Introduction
Vascular niches of cancer stem cells formed by
microvessels in a tumor have been considered a cause of local tumor
recurrence and metastasis (1,2).
Therefore, microvessels in stem cell niches constitute a new target
for cancer therapy and destruction of certain clusters of
microvessels may assist in eliminating cancer stem cells. Since
stem cells can be recruited to stem cell niches, a process termed
stem cell homing, the use of stem cells as vectors for gene therapy
has become an important topic of discussion in previous years
(3).
Adipose stem cells (ADSCs) isolated from adipose
tissue have previously been identified in adults (4) and have phenotypes and differentiation
potentials similar to those of bone marrow mesenchymal stem cells
(5). Numerous studies have
demonstrated that ADSCs possess an active homing capacity for
multiple types of cancer, including ovarian cancer, glioma and
metastatic lung cancer (4,6–8).
Additionally, ADSCs are easily isolated, cultured and transfected
in vitro and can survive following transplantation into
animals (3). Therefore, ADSCs are
good candidates for transporting anti-angiogenesis drugs in gene
therapy as they can migrate to the microvessels of cancer stem cell
niches, destroy the microvessels and ultimately eliminate the
cancer stem cells.
In a previous study, a DNA sequence expressing
Asn-Gly-Arg (NGR) and the cyclic peptide Cys-Asn-Gly-Arg-Cys
(CNGRC) was designed that could specifically penetrate breast
cancer cells (9). Additionally,
in vitro experiments have indicated that the CNGRC peptide
has antitumor activity (9). NGR is
a tri-peptide motif that can specifically bind to vascular
endothelial cells of newly generated blood vessels and such binding
is mediated by amino peptidase N (CD13). CD13 is a transmembrane
protein with a molecular weight of 140,000 Da. It is mainly
expressed in solid tumors and the blood vessels of new tumors, but
is rarely expressed in normal blood vessels. NGR peptide not only
binds to new vessels through CD13, but can also undergo deamination
of asparagine to form the isomer, isoDGR. isoDGR can regulate
adhesion and proliferation of epithelial cells and has a higher
affinity than NGR for blood vessels in new tumors (10). Following binding, NGR peptide can
be internalized into vascular endothelial cells by
receptor-mediated endocytosis. Based on this principle, NGR peptide
can carry compounds and particles, including cytotoxic drugs,
cytokines, virus particles and fluorescent compounds to new
vessels, which can markedly improve the efficacy of targeted
therapy (11). A number of studies
have used NGR motif containing peptides for ligand-mediated imaging
and treatment of tumors with new blood vessels (12–15).
For example, Corti (16) et
al used doxorubicin (DOX)-NGR conjugates to treat human
metastatic tumors in nude mice. Compared with free DOX, DOX-peptide
conjugates not only improved the efficacy of breast cancer
treatment, but also significantly reduced toxicity to the liver and
heart. Curnis et al (17)
demonstrated that use of tumor necrosis factor-NGR conjugates
improved drug permeability and the therapeutic effects of tumor
necrosis factor by 8–10-fold. However, stem cells expressing NGR
peptide and its conjugates were not available for assessment.
To establish an NGR expressing stem cell line, it is
essential to select a gene delivery vector that can stably express
CNGRC peptide for a long period of time. Eukaryotic viral vectors,
including adenovirus vectors, adeno-associated vectors, retrovirus
vectors and lentiviral vectors have high transduction efficiencies
and evident targeting capacities and are most commonly used in gene
therapy. However, retroviral vectors can only infect dividing cells
and cannot accommodate DNA fragments >8 kb (18). Additionally, adenovirus vectors
cannot achieve long-term expression of exogenous genes and repeated
application of the adenovirus can lead to immune responses
(19). By contrast, the human
immunodeficiency virus derived lentiviral vector can infect
dividing cells and non-dividing cells and produce long-term stable
expression of exogenous genes, without inducing an immune response
(20). Compared with other
conventional transduction methods, including liposome transduction,
calcium phosphate transduction and electroporation, use of a
lentivirus can also efficiently infect cells that are difficult to
transfect, including nerve cells, myocardial cells, endothelial
cells and cells grown in suspension (21,22).
Therefore, lentiviral vectors are the most satisfactory gene
transfer vectors for use in gene therapy.
In the present study, an enzymatic digestion and
repeat adherence methods were used to isolate highly purified ADSCs
and the feasibility of using a lentiviral vector to express CNGRC
peptide in ADSCs was investigated.
Materials and methods
Animal care and maintenance
Sprague Dawley (SD) rats aged 3 months and weighing
180–250 g were maintained at the Experimental Animal Center of
Medical School of Xi’an Jiaotong University (Xi’an, China). All
experimental manipulations were performed in accordance with
general guidelines of the Association for Assessment and
Accreditation of Laboratory Animal Care. The Animal Care and Use
Committee of Xi’an Jiaotong University reviewed and approved all
animal-related procedures.
Isolation and culture of primary
ADSCs
Bilateral samples of inguinal subcutaneous adipose
tissue (400–600 ml) were collected from adult male SD rats. The
samples were washed, cut into sections (13 mm) and
digested in 0.1% collagenase I at 37°C on a shaker (100 × g) for 60
min. Suspended fat was removed by centrifuging twice at 1,200 × g
for 12 min. The cells were then filtered and suspended in
Dulbecco’s modified Eagle’s medium (DMEM)/F12 containing 10% fetal
bovine serum and penicillin/streptavidin and cultured for 72 h at
37°C, in a 5% CO2 humidified incubator (Thermo Fisher
Scientific, Rockford, MA, USA). After 72 h, non-adherent red blood
cells were removed by changing the culture medium. Following
reaching 70–80% confluence, the fat cells were passaged at a ratio
of 1:2 into two T-25 cell culture flasks. The cells were then
cultured at 37°C in a 5% CO2 humidified culture
container. The culture medium was changed every 2–3 days.
Immunofluorescence staining
Surface markers CD29, CD34, CD106 and CD90 were used
to identify ADSCs. Following reaching 80% confluence, at the third
passage ADSCs were stained using fluorescence-labeled antibodies,
phycoerythrin (PE)-conjugated mouse-anti-rat CD106 monoclonal
antibody, PE mouse-anti-rat CD34 monoclonal antibody, PE
mouse-anti-rat CD90 monoclonal antibody and PE mouse-anti-rat CD29
monoclonal antibody (1:5,000; BioLegend, San Diego, CA, USA). Cells
were visualized and images were captured using an inverted
fluorescence microscope (ECLIPSE Ti; Nikon, Tokyo, Japan).
Adipogenic induction
ADSCs at the third passage were induced by addition
of an adipogenic inducer solution containing 10% fetal bovine
serum, 1 μmol/l dexamethasone, 0.5 mmol/l 3-isobutyl-1-methyl
xanthine and 1 μmol/l indomethacin in DMEM/F12. Control cells were
cultured in DMEM/F12 containing 10% fetal bovine serum. Cells were
maintained at 37°C in a 5% CO2 incubator and the medium
was changed every 3 days. Following a 2 week period of induction,
the cells were stained with Oil Red O and observed under an
inverted microscope (TMS; Nikon).
Osteogenic induction
ADSCs at the third passage were induced by addition
of induction media containing 1 μmol/l dexamethasone, 0.5 mmol/l
3-isobutyl-1-methylxanthine and 1 μmol/l indole indomethacin.
Control cells were cultured in DMEM/F12 containing 10% fetal bovine
serum. Cells were maintained at 37°C in a 5% CO2
incubator and the medium was changed every 3 days. Following a
3-week induction period, the cells were stained with alizarin red
and cells exhibiting calcium nodule formation were observed under
an inverted microscope (TMS; Nikon).
Lentivirus packaging and quantitative
fluorescence polymerase chain reaction (PCR)
Aliqots of 293T cells (GeneChem, Montreal, Canada)
were cultured in high-glucose DMEM supplemented with 10% fetal
bovine serum. Recombinant virus plasmid Ubi-CNGRC-3Flag-EGFP and
packaging plasmids (Helper 1.0 and Helper 2.0) were prepared using
an EndoFree Plasmid Maxi kit (Qiagen, Amsterdam, The Netherlands).
Three plasmids were co-transfected into 293T cells using
Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA).
After a 48 h transduction period, the supernatant containing
lentiviral particles was harvested and concentrated by super-speed
centrifugation at 4,000 × g for 10 min. Viral titers were measured
by quantitative PCR, using GAPDH as an internal control. The Ct
value was defined as the number of cycles when the fluorescent
signal reached a specified threshold. The sequences of primers were
as follows: EGFP, forward 5′-TGCTTCAGCCGCTACCC-3′ and reverse
5′-AGTTCACCTTGATGCCGTTC-3′; GAPDH, forward
5′-TGACTTCAACAGCGACACCCA-3′ and reverse
5′-CACCCTGTTGCTGTAGCCAAA-3′.
Lentiviral infection of ADSCs
Concentrated lentiviral solutions of LV-EGFP and
LV-CNGRC-3Flag-EGFP were added into two wells of cultured ADSCs,
respectively, once the cells reached 40% confluence. Enhanced
infection solution was then added to reach a total incubation
volume of 2 ml. After 12 h of incubation, the cell culture medium
was changed and 72 h later, images of the cells expressing EGFP
were captured under an inverted fluorescence microscope (ECLIPSE
Ti; Nikon). Images were processed using NIS-Elements imaging
software (Nikon Instruments, Inc., Tokyo, Japan).
Immunoblot analysis
Stable transfected ADSCs were selected with
puromycin (Sigma-Aldrich, St. Louis, MO, USA) and CNGRC expression
levels of cells at the third passage (12 days after transduction)
were identified by western blot analysis. Total protein was
extracted from ADSCs transfected with LV-EGFP and
LV-CNGRC-3Flag-EGFP, respectively. Following denaturation, the
protein samples were centrifuged at 12,000 × g for 2 min. The
target proteins were hybridized with mouse anti-flag tag monoclonal
antibody (1:1,000; Proteintech, Chicago, IL, USA), mouse
anti-β-actin monoclonal antibody (1:5,000; Proteintech) and
horseradish-peroxidase-conjugated goat anti-mouse IgG polyclonal
antibody (1:10,000; CWBio, Beijing, China) IgG. Enhanced
chemiluminescent substrates (Pierce Biotechnology, Inc., Rockford,
IL, USA) were used to detect the signals of targeted proteins.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard error of the mean. The paired-sample t-test was
used for comparisons among multiple groups and P<0.05 was
considered to indicate a statistically significant difference.
Results
Successful isolation and culture of
ADSCs
ADSCs were isolated from rat adipose tissue by use
of enzymatic digestion. Twenty-four hours after seeding, several
transparent adherent cells with round or oval morphology were
visible under a microscope and at 4 days after seeding, additional
ADSCs with fusiform and polygonal morphology were observed. At
10–11 days after seeding, the cell clones had significantly
expanded, become connected to each other and reached 80–90%
confluence. Cells with long spindle-shaped morphology were closely
arranged in a swirling pattern with multilayer cells in the center.
Following passage, the ADSCs demonstrated a higher growth rate.
Without alterations in cell morphology, the doubling time of these
cells was 70 h and the cells could be successfully sub-cultured
(Fig. 1).
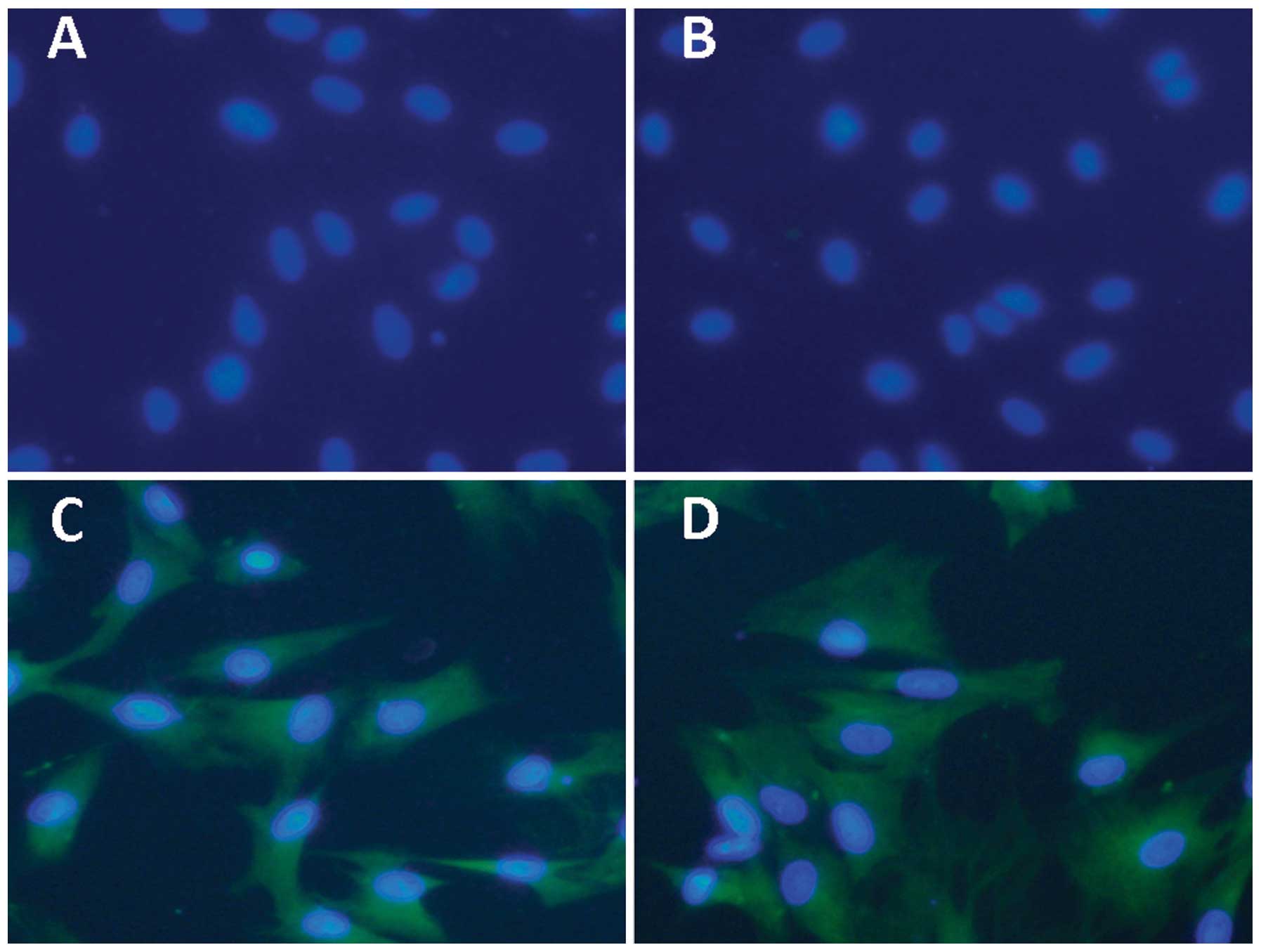
ADSCs express typical surface markers in
culture
ADSCs were identified by cell surface markers
(23). Previous studies reported
that ADSCs expressed the cell surface markers CD90, CD29, CD49e,
CD54, CD55, CD63, CD73 and CD105, but did not express CD11b, CD34,
CD31, HLA-DR and CD117 (24,25).
Zuk et al (26) revealed
that ADSCs expressed CD49d but not CD106, while bone marrow
mesenchymal stem cells expressed CD106 but not CD49d. In the
present study, >80% of cultured cells were CD29 and CD90 double
positive (Fig. 2C and D) and CD106
and CD34 double negative (Fig. 2A and
B). These findings indicated that ADSCs were successfully
isolated and cultured in vitro.
ADSCs differentiate to form adipocytes in
culture
Adipogenic inducers were added to cultures of ADSCs
to determine their capacity for adipogenesis. Following induction
for 24 h, the cells became smaller in size and the cytoplasm began
to retract and demonstrated a small square morphology. The
refractive index of these cells was also enhanced. At 3–4 days
after induction, cell proliferation slowed significantly and small,
round, shiny lipid droplets had formed in the cytoplasm.
Observations made at longer time periods following induction
revealed enlargement of lipid droplets in the cytoplasm and
increased cell numbers (Fig. 3A and
C). At 2 weeks after induction, ADSCs were stained with Oil Red
O, which revealed the presence of various sized round red particles
and fat droplets in the cytoplasm. By contrast, no significant
difference was identified in cell size or formation of small lipid
droplets in cells not treated with the adipogenic inducer (Fig. 3B and D).
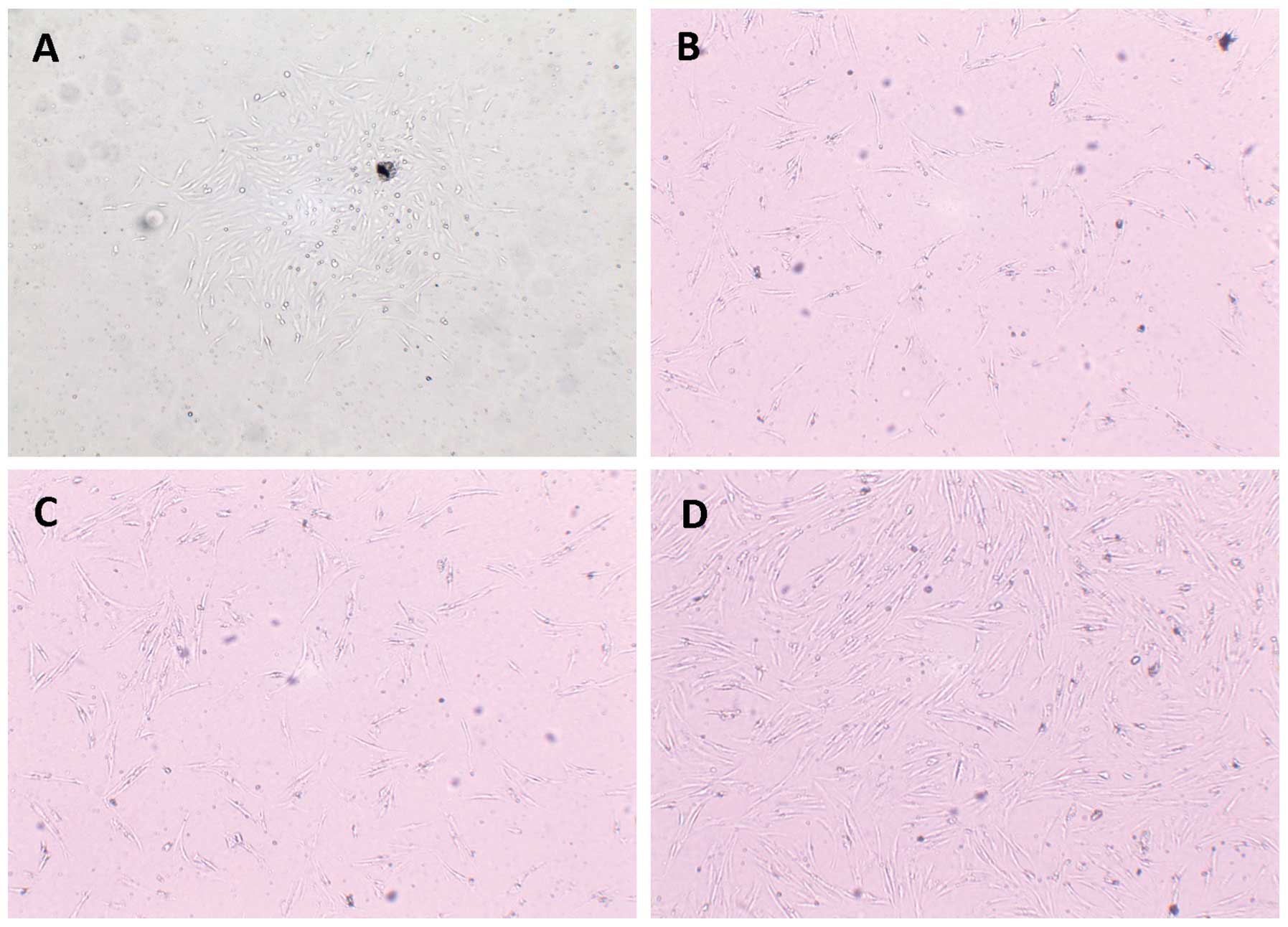
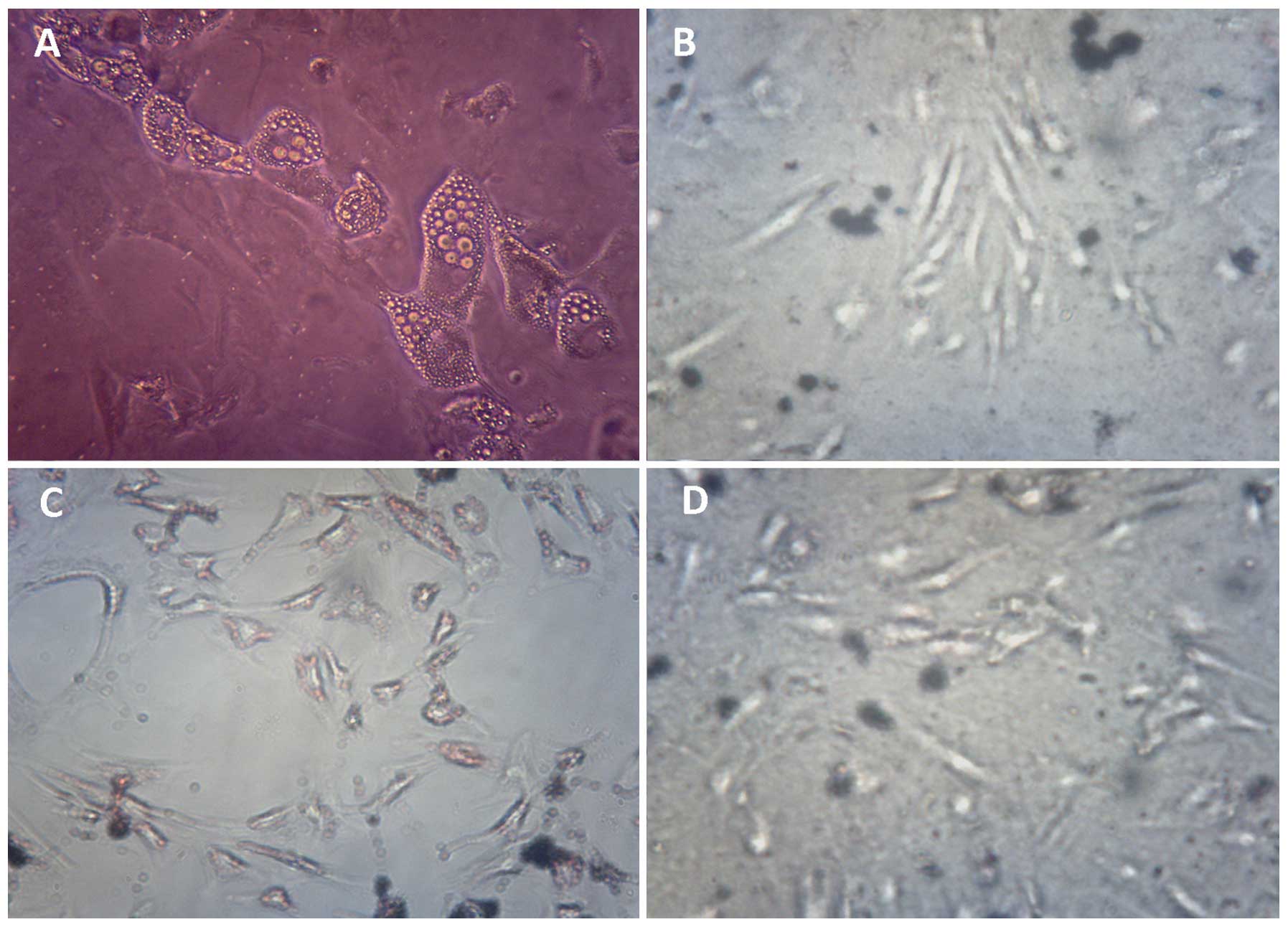 | Figure 3ADSCs following adipogenic induction.
(A and B) ADSCs were cultured in induction medium (A,
magnification, ×2,000) or normal medium (B, magnification, ×600)
for 1 week. (C and D) Oil Red O staining-labeled fat droplets in
ADSCs cultured in induction medium for 2 weeks (C, magnification,
×600) but not in cells cultured in normal medium (D, magnification,
×600). ADSCs, adipose stem cells. |
ADSCs differentiate to form osteocytes in
culture
Osteogenic inducers were added to cultures of ADSCs
to examine their capacity for osteogenesis. After 3 days of
induction, the morphology of ADSCs altered from a long spindle
shape into a round or irregular shape. Additionally, cell body size
increased, cellular projections disappeared and the cell
proliferation rate decreased. One week after induction, the cells
formed colonies, the nuclei became rounded and larger and
additional small black particles appeared in the cytoplasm.
Following longer periods of induction, the cells became distributed
surrounding the colonies and grew in an overlapping manner. At 3
weeks after induction, the cells had secreted large quantities of
extracellular matrix and formed growing granular calcium nodules of
varying sizes (Fig. 4A). The
mineralized nodules were distributed in colonies and had a
reddish-brown color following alizarin red staining, confirming
calcium deposition (Fig. 4C). By
contrast, there was no calcium deposition in cells that were not
subjected to induction (Fig. 4B and
D).
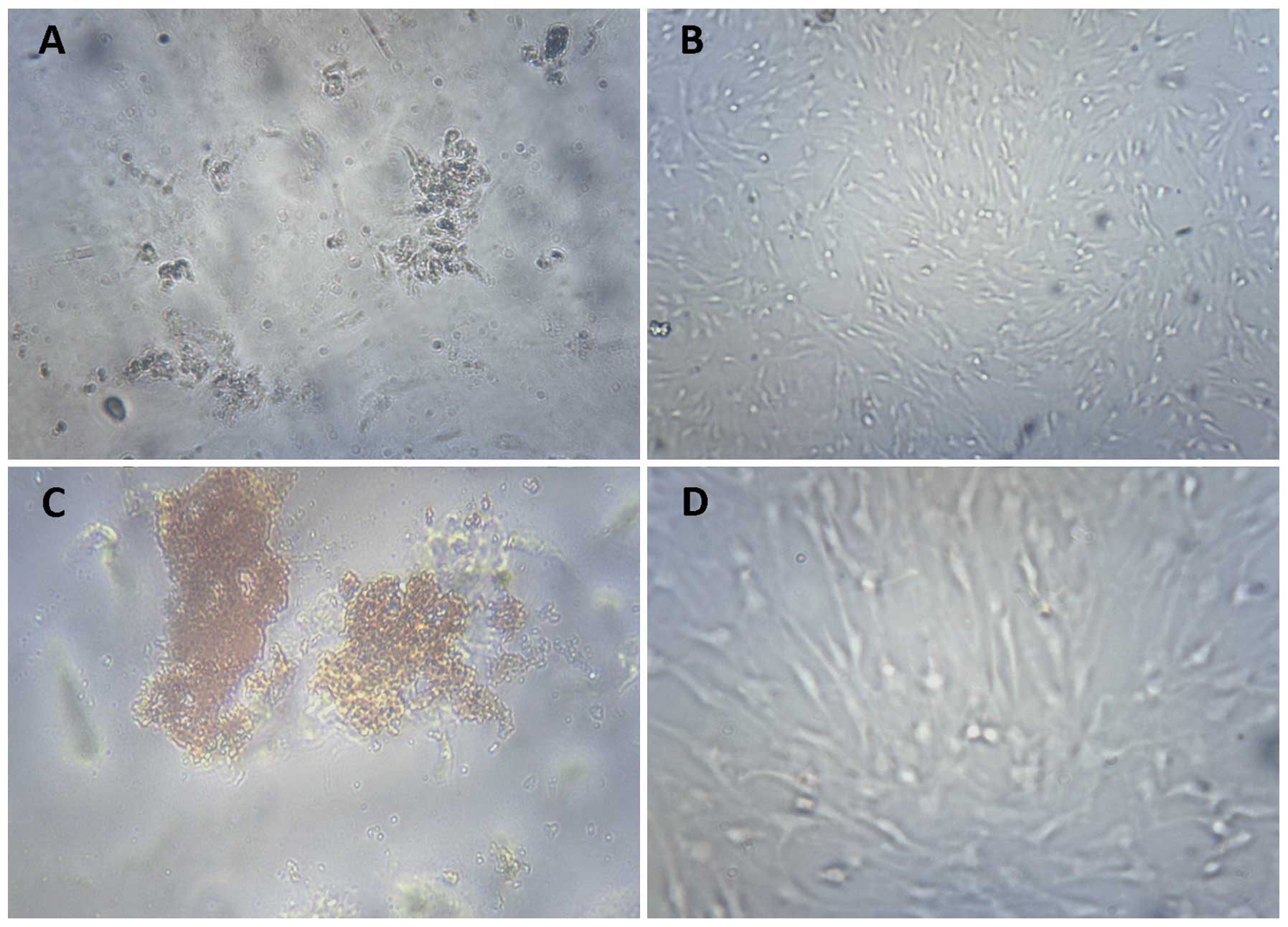 | Figure 4ADSCs following osteogenic induction.
(A and B) ADSCs were cultured in induction medium (A,
magnification, ×400) or normal medium (B, magnification, ×400) for
3 weeks. (C and D) Alizarin red stained calcium nodules in ADSCs
cultured in induction medium (C, magnification, ×2,000) but not in
cells cultured in normal medium (D, magnification, ×600). ADSCs,
adipose stem cells. |
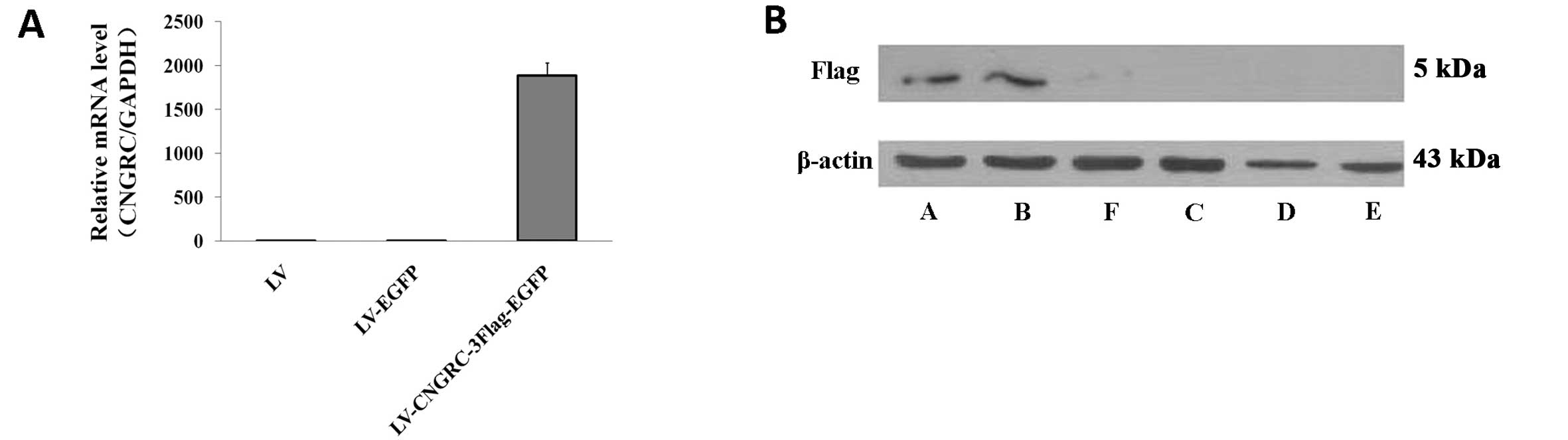
Expression of CNGRC in 293T cells and
successful packaging of lentiviral vectors
Lentiviral vectors LV-EGFP and LV-CNGRC-3Flag-EGFP
were co-transfected with Helper 1.0 and Helper 2.0 into 293T cells.
CNGRC RNA levels in transfected and non-transfected cells were
measured 72 h later by quantitative fluorescence PCR. CNGRC RNA
levels in transfected cells were significantly higher (P<0.05)
than levels in non-transfected and LV-EGFP-transfected cells
(Table I and Fig. 5A), indicating successful expression
of CNGRC in transfected cells. The titer of the lentivirus was
calculated as 2.00E+8TU/ml, based on the difference in Ct values
between non-transfected cells and transfected cells.
 | Table IRelative quantities of CNGRC mRNA in
non-transfected, LV-EGFP-transfected and
LV-CNGRC-3Flag-EGFP-transfected adipose stem cells. |
Table I
Relative quantities of CNGRC mRNA in
non-transfected, LV-EGFP-transfected and
LV-CNGRC-3Flag-EGFP-transfected adipose stem cells.
| Relative mRNA
level | LVa | LV-EGFPb |
LV-CNGRC-3Flag-EGFPab |
|---|
| (Average ±
STDEV) | 1.054±0.413 | 0.967±0.538 | 1883.897±145.304 |
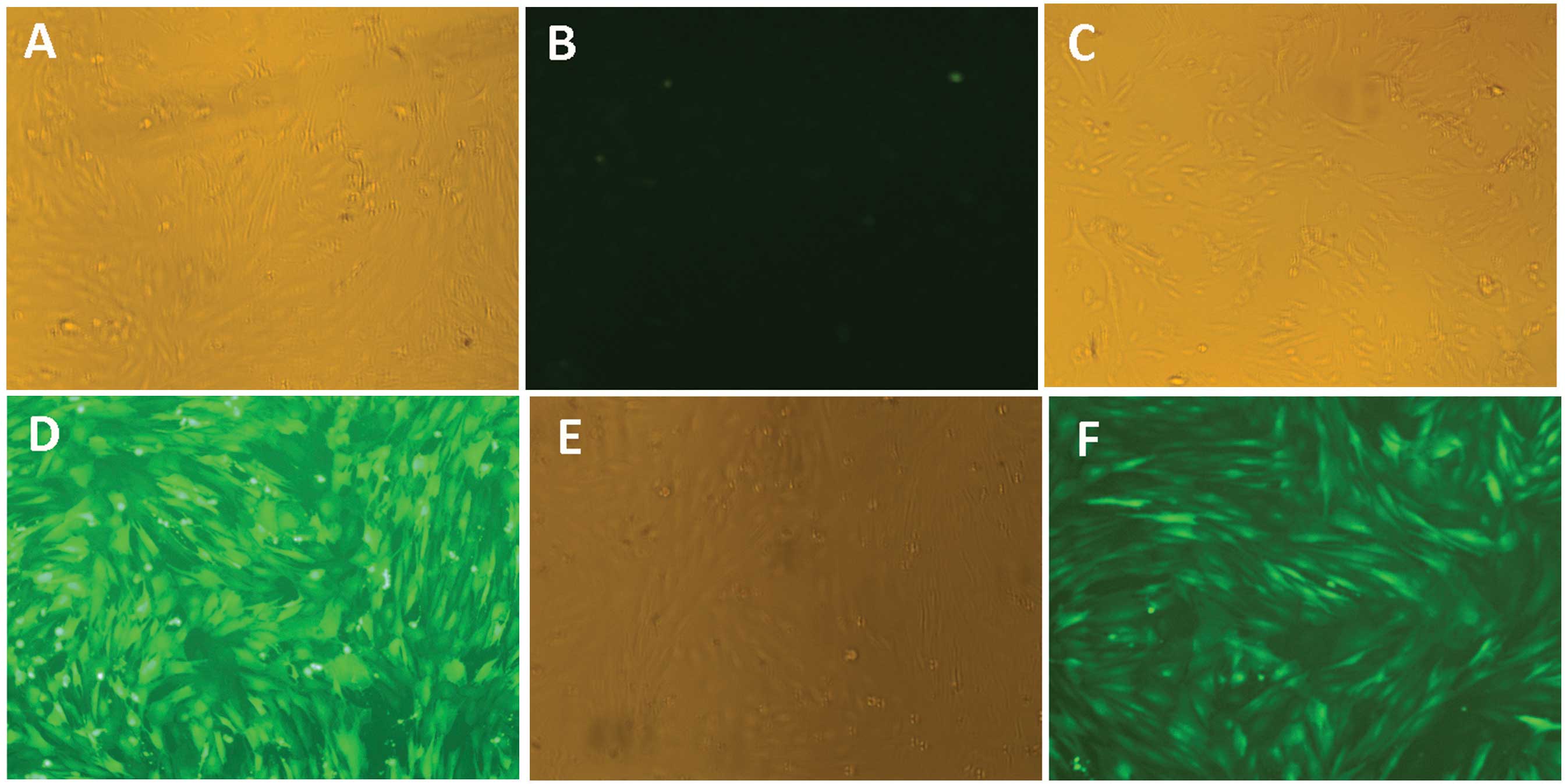
Expression of exogenous DNA in ADSCs
following lentivirus infection
To examine expression of exogenous DNA, transfected
ADSCs were observed under a fluorescence microscope to detect the
expression of EGFP after 72 h of transduction. LV-EGFP and
LV-CNGRC-3Flag-EGFP demonstrated transduction efficiencies >80%.
As shown in Fig. 6, EGFP was
highly expressed in ADSCs transfected with the lentivirus. By
contrast, no EGFP signal was detected in non-transfected cells.
These results indicated that the exogenous gene could be expressed
using lentiviral transduction.
Expression of CNGRC peptide in ADSCs
following lentiviral transduction
To confirm expression of the CNGRC peptide, total
proteins were extracted from ADSCs transfected with LV-EGFP,
LV-CNGRC-3Flag-EGFP and without transduction, respectively.
CNGRC-3Flag-EGFP was detected at the expected size by western blot
analysis using anti-flag antibody in LV-CNGRC-3Flag-EGFP
transfected cells, but was not detected in either non-transfected
or LV-EGFP-transfected cells (Fig.
5B). The results clearly demonstrated expression of CNGRC
peptide in ADSCs at the third passage, 12 days after infection with
the lentivirus.
Discussion
ADSCs and bone marrow mesenchymal stem cells have
similar phenotypes and differentiation potentials. However, it is
easier to obtain large numbers of ADSCs than large numbers of
mesenchymal stem cells (27).
Bacigalupo et al (28)
could obtain only 40 ml of human bone marrow under local
anesthesia, which yielded ~1.2×105 stem cells. To obtain
larger quantities of bone marrow mesenchymal stem cells, surgery
must be performed under whole-body anesthesia, which may cause
other complications. By contrast, Aust et al (29) revealed that up to 4×106
stem cells could be obtained from 200 ml of adipose tissue, which
was ~30-fold greater than the number of mesenchymal stem cells
obtained from bone marrow. In addition, previous studies have
reported successful transduction of ADSCs using a lentivirus and
identified that transduction efficiency was significantly greater
when using ADSCs rather than bone marrow mesenchymal stem cells
(30–32). In the present study, it was
demonstrated that ADSCs could be easily isolated from rat adipose
tissue and that transduction efficiency using lentiviral vectors
was >80%. Additionally, DNA expressing CNGRC peptide was
successfully transfected using lentiviral vectors.
Microvessels surrounding cancer stem cells (stem
cell niche) are important in regulating cancer stem cell numbers
and their functions (1,2). As mentioned previously, targeting of
microvessels in stem cell niches has been suggested as an efficient
method for treating tumors. NGR is a tri-peptide motif that can
specifically bind to vascular endothelial cells of newly generated
vessels in tumors (9). Based on
this biological function, NGR-conjugates have been used in tumor
diagnosis and treatment (12–17).
However, the efficiency achieved when using this approach has been
low due to the lack of a targeting vehicle. It was suggested that
the efficient homing capacity of ADSCs could be exploited by using
them as targeting vehicles to deliver NGR-conjugates specifically
to cancer stem cell niches (3). To
achieve this goal, it was first necessary to establish an ADSC cell
line that stably expresses NGR-conjugates. In the present study,
ADSC cell lines expressing the CNGRC peptide were established using
lentiviral transduction. The mechanism by which these
CNGRC-expressing ADSC cell lines migrate to cancer stem cell niches
and eliminate newly generated microvessels remains to be
elucidated. Future studies are to be conducted to assess the homing
capacity of these ADSC cell lines and the in vivo vascular
tropism effects of the CNGRC peptide.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81201680).
The authors would like to thank Medjaden Bioscience Limited for
assisting in the preparation of this manuscript.
References
|
1
|
Matsumoto Y, Iwasaki H and Suda T:
Maintenance of adult stem cells: Role of the stem cell niche. Adult
Stem Cells. Springer Science+Business Media, LLC; pp. 35–55. 2011,
View Article : Google Scholar
|
|
2
|
Takeda N, Jain R, LeBoeuf MR, et al:
Interconversion between intestinal stem cell populations in
distinct niches. Science. 334:1420–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuhbier JW, Weyand B, Radtke C, et al:
Isolation, characterization, differentiation and application of
adipose-derived stem cells. Adv Biochem Eng Biotechnol. 123:55–105.
2010.
|
|
4
|
Josiah DT, Zhu D, Dreher F, et al:
Adipose-derived stem cells as therapeutic delivery vehicles of an
oncolytic virus for glioblastoma. Mol Ther. 18:377–385. 2010.
View Article : Google Scholar :
|
|
5
|
Schäffler A and Büchler C: Concise review:
adipose tissue-derived stromal cells-Basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar
|
|
6
|
Cho JA, Park H, Lim EH, et al: Exosomes
from ovarian cancer cells induce adipose tissue-derived mesenchymal
stem cells to acquire the physical and functional characteristics
of tumor-supporting myofibroblasts. Gynecol Oncol. 123:379–386.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rowan BG, Gimble JM, Sheng M, et al: Human
adipose tissue-derived stromal/stem cells promote migration and
early metastasis of triple negative breast cancer xenografts. PLoS
One. 28:e895952014. View Article : Google Scholar
|
|
8
|
Park YM, Yoo SH and Kim SH:
Adipose-derived stem cells induced EMT-like changes in H358 lung
cancer cells. Anticancer Res. 33:4421–4430. 2013.PubMed/NCBI
|
|
9
|
Hou L, Zhao X, Wang P, et al: Antitumor
activity of antimicrobial peptides containing CisoDGRC in CD13
negative breast cancer cells. PLoS One. 8:e534912013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robak LA, Venkatesh K, Lee H, et al:
Molecular basis of the interactions of the Nogo-66 receptor and its
homolog NgR2 with myelin-associated glycoprotein: development of
NgROMNI-Fc, a novel antagonist of CNS myelin inhibition. J
Neurosci. 29:5768–5783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curnis F, Cattaneo A, Longhi R, et al:
Critical role of flanking residues in NGR-to-isoDGR transition and
CD13/integrin receptor switching. J Biol Chem. 285:9114–9123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ndinguri MW, Solipuram R, Gambrell RP, et
al: Peptide targeting of platinum anti-cancer drugs. Bioconjug
Chem. 20:1869–1878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yokoyama Y and Ramakrishnan S: Addition of
an aminopeptidase N-binding sequence to human endostatin improves
inhibition of ovarian carcinoma growth. Cancer. 104:321–331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Wei H, Zhen Y, et al: Relationship
between receptor expression on cell surface of several solid tumors
and tumor sensitivity to IFN-α 2a. Journal of the Fourth Military
Medical University. 10:865–868. 2008.(In Chinese).
|
|
15
|
Meng J, Ma N, Yan Z, et al: NGR enhanced
the anti-angiogenic activity of tum-5. J Biochem. 140:299–304.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corti A, Pastorino F, Curnis F, et al:
Targeted drug delivery and penetration into solid tumors. Med Res
Rev. 32:1078–1091. 2012. View Article : Google Scholar
|
|
17
|
Curnis F, Sacchi A and Corti A: Improving
chemotherapeutic drug penetration in tumors by vascular targeting
and barrier alteration. J Clin Invest. 110:475–482. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Podolska K, Stachurska A, Hajdukiewicz K,
et al: Gene therapy prospects - intranasal delivery of therapeutic
genes. Adv Clin Exp Med. 21:525–534. 2012.PubMed/NCBI
|
|
19
|
Christ M, Lusky M, Stoeckel F, et al: Gene
therapy with recombinant adenovirus vectors: evaluation of the host
immune response. Immunol Lett. 57:19–25. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou YF: In vitro study of HCN4
transfected biological pacemaker cells using lentivirus. Soochow
University. 47–49. 2009.
|
|
21
|
Zhao L, Wu J, Zhou H, et al: Local gene
delivery for cancer therapy. Curr Gene Ther. 11:423–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YP and Berkhout B: miRNA cassettes in
viral vectors: problems and solutions. Biochim Biophys Acta.
1809.732–745. 2011.
|
|
23
|
Nery AA, Nascimento IC, Glaser T, et al:
Human mesenchymal stem cells: from immunophenotyping by flow
cytometry to clinical applications. Cytometry A. 83:48–61. 2013.
View Article : Google Scholar
|
|
24
|
Zuk PA, Zhu M, Ashjian PH, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rangappa S, Fen C, Lee EH, et al:
Transformation of adult mesenchymal stem cells isolated from the
fatty tissue into cardiomyocytes. The Annals of thoracic surgery.
75:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: Implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Araña M, Mazo M, Aranda P, et al: Adipose
tissue-derived mesenchymal stem cells: isolation, expansion and
characterization. Methods Mol Biol. 1036:47–61. 2013. View Article : Google Scholar
|
|
28
|
Bacigalupo A, Socié G, Schrezenmeier H, et
al: Bone marrow versus peripheral blood as the stem cell source for
sibling transplants in acquired aplastic anemia: survival advantage
for bone marrow in all age groups. Haematologica. 97:1142–1148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aust L, Devlin B, Foster SJ, et al: Yield
of human adipose-derived adult stem cells from liposuction
aspirates. Cytotherapy. 6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dragoo JL, Choi JY, Lieberman JR, et al:
Bone induction by BMP-2 transduced stem cells derived from human
fat. J Orthop Res. 21:622–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun XZ, Liu GH, Wang ZQ, et al:
Over-expression of VEGF165 in the adipose tissue-derived stem cells
via the lentiviral vector. Chin Med J (Engl). 124:3093–3097.
2011.
|
|
32
|
Liu Y, Chen C, He H, et al:
Lentiviral-mediated gene transfer into human adipose-derived stem
cells: role of NELL1 versus BMP2 in osteogenesis and adipogenesis
in vitro. Acta Biochim Biophys Sin (Shanghai). 44:856–865. 2012.
View Article : Google Scholar
|