. 60:277–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie XJ, Wang JA, Cao J and Zhong X:
Differentiation of bone marrow mesenchymal stem cells induced by
myocardial medium under hypoxic conditions. Acta Pharmacol Sin.
27:1153–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forte G, Minieri M, Cossa P, et al:
Hepatocyte growth factor effects on mesenchymal stem cells:
proliferation, migration, and differentiation. Stem Cells.
24:23–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Ma N, Ong LL, et al: Bcl-2
engineered MSCs inhibited apoptosis and improved heart function.
Stem Cells. 25:2118–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu X, Wang J, Chen J, et al: Optimal
temporal delivery of bone marrow mesenchymal stem cells in rats
with myocardial infarction. Eur J Cardiothorac Surg. 31:438–443.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mislow JM, Holaska JM, Kim MS, et al:
Nesprin-1alpha self-associates and binds directly to emerin and
lamin A in vitro. FEBS Lett. 525:135–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mislow JM, Kim MS, Davis DB, et al:
Myne-1, a spectrin repeat transmembrane protein of the myocyte
inner nuclear membrane, interacts with lamin A/C. J Cell Sci.
115(Pt 1): 61–70. 2002.PubMed/NCBI
|
|
31
|
Starr DA and Han M: Role of ANC-1 in
tethering nuclei to the actin cytoskeleton. Science. 298:406–409.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Starr DA and Han M: ANChors away: an actin
based mechanism of nuclear positioning. J Cell Sci. 116(Pt 2):
211–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gough LL, Fan J, Chu S, et al: Golgi
localization of Syne-1. Mol Biol Cell. 14:2410–2424. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Q, Ragnauth C, Greener MJ, et al:
The nesprins are giant actin-binding proteins, orthologous to
Drosophila melanogaster muscle protein MSP-300. Genomics.
80:473–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pare GC, Easlick JL, Mislow JM, et al:
Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac
myocyte nuclear envelope. Exp Cell Res. 303:388–399. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Puckelwartz MJ, Kessler EJ, Kim G, et al:
Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell
Cardiol. 48:600–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Randles KN, Lam le T, Sewry CA, et al:
Nesprins, but not sun proteins, switch isoforms at the nuclear
envelope during muscle development. Dev Dyn. 239:998–1009. 2010.
View Article : Google Scholar : PubMed/NCBI
|