Introduction
Rheumatoid arthritis (RA) is a systemic disease
characterized by synovial inflammation. The combination of the
proliferation of synovial lining cells and the infiltration of
inflammatory cells, including monocytes and activated leukocytes,
into joint tissues contributes to ‘pannus’ tissue formation,
tumor-like growth and eventually to extensive synovial inflammation
and joint destruction (1,2). Thus, the continued neovascularization
of pannus tissues may facilitate the penetration of inflammatory
cells into the synovium and thereby stimulate pannus formation
(3). Increased angiogenesis is one
characteristic of RA, and rheumatoid joints also contain elevated
levels of pro-angiogenic molecules, including vascular endothelial
growth factor (VEGF), basic fibroblast growth factors (FGF),
hypoxia-inducible factor-1, and angiopoietins (4). Furthermore, preclinical studies have
indicated that angiogenesis inhibitors are able to reduce pannus
formation, inflammation and joint erosion; and that therapeutic
targeting of angiogenesis has demonstrated beneficial effects in
the treatment of diseases, including colorectal, kidney and lung
cancer (4,5). Thus, angiogenesis inhibitors may be
developed as potential therapies.
Endocan, which was previously named endothelial
cell-specific molecule (ESM-1), has been studied as a
pro-angiogenic factor in tumor tissues (6). It was originally cloned from human
endothelial cells in 1996 by Lassalle et al (7). Structurally, endocan is a 50 kDa
secretory proteoglycan composed of a mature polypeptide of 165
amino acids with a single dermatan sulfate chain covalently linked
to Ser137 (6). It is a
relatively unusual molecule in that it is able to freely circulate
in the blood and carries only one GAG chain. Endocan binds
CD11a/CD18 integrin (also known as leukocyte function-associated
antigen-1) on human leukocytes, inhibiting its binding to
intercellular adhesion molecule 1 and thereby preventing
transendothelial migration (7–9). The
silencing of endocan in hepatocellular carcinoma resulted in
decreased cell migration, invasion and survival (10). Furthermore, endocan has been
suggested to be a specific biomarker of tip cells during
neoangiogenesis (6). The
expression of endocan is upregulated by pro-inflammatory molecules,
including tumor necrosis factor alpha (TNF-α), as well as
pro-angiogenic molecules, including VEGF and FGF-2 (6,11,12).
These physiological functions of endocan may
additionally be involved in the angiogenesis of pannus in
rheumatoid arthritic joints, which are characterized by tumor-like
growth. To the best of our knowledge, the relevance of endocan to
RA has not previously been studied. In addition, a previous study
by our group indicated that adiponectin, which was recently
demonstrated to be involved in RA pathogenesis, stimulated the
expression of VEGF in FLSs to the same extent as interleukin-1 β
(IL-1β), one of the most important stimulators of FLSs. This result
suggested that adiponectin was also an important stimulant of
angiogenesis in arthritic joints (13). In the present study, endocan
expression in arthritic joints was evaluated and those cells which
contributed most to endocan production were identified.
Furthermore, the effects of adiponectin on the expression of
endocan in the FLSs of arthritic joints were examined. In the
present study, for the first time, to the best of our knowledge, it
is concluded that endocan expression is increased in arthritic
synovial tissues and that adiponectin is an important factor
involved in mediating the increased endocan expression observed in
synovial cells.
Materials and methods
Synovial tissues and joint fluid
collection
Synovial tissues were collected from patients with
RA or OA following the attainment of informed consent. Patients met
the 1987 American College of Rheumatology criteria for the
diagnosis of RA, had been treated with non-biological,
disease-modifying anti-rheumatic drugs and had undergone
therapeutic joint surgery. Ethical approval was obtained from the
Institutional Review Board for Human Research of Kyung Hee
University Hospital at Gangdong, Republic of Korea.
Cell culture
FLSs from patients with RA were isolated and grown
in Dulbecco’s modified Eagle’s medium (DMEM, low glucose; Gibco
Invitrogen Inc., Grand Island, NY, USA) as described previously
(13). Human umbilical vein
endothelial cells were obtained from Cell Applications Inc. (San
Diego, CA, USA). Once the cells had grown to confluence, they were
split at a 1:4 ratio. Passages three to six were used for all
experiments. The cells were treated with IL-1β (0.1 or 1 ng/ml;
ProSpec, Rehovot, Israel) or adiponectin (1 or 10 μg/ml; ProSpec).
Culture supernatants were collected for the analysis of VEGF and
endocan by ELISA, and the cells were used for total RNA
extraction.
Measurement of gene expression by
ELISA
The cells (2.5×105 cells/60 mm dish/2 ml
serum-free media) were treated with recombinant adiponectin (1 or
10 μg/ml) or IL-1β (0.1 or 1 ng/ml; ProSpec, Rehovot, Israel).
Conditioned media was collected following 24 h. Briefly, culture
supernatants were centrifuged and the supernatants were collected
and analyzed for endocan (Lunginnov, Lille, France) and VEGF
(R&D Systems Inc., Minneapolis, MN, USA) with ELISA kits. Three
independent experiments were performed in quadruplicate.
Polymerase chain reaction (PCR) analysis
of messenger RNA (mRNA) expression levels
Culture supernatants were harvested and the cells
were subsequently used to measure gene expression levels, as
described previously (14).
Briefly, complementary DNA was synthesized from 1 μg of total RNA
in a 20 μl reverse transcription reaction mixture. For
semi-quantitative PCR, aliquots of cDNA were amplified with the
primers in a 25-μl PCR mixture containing 1X PCR buffer, 0.625
units TaKaRa Ex TaqTM HS and 0.2 μM specific upstream primers,
according to the manufacturer’s instructions (TaKaRa Bio, Kyoto,
Japan). The PCR conditions for VEGF and endocan were as follows:
95°C for 45 sec, 55–60°C for 45 sec and 72°C for 45 sec; repeated
for 30–33 cycles. The PCR products were subjected to
electrophoresis on a 1.5% agarose gel containing ethidium bromide
(Bio-Rad, Hercules, CA, USA) and the bands were visualized under
ultraviolet light. For quantitative PCR, the reaction was performed
using the LightCycler PCR system (Roche Diagnostics, Meylan,
France) and the DNA binding SYBR Green I dye was used to detect the
PCR products. Product specificity was determined by melting curve
analysis as described in the LightCycler manual. Results are
expressed as ratios of endocan transcripts to β-actin transcripts,
with the quantity of transcripts in each sample expressed as a copy
number. The primers were synthesized by Bioneer Co. Ltd. (Seoul,
Korea), and their sequences are listed in Table I.
 | Table IThe sequence of polymerase chain
reaction primers used in this experiment. |
Table I
The sequence of polymerase chain
reaction primers used in this experiment.
| Primer name | Primer sequence | Product size |
|---|
| MMP-1 sense | 5′-CCT AGC TAC ACC
TTC AGT GG-3′ | 338 bp |
| MMP-1 antisense | 5′-GCC CAG TAC TTA
TTC CCT TT-3′ | |
| MMP-13 sense | 5′-TTG AGG ATA CAG
GCA AGA CT-3′ | 311 bp |
| MMP-13 antisense | 5′-TGG AAG TAT TAC
CCC AAA TG-3′ | |
| MMP-2 sense | 5′-ACT TCA GGC TCT
TCT CCT TT-3′ | 288 bp |
| MMP-2 antisense | 5′-TTC AGA CAA CCT
GAG TCC TT-3′ | |
| Endocan sense | 5′-TGC CTG AAA TTC
CCC TTC TT-3′ | 152 bp |
| Endocan
antisense | 5′-TTC CTC ATT ACG
GGA GAC CC-3′ | |
| β-actin sense | 5′-TCA TGA GGT AGT
CAG TCA GG-3′ | 305 bp |
| β-actin
antisense | 5′-CTT CTA CAA TGA
GCT GCG TG-3′ | |
Transfection of small interfering RNA
(siRNA)
FLSs were transiently transfected with siRNA that
targeted endocan (Bioneer Co. Ltd.) in Lipofectamine 2000 (Gibco),
according to the manufacturer’s instructions. Briefly, siRNA (1 μg)
for endocan (GenBank accession number NM_007036.3) was suspended in
100 μl Lipofectamine solution and mixed with an equal volume of
serum-free DMEM (Gibco). The mixture was added to 5×105
FLSs cultured in 100-mm dishes. (BD Biosciences, Franklin Lakes,
NJ, USA) Control siRNA was used as a negative control. Following 6
h of incubation, the transfected cells were washed twice with
phosphate-buffered saline, replenished with fresh medium and grown
under IL-1β stimulation (10 ng/ml) for 24 h. The knockdown of
endocan was determined by reverse transcription PCR. Three
independent experiments using the synovial cells of one patient
with RA were performed in quadruplicate.
Cell migration and invasion assays
Migration and invasion were examined by Transwell
assay using a CytoSelectTM 24-Well kit (Cell Biolabs, Inc., San
Diego, CA, USA), according to the manufacturer’s instructions. For
the migration assay, briefly, the inner chambers of the transwells
containing polycarbonate membrane inserts were seeded with 0.3 ml
synovial cells (0.6×105 cells/well) that were
transfected with endocan siRNA or control siRNA. Media containing
10% fetal bovine serum (Sigma-Aldrich St. Louis, MO, USA) was added
to the lower well of the migration plate. IL-1β was added to the
upper well containing the cells which were activated with IL-1β for
24 h. The migrated cells were stained with a cell staining solution
and extracted with an extraction solution (both Cell Biolabs, San
Diego, CA, USA) according to the manufacturer’s instructions. The
optical density of the extracted solution was measured at 560 nm
using an Emax Microplate Reader (Molecular Devices, Sunnyvale, CA,
USA). For the invasion assay, the kit required a 24-well plate
containing polycarbonate membrane inserts; the upper surface of the
insert membrane was coated with a uniform layer of dried basement
membrane matrix solution. This basement membrane layer served as a
barrier to discriminate invasive from non-invasive cells. The
invasion assay was performed simultaneously using an identical
protocol to that used for the migration assay but with a different
insert.
Histopathology
Specimens were fixed in 10% buffered formalin (DNA
Korea, Incheon, Korea), processed routinely and embedded in
paraffin. Sections (4 μm) of paraffin blocks were cut and
subsequently stained with hematoxylin and eosin (H&E) and
immunohistochemical stain. Immunohistochemical staining was
performed in a Bond-Max automated slide stainer (Leica
Microsystems, Newcastle, UK) using monoclonal mouse endocan/ESM-1
antibody (1/5,000; LIA-0901, Lunginnov, Lille, France). Antigens
(Leica Microsystems) were retrieved with epitope retrieval solution
1 (Leica Microsystems). Slides were incubated with the antibody at
room temperature for 20 min and subsequently incubated with a
biotinylated secondary antibody for 8 min. The resulting complexes
were detected using avidin-peroxidase conjugate polymer. Color was
developed using 3,3′-diaminobenzidine (ScyTek, Logan, UT, USA).
Mayer’s hematoxylin (Leica Microsystems) was used as a
counterstain. Positive and negative control staining were used. For
the evaluation of endocan and VEGF expression, the area of
cytoplasmic staining was determined as a percentage and scored as
follows: 1, staining in <10% of cells; 2, staining in 10–50% of
cells and 3, staining in >50% of cells.
Statistical analysis
The in vitro experimental data were expressed
as the mean ± standard error of the mean (SEM) of quadruplicate
samples. Differences between groups were assessed using repeated
analysis of variance followed by the Dunnett multiple comparison
test. The degree of inflammation observed in H&E-stained
sections, mRNA expression levels determined by PCR and the cell
migration and invasion tests in endocan siRNA-transfected cells
were compared between groups with the Mann-Whitney U test. Prism
software 5.02 (GraphPad Software, Inc., San Diego, CA, USA) was
used for statistical analysis and graphing. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Increased expression of endocan and VEGF
in inflammatory arthritic joints
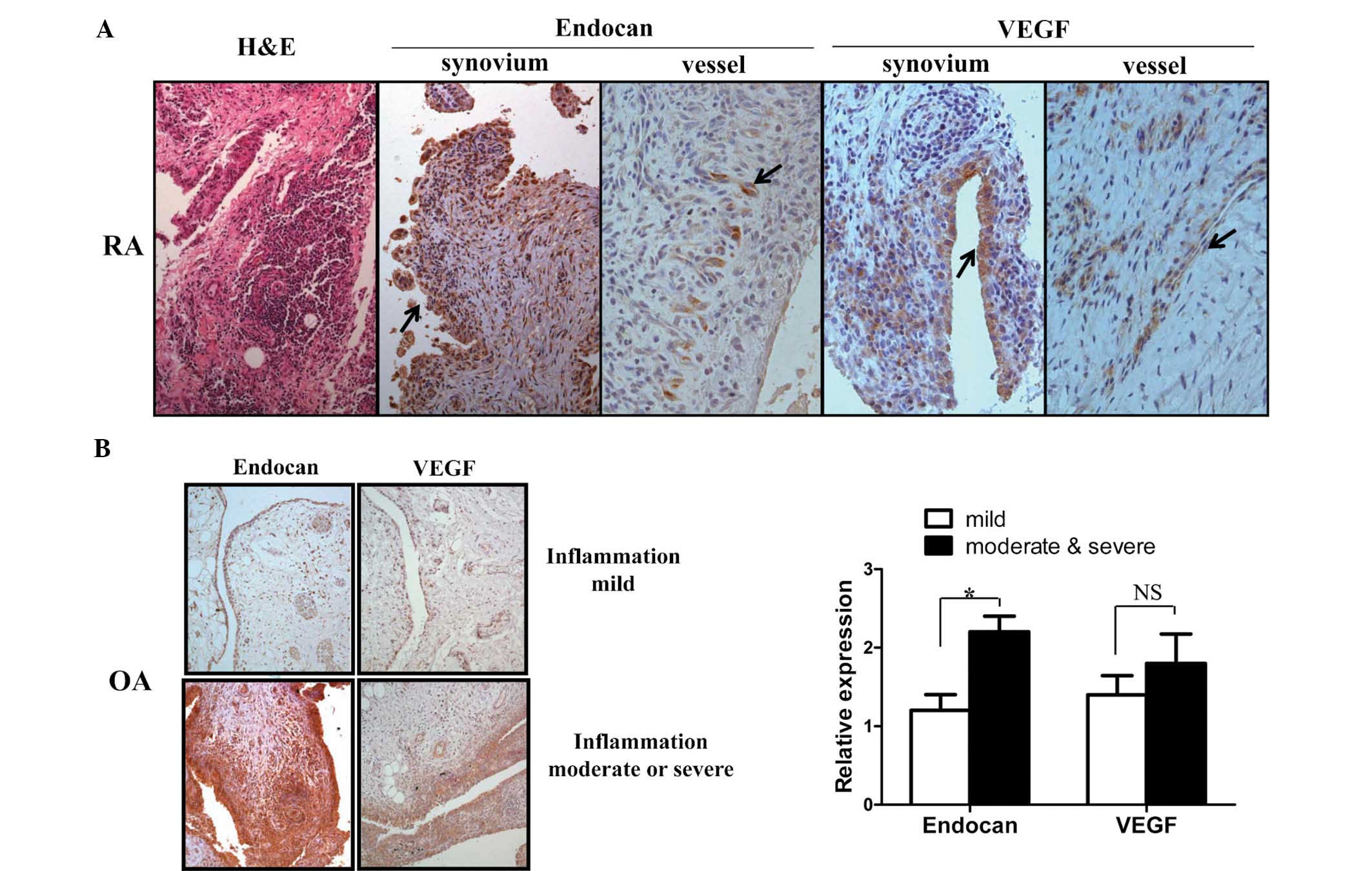
Synovial tissues from patients with RA (n=3) and OA
(n=10) were immunohistochemically stained with antibodies to
endocan and VEGF in order to examine their expression in arthritic
joints. Endocan expression was detected in vascular endothelial
cells, infiltrated lymphocytes and proliferating synovial cells in
synovial tissues of RA (Fig. 1A).
Based on synovial tissues from three RA patients, expression of
endocan in synovial cells was correlated with the degree of
inflammation, though its expression remained unaltered in vessels
regardless of the degree of inflammation. Similarly, VEGF
expression was also detected in these cells; however, endocan
expression in RA synovial cells was markedly higher than that of
VEGF. Subsequently, to evaluate endocan and VEGF expression in
synovial tissues of OA patients and determine their association
with the degree of inflammation, OA synovial tissues (n=10) were
divided into mild, moderate, and severe groups according to the
degree of inflammation observed. The degree of inflammation was
evaluated by the number of infiltrated immune cells, based on a
relative degree of inflammation. The endocan expression levels in
OA tissues with moderate and severe inflammation were approximately
two-fold higher than those of tissues with mild inflammation.
Similarly, VEGF expression was higher in tissues exhibiting
moderate and severe inflammation; however, this had no statistical
significance (Fig. 1B). These
results suggested that endocan expression was significantly
upregulated in inflamed arthritic synovial tissues.
Comparison of endocan and VEGF expression
in endothelial cells and FLSs under pro-inflammatory
stimulation
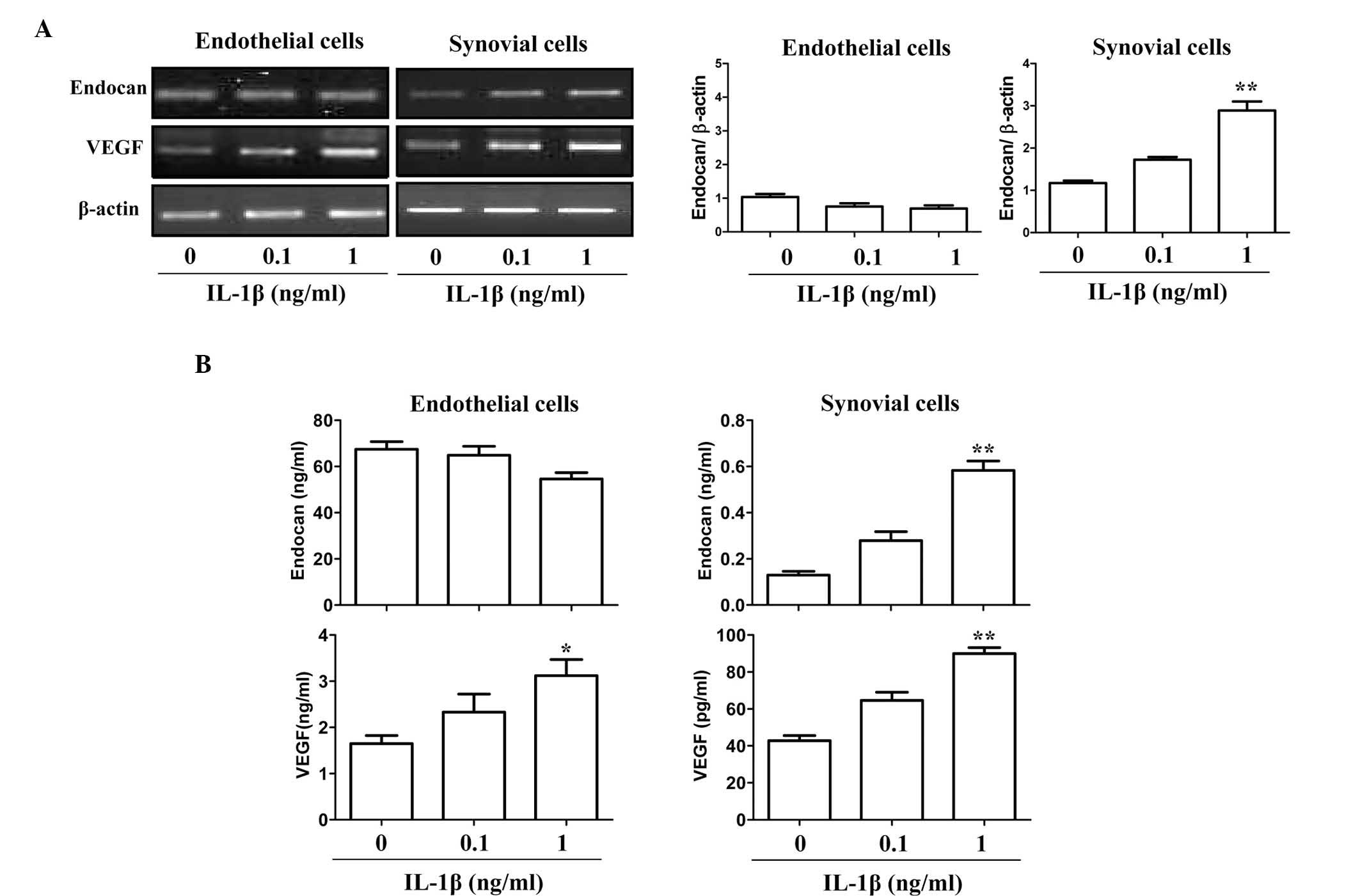
To further provide indirect evidence of the
expression patterns of endocan and VEGF in arthritic tissues, two
gene expression patterns in cultured endothelial cells and FLSs
under pro-inflammatory stimulation were investigated in
vitro by PCR. The endocan gene was constitutively expressed in
cultured endothelial cells, and its expression was not
significantly increased in response to IL-1β (0.1–1 ng/ml)
(Fig. 2A). By contrast, VEGF
expression was significantly increased following IL-1β stimulation
in endothelial and synovial cells. Consistent with the observed RNA
expression levels, endocan protein was constitutively expressed in
cultured endothelial cells and its expression levels (mean ± SEM)
were not increased in response to stimulation with 1 ng/ml IL-1β
(67.44±3.31 ng/ml vs. 54.63±2.75 ng/ml) (Fig. 2B). However, the protein expression
levels of endocan in synovial cells in response to IL-1β (1 ng/ml)
were increased ~5-fold compared to those with no stimulation
(0.58±0.04 vs. 0.12±0.01 ng/ml). The relative protein expression
levels of endocan in endothelial cells were ~100-fold greater than
those of FLSs (54.63±2.75 vs. 0.58±0.04 ng/ml); however, IL-1β
significantly increased the expression of endocan in synovial cells
(Fig. 2B). Conversely, the
relative VEGF protein expression levels in synovial cells were
~30-fold higher than those in endothelial cells (89.94±3.29 vs.
3.12±0.34 pg/ml), although IL-1β stimulation induced VEGF
expression in both cell types. These results suggested that endocan
was mainly produced in endothelial cells and partly produced in
synovial cells of arthritic joints under inflammatory stimulation,
whereas VEGF was produced equally in both cell types.
Effect of adiponectin on the expression
of endocan in synovial cells
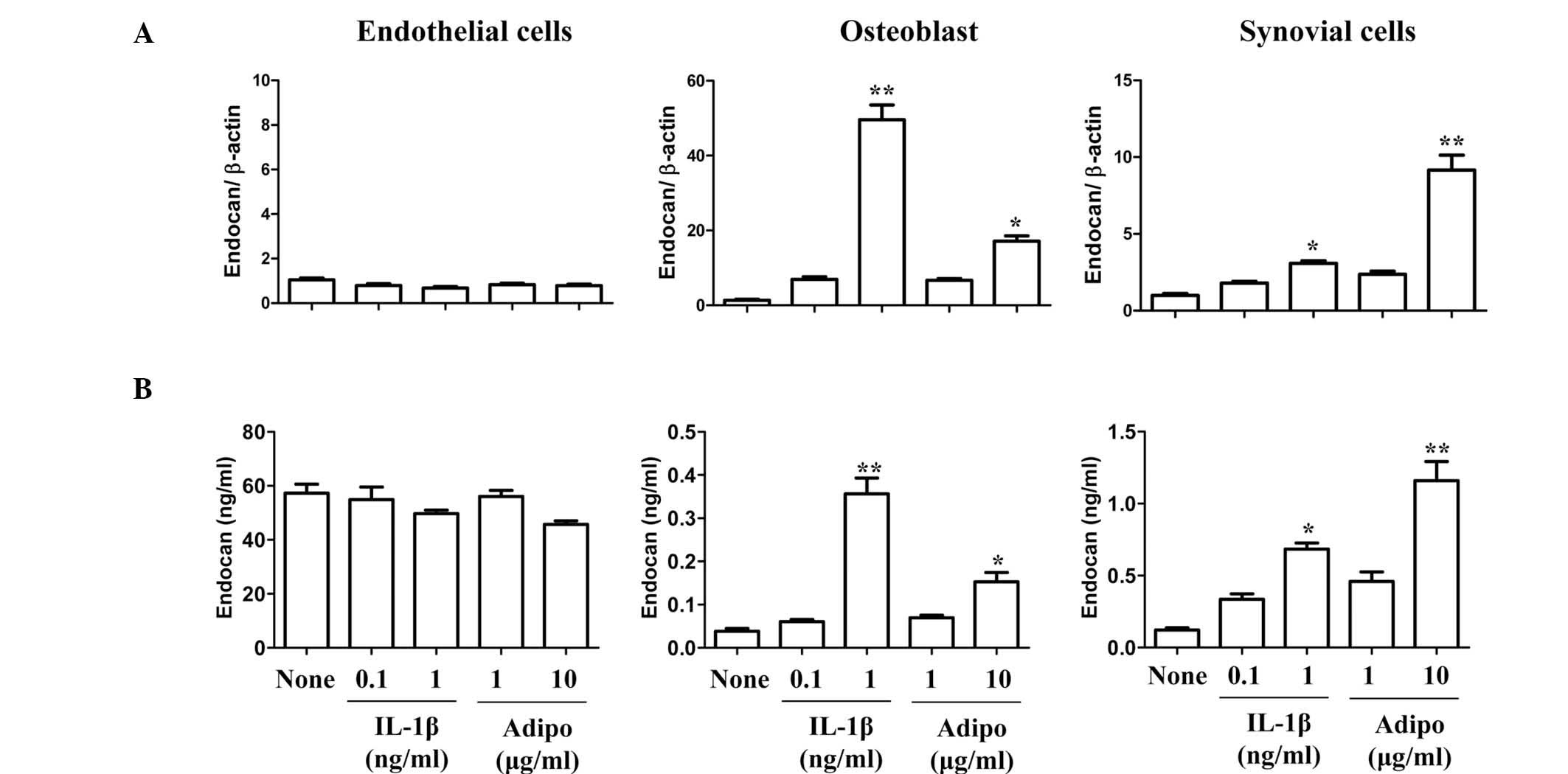
In previous studies by our group, adiponectin was
shown to have an important function in angiogenesis, as it was a
potent stimulant of VEGF in synovial cells, similarly to IL-1β
(13). Therefore, the stimulatory
effect of adiponectin on endocan expression in arthritic joints was
investigated. FLSs and endothelial cells were stimulated with
adiponectin or IL-1β (Fig. 3). The
expression pattern of endocan was compared in three different cell
types. Endocan expression in adiponectin (10 μg/ml)-stimulated
synovial cells was ~4-fold and ~3-fold higher than in
IL-1β-stimulated cells at concentrations of 0.1 and 1 ng/ml,
respectively. Considering the physiological concentrations of IL-1β
(<0.1 ng/ml) and adiponectin (1–10 μg/ml) in joint fluids,
adiponectin is more likely to be significantly involved in the
production of endocan in synovial cells. However, endocan
expression was not stimulated by adiponectin in endothelial cells.
In addition, the expression of VEGF and endocan in osteoblasts was
stimulated by adiponectin in a similar pattern to that of synovial
cells (data not shown). These results suggested that endocan was
constitutively expressed in endothelial cells, and that adiponectin
was a more potent stimulant of endocan production in synovial cells
and osteobalsts than IL-1β at physiological concentrations.
Endocan gene knockdown by siRNA inhibits
the invasiveness and migration of synovial cells
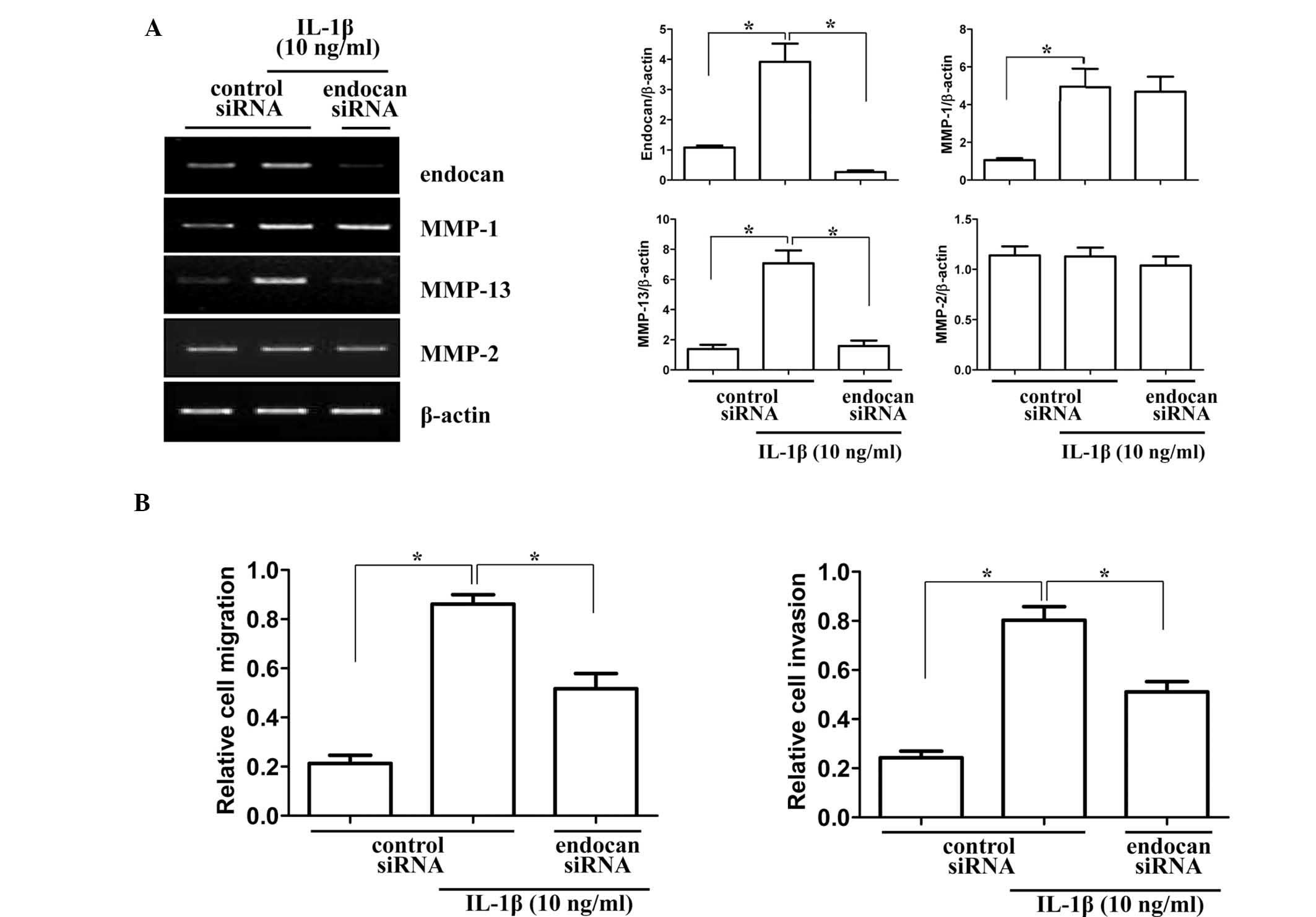
To provide insight into the role of the endocan gene
in synovial cell migration and invasion in arthritic joints, the
gene was silenced in vitro by siRNA transfection. Matrix
metalloproteinase (MMP) genes, which are associated with invasion
and migration, were analyzed for mRNA expression levels in endocan
gene-silenced synovial cells (Fig.
4A). Gene levels of collagenase (MMP-1 and MMP-13) and
gelatinase (MMP-2) were investigated by RT-PCR. As indicated in
Fig. 4A, mRNA levels of MMP-1 and
MMP-2 were not significantly altered, but MMP-13 levels were
significantly decreased in cells transfected with endocan siRNA and
treated with IL-1β (10 ng/ml) for 24 h. Subsequently, the effects
of endocan gene knockout on the invasion and migration of FLSs were
investigated. As demonstrated in Fig.
4B, endocan gene silencing significantly decreased cell
migration and invasion of FLSs under inflammatory conditions. These
results suggested that the endocan gene may have an important role
in FLSs in mediating the pannus invasion of cartilage and bone in
arthritic joints.
Discussion
In the current study, we hypothesized that the
previously reported physiological roles of endocan in tumors were
involved in the pathogenesis and progression of RA. In particular,
endocan was reported to be involved in angiogenesis and tumor
invasion (15,16). RA is characterized by excessive
angiogenesis, which may be essential in the pathogenesis of the
disease (17). Furthermore, the
pannus tissue in RA joints exhibits aggressive, tumor-like growth
and invades and erodes the surrounding cartilage and subchondral
bones (18). Thus, the present
study investigated whether endocan expression was increased in
arthritic tissues. Immunohistochemistry revealed that its
expression was increased in severe inflammatory arthritic tissues.
The increased expression of endocan in arthritic joints may be
responsible for excessive angiogenesis and pannus invasion as well
as in the recruitment of circulating lymphocytes to inflammatory
sites and leukocyte adhesion and activation (9).
Subsequently, which cells in arthritic joints are
mainly responsible for the production of endocan and how they are
regulated by inflammatory stimuli was examined. The results
suggested that endocan was mainly produced in the endothelial cells
of arthritic joints, even following inflammatory stimulation.
Endocan was previously reported to be preferentially expressed in
the tumor endothelium in vivo, which supported this result
(19). VEGF is an important factor
that stimulates endocan expression in the endothelium (15). Furthermore, endocan secretion was
significantly increased in response to TNF-α, and the spontaneous
and TNF-α-induced secretion of endocan-1 was inhibited by
interferon-γ (20). However, the
results of the present study indicated that endocan expression was
not significantly increased in response to IL-1β (1 ng/ml). In
addition, endocan expression was elevated in bronchial and renal
epithelia (20). Adipocytes, which
actively produce endocan (21),
also increased their endocan expression in response to phorbol
ester, an activator of protein kinase C, and retinoic acid
(22). In the present study,
synovial cells significantly increased the expression of endocan in
response to inflammatory stimuli, including IL-1β. Meanwhile,
adiponectin, an adipokine, was involved in the pathogenesis and
progression of arthritis in joints. Thus, the role of adiponectin
in the stimulation of endocan expression in arthritic joints was
studied. To the best of our knowledge, the present study was the
first to describe the effect of adiponectin on the expression of
endocan.
To evaluate the role of endocan in arthritic joints,
the endocan gene was knocked down by endocan siRNA in FLSs.
Consistent with previous results in other cell types (10), endocan silencing in FLSs decreased
the levels of cell migration and invasion in in vitro
assays. The results of the present study indicated that the
decrease in cell migration and invasion may be associated with
downregulation of the MMP-13 gene caused by endocan silencing,
similar to results observed in a previous study (23).
In conclusion, to the best of our knowledge, the
present study indicated, for the first time, that endocan
expression was detected in arthritic joint tissues and that the
expression was higher at severe inflammatory sites than at mild
inflammatory sites. Therefore, endocan may be involved in synovial
cell migration and invasion of pannus tissue in arthritic joints.
The expression regulation of endocan and its major sources in
arthritic joints remain to be further investigated. Furthermore, in
order to present a potential therapeutic target against rheumatoid
arthritis, the in vivo role of endocan should be
investigated in animal models of arthritis through knockout of the
endocan gene.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
and funded by the Ministry of Education, Science and Technology
(Korea; grant nos. 2011-0009061 and 2010-0024089).
Abbreviations:
|
HUVEC
|
human umbilical vascular endothelial
cells
|
|
RA
|
rheumatoid arthritis
|
|
ESM-1
|
endothelial cell-specific molecule
|
|
FLSs
|
fibroblast-like synoviocytes
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Gravallese EM and Monach PA: Rheumatoid
synovitis and pannus. Rheumatology. Hochberg MC, Silman AJ, Smolen
JS, Weinblatt ME and Weisman MH: 4th edition. Elsevier Ltd; London,
UK: pp. 841–865. 2008
|
|
2
|
Bresnihan B: Pathogenesis of joint damage
in rheumatoid arthritis. J Rheumatol. 26:717–719. 1999.PubMed/NCBI
|
|
3
|
Szekanecz Z and Koch AE: Mechanisms of
disease: angiogenesis in inflammatory diseases. Nat Clin Pract
Rheumatol. 3:635–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schoettler N and Brahn E: Angiogenesis
inhibitors for the treatment of chronic autoimmune inflammatory
arthritis. Curr Opin Investig Drugs. 10:425–433. 2009.PubMed/NCBI
|
|
5
|
Lainer-Carr D and Brahn E: Angiogenesis
inhibition as a therapeutic approach for inflammatory synovitis.
Nat Clin Pract Rheumatol. 3:434–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarrazin S, Adam E, Lyon M, Depontieu F,
Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P and
Delehedde M: Endocan or endothelial cell specific molecule-1
(ESM-1): a potential novel endothelial cell marker and a new target
for cancer therapy. Biochim Biophys Acta. 1765:25–37. 2006.
|
|
7
|
Lassalle P, Molet S, Janin A, Heyden JV,
Tavernier J, Fiers W, Devos R and Tonnel AB: ESM-1 is a novel human
endothelial cell-specific molecule expressed in lung and regulated
by cytokines. J Biol Chem. 271:20458–20464. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Béchard D, Gentina T, Delehedde M,
Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P,
Jude B, et al: Endocan is a novel chondroitin sulfate/dermatan
sulfate proteoglycan that promotes hepatocyte growth factor/scatter
factor mitogenic activity. J Biol Chem. 276:48341–48349.
2001.PubMed/NCBI
|
|
9
|
Béchard D, Scherpereel A, Hammad H,
Gentina T, Tsicopoulos A, Aumercier M, Pestel J, Dessaint JP,
Tonnel AB and Lassalle P: Human endothelial-cell specific
molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and
blocks binding to intercellular adhesion molecule-1. J Immunol.
167:3099–3106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang YH, Ji NY, Lee CI, Lee HG, Kim JW,
Yeom YI, Kim DG, Yoon SK, Kim JW, Park PJ and Song EY: ESM-1
silencing decreased cell survival, migration, and invasion and
modulated cell cycle progression in hepatocellular carcinoma. Amino
Acids. 40:1003–1013. 2011. View Article : Google Scholar
|
|
11
|
Maurage CA, Adam E, Minéo JF, Sarrazin S,
Debunne M, Siminski RM, Baroncini M, Lassalle P, Blond S and
Delehedde M: Endocan expression and localization in human
glioblastomas. J Neuropathol Exp Neurol. 68:633–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grigoriu BD, Depontieu F, Scherpereel A,
Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB and
Lassalle P: Endocan expression and relationship with survival in
human non-small cell lung cancer. Clin Cancer Res. 12:4575–4582.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH,
Choi SY, Yang HI, Yoo MC and Kim KS: Adiponectin may contribute to
synovitis and joint destruction in rheumatoid arthritis by
stimulating vascular endothelial growth factor, matrix
metalloproteinase-1, and matrix metalloproteinase-13 expression in
fibroblast-like synoviocytes more than proinflammatory mediators.
Arthritis Res Ther. 11:R1612009. View
Article : Google Scholar
|
|
14
|
Kim KS, Park EK, Ju SM, Jung HS, Bang JS,
Kim C, Lee YA, Hong SJ, Lee SH, Yang HI and Yoo MC: Taurine
chloramine differentially inhibits matrix metalloproteinase 1 and
13 synthesis in interleukin-1beta stimulated fibroblast-like
synoviocytes. Arthritis Res Ther. 9:R802007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roudnicky F, Poyet C, Wild P, Krampitz S,
Negrini F, Huggenberger R, Rogler A, Stöhr R, Hartmann A,
Provenzano M, et al: Endocan is upregulated on tumor vessels in
invasive bladder cancer where it mediates VEGF-A-induced
angiogenesis. Cancer Res. 73:1097–1106. 2013. View Article : Google Scholar
|
|
16
|
Aitkenhead M, Wang SJ, Nakatsu MN, Mestas
J, Heard C and Hughes CC: Identification of endothelial cell genes
expressed in an in vitro model of angiogenesis: induction of ESM-1,
(beta)ig-h3, and NrCAM. Microvasc Res. 63:159–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weber AJ and De Bandt M: Angiogenesis:
general mechanisms and implications for rheumatoid arthritis. Joint
Bone Spine. 67:366–383. 2000.
|
|
18
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abid MR, Yi X, Yano K, Shih SC and Aird
WC: Vascular endocan is preferentially expressed in tumor
endothelium. Microvasc Res. 72:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bechard D, Meignin V, Scherpereel A, Oudin
S, Kervoaze G, Bertheau P, Janin A, Tonnel A and Lassalle P:
Characterization of the secreted form of endothelial-cell-specific
molecule 1 by specific monoclonal antibodies. J Vasc Res.
37:417–425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Janke J, Engeli S, Gorzelniak K,
Feldpausch M, Heintze U, Böhnke J, Wellner M, Herse F, Lassalle P,
Luft FC and Sharma AM: Adipose tissue and circulating endothelial
cell specific molecule-1 in human obesity. Horm Metab Res.
38:28–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellner M, Herse F, Janke J, Gorzelniak K,
Engeli S, Bechart D, Lasalle P, Luft FC and Sharma AM: Endothelial
cell specific molecule-1 - a newly identified protein in
adipocytes. Horm Metab Res. 35:217–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang YH, Ji NY, Han SR, Lee CI, Kim JW,
Yeom YI, Kim YH, Chun HK, Kim JW, Chung JW, et al: ESM-1 regulates
cell growth and metastatic process through activation of NF-κB in
colorectal cancer. Cell Signal. 24:1940–1949. 2012. View Article : Google Scholar : PubMed/NCBI
|