Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
autoimmune disease which primarily affects the synovia of joints.
The synovium consists of a lining layer of fibroblast-like
synoviocytes (FLS) that overlies the connective tissue of the
synovial sublining (1). During the
course of RA, massive leukocyte infiltration activates the FLS to
produce proinflammatory mediators, including cytokines and
chemokines (2,3). Activated FLS become hyperplastic and
form a pannus tissue that invades articular cartilage and bone
(3,4). Therefore, FLS perpetuates
inflammation and destroys cartilage and bone.
CD11b+Gr-1+ myeloid-derived
suppressor cells (MDSCs) consist of a heterogeneous population of
early myeloid progenitors, with potent immunosuppressive abilities
(5). MDSCs were discovered in 1987
and previous studies have identified an important role for MDSCs in
autoimmunity and inflammation (6–8).
Expansion of MDSCs has been reported to reduce experimental
autoimmune encephalomyelitis and the transfer of MDSCs has been
demonstrated to reduce disease severity and significantly inhibit
Th1 and Th17 responses (9–11). Notably, previous studies have
implicated a role for MDSCs in RA. MDSCs potently suppress the
expansion of autoreactive T cells, and inhibit collagen-induced
arthritis (CIA) (12). It has been
reported that an increase in circulating MDSCs negatively
correlates with the levels of Th17 cells in patients with RA
(13).
Piperlonguminine (PL), a major alkaloid isolated
from Piper longum fruits, exhibits several biological
activities, including anti-tumor and anti-inflammatory effects
(14). However, the potential
effect of PL on RA has not yet been investigated to the best of our
knowledge. The aim of the present study was to investigate the
effect of PL treatment on the migration of FLS and the progression
of RA.
Materials and methods
Mice
Six to seven week-old male DBA/1 mice were bought
from the Chinese Academy of Sciences (Shanghai, China) and kept
under standard conditions (15) at
the Tongji University School of Medicine (Shanghai, China). All
experimental procedures were approved by the Institutional Animal
Care and Use Committee of Tongji University.
Human patients with RA
Ten newly diagnosed patients with active RA who had
not receive any treatment and fulfilled the 2010 American College
of Rheumatology/European League Against Rheumatism criteria for RA
(16) were recruited at the
Shanghai Tenth People’s Hospital of Tongji University (Shanghai,
China). Patients with active disease were defined as those with a
disease activity score in 28 joints of >3.2. This study was
performed with the approval of the Ethics Committee of Tongji
University in accordance with the Declaration of Helsinki. Informed
consent was obtained from all patients.
Induction of CIA and PL treatment
CIA was induced in male DBA/1 mice via an
intradermal injection of 100 μg bovine type II collagen (CII;
Chondrex, Inc., Redmond, WA, USA) emulsified with Complete Freund’s
adjuvant containing Mycobacterium tuberculosis (Chondrex,
Inc.). Mice were boosted 21 days later with an injection (100 μg
bovine type II collagen emulsified in Incomplete Freund’s adjuvant
(Chondrex, Redmond, WA, USA).
On day 28 following the first immunization,
arthritis was observed in all mice and treatment with PL was
initiated. Mice were randomly divided into two groups (n=8 per
group) and intraperitoneally injected with 2.4 mg/kg/day PL (Sigma,
St. Louis, MO, USA) or a vehicle control (dimethylsulfoxide; DMSO;
Sigma, St. Louis, MO, USA) as described by Raj et al
(17). PL treatment was continued
until day 47.
Assessment of arthritis
Clinically, arthritic severity was characterized via
the observation of joint properties and the inflammation of the
surrounding tissue. The clinical arthritis was assessed every three
days and scored as previously described (18). The maximal arthritis score per paw
is 4, and the maximal disease score per mouse is 16.
Histopathologic analysis
The mouse joints were fixed in 4% paraformaldehyde
(Sigma), decalcified in EDTA (Sigma) and embedded in paraffin.
Sections (thickness, 7 μm) were prepared and stained with
hematoxylin and eosin (H&E) and Safranin O (Promega
Corporation, Madison, WI, USA to detect proteoglycans. On day 47
following immunization, the mice were sacrificed under
CO2 anaesthesia and the hind paws were fixed in 10%
buffered formalin, decalcified and embedded in paraffin. Joint
sections (thickness, 6 μm) were prepared and stained with
hematoxylin. Images were captured using an Olympus BX41 microscope
(Olympus, Center Valley, PA, USA). The histological scores were
evaluated, as previously described (18).
Measurement of anti-CII and serum
cytokine levels
Serum concentrations of anti-CII IgG and IgG2a were
measured using an enzyme-linked immunosorbent assay (ELISA). Blood
was collected at the end of study, immediately following sacrifice.
Microtiter plates were coated with CII (10 μg/ml), blocked and
incubated with serially diluted test sera. Bound IgG was detected
via incubation with horseradish peroxidase (HRP)-conjugated rat
anti-mouse IgG or IgG2a, and tetramethylbenzidine substrate.
Absorbance (wavelength, 450 nm) was measured with an ELISA plate
reader (3550; Bio-Rad, Hercules, CA, USA), and the values were
represented in arbitrary optical density (OD) units.
Serum and supernatant TNF-α, IL-1β, IL-23 and IL-17
were measured using the BD Cytometric Bead Array (CBA) and specific
ELISA kits for TNF-α, IL-1β, IL-23 and IL-17 (BD Biosciences, San
Jose, CA, USA) according to the manufacturer’s instructions. (BD
Biosciences, San Jose, CA, USA) according to the manufacturer’s
instructions.
Flow cytometry
Draining lymph node (DLN) cells from each treatment
group were cut and removed following sacrifice and made into a
single cell suspension. The cells were washed, incubated with
antibodies for fluorescence-activated cell sorting (FACS) and
analyzed with a FACS Canto II flow cytometer. The following
monoclonal antibodies were obtained from eBiosciences, Inc. (San
Diego, CA, USA): Anti-CD11b, anti-Gr-1, anti-CD4 (phycoerythrin;
PE), anti-CD25 (fluorescein isothiocyanate; FITC), anti-Foxp3
(PE-cyanine 5) and anti-IL-17 (FITC). Cells were restimulated with
50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin in
the presence of GolgiPlug™ for 5 h followed by intracellular
staining. Cells were fixed, permeabilized and stained with
fluorochrome-conjugated antibodies. The stained cells were then
analyzed using a FACSCalibur or BD FACSAria instrument. Unless
otherwise stated, all reagents and equipment was purchased from BD
Biosciences.
Human RA FLS collection and culture
Synovial tissues were obtained from patients with RA
during total knee replacement surgery or arthroscopic synovectomy.
The FLS from five patients were treated with PL (100 μg/ml) or a
vehicle. Tissues were digested with 4 mg/ml collagenase II (Sigma)
in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Grand Island< NY, USA) for ≥4 h at 37°C. Cell suspensions were
passed through a nylon mesh and the cell suspensions were collected
via centrifugation at 500 × g for 5 min and re-suspended in DMEM
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL).
Harvested cells were cultured in 75-cm2 culture flasks
(Costar, Cambridge, MA, USA) with DMEM supplemented with 10% FBS at
37°C in 5% CO2. Once the cells had grown to confluence
they were detached with 0.25% trypsin, split at a ratio of 1:3 and
re-cultured in DMEM under the same conditions. Cells obtained from
the fourth to sixth passages were used in the following
experiments.
Human RA FLS migration and invasion
In vitro migration assay of FLS chemotaxis
was performed using the Boyden chamber method with an 8.0-μm pore
size (Transwell; Corning Inc., Corning, NY, USA). DMEM containing
10% FBS as a chemoattractant was placed in the lower wells. FLS
(5×104 cells/ml) were suspended in serum-free DMEM in
the upper wells. The chamber was incubated at 37°C under 5%
CO2 for 6 h. Following incubation, the non-migrating
cells were removed from the upper surface of the filter using a
cotton swab. The filters were fixed in methanol for 20 min and
stained with 0.1% crystal violet for 20 min. Chemotaxis was
quantified by counting the stained cells which had migrated to the
lower side of the filter using an optical fluorescence microscope
(System Microscope BX53; Olympus, Center Valley, PA, USA)
For the in vitro invasion assay, similar
experiments were performed using inserts coated with a Matrigel
basement membrane matrix (BD Biosciences, Oxford, UK). To
investigate the effect of PL on RA FLS invasion, PL (100 μg/ml) was
added to the upper and lower wells. Following treatment with TNF-α
(100 ng/ml), cells were observed under a fluorescence microscope
(Olympus).
Statistical analysis
The differences between groups were determined by
analysis of variance and comparison between two groups was analyzed
with the Student’s t-test using SPSS, version 16.0 (SPSS, Inc.,
Chicago, IL, USA). Data are presented as the mean ± the standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
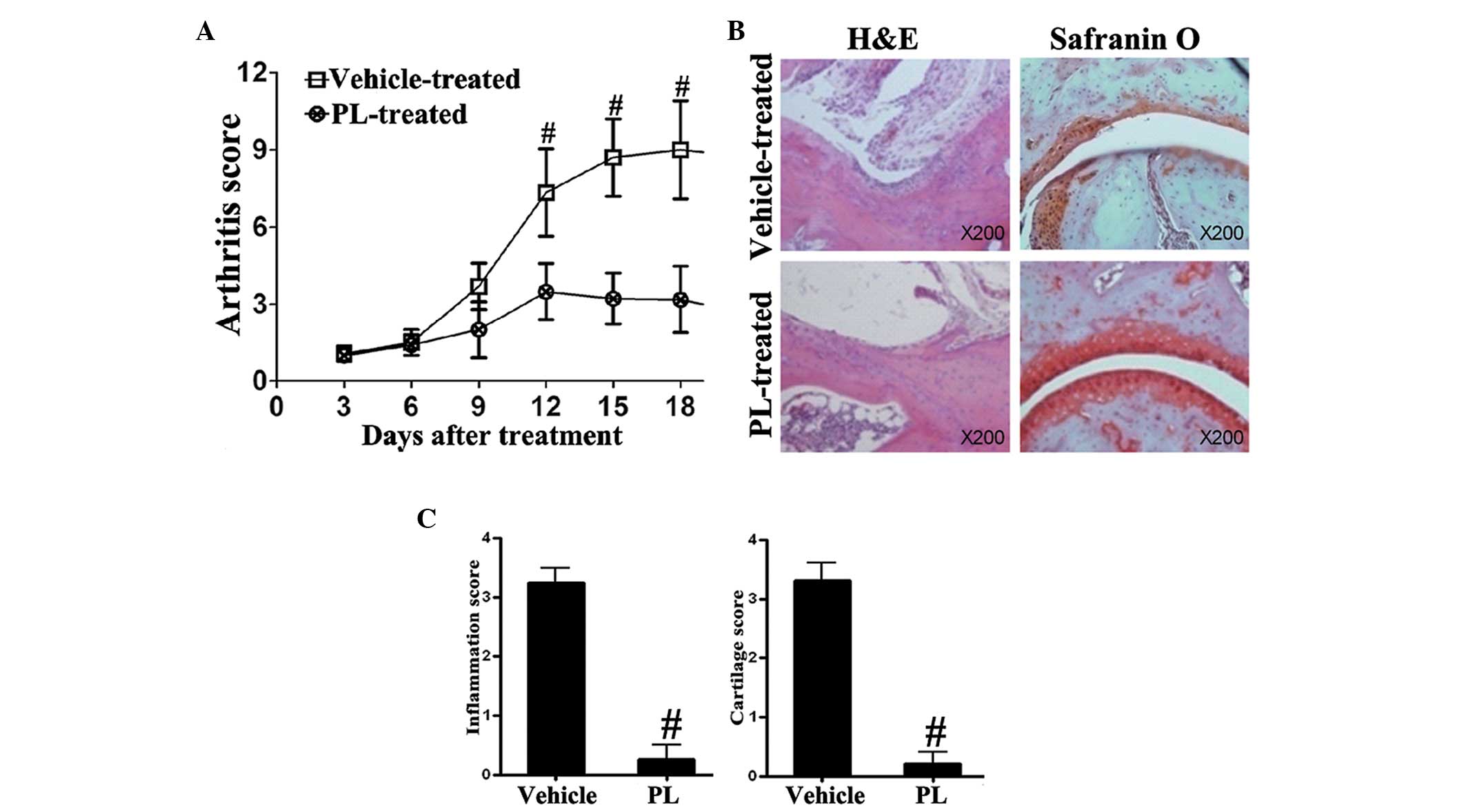
PL reduces arthritis and histopathologic
lesions in CIA mice
The clinical arthritis was assessed every three days
and scored. PL was observed to attenuate the clinical score of CIA
(Fig. 1A). In addition, H&E
and Safranin O staining were used to detect proteoglycans. It was
revealed that the histopathologic damage, including inflammatory
cell infiltration and cartilage destruction, was significantly
attenuated in PL-treated CIA mice in comparison with that in the
vehicle-treated CIA control mice (Fig.
1B and C).
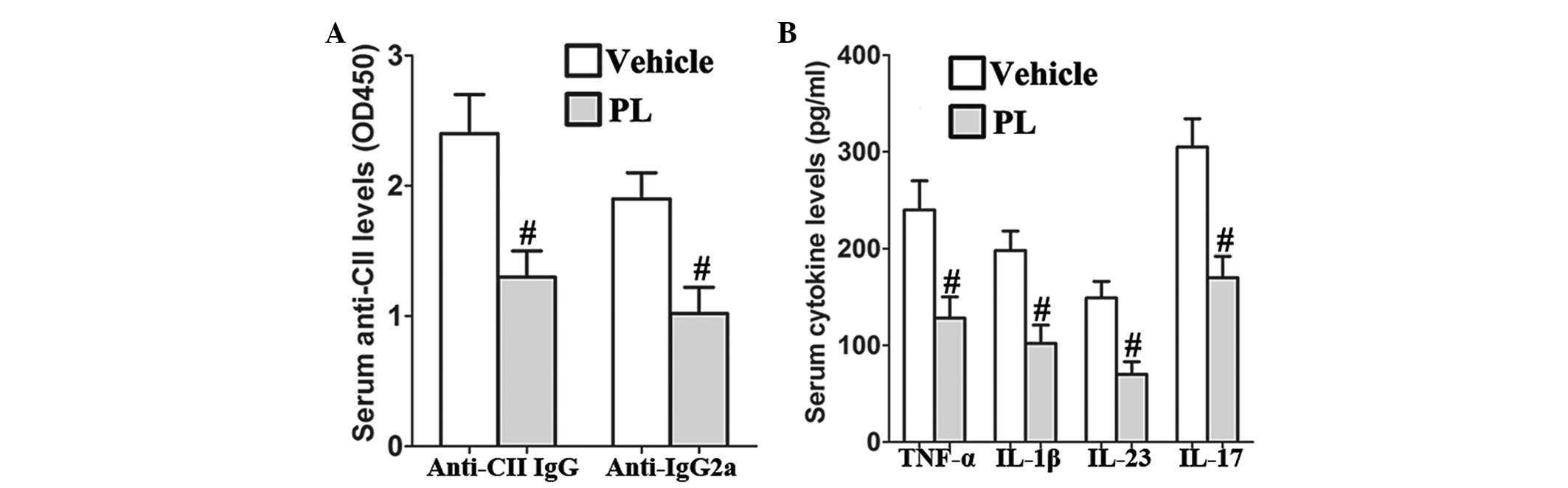
PL reduces the levels of serum anti-CII
and proinflammatory cytokines in CIA mice
PL significantly reduced the serum anti-CII IgG and
IgG2a levels as compared with those of the vehicle-treated CIA
control mice (Fig. 2A). In
addition, the results revealed that PL inhibits the production of
serum TNF-α, IL-1β, IL-23 and IL-17 in CIA mice as compared with
that in the vehicle-treated CIA control mice (Fig. 2B).
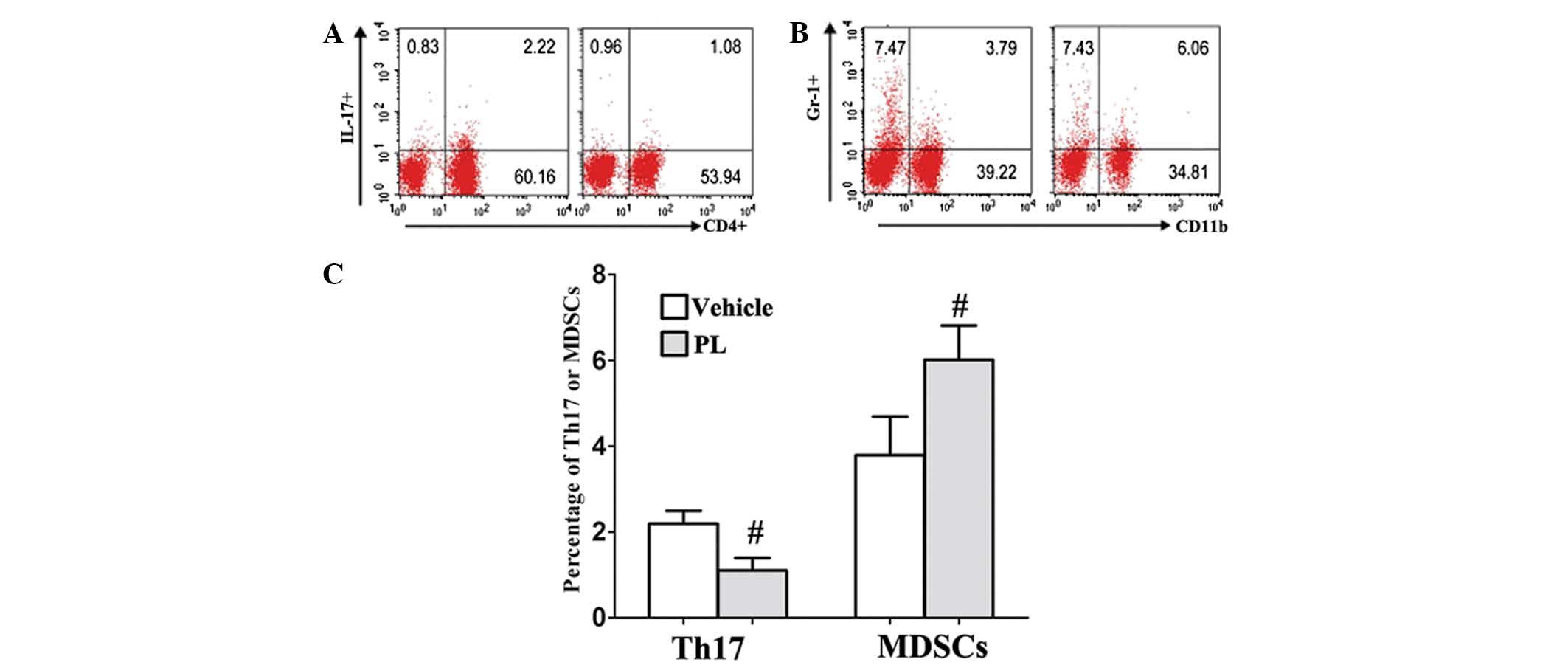
PL increases the levels of MDSCs and
decreases the levels of Th17
To evaluate the effect of PL on immune cell
responses in vivo, the frequencies of MDSCs,
Tregs and Th17 cells were examined in the DLNs of the
control and PL-treated CIA mice. The percentage of Th17 cells was
markedly decreased in the DLNs of the PL-treated CIA mice compared
with that of the vehicle-treated CIA control mice (Fig. 3A and C). Conversely, the number of
CD11b+Gr-1+ MDSCs showed a marked increase in
the DLN of the PL-treated CIA mice compared with that of the
vehicle-treated CIA control mice (Fig.
3B and C). In addition, the Tregs CD4+,
CD25+ and Foxp3+ frequency were not affected
by PL treatment (9.8±1.27, vs. 10.1±1.31%; P>0.05).
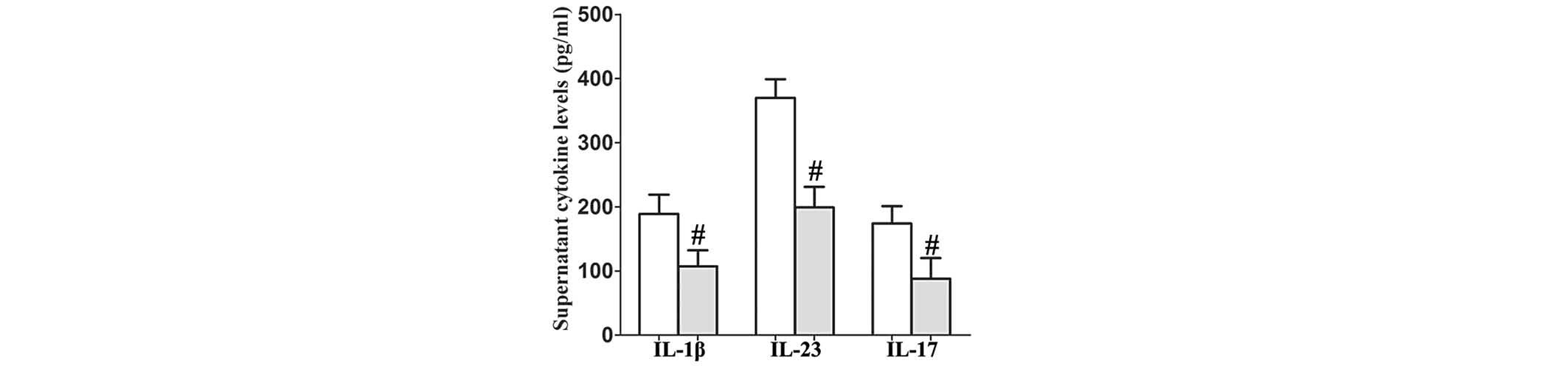
PL reduces the secretion of IL-1β, IL-23
and IL-17 by TNF-α-stimulated human RA FLS
To determine whether PL affects human RA FLS in a
similar way to that observed in mice, the human RA FLS were
pretreated with PL for 24 h and then stimulated by human TNF-α (100
ng/ml) for 48 h. The supernatants were then collected. It was
revealed that PL reduced the levels of secretion of IL-1β, IL-23
and IL-17 by human RA FLS as detected in the supernatants compared
with those in the controls (Fig.
4).
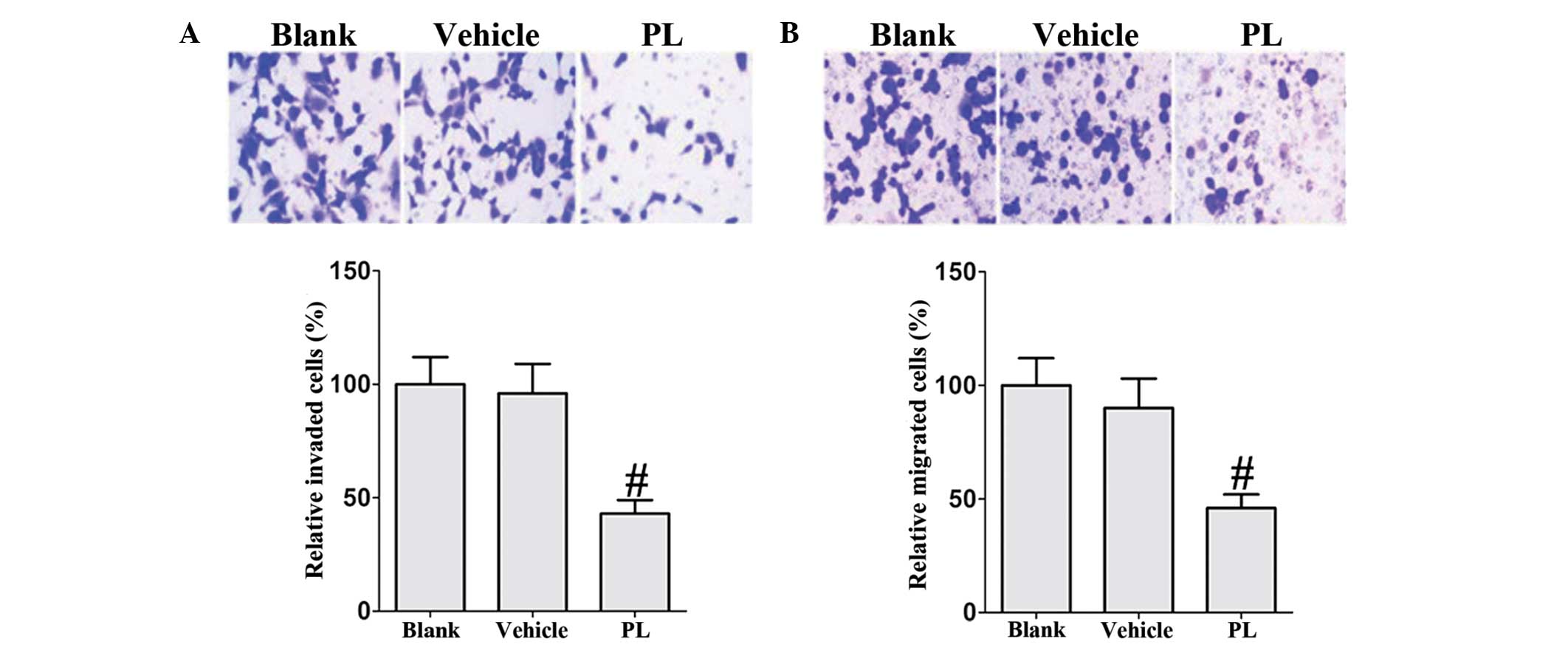
PL restricts the migration and invasion
of human RA FLS
Subsequently, the effect of PL on migration and
invasion of human RA FLS was investigated. Treatment with PL
substantially inhibited the migration and invasion of TNF-α-induced
RA FLS compared with that of the vehicle controls (Fig. 5). These results indicate that PL
inhibits the migratory behavior of human RA FLS.
Discussion
The results of the present study demonstrated the
effects of PL in a mouse model of CIA and in human RA FLS. PL
effectively alleviated CIA in mice, as indicated by the notable
reductions in the clinical arthritis score and the histological
lesions of CIA. The therapeutic effects of PL may be due to
immunomodulatory and anti-inflammatory activities. Immunomodulatory
activity was observed via the enhancement of MDSCs and the
suppression of the Th17 response. Anti-inflammatory activity was
demonstrated through the reduction of systemic levels of
pro-inflammatory cytokines. These therapeutic effects of PL are
also mediated by a decrease in migratory behavior as demonstrated
in human RA FLS.
Although the etiology of RA remains unknown and
controversy persists concerning the role of humoral and cellular
autoimmunity in the pathogenesis of RA, CD4+ T cells and
pro-inflammatory cytokines have been shown to have an important
role in CIA (19,20). MDSCs are of myeloid origin and are
able to suppress T cell responses. The roles of MDSCs in autoimmune
diseases have recently been elucidated in autoimmune arthritis. In
a mouse model of CIA, MDSCs inhibited the proliferation of
CD4+ T cells and their differentiation into Th17 cells
in addition to inhibiting the production of IFN-γ, IL-2, TNF-α, and
IL-6 by CD4+ T cells in vitro, while promoting
the production of IL-10 (19).
Adoptive transfer of MDSCs reduced the severity of CIA in
vivo, which was accompanied by a decrease in the number of
CD4+ T cells and Th17 cells in the DLNs (21). A subsequent study indicated that
the transfer of MDSCs significantly reduces the clinical score of
arthritis and alleviates joint inflammation and the progression of
CIA in antigen-induced arthritis and CIA models via the inhibition
of the proinflammatory immune response of CD4+ T cells
(22). These observations indicate
that MDSCs have crucial roles in the regulation of autoimmune
arthritis. In addition, Th17 cells are of critical importance in
immune response. Th17 cells produce cytokines with pro-inflammatory
effects, including IL-17, IL-6, IL-21, IL-22 and TNF-α, all of
which have a role in the immunopathogenesis of RA (23). IL-23 is involved in the maintenance
of Th17 cells and the IL-23/Th17 axis has been demonstrated to be a
potential target for the treatment of human RA (24). In the current study, it was
determined that PL treatment inhibits the production of serum
anti-CII, TNF-α, IL-1β, IL-23 and IL-17 in CIA mice. Additionally,
PL treatment reduces the secretion of IL-1β, IL-23 and IL-17 by
TNF-α-stimulated human RA FLS.
Notably, FLS are one of the dominant types of cell
in the hyperplastic rheumatoid synovium and they have an important
role in the pathogenesis of RA (25). A previous study indicated that the
capacity of FLS to migrate from joint to joint, and the mechanisms
by which FLS mediate persistence may offer disease-specific
therapeutic targets in human RA (26). In the present study, it was
revealed that PL significantly inhibits the TNF-α-induced migration
and invasion of RA FLS.
In conclusion, the results of the current study
revealed that PL reduces the arthritis score and joint destruction
via the expansion of MDSCs and the inhibition of the activation of
FLS. Hence, it is hypothesized that PL may be a candidate
therapeutic agent for RA.
References
|
1
|
Jang E, Kim HR, Cho SH, Paik DJ, Kim JM,
Lee SK and Youn J: Prevention of spontaneous arthritis by
inhibiting homeostatic expansion of autoreactive CD4+ T
cells in the K/BxN mouse model. Arthritis Rheum. 54:492–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanford SM, Maestre MF, Campbell AM,
Bartok B, Kiosses WB, Boyle DL, Arnett HA, Mustelin T, Firestein GS
and Bottini N: Protein tyrosine phosphatase expression profile of
rheumatoid arthritis fibroblast-like synoviocytes: a novel role of
SH2 domain-containing phosphatase 2 as a modulator of invasion and
survival. Arthritis Rheum. 65:1171–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang SK, Gu Z and Brenner MB:
Fibroblast-like synoviocytes in inflammatory arthritis pathology:
the emerging role of cadherin-11. Immunol Rev. 233:256–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HS, Ka SO, Lee SM, Lee SI, Park JW and
Park BH: Overexpression of sirtuin 6 suppresses inflammatory
responses and bone destruction in mice with collagen-induced
arthritis. Arthritis Rheum. 65:1776–1785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goh C, Narayanan S and Hahn YS:
Myeloid-derived suppressor cells: the dark knight or the joker in
viral infections? Immunol Rev. 255:210–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Young MR, Newby M and Wepsic HT:
Hematopoiesis and suppressor bone marrow cells in mice bearing
large metastatic Lewis lung carcinoma tumors. Cancer Res.
47:100–105. 1987.PubMed/NCBI
|
|
7
|
Monu NR and Frey AB: Myeloid-derived
suppressor cells and anti-tumor T cells: a complex relationship.
Immunol Invest. 41:595–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong YY, Fuchsberger M, Xiang SD,
Apostolopoulos V and Plebanski M: Myeloid derived suppressor cells
and their role in diseases. Curr Med Chem. 20:1437–1444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ioannou M, Alissafi T, Boon L, Boumpas D
and Verginis P: In vivo ablation of plasmacytoid dendritic cells
inhibits autoimmunity through expansion of myeloid-derived
suppressor cells. J Immunol. 190:2631–2640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alabanza LM, Esmon NL, Esmon CT and Bynoe
MS: Inhibition of endogenous activated protein C attenuates
experimental autoimmune encephalomyelitis by inducing
myeloid-derived suppressor cells. J Immunol. 191:3764–3777. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ioannou M, Alissafi T, Lazaridis I, Deraos
G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A,
Boumpas D and Verginis P: Crucial role of granulocytic
myeloid-derived suppressor cells in the regulation of central
nervous system autoimmune disease. J Immunol. 188:1136–1146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egelston C, Kurkó J, Besenyei T,
Tryniszewska B, Rauch TA, Glant TT and Mikecz K: Suppression of
dendritic cell maturation and T cell proliferation by synovial
fluid myeloid cells from mice with autoimmune arthritis. Arthritis
Rheum. 64:3179–3188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao Z, Hua S, Wang W, Wang H, Gao J and
Wang X: Increased circulating myeloid-derived suppressor cells
correlated negatively with Th17 cells in patients with rheumatoid
arthritis. Scand J heumatol. 42:85–90. 2013. View Article : Google Scholar
|
|
14
|
Ku SK, Kim JA and Bae JS: Piperlonguminine
downregulates endothelial protein C receptor shedding in vitro and
in vivo. Inflammation. 37:435–442. 2014. View Article : Google Scholar
|
|
15
|
Khathi A, Masola B and Musabayane CT:
Effects of Syzygium aromaticum-derived oleanolic acid on glucose
transport and glycogen synthesis in the rat small intestine. J
Diabetes. 5:80–87. 2013. View Article : Google Scholar
|
|
16
|
Nakagomi D, Ikeda K, Okubo, et al:
Ultrasound can improve the accuracy of the 2010 American College of
Rheumatology/European League Against Rheumatism classification
criteria for rheumatoid arthritis to predict the requirement for
methotrexate treatment. Arthritis Rheum. 65:890–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, et al: Selective killing of cancer cells by a small
molecule targeting the stress response to ROS. Nature. 475:231–234.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mauri C, Mars LT and Londei M: Therapeutic
activity of agonistic monoclonal antibodies against CD40 in a
chronic autoimmune inflammatory process. Nat Med. 6:673–679. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotzin B and Kappler J: Targeting the T
cell receptor in rheumatoid arthritis. Arthritis Rheum.
41:1906–1910. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malemud CJ and Miller AH: Pro-inflammatory
cytokine-induced SAPK/MAPK and JAK/STAT inrheumatoid arthritis and
the new anti-depression drugs. Expert Opin Ther Targets.
12:171–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujii W, Ashihara E, Hirai H, Nagahara H,
Kajitani N, Fujioka K, Murakami K, Seno T, Yamamoto A, Ishino H,
Kohno M, Maekawa T and Kawahito Y: Myeloid-derived suppressor cells
play crucial roles in the regulation of mouse collagen-induced
arthritis. J Immunol. 191:1073–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhang Z, Zhang H, Wu M and Wang
Y: Myeloid-derived suppressor cells protect mouse models from
autoimmune arthritis via controlling inflammatory response.
Inflammation. 37:670–677. 2014. View Article : Google Scholar
|
|
23
|
Noack M and Miossec P: Th17 and regulatory
T cell balance in autoimmune and inflammatory diseases. Autoimmun
Rev. 13:668–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neumann E, Lefèvre S, Zimmermann B, Gay S
and Müller-Ladner U: Rheumatoid arthritis progression mediated by
activated synovial fibroblasts. Trends Mol Med. 16:458–468. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filer A: The fibroblast as a therapeutic
target in rheumatoid arthritis. Curr Opin Pharmacol. 13:413–419.
2013. View Article : Google Scholar : PubMed/NCBI
|