Introduction
Lung cancer is one of the most common causes of
cancer-related mortalities in the world (1,2).
Lung adenocarcinoma (LAC) is one of the most common histological
types of this disease, and its incidence has been gradually
increasing worldwide (3, 4). Patients with LAC often experience
chronic physical and psychological symptoms, including physical
impairment, and the majority of patients succumb to the disease
within 2 years (5). Therefore,
there is an emergent requirement for novel interventions for the
treatment of LAC.
CD151 is a member of the tetraspanin or
transmembrane-four superfamily (TM4SF), which was first discovered
in a megakaryocyte cell line (MO7e) (6). It is frequently highly expressed in
tumor cells. Regulating cell progression and movement is the most
prominent feature of this protein (7–9). A
number of previous studies have demonstrated that CD151 has a key
role in cell proliferation, migration, colony formation and
signaling transduction(10–12).
Increased CD151 protein expression is associated with a poor
prognosis in a number of different types of cancer. It has been
shown that CD151 mediates positive effects on
pro-carcinogenesis(13–15) and previous studies have indicated
that inhibiting CD151 expression may significantly reduce cancer
cell progression (16,17). In benign prostatic hyperplasia, the
level of CD151 expression has been reported to be very low, however
the opposite has been observed in prostate cancer (14,18).
In addition, previous studies have detected that the expression
level of CD151 was positively correlated with tumor progression,
degree of tumor differentiation and clinical prognosis of lung
cancer (13,19). A positive correlation was also
found in hepatocarcinoma, prostate cancer and colon cancer,
demonstrating that CD151 may function in tumor progression and
metastasis. An earlier study from our group had shown that the
capacity of migration was significantly upregulated in prostate
cancer cells that were transfected with CD151. This effect could be
inhibited by mutations to CD151 (20). Thus, tetraspanin, and especially
the critical role of CD151, may be a potential target for gene
therapy in human LAC.
RNA interference (RNAi) refers to the degradation of
homologous mRNA induced by double-stranded small interfering RNA,
which is highly conserved throughout evolution. Through the use of
RNAi technology it is possible specifically remove or turn off the
expression of specific genes. RNAi technology has been used widely
in recent years to explore gene function and the treatment of
infectious diseases and cancer (21,22).
As such, RNAi has broad applications in the treatment of
disease.
In the present study, siRNA was used to silence
endogenous CD151 expression in A549 cells, to perturb the
expression of downstream signaling molecules and the activation of
specific cytokines. The experiments of this study analyzed the
proliferation, migration, invasion and colony formation capacity of
CD151-deficient A549 cells as well as the level of apoptosis. In
addition, an investigation into the molecular mechanisms by which
CD151 knockdown induces anti-tumor effects was performed. An
understanding of the functional mechanism of CD151 in LAC may
provide a novel approach for gene therapy in this disease.
Materials and methods
Materials
The human LAC cell line, A549, was purchased from
the China Center for Type Culture Collection (Wuhan, China). Fetal
bovine serum (FBS), cell culture medium, and TRIzol®
reagent were purchased from Gibco-BRL (Carlsbad, CA, USA).
Lipofectamine™ 2000 reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA) for transfection of siRNAs
obtained from Genepharma (Shanghai, China). MTT was purchased from
Sigma-Aldrich. (St. Louis, MO, USA). The prestained molecular
weight standards were obtained from Bio-Rad (Hercules, CA, USA),
and polyvinylidene difluoride (PVDF) membranes from Schleicher and
Schuell GmbH (Dassel, Germany). Borden chamber plates (Costar
Corning, Cambridge, MA, USA) were prepared for the Matrigel
invasion assay. The AnnexinV/fluorescein isothiocyanate (FITC)
apoptosis detection kit was purchased from KeyGen Biotech Co.
(Nanjing, China). Antibodies against CD151, PI3K, p-FAK, p-Akt,
p-Erk1/2, p-MEK, VEGF, MEK, MMP-2, MMP-9 and β-actin were bought
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Antibodies against FAK and Akt were obtained from Cell Signaling
Technology (Beverly, MA, USA). Enhanced chemiluminescence (ECL)
reagent was purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA). All other reagents were obtained from standard commercial
suppliers unless otherwise specified.
Cell line and cell culture
The A549 cells were cultured in RPMI-1640 medium,
supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml
streptomycin and incubated at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air. Throughout the
experiments, the cells were used in the logarithmic phase of
growth.
Transfection of synthetic siRNA
The A549 cells were cultured in 6-well plates for 24
h. Once the cells reached an 80% density/well, they were
transfected with either 50, 75, and 100 nm siRNA using
Lipofectamine 2000 reagent, according to the manufacturer’s
instructions. The cells were harvested 24 h after transfection for
analyses. A549 cells that had been either untreated or treated only
with Lipofectamine 2000 reagent were used as controls.
Proliferation and apoptosis assay
The A549 cells were transfected with siRNA in 6-well
plates, using Lipofectamine 2000. The cells were trypsinized 24 h
following transfection, and seeded in 96-well plates in triplicate
(1×104 cells/well). Following adherence, the cells were
exposed to RPMI-1640 medium with 0.5% FBS for 24 h, and then the
effects of downregulated CD151 on A549 cell proliferation was
evaluated by MTT colorimetric assay. The medium was removed and
replaced with medium containing 5 mg/ml MTT and then incubated for
4 h. The medium was aspirated, and the product was solubilized
using dimethyl sulfoxide. Finally, the absorbance (A) was
measured at 490 nm for each well using a microplate reader (Bio Tek
Instruments Inc., Winooski, VT, USA) according to the
manufacturer’s instructions. To measure the effects on apoptosis,
cells were harvested 24 h after transfection and resuspended in
binding buffer. FITC-conjugated Annexin V and propidium iodide (PI)
(KeyGen Biotech Co.) was added to the cells, and all samples were
incubated for 15 min at room temperature. Cells were then analyzed
using a FACStar-Plus flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) to determine the percentage of apoptotic cells. The
results were calculated using the percentage of apoptotic cells by
the percentage of cells in the upper-right quadrant (Annexin
V-positive, PI-positive) plus cells in the lower right quadrant
(Annexin V-positive, PI-negative) to the total number of cells.
Western blotting
The A549 cells were incubated with
radioimmunoprecipitation assay (RIPA) buffer containing 50 mmol/l
Tris-HCl (pH 8.0), 0.5% deoxycholic acid, 1% Nonidet-P40, 150
mmol/l NaCl and 0.1% sodium dodecyl sulfate for 30 min on ice. The
cells were then detached from the plates using a cell scraper. The
concentration of protein from the lysates of each group was
obtained. Subsequently, 40 μg protein was separated by SDS-PAGE and
transferred to a PVDF membrane. The PVDF membranes were blocked for
2 h at room temperature with 5% fat-free dried milk in
tris-buffered saline with tween (10 mm Tris-HCl, 100 mm NaCl, and
0.1% Tween-20). The membranes were then incubated with primary
antibodies overnight at 4°C, followed by secondary antibodies
against rabbit or mouse IgG conjugated to horseradish peroxidase
(1:3000) for 2.5 h at room temperature. Finally, ECL was used to
detect the intensities of the various protein bands, which were
quantified by densitometry. β-actin FAK, t-AKT, MEK and ERK were
used as loading controls.
Migration and invasion assay
A549 cells were transfected with siRNAs in 6-well
plates. Following 24 h transfection, when the cells reached 90%
confluency/well, the cells were scratched with a 200 μl pipette
tip. The plates were then washed twice with phosphate-buffered
saline to remove the detached cells and incubated in FBS-free
medium. Cells that migrated into the wounded area were imaged
immediately (0 h) and at 24 h. The invasion assay was performed
using a transwell chamber with 8 μm pore size polycarbonate
membrane (Costar Corning), using serum-free RPMI-1640 media diluted
extracellular matrix (ECM) to coat the membrane. The A549 cells
transfected with siRNA were trypsinized and seeded in the upper
compartment of the upper wells at a density of 1×104
cells/well in 200 μl of the serum-free media. The lower chamber was
filled with media containing 20% FBS. Subsequently, the cells were
able to invade the membrane for 24 h. Following this, the
non-migrated cells were removed from the upper surface of the
polycarbonate membrane, and the invaded cells were stained with
gentian violet. Invasion analysis was determined by counting the
invaded cells under a microscope. Six visual fields were chosen at
random from each of the weels and the number of the invaded cells
in the six fields was averaged.
Clonogenic assay
Cells were seeded in 6-well plates at a
concentration of 200–300 cells/well. Following transfection for 24
h, the cells were incubated for 14 days at 37°C in a humidified
atmosphere of 5% CO2. The colonies were fixed in 4%
paraformaldehyde at room temperature for 30 min, stained with 0.1%
crystal violet for 10 min. At the end of the experiment, the
positive colonies formed (>50 cells/colony) were counted and the
colony formation rate was calculated. This experiment was repeated
three times.
Statistical analysis
All the experiments were repeated at least three
times, and the results are expressed as the mean ± standard error
of the mean. Comparisons between the two groups were performed by
Fisher t-test and those among three groups were followed by
one-way analysis of variance. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
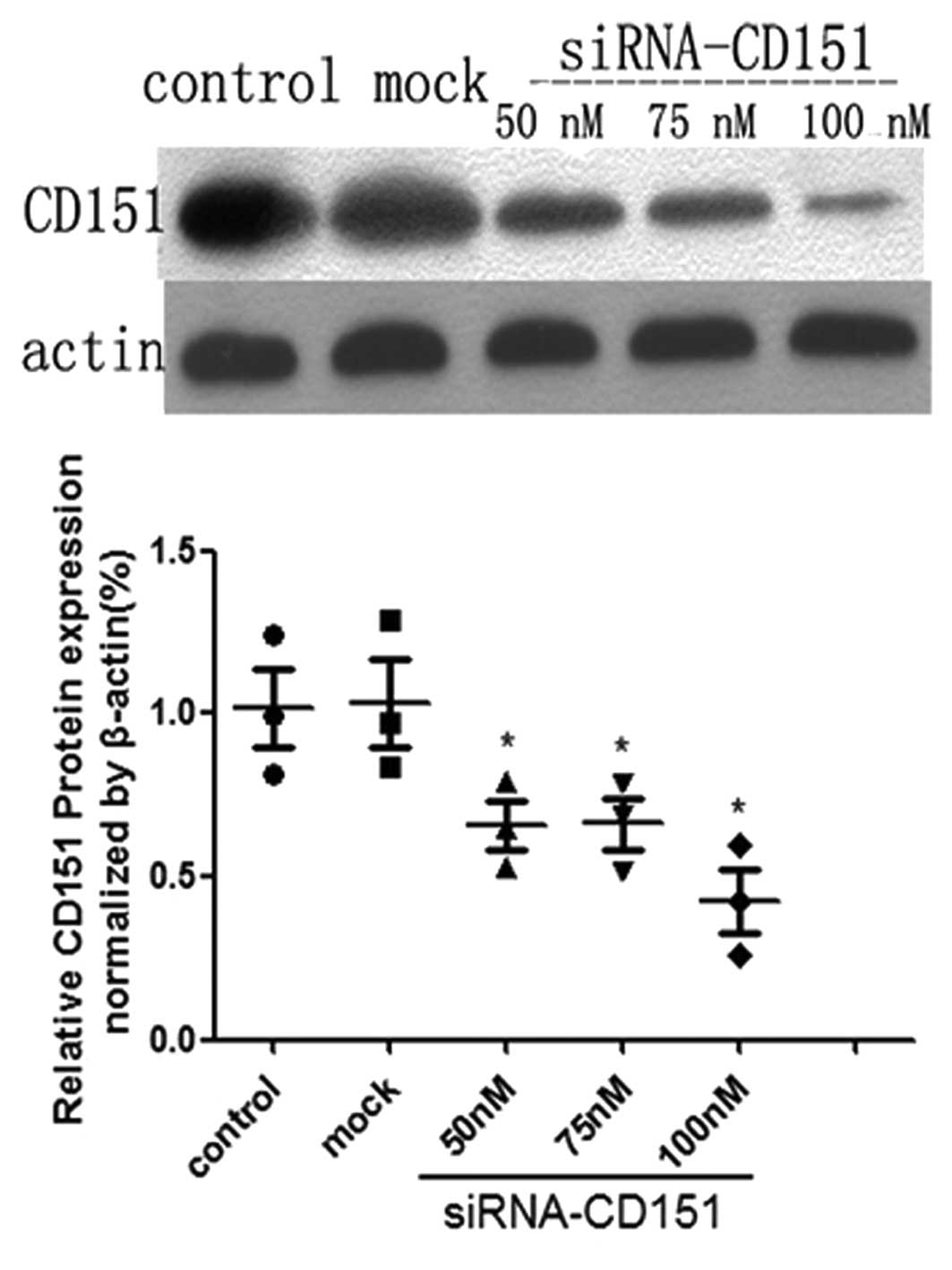
CD151-siRNA targets the endogenous CD151
gene and effectively inhibits its protein expression in A549 cells
in vitro
To determine whether CD151 protein was effectively
decreased in A549 cells following siRNA transfection, the protein
expression was examined by western blot analyses. The degree of
CD151 protein inhibition in A549 cell lines was different according
to different doses of siRNA. The protein inhibition occurred in a
dose-depended manner. A549 cells that had been either untreated or
treated only with Lipofectamine 2000 reagent were used as controls.
The results of the western blot analysis revealed that the CD151
protein was significantly downregulated in A549 cells as compared
with the control and mock group (P<0.05) (Fig. 1). When a 100 nM dose of siRNA was
used, the transfection efficiency was at its highest.
CD151 gene inhibition directly decreases
the proliferation rate of A549 cells, but the percentage of
apoptotic cells in the siRNA treated group is increased
To investigate whether endogenous CD151 inhibition
affected the proliferation and apoptosis of A549 cells, cells were
transfected with CD151 siRNA and 24 h after transfection, the
proliferation rates were determined by MTT assay. As shown in
Fig. 2A, the growth rate of
CD151-siRNA treated A549 cells was significantly decreased after
24, 48 and 72 h (P<0.05, respectively), when compared to the
control and mock cells. No significant difference was observed
between the control and mock groups. These data demonstrated that
knockdown of CD151 may have a significant negative effect on the
proliferation of A549 cells. Apoptosis, measured by Annexin V and
PI staining, was significantly increased in cells treated with
CD151-siRNA (Fig. 2B). There was
no significance difference between the control and mock group
cells. These data suggested that knockdown of CD151 expression
resulted in decreased proliferation and activated apoptosis in A549
cell lines.
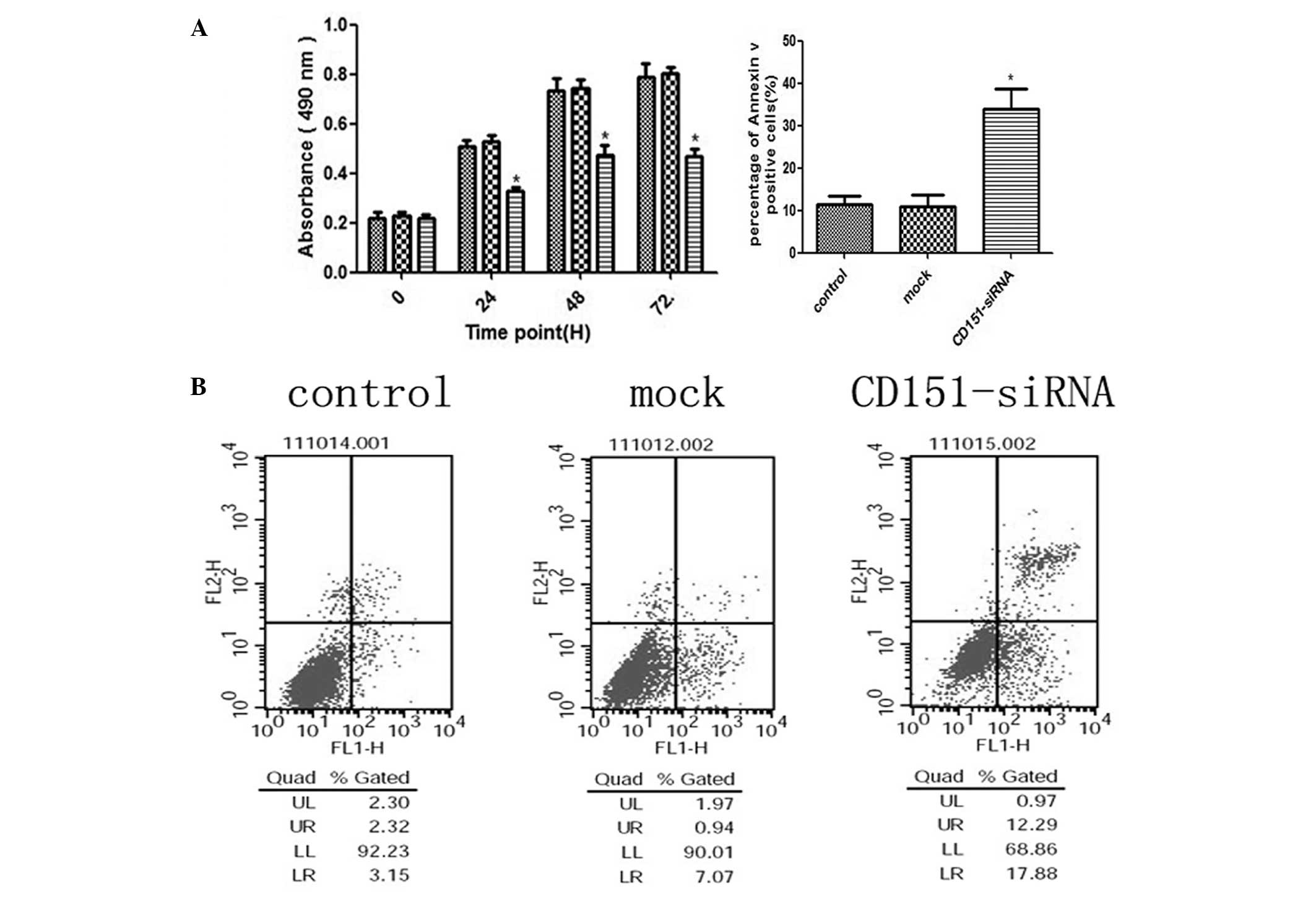 | Figure 2Inhibition of the endogenous CD151
protein expression with CD151-siRNA significantly inhibits A549
cell proliferation, but the apoptosis rate of A549 cells was
directly increased. (A) A549 cells were seeded into 96-well plates
and transfected with CD151 and scrambled siRNAs (mock) at 100 nm.
Untreated cells were used as a control. The three groups of cells
were incubated at 37°C in a humidified atmosphere containing 5%
CO2 and 95% air for 0–72 h and were analyzed for 0, 24,
48, and 72 h by MTT assay. CD151-siRNA-treated A549 cell growth was
significantly inhibited at 24, 48 and 72 h (P<0.05) as compared
with the mock cells. (B) A549 cells were seeded in 6-well plates
and transfected with CD151-siRNA for 24 h. The cells stained with
Annexin V/fluorescein isothiocyanate and propidium iodide were used
for subsequent analysis by flow cytometry. The percentage of
apoptotic cells was increased in CD151-siRNA treated cells, whereas
there was no increase in the cells treated with scrambled siRNA
sequence. The percentage of cells in the upper-right quadrant
(propidium iodide-positive, Annexin V-positive) plus the cells in
the lower-right quadrant (propidium iodide-negative, Annexin
V-positive) from the total cell number are represented under the
relevant graph. The columns represent the mean of three experiments
and the error bars represent the standard deviation.
*P<0.05 vs control and mock. UL, upper left; UR,
upper right; LL, lower left; LR, lower right; siRNA, small
interfering RNA. |
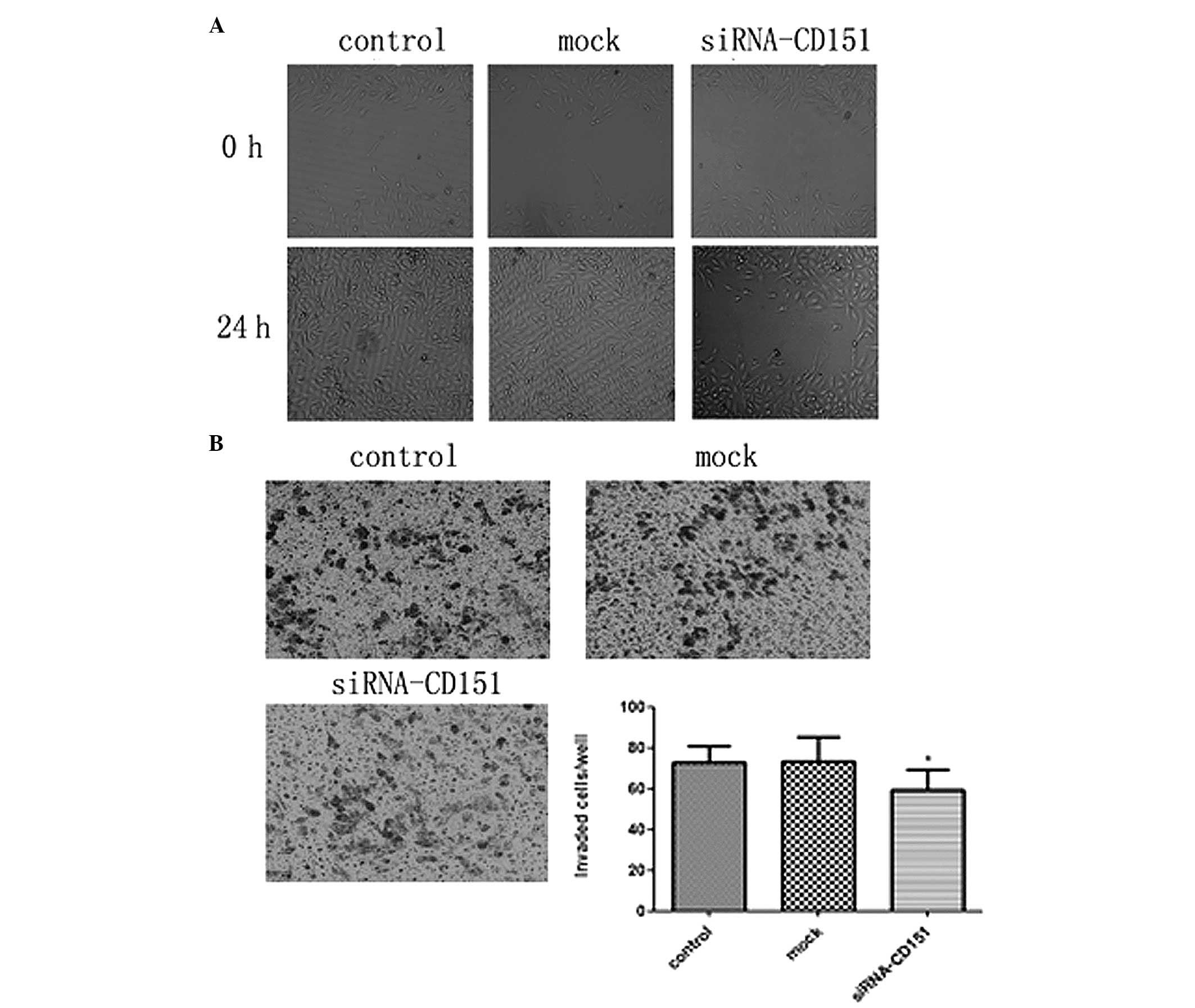
Targeting endogenous CD151 siRNA weakens
the migratory/invasive ability of A549 cells
CD151 is associated with the biological behavior of
tumor cells including metastasis, for which two critical steps are
migration and invasion. To investigate the effects of CD151 on the
metastasis of A549 cells, siRNA was used to silence the endogenous
CD151 expression in A549 cells and wound healing migration and
Boyden chamber invasion assays were performed. In the wound healing
assay, CD151 siRNA-treated cells exhibited a significantly
decreased migration rate and were unable to repair the wound as
compared with the scrambled siRNA mock cells. The control and mock
cells achieved almost complete wound healing (Fig. 3A). Furthermore, in the Boyden
chamber assay, the number of invasive cells among those treated
with CD151 siRNA was significantly decreased as compared to the
mock cells. These results indicated that CD151 inhibition decreased
the invasive capacity of A549 cells, but there was no significant
difference between the control and mock cells (Fig. 3B). These data showed that targeting
of CD151 siRNA weakens the migratory and invasive abilities of A549
cells in vitro.
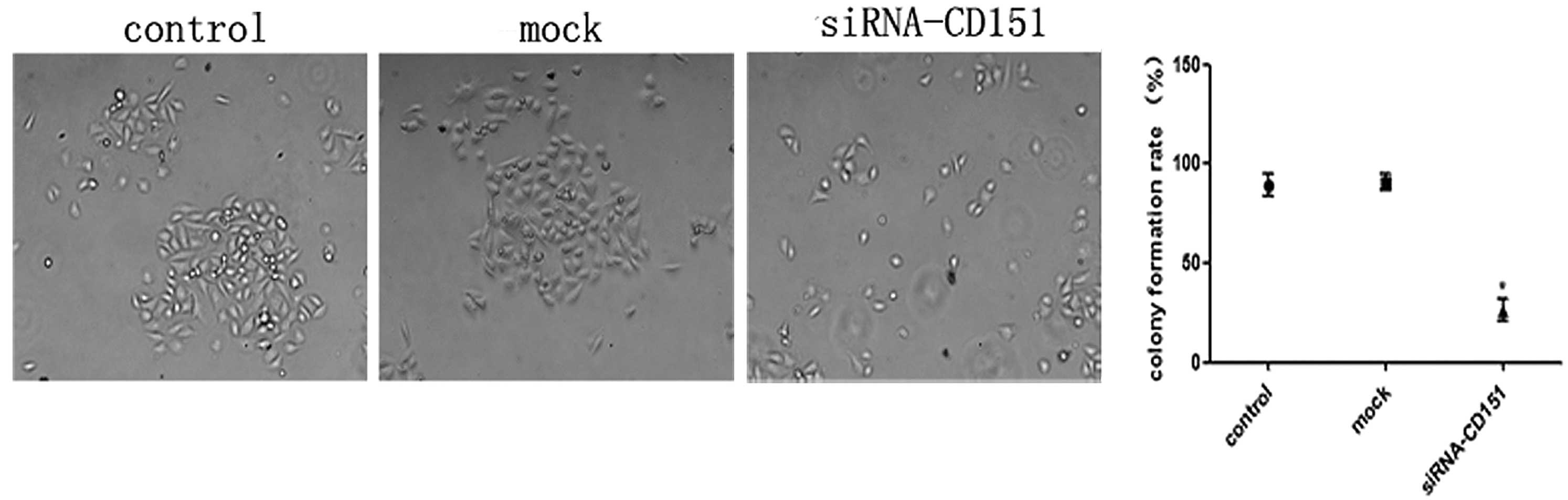
Inhibition of colony formation in CD151
knockdown A549 cells
To further study the role of CD151 inhibition on the
growth properties of A549 cells, a colony formation (>50
cells/colony) assay was performed. Control, mock and CD151-siRNA
cells were seeded at a density of 300 cells/ml in 6-well plates.
Following 24 h transfection, the cells were incubated at 37°C in a
humidified atmosphere of 5% CO2 for 14 days. Finally,
positive colonies were counted. As shown in Fig. 4, siRNA-treated cells exhibited a
significantly lower colony formation rate as compared with the
control and mock cells. There was no significant difference between
the control and mock group. CD151 downregulation resulted in a
marked inhibition in the capacity of A549 cells to form
colonies.
Molecular mechanisms by which CD151
knockdown exhibits the anti-tumor effects
Protein samples from the three groups of cells were
extracted to investigate the role of the CD151 knockdown in the
expression and phosphorylation changes of signaling molecules and
specific cytokines. It was observed that the protein expression
levels of phosphorylated FAK, PI3K, phosphorylated Akt,
phosphorylated MEK, and phosphorylated Erk1/2, were downregulated
in the CD151 siRNA-treated cells, whereas those in the control and
mock cells had no significant difference (Fig. 5A and B). These results demonstrated
that CD151 inhibition had significantly decreased expression of
intracellular growth factor signaling molecules in A549 cells. In
addition, CD151 knockdown also disturbed the protein expression of
VEGF and MMP-9, which have been documented to directly promote
carcinogenesis and tumor progression (23,24).
However, a difference in expression of MMP-2 protein was not
observed (Fig. 5C). Together,
these data indicated that CD151 knockdown in A549 cells functions
in anti-tumor effects through the inhibition of downstream
proliferation and metastasis pathways.
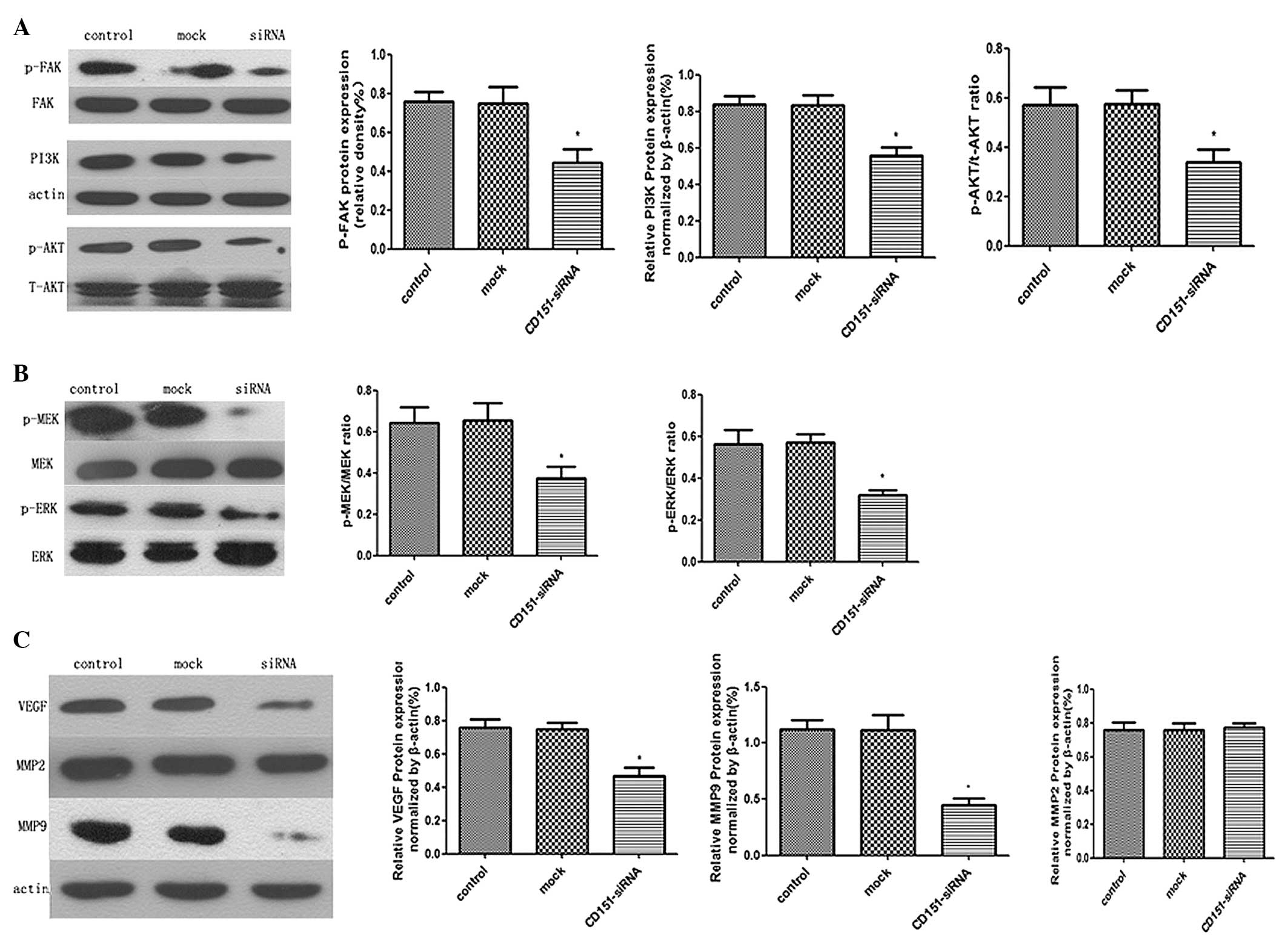 | Figure 5Western blot analysis of the
anti-tumor effects of CD151 knockdown using siRNA in A549 cells.
A549 cells were treated with CD151-siRNA or scrambled siRNA for 24
h, and then total cell protein was extracted. (A and B) The levels
of phosphorylated FAK, PI3K, phosphorylated Akt, phosphorylated MEK
and phosphorylated Erk1/2 were examined by western blotting.
CD151-siRNA-treated A549 cells exhibited decreased activation of
FAK, PI3K/Akt and MEK/Erk1/2 MAPK as compared with the control and
mock group. (C) The effects of CD151 downregulation on MMPs and
VEGF expression. The MMP-9 and VEGF protein expression in
CD151-siRNA-treated A549 cells was relatively diminished as
compared with the control and mock group. There was no difference
in the protein expression level of MMP-2 among these three groups.
The columns represent the mean of three experiments and the error
bars represent the standard deviation. *P<0.05 vs
control and mock. MMP, matrix metalloproteinase; FAK, focal
adhesion kinase; PI3K, phosphoinositide 3-kinase; MEK,
mitogen-activated protein kinase kinase; ERK, extracellular
signal-regulated kinase; VEGF, vascular endothelial growth factor;
p, phosphorylated; siRNA, small interfering RNA. |
Discussion
Despite the advances in medical and surgical
treatments, lung cancer remains the leading cause of cancer-related
death (2). Because of the
intrinsic properties of LAC and the ability of tumor cells to
rapidly progress, the prognosis in LAC is poor. Tumor progression
involves tumor cell proliferation, migration, invasion and vascular
intravasation or extravasation, establishment of a metastatic
niche, and angiogenesis (25,26).
Therefore, controlling the biological behavior of LAC cells in
proliferation, migration and invasion ability is crucial for LAC
therapy.
CD151 is a member of the tetraspanins, transmembrane
4 superfamily (TM4SF). It has been considered to have important
roles in various cellular functions, including cell proliferation,
differentiation, adhesion, and motility (16). Previous studies have indicated that
CD151 is highly expressed in numerous cancers, thus contributing to
the malignancy of human carcinoma. Overexpression of CD151 clearly
induced tumorigenicity (16,27,28).
Data has shown that CD151 overexpression enhanced the invasive and
metastatic capability of numerous cancer cell lines, however, the
treatment of cells with anti-CD151 suppressed this ability
(10). Anti-CD151 antibodies or
CD151 mutants could selectively inhibit integrin-α3/α6-dependent
cell metastasis, whereas upregulation of CD151 enhanced
experimental migration of colon carcinoma and fibrosarcoma cells
(24). Although the biochemical
functions of CD151 remain elusive, the ability of tetraspanin
proteins to associate with each other and other membrane proteins
demonstrates that CD151 may have a role as a transmembrane
connector that links intercellular molecular signaling. It has been
reported that in various normal cells, CD151 controls intercellular
signaling balance and regulates cell survival, proliferation,
movement, differentiation and morphology (29). Nevertheless, under pathological
conditions, CD151 may promote tumor progression on account of its
ability to promote cell survival, proliferation, movement, colony
formation and signaling transduction. All of its abilities may
significantly alter the cellular environment, and consequently
result in tumor progression. However, the definitive role of CD151
in cancer progression and metastasis remains to be determined, and
it remains undetermined whether CD151 inhibition has an important
role in LAC..
In the present study, siRNA was used for targeted
dowregulation of CD151 expression in the LAC cell line, A549, to
investigate a novel anti-tumor gene therapy. CD151-siRNAs were
synthesized to specifically target endogenous CD151 in A549 cells,
according to the literature (30).
The transfection efficiency was ~75% and the siRNA efficacy was
sustained for >24 h without degradation. Furthermore, western
blot analysis demonstrated that the efficiency of gene silencing
occurred in a dose-dependent manner. When the concentration of
CD151-siRNA was at 100 nm, the transfection efficacy was highest.
This method of siRNA-induced gene silencing was appropriate based
on the specificity of the siRNA designed against CD151. It was
identified that inhibiting the expression of CD151 resulted in
restraint of proliferation in A549 cells, in a time-dependent
manner. Furthermore, tumor colony-forming analysis indicated that
the percentage of positive colonies formed following CD151
knockdown in A549 cells was significantly reduced. The percentage
of apoptotic A549 cells following CD151-siRNA transfection was
significantly increased. Therefore, knockdown of CD151 expression
effectively diminished the capacity of A549 cell survival and
colony formation. Moreover, we investigated the signaling pathways
related to cell growth, including FAK, and other independent
pathways, including PI3K/AKT and MEK-ERK/MAPK. The results
indicated that knockdown of CD151 expression in A549 LAC cells, led
to a decrease in FAK, PI3K/Akt, MEK/Erk1/2 phosphorylation. There
was no significant difference between the control and mock cells.
The chemically synthesized CD151-siRNAs targeting CD151 may
therefore restrain the uncontrolled growth of A549 cells and
promote cell apoptosis through downstream intercellular signaling
molecules.
Beyond participating in the growth of A549 cells, it
was identified that the capabilities of metastasis and invasion
were impaired in cells undergoing CD151-siRNA treatment. This was
consistent with our previous hypothesis that CD151 is required for
LAC metastasis and invasion, similar to other cancer types
(13). Tetraspanin proteins have
been considered to be associated with signal transduction by
modulating the organization and assembly of signaling complexes in
membrane microdomains, known as the ‘tetraspanin web’ (31–33).
CD151 has a strong lateral association with laminin-banding
integrins, including α3β1, α6β1, α6β4 and α7β1. In contrast to
previously confirmed roles of CD151 as an adaptor for
integrin-delivered signaling molecular, some previous research has
indicated that CD151 can modulate its own signals such as
upregulation of MMPs, cell motility, and invasiveness. Although it
has been demonstrated that increased expression of MMP-2 and MMP-9
function in lung cancer in cell invasion and metastasis (24), the manner in which CD151 modulates
MMPs in LAC remains elusive. To the best of our knowledge, the
present study confirmed for the first time that CD151 knockdown
markedly decreased the expression level of MMP9 but not MMP2. In
addition, angiogenesis also functions in LAC progression and
metastasis and VEGF has a key function in the process of
neovascularization. It was identified that VEGF expression was
significantly decreased in A549 cells treated with CD151-siRNA.
Thus, it was hypothesized that CD151 may directly regulate MMP-9
and VEGF expression in A549 cells and promote tumor progression and
invasion, however, the molecular mechanism remains to be
discovered.
In summary, the results of the present study have
confirmed that downregulation of CD151 by targeted siRNA inhibited
the proliferation, metastasis, invasion and colony formation, but
induced apoptosis in A549 cells. Downregulation of CD151 by siRNA
may be a novel method for gene therapy in LAC. Further studies are
warranted to verify the effectiveness and safety for anti-LAC
therapy in vivo.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (nos. 81000047 and 81000139).
References
|
1
|
Bennett A and White J: Improving care and
quality of life for patients with lung cancer. Nurs Stand.
28:50–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J, et al: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oken MM, Hocking WG, Kvale PA, et al:
Screening by chest radiograph and lung cancer mortality: the
Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial.
JAMA. 306:1865–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanagiri T, Baba T, So T, et al: Time
trends of surgical outcome in patients with non-small cell lung
cancer. J Thorac Oncol. 5:825–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X and Zheng Y: Retreatment with
icotinib in a patient with metastatic lung adenocarcinoma. Tumori.
99:e124–e126. 2013.PubMed/NCBI
|
|
6
|
Hashida H, Takabayashi A, Tokuhara T, et
al: Clinical significance of transmembrane 4 superfamily in colon
cancer. Br J Cancer. 89:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang BW, Lee D, Chung HY, et al:
Tetraspanin CD151 expression associated with prognosis for patients
with advanced gastric cancer. J Cancer Res Clin Oncol.
139:1835–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HX, Li Q, Sharma C, et al:
Tetraspanin protein contributions to cancer. Biochem Soc Trance.
39:547–552. 2011. View Article : Google Scholar
|
|
9
|
Yue S, Mu W and Zöller M: Tspan8 and CD151
promote metastasis by distinct mechanisms. Eur J Cancer.
49:2934–2948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Copeland BT, Bowman MJ and Ashman LK:
Genetic ablation of the tetraspanin CD151 reduces spontaneous
metastatic spread of prostate cancer in the TRAMP model. Mol Cancer
Res. 11:95–105. 2013. View Article : Google Scholar
|
|
11
|
Chernousov MA, Stahl RC and Carey DJ:
Tetraspanins are involved in Schwann cell-axon interaction. J
Neurosci Res. 91:1419–1428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spring FA, Griffiths RE, Mankelow TJ, et
al: Tetraspanins CD81 and CD82 facilitate α4β1-mediated adhesion of
human erythroblasts to vascular cell adhesion molecule-1. PLoS One.
8:e626542013. View Article : Google Scholar
|
|
13
|
Kwon MJ, Seo J, Kim YJ, et al: Prognostic
significance of CD151 overexpression in non-small cell lung cancer.
Lung Cancer. 81:109–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ang J, Lijovic M, Ashman LK, et al: CD151
protein expression predicts the clinical outcome of low-grade
primary prostate cancer better than histologic grading: a new
prognostic indicator? Cancer Epidemiol Biomarkers Prev.
13:1717–1721. 2004.PubMed/NCBI
|
|
15
|
Lee D, Suh YL, Park TI, et al: Prognostic
significance of tetraspanin CD151 in newly diagnosed glioblastomas.
J Surg Oncol. 107:646–652. 2013. View Article : Google Scholar
|
|
16
|
Haeuw JF, Goetsch L, Bailly C, et al:
Tetraspanin CD151 as a target for antibody-based cancer
immunotherapy. Biochem Soc Trans. 39:553–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minner S, De Silva C, Rink M, et al:
Reduced CD151 expression is related to advanced tumour stage in
urothelial bladder cancer. Pathology. 44:448–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ang J, Fang BL, Ashman LK, et al: The
migration and invasion of human prostate cancer cell lines involves
CD151 expression. Onco Rep. 24:1593–1597. 2010.
|
|
19
|
Woegerbauer M, Thurnher D, Houben R, et
al: Expression of the tetraspanins CD9, CD37, CD63, and CD151 in
Merkel cell carcinoma: strong evidence for a posttranscriptional
fine-tuning of CD9 gene expression. Mod pathol. 23:751–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang W, Li P, Lin J, et al: CD151 promotes
proliferation and migration of PC3 cells via the formation of
CD151-integrin α3/α6 complex. J Huazhong Univ Sci Technolog Med
Sci. 32:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gottumukkala SN, Dwarakanath CD and
Sudarsan S: Ribonucleic acid interference induced gene knockdown. J
Indian Soc Periodontol. 17:417–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujishima S, Shiomi T, Yamashita S, et al:
Production and activation of matrix metalloproteinase 7 (matrilysin
1) in the lungs of patients with idiopathic pulmonary fibrosis.
Arch Pathol Lab Med. 134:1136–1142. 2010.PubMed/NCBI
|
|
24
|
Hong IK, Jin YJ, Byun HJ, et al:
Homophilic interactions of Tetraspanin CD151 up-regulate motility
and matrix metalloproteinase-9 expression of human melanoma cells
through adhesion-dependent c-Jun activation signaling pathways. J
Biol Chem. 281:24279–24292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ogorevc E, Kralj-Iglic V and Veranic P:
The role of extracellular vesicles in phenotypic cancer
transformation. Radiol Oncol. 47:197–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olbryt M: Role of tumor microenvironment
in the formation and progression of skin melanoma. Postepy Hig Med
Dosw (Online). 67:413–432. 2013.(In Polish). View Article : Google Scholar
|
|
27
|
Wang HX, Li Q, Sharma C, et al:
Tetraspanin protein contributions to cancer. Biochem Soc Trans.
39:547–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Romanska HM and Berditchevski F:
Tetraspanins in human epithelial malignancies. J Pathol. 223:4–14.
2011. View Article : Google Scholar
|
|
29
|
Takeda Y, Kazarov AR, Butterfield CE, et
al: Deletion of tetraspanin Cd151 results in decreased pathologic
angiogenesis in vivo and in vitro. Blood. 109:1524–1532. 2007.
View Article : Google Scholar
|
|
30
|
Yañez-Mó M, Barreiro O, Gonzalo P, et al:
MT1-MMP collagenolytic activity is regulated through association
with tetraspanin CD151 in primary endothelial cells. Blood.
112:3217–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devbhandari RP, Shi GM, Ke AW, et al:
Profiling of the tetraspanin CD151 web and conspiracy of
CD151/integrin β1 complex in the progression of hepatocellular
carcinoma. PLoS One. 6:e249012011. View Article : Google Scholar
|
|
32
|
Colin S, Guilmain W, Creoff E, et al: A
truncated form of CD9-partner 1 (CD9P-1), GS-168AT2, potently
inhibits in vivo tumour-induced angiogenesis and tumour growth. Br
J Cancer. 105:1002–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Charrin S, Manié S, Billard M, et al:
Multiple levels of interactions within the tetraspanin web. Biochem
Biophys Res Commun. 304:107–112. 2003. View Article : Google Scholar : PubMed/NCBI
|