Introduction
Parkinson’s disease (PD) is the most common
neurodegenerative disorder and for which no cure is currently
available (1). PD is characterized
pathologically by the selective and progressive loss of
dopaminergic (DA) neurons of the substantia nigra pars compacta
(SNc) (1). Despite significant
developments in available therapies, including drug treatment and
deep brain stimulation, these treatments are only able to relieve
the movement-associated symptoms of PD, rather than its
pathological progression (1). The
development of treatments to prevent or rescue the pathological
deterioration remains the ultimate goal in PD research.
Although the pathogenesis of PD remains elusive,
numerous neurotrophic factors have been identified which protect
and rescue DA neurons from numerous assaults in vitro and
in vivo (2–4).
Conserved dopamine neurotrophic factor (CDNF) is a
novel neurotrophic factor for DA neurons and is a secreted protein
containing eight conserved cysteine residues (5). Lindholm et al (5) demonstrated that CDNF was able to
protect and rescue midbrain DA neurons following 6-hydroxydopamine
(6-OHDA)-induced neurotoxicity in vivo, as well as
facilitating the recovery of the resulting behavioral deficits.
Subsequently, several studies have demonstrated that administration
of CDNF into the brain protected DA neurons from injury, rescued
dying DA neurons in mice and facilitated the recovery of behavioral
deficits in nonhuman primate models of PD (5–8).
However, the beneficial effects of CDNF administration were not
sustained following its withdrawal, indicating a requirement for
continuous CDNF administration to maintain its neuroprotective
effects (6). However, chronic
infusion is technically challenging in a clinical setting (7). A cell-mediated gene therapy has the
potential to improve the therapeutic efficacy of CDNF
administration (9).
Previous studies have indicated that mesenchymal
stem cells (MSCs) have the capacity to differentiate into numerous
non-mesenchymal cell types in vivo (8,10)
and migrate extensively throughout the adult animal (11,12).
In addition, studies have reported that bone marrow-derived cells
migrate preferentially to the sites of brain injury, for example
ischemia, in rats (2,13). These characteristics suggest that
bone marrow stromal cells (BMSCs) may be a potential vehicle for
the delivery of therapeutic genes to the diseased brain. In the
present study, BMSCs were transfected with CDNF complementary DNA
(cDNA) and transplanted into the brains of model rats with
6-OHDA-induced PD by intrastriatal infusion. The potential
neuroprotective effects of the transplanted CDNF-expressing BMSCs
(CDNF-BMSCs) on DA neurons were investigated in vivo.
Materials and methods
Recombinant plasmid construction
Wistar rats were obtained from Anhui Provincial
Hospital Research Center (Hefei, China). The total RNA of the
CDNF gene was isolated from the rats (564 bases) using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
The first-strand cDNAs were subsequently synthesized by reverse
transcription (RT), followed by polymerase chain reaction (PCR)
using the following primers: Forward,
5′-ACCATGCGGTGCATCAGTCCAACTGC-3′ and reverse,
5′-GAGCTCCGTTTGGGGGTATATC-3′. The pEGFP-N1-CDNF construct was
created by digestion by double restriction enzymes (BamHI
and XhoI), ligation and transfection into E. coli
DH5α-competent cells (China Science Institute of Shanghai Life
Science College, Shanghai, China). Purified pEGFP-N1-CDNF
recombinant plasmid was transfected into BMSCs using
Lipofectamine® 2000 (Life Technologies, Grand Island,
NY, USA) as described previously (2). At 72 h following the transfection,
the BMSCs were harvested ready for transplantation.
Experimental design
Four separate groups of rats received four
intrastriatal transplants, followed by PD modeling induced by
6-OHDA lesioning at the ipsilateral striatum one week later. The
groups were as follows: Group 1 (n=16), sham operation of
intrastriatal transplantation followed by saline injection into
striatum; group 2 (n=16), sham operation of intrastriatal
transplantation followed by 6-OHDA (Sigma-Aldrich, St. Louis, MO,
USA) lesioning at the ipsilateral striatum; group 3 (n=16),
intrastriatal BMSC transplantation followed by 6-OHDA lesioning at
the ipsilateral striatum; group 4 (n=16) intrastriatal CDNF-BMSC
transplantation followed by 6-OHDA lesioning at the ipsilateral
striatum. Rats were evaluated 2, 4 and 6 weeks following 6-OHDA
lesioning. Six weeks following 6-OHDA lesioning, the rats were
sacrificed for immunohistochemical analysis by TH immunostaining in
SNc and striatum.
Animals and surgical procedures
A total of 64 female Sprague-Dawley rats weighing
200–250 g were obtained from the Anhui Provincial Hospital Research
Center (Hefei, China). The animals received food and water ad
libitum and were kept under controlled environmental conditions
(12-h light/dark cycle, with light on between 7:00 and 19:00 h;
room temperature, 21°C). During all experimental procedures, rats
were treated in accordance with the Guidelines for Animal Care and
Use of the National Institutes of Health (Bethesda, MD, USA).
Surgical procedures were performed under chloral
hydrate [300 mg/kg, intraperintoneal injection (i.p.)] and executed
in a Kopf stereotaxic apparatus (Narishige, Tokyo, Japan). Animals
received three unilateral stereotaxic injections of variable
amounts of 6-OHDA (Sigma-Aldrich) in various locations within the
left striatum using a 10-μl Hamilton microsyringe driven by a
microinfusion pump (Shanghai Precision and Scientific Instrument
Co., Ltd., Shanghai, China). The injection rate was 1 μl/min and
the cannula was left in place for an additional two minutes prior
to being slowly retracted. The doses and coordinates used were
selected based on a previous study (14). The coordinates (mm) of the surgical
procedure were as follows: Anteroposterior (AP), +0.48;
mediolateral (ML), ±3.0; dorsoventral (DV), −5.6/−4.3/−3.5 by
injection of pt aequ 6-OHDA (total amount, 20 μg/6 μl) (15). All solutions were 0.02% ascorbic
acid in 0.9% saline and 6-OHDA solutions were freshly prepared,
kept on ice and protected from exposure to light to minimize
variability and degradation of the toxin.
Rotational behavior
At 2, 4 and 6 weeks following 6-OHDA lesion, the
rats were assessed by apomorphine (APO; Sigma-Aldrich)-induced
rotational asymmetry over 30 min. Rotational behavior was monitored
in automated rotameter (Panlab; DL Naturegene Life Sciences, Inc.;
Beijing; China)bowls following APO administration (1). The number of rotations to the
ipsilateral side were counted for 30 min following intraperitoneal
administration of APO (0.5 mg/kg).
Histology
Perfusion and tissue processing for
histology
Six weeks following 6-OHDA lesion, the rats were
sacrificed. The animals were deeply anesthetized by an intravenous
injection of chloral hydrate (300 mg/kg) and transcardially
perfused with 0.9% saline followed by 4% buffered formaldehyde
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China). The brains were removed, blocked, immersed in an identical
fixative for 24 h and placed in saline (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) with the addition of 30% sucrose
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) until the
block sank. The brains were subsequently sectioned coronally using
a cryostat (RM2015; Leica Microsystems GmbH, Wetzlar, Germany) at a
thickness of 50 μm. Sections were collected in sequence and two
series were collected onto gelatin-coated slides. The sections were
deparaffinized and rehydrated. Following the addition of 3%
H2O2, the sections were washed with
phosphate-buffered saline (PBS; ZLI-9062; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China), incubated in
citrate buffer (0.1M, pH 5.8; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) and washed repeatedly with PBS for
immunohistochemistry (14).
Immunohistochemistry
Sections were immersed in a solution of 10% normal
goat serum and 1% bovine serum albumin (in PBS) (both purchased
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 1
h. Alternate sections were incubated with anti-tyrosine hydroxylase
(TH; 1:500; Sigma-Aldrich) overnight at 4°C. The sections were
subsequently incubated with biotinylated goat anti-mouse
immunoglobulin G secondary antibody (dilution, 1:3,000; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 15 min at room
temperature. Finally, sections were incubated in
avidin-biotin-peroxidase complex (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 15 min at room temperature. The bound
peroxidase molecules were visualized using 3,3′-diaminobenzidine
(Sigma-Aldrich). Between incubations, the sections were washed with
PBS multiple times. Each of the antibodies, as well as the
avidin-biotin-peroxidase complex, were diluted with PBS. Sections
were placed on gelatin-coated slides, dried overnight, dehydrated
in ascending alcohols, cleared in Histo-clear and mounted with
distrene, plasticiser and xylene (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.). For the control experiments, antibodies
were replaced with equal volumes of PBS and the procedure was
performed as above. Control sections were immunonegative.
Morphological analysis
SNc cell counts
The number of DA cells was determined using TH
immunoreactivity. The total number of cells in the midbrain on each
side of the brain was counted from ten sections in each animal (one
section every five sections from the midbrain in sequence). Counts
were made from comparable sections across the rostrocaudal extent
of the ventral SNc. This region was the most clearly defined part
of the nucleus and hence formed the focus of analysis. The present
study did not aim to report on the total number of cells in the SNc
of rats, instead, the number of cells in the nuclei of
corresponding sections in various individual cases was compared.
This strategy has been used in a study examining questions
analagous to those addressed in the present study (16).
Striatal fiber density
measurements
The optical densities of TH-immunoreactive fibers in
the striatum were assessed using the image processing system of the
Olympus BX51 (Olympus Corp., Tokyo, Japan). For each animal the
optical density (OD) was measured at rostro-caudal levels according
to the atlas of Paxinos and Watson (17) over the whole striatum: i)
AP, 11.6; ii) AP, 11.0; iii) AP, 10.2; iv) AP,
20.3; v) AP, 20.9; vi) AP, 21.4 and vii) AP:
22.1 relative to the bregma. To estimate the specific TH staining
density, the optical density readings were corrected for
nonspecific background density, as measured from the completely
denervated parts of the striatum in the animals with saline lesion.
Data are presented as a percentage of the intact side.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean of n separate experiments. Statistical analysis between
two groups was performed using Student’s t-test and more than two
groups were analyzed using analysis of variance, with SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Generating CDNF-expressing BMSCs for
transplantation
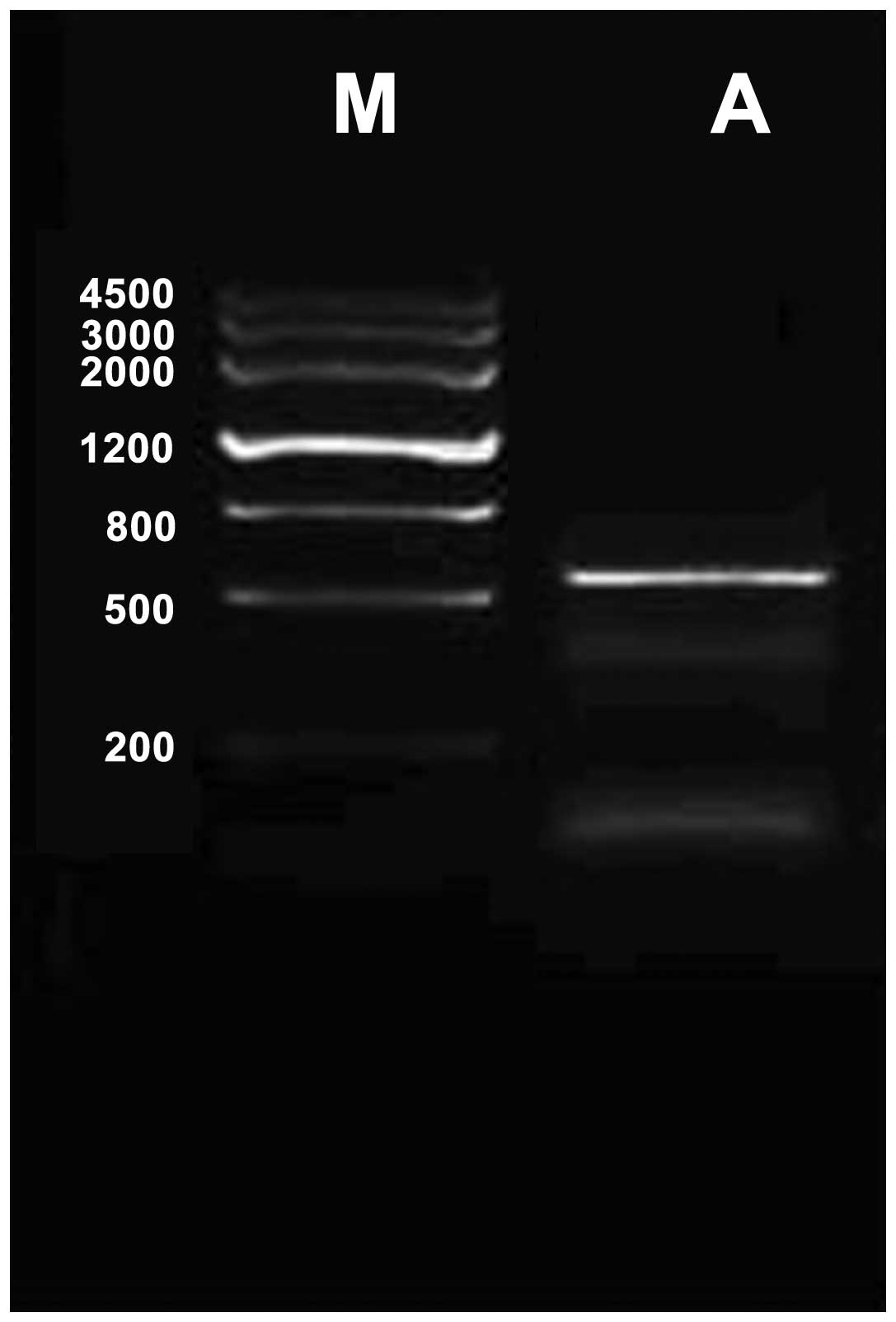
Mouse CDNF cDNA was amplified by RT-quantitative PCR
and cloned into pEGFP-N1 vectors for expressing CDNF (Fig. 1). The BMSC primary culture was
identified by fusiform cell shape and colony spread. The
recombinant plasmid pEGFP-N1-CDNF was constructed and transfected
into BMSCs and CDNF expression in BMSCs was confirmed by
fluorescence microscopy (Fig. 2),
the transfection efficiency was up to 56.8%.
BMSC transplantation reduces APO-induced
rotations
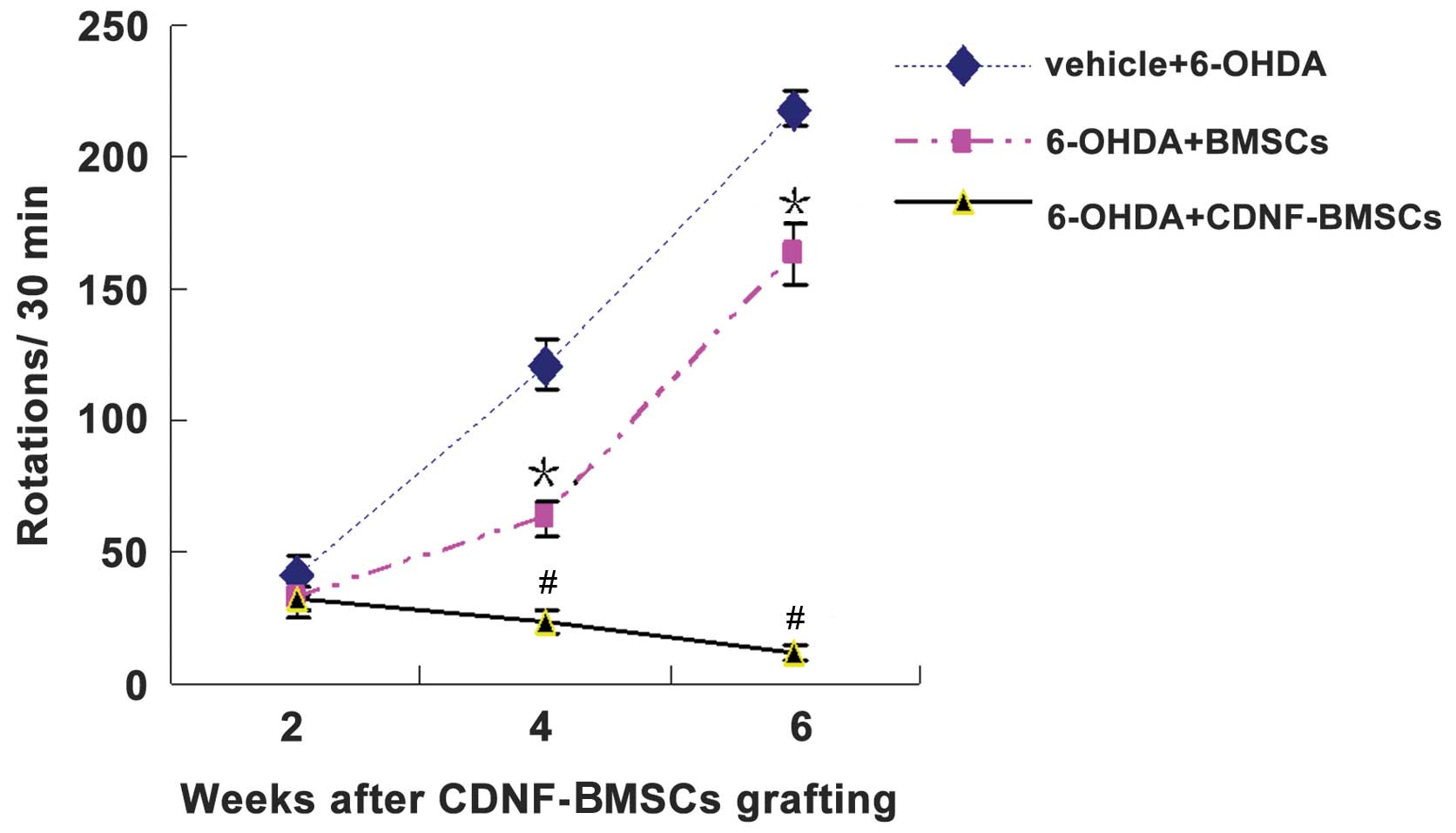
The results of the behavioral testing are exhibited
in Table I and Fig. 3. In brief, two weeks following
6-OHDA administration, transplantation of BMSCs had significantly
reduced APO-induced rotations compared with those of
non-transplanted rats. Furthermore, the rats that had received a
transplantation of CDNF-BMSCs had a significantly greater reduction
in rotations at two, four and six weeks following lesion, compared
to those of the rats which received BMSCs (Table I). This indicated that CDNF
expression by transplanted BMSCs had significant neuroprotective
effects against 6-OHDA-induced toxicity.
 | Table IChanges in the number of
apomorphine-induced rotations per 30 min following intrastriatal
CDNF-engineered BMSC transplantation and 6-OHDA intrastriatal
induction. |
Table I
Changes in the number of
apomorphine-induced rotations per 30 min following intrastriatal
CDNF-engineered BMSC transplantation and 6-OHDA intrastriatal
induction.
| Time-point following
6-OHDA induction |
|---|
|
|
|---|
| Group | 2 weeks | 4 weeks | 6 weeks |
|---|
| 1: Control
(sham-operation) | 0 | 0 | 0 |
| 2: Vehicle +
6-OHDA | 36.83±4.97 | 120.92±9.89 | 218.33±6.37 |
| 3: 6-OHDA +
BMSCs | 34.67±2.90a | 63.08±6.58b | 162.67±11.63b |
| 4: 6-OHDA +
CDNF-BMSCs | 32.83±4.69cd | 23.42±4.56be | 12.00±2.89be |
BMSC transplantation reduces
6-OHDA-induced loss of TH-positive fibers
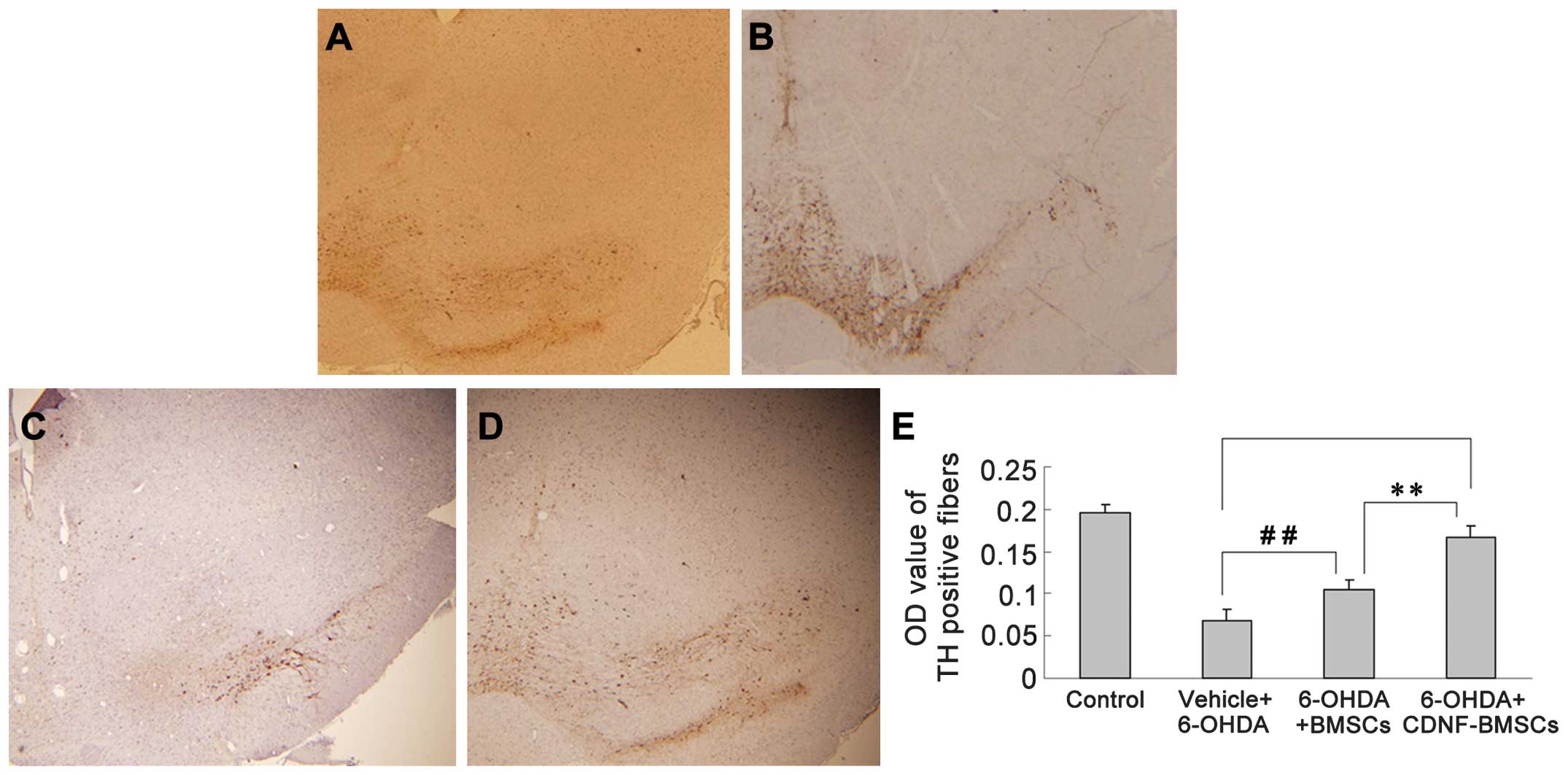
Six weeks following 6-OHDA administration,
immunohistochemistry was used to analyze TH-positive fibers in the
striatum and differentiated DA neurons in the SNc (Fig. 4). The OD of TH immunoreactivity in
the striatum of 6-OHDA-treated rats was significantly reduced (from
0.1963±0.0086 to 0.0687±0.0123). Transplantation of BMSCs
significantly attenuated the reduction in TH-positive fibers
(P<0.01). Transplantation of CDNF-BMSCs almost eliminated the
6-OHDA-induced loss of TH-positive fibers (OD, 0.1893±0.0690 vs.
0.1963±0.0086) (Table II).
 | Table IITH immuno-positive expression
following intrastriatal injection of CDNF-BMSCs and BMSCs six weeks
following 6-OHDA administration. |
Table II
TH immuno-positive expression
following intrastriatal injection of CDNF-BMSCs and BMSCs six weeks
following 6-OHDA administration.
| Group | TH-positive cells
in lesioned substantia nigra | Optical density
value of TH-positive fibers in lesioned striatum |
|---|
| 1: Control | 616.67±26.67 | 0.1963±0.0086 |
| 2: Vehicle +
6-OHDA |
128.00±11.86a |
0.0687±0.0123a |
| 3: 6-OHDA +
BMSCs |
371.33±28.31a |
0.1140±0.0125a |
| 4: 6-OHDA +
CDNF-BMSCs |
599.25±15.50bc |
0.1893±0.0690cd |
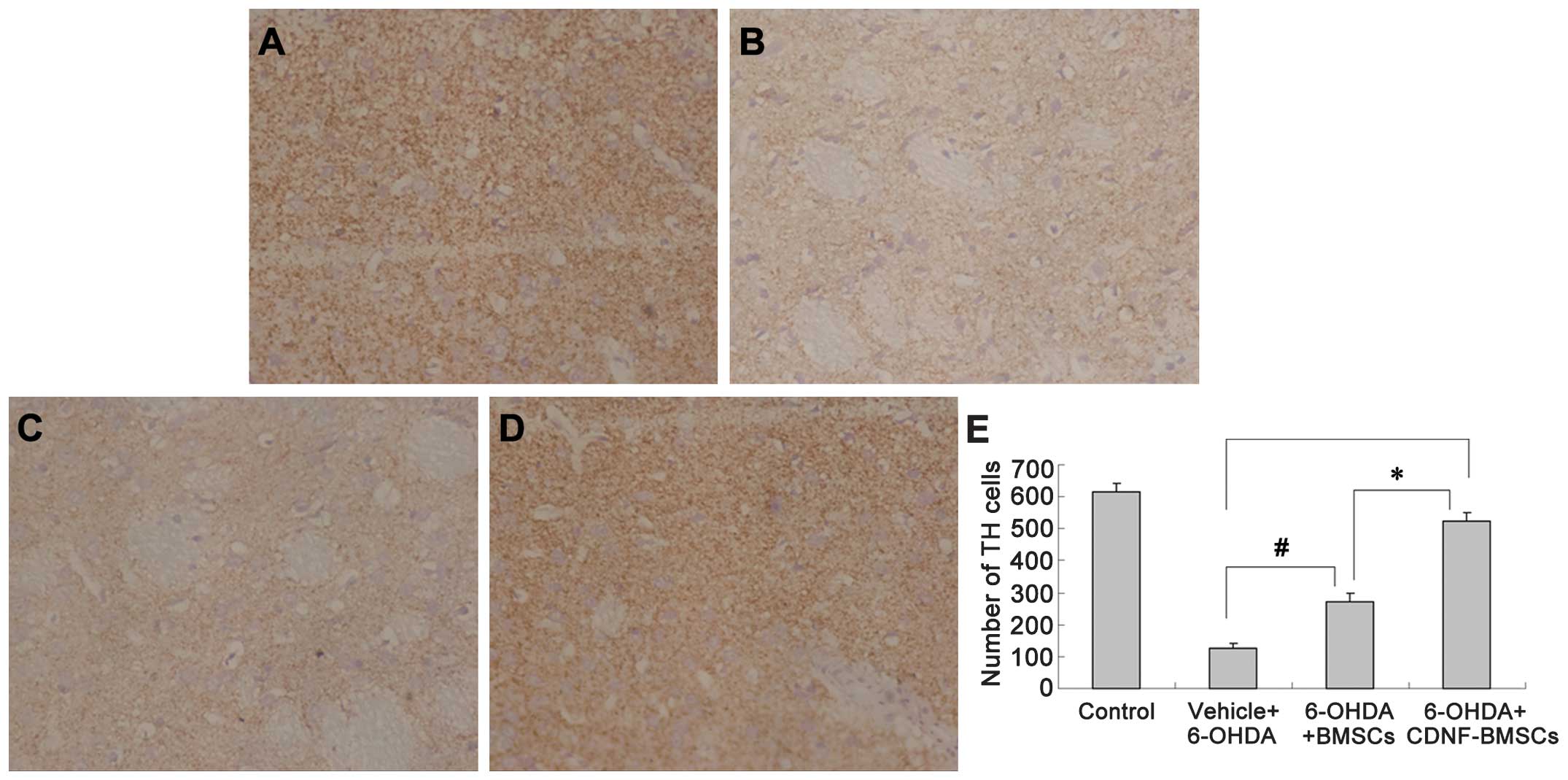
In the 6-OHDA lesioned striatum group, 6-OHDA
treatment caused the number of TH-positive neurons to decrease from
616.67±26.67 (sham-operation group) to 128.00±11.86 in the
ipsilateral SNc, with somal collapse and unclear cytoarchitectonics
(Fig. 5). The groups of rats which
received BMSC or CDNF-BMSC transplantation exhibited a greater
number of TH-positive cells in the SNc compared with that in the
sham operation group (P<0.01). Furthermore, the number of
TH-positive cells in the CDNF-BMSCs group (599.25±15.50) was
significantly greater than that in the BMSCs group (371.33±28.31,
P<0.01; Fig. 5).
Discussion
PD is a progressive movement disorder characterized
pathologically by DA neuron degeneration in the SNc (1). Direct transfection with neurotrophic
genes presented a potential therapeutic strategy. The ideal
cellular vehicle for gene transfer into the brain was considered to
be an autologous cell that does not disrupt brain circuitry
(18–20). In previous studies, BMSCs have been
successfully used as vehicles to deliver neurotrophin genes to the
disease tissue (3,21,22).
In the present study, it was demonstrated that intrastriatal
transplantation of CNDF-BMSCs significantly protected DA neurons
against 6-OHDA-induced toxicity, resulting in behavioral recovery
and attenuating the pathological degeneration of the substantia
nigra (SN) and striatum. These data indicated the suitability of
BMSCs as a vector for gene therapy and demonstrated that
CDNF-expressing BMSCs as a subsidiary therapy may be beneficial in
the treatment of PD.
DA axon terminal lesions, induced by intrastriatal
injection of the neurotoxin 6-OHDA, have been developed as a tool
for obtaining selective partial lesion of the nigrostriatal DA
system in the rat (23–25). The stereotactic administration of
6-OHDA into the striatum initially induced direct toxic damage to
the DA axons surrounding the injection site, followed by a gradual
loss of DA neurons in the ipsilateral SN (23). The functional effects induced by
intrastriatal 6-OHDA administration depend not only on the total
dose of injection, but additionally on the site of injection
(15,26,27).
While a single high dose of 6-OHDA (20 g) failed to induce
significant APO-induced rotation, pronounced behavioral symptoms
were produced following distribution of an identical dose of toxin
over three injection sites (27).
In the present study, a three-site injection of 6-OHDA protocol was
utilized, which induced an ideal PD model as indicated by the
behavioral and pathological changes observed. It is advantageous to
target the striatum, as opposed ot the SN, since intrastriatal
induction may avoid damage to the SN by direct toxin lesion and
mechanical insult (15).
Therefore, rats with intrastriatal 6-OHDA lesions provide a model
of progressive DA neuron degeneration, which is useful for the
evaluation of potential neuroprotective therapies and additionally
for the study of mechanisms underlying functional and structural
recovery following damage to the nigrostriatal dopamine system.
The novel dopaminergic neurotrophic factor CDNF, a
member of the highly conserved mesencephalic astrocyte-derived
neurotrophic factor protein family, has been demonstrated to
protect DA neurons from injury and rescue dying DA neurons
following intrastriatal injection, as well as facilitate the
recovery of behavioral deficits in 6-OHDA and
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced rat models of
PD (5–7). Further studies have indicated that
chronic infusion of CDNF into the striatum by micro-pump,
retrogradely transported from the striatum to the SN, was able to
prevent 6-OHDA-induced deficits in a rat model of PD (5–7). In
general, intrastriatially injected CDNF was rapidly degraded in the
brain and therefore, CDNF was unable to sustain its role in
neuroprotection (7). Although
chronic infusion of CDNF into the striatum has been used in animal
models of PD, it is difficult to implement this process for
clinical application. Therefore, gene therapy may provide an
alternative therapeutic route.
Several studies have demonstrated that bone
marrow-derived cells migrate preferentially to the sites of brain
insults, including ischemia (13,28)
and diseased brain tissue in PD and Huntington’s disease (29). Studies have shown that cDNA for
glial cell line-derived neurotrophic factor transfected via BMSC
vectors exerts neuroprotective and neurorestorative effects on DA
neurons by engrafting into the striatum (4,21).
These characteristics indicated that the use of BMSCs as vehicles
to deliver therapeutic genes into the sites of brain injury had the
potential to improve functional deficits. In the present study,
CDNF-BMSCs were delivered stereotactically into the striatum and
exerted a significant neuroprotective effect. It was also revealed
that the delivery of BMSCs produced significant neuroprotective
effects. It was therefore hypothesized that the transplanted BMSCs
may secrete CDNF or other neurotrophic factors. However,
transplantation of CDNF-BMSCs produced a more significant
neuroprotective effect than that of BMSCs, suggesting that the
ectopic expression of CDNF by CDNF-BMSCs was responsible for the
differences observed. Greater therapeutic efficacy and safety
issues represent a significant challenge in the development of
practical applications of such therapies for PD in the future.
The neuroprotective effects of CDNF on DA neurons
have gradually been established. BMSCs have the potential for
multilineage differentiation and represent potentially useful
vectors for the transfer of gene fragments to injury sites of the
brain(11–13,18,19).
PD is characterized by the selective and progressive loss of DA
neurons in the SN. Therefore, in the present study, BMSCs were
utilized as a vector to deliver CDNF cDNA fragments into the SN,
utilizing the characteristic of BMSCs of migrating to the brain
injury site. A significant neuroprotective effect was revealed in a
6-OHDA-induced rat model following transplantation of CDNF-BMSCs
into the striatum. Significant behavioral recovery and a reduction
in the loss of TH-positive fibers in the SNc and striatum were
observed. In conclusion, the results of the present study indicated
that CDNF transfer via BMSC transplantation represent a potential
therapeutic strategy for the treatment of PD.
Acknowledgements
The present study was supported by the Foundation of
The Department of Education of Anhui Province (no. KJ2010B381), the
Foundation of Natural Science of Anhui Province (no. 11040606Q11)
and The National Natural Science Fund of China (no. 81100960).
References
|
1
|
Niu C, Mei J, Pan Q and Fu X: Nigral
degeneration with inclusion body formation and behavioral changes
in rats after proteasomal inhibition. Stereotact Funct Neurosurg.
87:69–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su YR, Wang J, Wu JJ, Chen Y and Jiang YP:
Overexpression of lentivirus-mediated glial cell line-derived
neurotrophic factor in bone marrow stromal cells and its
neuroprotection for the PC12 cells damaged by lactacystin. Neurosci
Bull. 23:67–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong N, Zhang Z, Huang J, et al:
VEGF-expressing human umbilical cord mesenchymal stem cells, an
improved therapy strategy for Parkinson’s disease. Gene Ther.
18:394–402. 2011. View Article : Google Scholar
|
|
4
|
Park KW, Eglitis MA and Mouradian MM:
Protection of nigral neurons by GDNF-engineered marrow cell
transplantation. Neurosci Res. 40:315–323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindholm P, Voutilainen MH, Laurén J, et
al: Novel neurotrophic factor CDNF protects and rescues midbrain
dopamine neurons in vivo. Nature. 448:73–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Airavaara M, Harvey BK, Voutilainen MH, et
al: CDNF protects the nigrostriatal dopamine system and promotes
recovery after MPTP treatment in mice. Cell Transplant.
21:1213–1223. 2012. View Article : Google Scholar
|
|
7
|
Voutilainen MH, Bäck S, Peränen J, et al:
Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat
model of Parkinson’s disease. Exp Neurol. 228:99–108. 2011.
View Article : Google Scholar
|
|
8
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Liu Y, Zhao H, Zhang W, Guo YJ
and Nie L: Lentiviral-mediated transfer of CDNF promotes nerve
regeneration and functional recovery after sciatic nerve injury in
adult rats. Biochem Biophys Res Commun. 18:330–335. 2013.
View Article : Google Scholar
|
|
10
|
Li JM, Zhu H, Lu S, et al: Migration and
differentiation of human mesenchymal stem cells in the normal rat
brain. Neurol Res. 33:84–92. 2011. View Article : Google Scholar
|
|
11
|
Brazelton TR, Rossi FM, Keshet GI and Blau
HM: From marrow to brain: expression of neuronal phenotypes in
adult mice. Science. 290:1775–1779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muñoz-Elias G, Marcus AJ, Coyne TM,
Woodbury D and Black IB: Adult bone marrow stromal cells in the
embryonic brain: engraftment, migration, differentiation, and
long-term survival. J Neurosci. 24:4585–4595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eglitis MA, Dawson D, Park KW and
Mouradian MM: Targeting of marrow-derived astrocytes to the
ischemic brain. Neuroreport. 10:1289–1292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CS, Sauer H and Bjorklund A:
Dopaminergic neuronal degeneration and motor impairments following
axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat.
Neuroscience. 72:641–653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirik D, Rosenblad C and Björklund A:
Characterization of behavioral and neurodegenerative changes
following partial lesions of the nigrostriatal dopamine system
induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol.
152:259–277. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lundblad M, Andersson M, Winkler C, Kirik
D, Wierup N and Cenci MA: Pharmacological validation of behavioural
measures of akinesia and dyskinesia in a rat model of Parkinson’s
disease. Eur J Neurosci. 15:120–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 2nd edition. Academic Press; San Diego:
1986
|
|
18
|
Rice CM and Scolding NJ: Autologous bone
marrow stem cells - properties and advantages. J Neurol Sci.
265:59–62. 2008. View Article : Google Scholar
|
|
19
|
Benabdallah BF, Allard E, Yao S, et al:
Targeted gene addition to human mesenchymal stromal cells as a
cell-based plasma-soluble protein delivery platform. Cytotherapy.
12:394–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwarz SC and Schwarz J: Translation of
stem cell therapy for neurological diseases. Transl Res.
156:155–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Yu W, Chen Y, et al: Intrastriatal
transplantation of GDNF-engineered BMSCs and its neuroprotection in
lactacystin-induced Parkinsonian rat model. Neurochem Res.
35:495–502. 2010. View Article : Google Scholar
|
|
22
|
Moloney TC, Rooney GE, Barry FP, Howard L
and Dowd E: Potential of rat bone marrow-derived mesenchymal stem
cells as vehicles for delivery of neurotrophins to the Parkinsonian
rat brain. Brain Res. 1359:33–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haleagrahara N, Siew CJ and Ponnusamy K:
Effect of quercetin and desferrioxamine on 6-hydroxydopamine
(6-OHDA) induced neurotoxicity in striatum of rats. J Toxicol Sci.
38:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Um JW, Park HJ, Song J, Jeon I, Lee G, Lee
PH and Chung KC: Formation of parkin aggregates and enhanced PINK1
accumulation during the pathogenesis of Parkinson’s disease.
Biochem Biophys Res Commun. 393:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zafar KS, Siddiqui A, Sayeed I, Ahmad M,
Saleem S and Islam F: Protective effect of adenosine in rat model
of Parkinson’s disease: neurobehavioral and neurochemical
evidences. J Chem Neuroanat. 26:143–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richter F, Hamann M and Richter A:
Moderate degeneration of nigral neurons after repeated but not
after single intrastriatal injections of low doses of
6-hydroxydopamine in mice. Brain Res. 1188:148–156. 2008.
View Article : Google Scholar
|
|
27
|
Przedborski S, Levivier M, Jiang H,
Ferreira M, Jackson-Lewis V, Donaldson D and Togasaki DM:
Dose-dependent lesions of the dopaminergic nigrostriatal pathway
induced by intrastriatal injection of 6-hydroxydopamine.
Neuroscience. 67:631–647. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou Z, Jiang X, Zhang W, Zhou Y, Ke Y,
Zhang S and Xu R: Efficacy of Tyrosine Hydroxylase gene modified
neural stem cells derived from bone marrow on Parkinson’s disease -
a rat model study. Brain Res. 1346:279–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sadan O, Shemesh N, Cohen Y, Melamed E and
Offen D: Adult neurotrophic factor-secreting stem cells: a
potential novel therapy for neurodegenerative diseases. Isr Med
Assoc J. 11:201–204. 2009.PubMed/NCBI
|