Introduction
Osteosarcoma (OS) is a rare, highly malignant tumor
of the bone. It is the most common primary bone malignancy in
childhood and adolescence (1–3). OS
is primarily a malignant neoplasm of the long bones, with the
greatest predilection for the metaphyses of the distal femur and
proximal tibia (4). OS is a highly
aggressive tumor that metastasizes primarily to the lung (5). Metastasis is not only a sign of
deterioration but also the major cause of treatment failure and
mortality (6). The prognosis is
poor due to lack of effective treatment methods (7). Therefore, innovative approaches that
target the invasion and metastasis of osteosarcoma are urgently
required. To date, the molecular mechanisms behind osteosarcoma
development and metastasis have remained elusive. Therefore,
broadening our understanding of the pathogenesis and biology of
metastatic osteosarcoma is a key factor for improving treatment
results and identifying potential therapeutic targets (2).
Activin A, belonging to the TGF-β protein
superfamily, interacts with two structurally similar
serine/threonine kinase receptors and initiates downstream
signaling via Smads to regulate gene expression (8). Activin is a pleiotropic cytokine with
broad tissue distributions. Activin was initially described as a
protein, which induces the release of follicle stimulating hormone
from the pituitary gland. In recent years, activin has exhibited
various effects on multiple physiological and pathological
processes, including inflammation, metabolism, homeostasis, repair,
cytoprotection, immune responses and endocrine function (9,10).
Recent studies have demonstrated that activin has an important role
in cell proliferation, differentiation, apoptosis and
carcinogenesis (8,11,12).
Activins are homo- or heterodimers composed of four
different β subunits termed βA, βB, βC and βE, respectively.
Activin A, the dimer of two A subunits, is critically involved in
the regulation of cell growth and apoptosis (13). Activin A is a multi-functional
cytokine. Matsuo et al (14) demonstrated that activin A had an
antiproliferative effect on thyroid papillary carcinoma cells and
had a pivotal effect on the control of thyroid tumorigenesis.
Kaneda et al (15) revealed
that activin A inhibited vascular endothelial cell growth and
suppressed tumor angiogenesis in gastric cancer. Activin A
exhibited an inhibitory role in the proliferation of breast cancer
cells through the activation of Smads (16). Activin A is a potent inhibitor of
proliferation of certain epithelial ovarian cancer cell lines
(17). Activin A normally inhibits
cancer development and progression; however, cancer cell growth in
high-grade prostate cancer is not inhibited by activin A (18). Recently, activin A has been
revealed to be overexpressed in various types of cancer (19). Several studies have revealed that
activin A may enhance tumor formation and progression through its
effect on the tumor microenvironment (10). Hoda et al (20) revealed that activin A was
overexpressed in malignant pleural mesothelioma (MPM) cells and
contributed to the malignant phenotype of MPM cells via regulation
of cyclin D. The elevated levels of activin A are responsible for
the development of gonadal tumors and a cachexia-like weight loss
syndrome (21). The overexpression
of activin A was also correlated with positive node stage, poor
histological differentiation and perineural invasion. Yoshinaga
et al (22) demonstrated
that activin A enhanced matrix metalloproteinase (MMP)-7 activity
via the transcription factor activator protein 1 in an oesophageal
squamous cell carcinoma cell line. Suppression of activin A in OC3
oral carcinoma cells using small interfering (si)RNA may attenuate
cell proliferation, migration and invasiveness (18). Inhibition of activin A action is a
promising strategy for the treatment of the types of cancer
overexpressing this factor (23).
Advanced myeloma is associated with high circulating levels of
activin A, supporting the theory for the use of activin A
antagonists in myeloma, such as sotatercept (24). Despite its pluripotent effects, the
roles of activin A signaling in osteosarcoma pathogenesis remain to
be elucidated. Therefore, the present study examined activin A
expression in osteosarcoma cell lines (MG63, SaOS-2 and U2OS) and a
human osteoblastic cell line (hFOB1.19) by reverse transcription
quantitative polymerase chain reaction (RT-qPCR) and western blot
analysis and observed changes in the viability, cell cycle as well
as invasion and migration ability of MG-63 cells following up- or
downregulation of activin A expression in human osteosarcoma MG-63
cells. The present study provided information which may aid in the
development of prognosis prediction tools and targeted therapy for
osteosarcoma.
Materials and methods
Reagents
Cell culture reagents were purchased from Gibco-BRL
(Gaithersburg, MD, USA). Osteosarcoma cell lines (MG63, SaOS-2,
U2OS) and a human osteoblastic cell line (hFOB1.19) were attained
from the American Type Culture Collection (Manassas, VA, USA).
Lipofectamine™2000, pcDNA3.1vector and pGEM-T vector were from
Invitrogen (Carlsbad, CA, USA). Restriction endonucleases
HindIII and BamHI were purchased from Promega
(Madison, WI, USA). T4 DNA Ligation agent was also from Promega.
Taq DNA polymerase was purchased from Fermentas (Vilnius,
Lithuania). The activin A siRNA was purchased from RiboBio Co.
(Guangzhou, China). Protein extraction buffer and propidium iodide
(PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
enhanced chemiluminescence kit was purchased from Pierce (Rockford,
IL, USA). Matrigel was purchased from Collaborative Research, Inc.
(Bedford, MA, USA). The Transwell invasion chamber was purchased
from Costar (Cambridge, MA, USA). The rabbit anti-activin A
polyclonal antibody was purchased from Biorbyt (San Francisco, CA,
USA). The rabbit anti-MMP-9 polyclonal antibody was purchased from
Abnova (Taipei, Taiwan). The rabbit anti-β-actin, rabbit
anti-cyclin D1 and rabbit anti-MMP-2 polyclonal antibodies were
purchased from Abbiotec (San Diego, CA, USA). The horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig)G was
purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA).
Cell culture
Osteosarcoma cell lines (MG63, SaOS-2 and U2OS) were
maintained in RPMI 1640 (Gibco-BRL) supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL) and 1% penicillin/streptomycin
(Gibco-BRL) at 37˚C. The human osteoblast cell line hFOB1.19 was
maintained in Dulbecco’s modified Eagle’s medium (DMEM):Ham’s F-12
(Gibco-BRL) containing 10% FBS and geneticin (400 μg/ml;
Gibco-BRL) at 34˚C in a humidified 5% CO2 incubator.
RT-qPCR
Total RNA was extracted from cells according to the
manufacturer’s instructions. RNA was reverse-transcribed and cDNA
was synthesized using an iScript cDNA synthesis kit (Bio-Rad,
Hercules, CA, USA). cDNA was stored at −80˚C until further use. All
primers were purchased from Shanghai Generay Biotech Co., Ltd.
(Shanghai, China). The primer sequences used were as follows:
Activin A forward, 5′-ATAGCCCCTTTGCCAACCTC-3′ and reverse,
5′-AGCACCTTAACGAAATGTAACTTGG-3′; and β-actin forward,
5′-GGCGGCCAACGCCAAAACTC-3′ and reverse, 5′-GCCTCCGCCCGGTTCAAACA-3′.
The qPCR reactions were performed using the iTaq Fast SYBR Green
Supermix (Bio-Rad) according to the manufacturer’s instructions.
The cycle threshold (Ct) values were determined and the relative
mRNA expression was calculated using the 2−ΔΔCt method
(25).
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation buffer and quantified through a
bicinchoninic acid assay kit (Sigma-Aldrich). Subsequently, the
total proteins were separated using 12% SDS-PAGE (Bio-Rad) and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Amresco LLC, Solon, OH, USA). The PVDF membranes were blocked with
5% milk/TBS-0.1%-Tween (TBST; Bio-Rad) for 1 h at room temperature
and probed with the appropriate primary antibody (1:1,000) at 4˚C
overnight. Subsequently, the PVDF membranes were washed in TBST for
3×5 min and probed with the corresponding secondary antibody for 2
h at room temperature. Following washing with TBST, autoradiography
was conducted with enhanced chemiluminescence reagents. The
relative expression of activin A was evaluated with the grey value
ratio of activin A content to β-actin content (activin
A/β-actin).
Cell transfection
MG63 cells were transfected with plasmid containing
pcDNA3.1-activin A (Invitrogen Life Technologies) or with activin A
siRNA (RiboBio Co.) and Lipofectamine™2000 (Invitrogen Life
Technologies), used as the overexpression group and knockdown
group, respectively. MG63 cells without any treatment were used as
the control group. Transient transfection was performed using a
high performance transfection reagent (Ribo FECT™ CP Transfection
kit; RiboBio Co.). Each stable transfectant clone was screened for
overexpression or knockdown of activin A by RT-qPCR and western
blot analysis.
MTT assay
MG63 cells were cultured on 96-well plates. MG63
cells in each well were incubated with 10 μl MTT (5 mg/ml;
Beyotime, Haimen, China) for 4 h The formazan crystals formed from
MTT by the living cells were dissolved in lysis buffer
(Sigma-Aldrich). The purple solution of the formazan was detected
using an OSE-260 spectrophotometer (Tiangen Biotech Co, Ltd,
Beijing, China) to measure absorbance (A) at 570 nm, and a 690-nm
measurement was used as a reference. The relative cell
proliferation (%) was calculated by the following equation:
Relative proliferation rate(%) = [study group(A570
nm−A690 nm)/control group(A570
nm−A690 nm)]×100%, as described in a previous
study (26) and the experiment was
performed in triplicate.
Cell cycle assay
MG63 cells were cultured in serum-free medium for 24
h for synchronization and then cultured in complete medium for 24
h. Subsequently, the MG63 cells were detached with trypsin, washed
and fixed in 70% cold ethanol overnight at −20˚C. The fixed MG63
cells were washed with phosphate-buffered saline (Beyotime) and
then treated with 1 mg/ml RNAse (Beyotime) for 30 min at 37˚C.
Subsequently, the MG63 cells were incubated with PI at a final
concentration of 100 μg/ml at room temperature for 30 min. The cell
cycle was evaluated through flow cytometry (BD FACSCalibur™; BD
Biosciences, San Jose, CA, USA) and the experiment was performed in
triplicate.
Cell invasion assay
The Transwell invasion chamber was washed and then
50 μl Matrigel (1 mg/ml) was added to evenly cover the
Transwell inserts with 8-μm pores to create the Matrigel membrane.
The Transwell invasion chamber was divided into the outer chamber
and the inner chamber by the Matrigel membrane. For invasion
assays, MG63 cells (4×105) were serum-starved overnight
and seeded in starvation medium on the outer chamber. The inner
chamber contained 10% FBS in RPMI 1640 medium. After 48 h
incubation, MG63 cells from the outer chamber were removed from the
surface of the matrigel membrane using a cotton swab. MG63 cells
that had invaded into the lower compartment and attached to the
lower surface of the filter were stained with Hoechst 33258 for 10
min. Images of the invading cells were captured using an inverted
microscope (XDS-1B; Wuzhou New Found Instrument Co., Ltd, Guangxi,
China) and total cell numbers were counted and quantified.
Cell migration assay
The Transwell invasion chamber was washed with
serum-free medium. The chamber was divided into upper and bottom
chambers by the Transwell insert. For migration assays, MG63 cells
(4×105) were serum-starved overnight and seeded in
starvation medium on the upper chamber. The bottom chamber
contained 10% FBS in RPMI 1640 medium, which acted as a
chemoattractant. After 48 h incubation, cells on the upper surface
of the chamber were wiped off with cotton swabs. Cells in the
Transwell inserts and on the lower surface of the inserts were
washed into the bottom chamber with medium until no cells were on
the inserts. Cells in the bottom chamber were incubated with MTT.
The formazan crystals formed from MTT by the living cells were
dissolved in lysis buffer. The relative number of cells was
calculated from the optical density (OD) value using an OSE-260
spectrophotometer.
Statistical analysis
Values are presented as the mean ± standard
deviation of three individual experiments. The SPSS 17.0
statistical software (International Business Machines, Armonk, N Y,
USA) was used to analyze the quantitative data by one-way analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
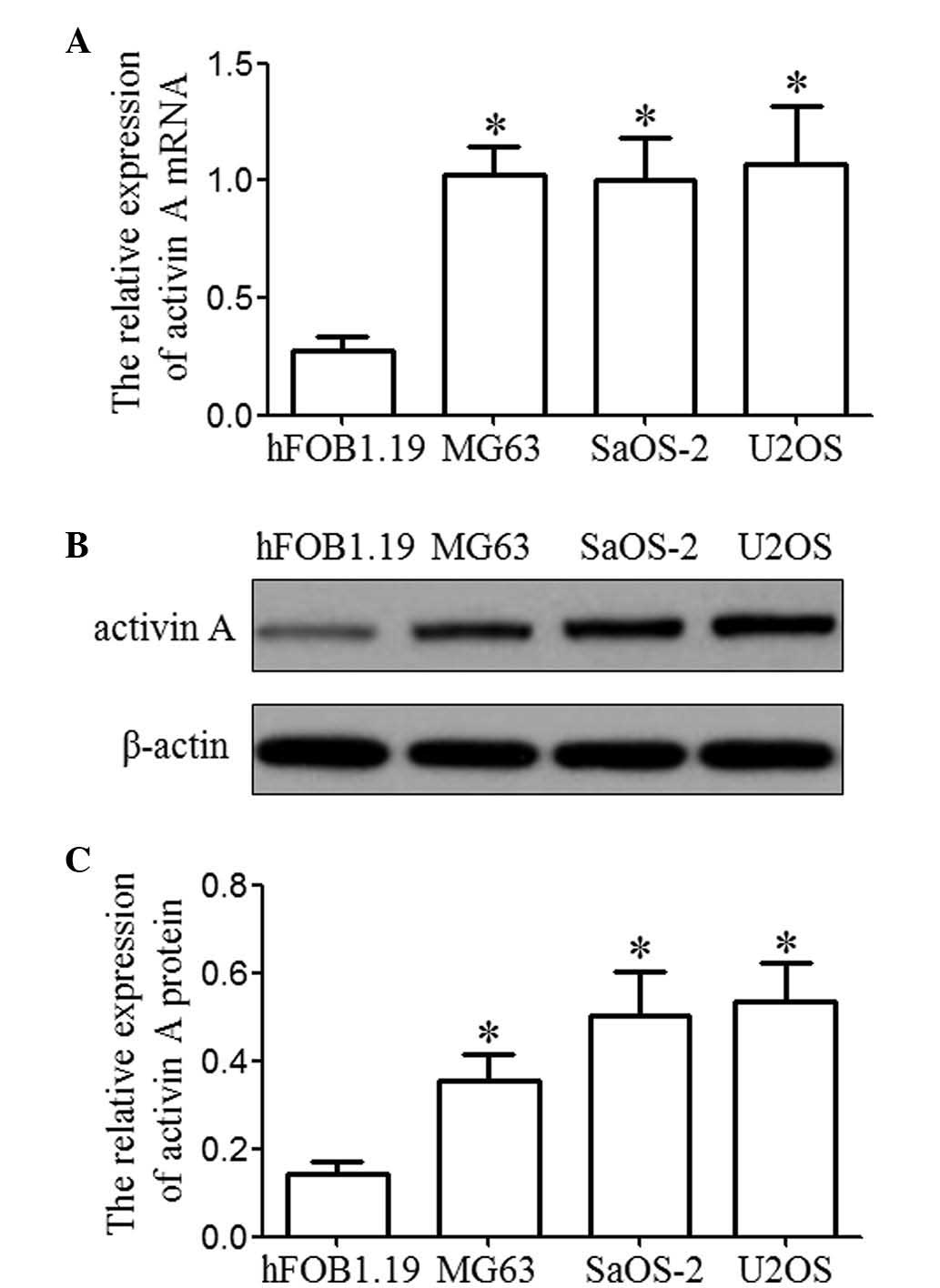
Activin A is overexpressed in
osteosarcoma cell lines
In order to address the expression of activin A in
osteosarcoma cells, activin A mRNA was detected by RT-qPCR and
western blotting in three osteosarcoma cell lines (MG63, SaOS-2 and
U2OS) and a human osteoblastic cell line (hFOB1.19). The expression
levels of activin A mRNA and protein in the osteosarcoma cell lines
was higher than that in a normal human osteoblastic cell line,
hFOB1.19 (P<0.05), as shown in Fig.
1.
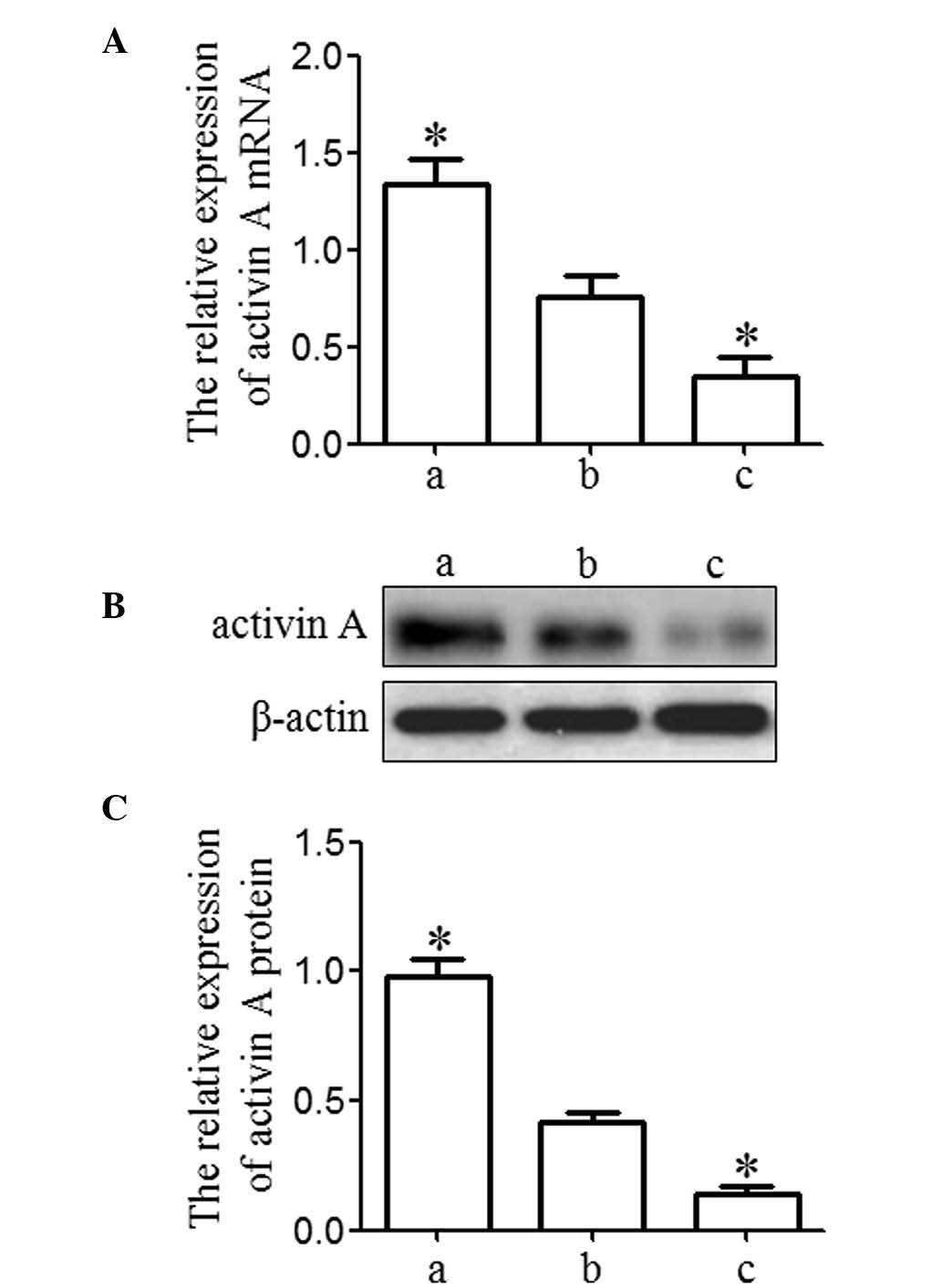
In order to examine the effect of activin A on
osteosarcoma cells, MG63 cells were selected for further study.
MG63 cells were transfected with activin A overexpression vector
(overexpression group) and activin A siRNA (knockdown group) to
enforce activin A expression or inhibit activin A expression in
MG63 cells, respectively. MG63 cells without any treatment were
used as the blank group. The results from RT-qPCR and western blot
analysis demonstrated that activin A exhibited a significant
upregulation in the overexpression group and a significant
downregulation in the knockdown group compared with that in the
blank group (P<0.05), which indicated that the expression of
activin A was effectively enforced or inhibited in MG63 cells
(Fig. 2).
Activin A improves MG63 cell
viability
The MTT assay demonstrated that MG63 cell viability
in the knockdown group was significantly lower than that in the
blank group and that MG63 cell viability in the overexpression
group was significantly higher than that in the blank group
(P<0.05; Fig. 3). These results
suggested that activin A may have an important role in the
improvement of MG63 cell viability.
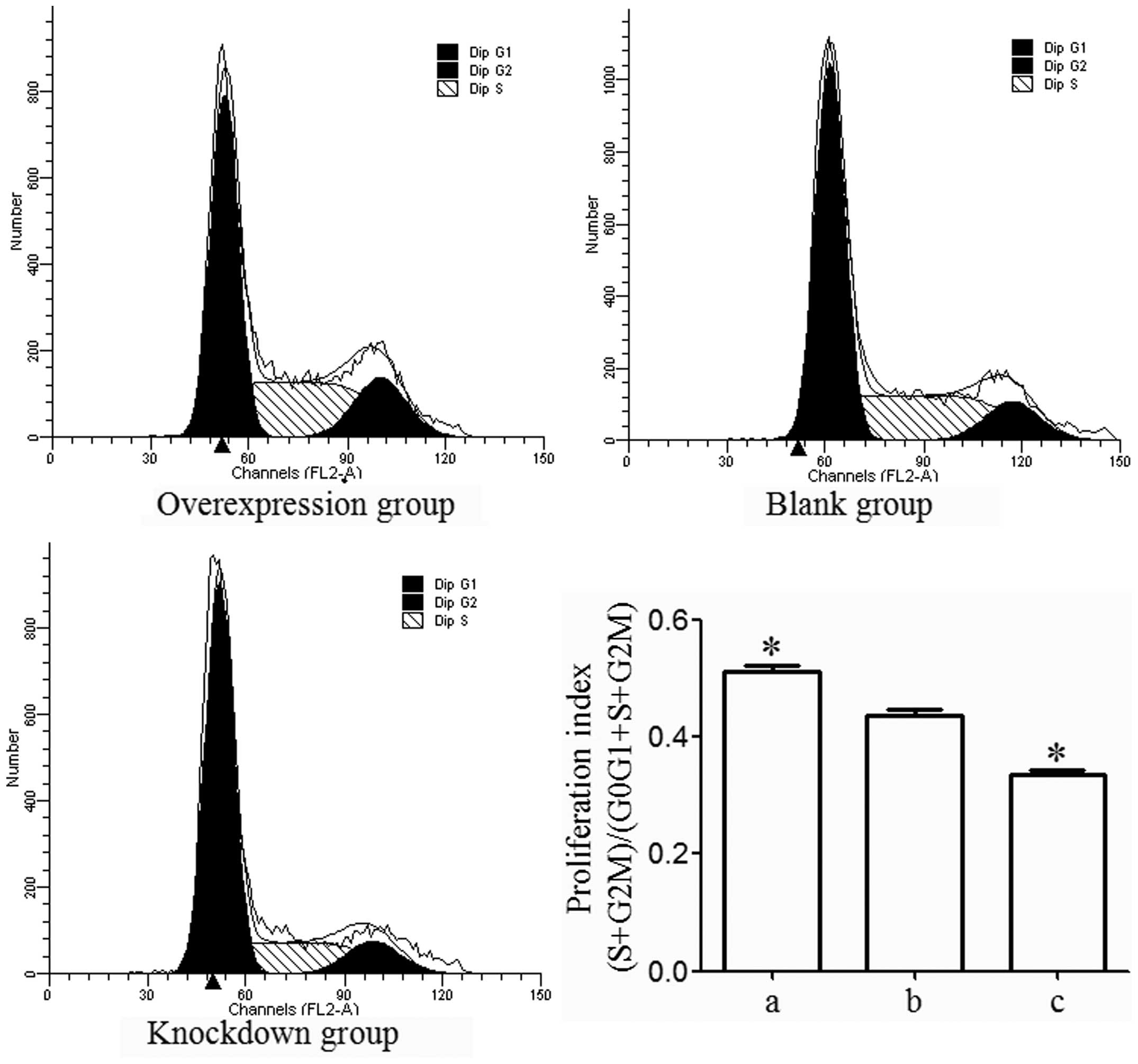
Activin A promotes MG63 cell
proliferation
Flow cytometric analysis indicated that the number
of MG63 cells from the overexpression group in
G0/G1 phase was lower than that from the
blank group (P<0.05) and that the number of MG63 cells from the
knockdown group in G0/G1 phase was higher
than that from the blank group (P<0.05). Furthermore, the
results also demonstrated that the number of MG63 cells from the
overexpression group in S and G2/M phase was higher than
that from the blank group (P<0.05) and that the number of MG63
cells from the knockdown group in S and G2/M phase was
lower than that from the blank group (P<0.05). The proliferation
index [(S+G2/M) / (G0/G1 + S +
G2/M)] was higher in the overexpression group
(P<0.05) and was lower in the knockdown group than that in the
blank group (P<0.05; Fig. 4),
which led to the conclusion that activin A promotes MG63 cell
proliferation.
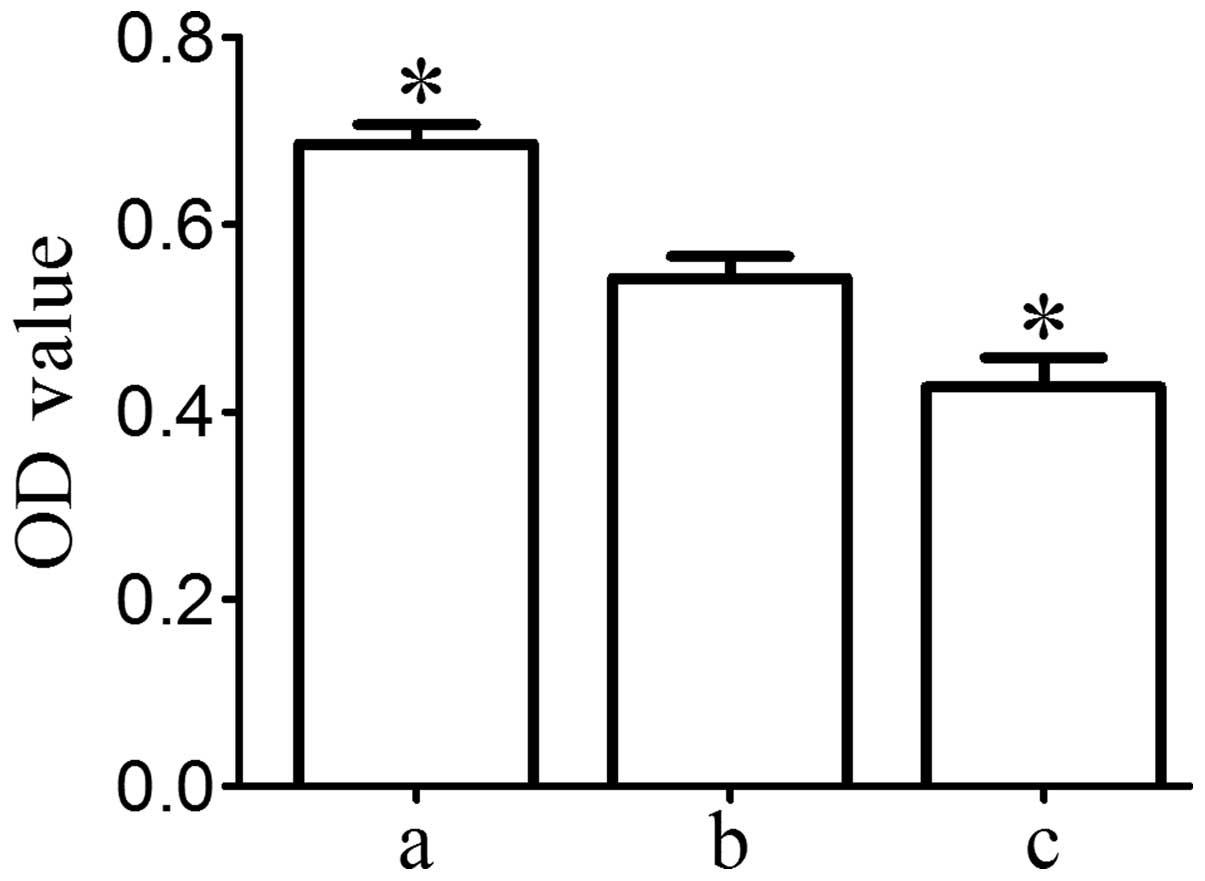
Activin A improves MG63 cell invasion and
migration
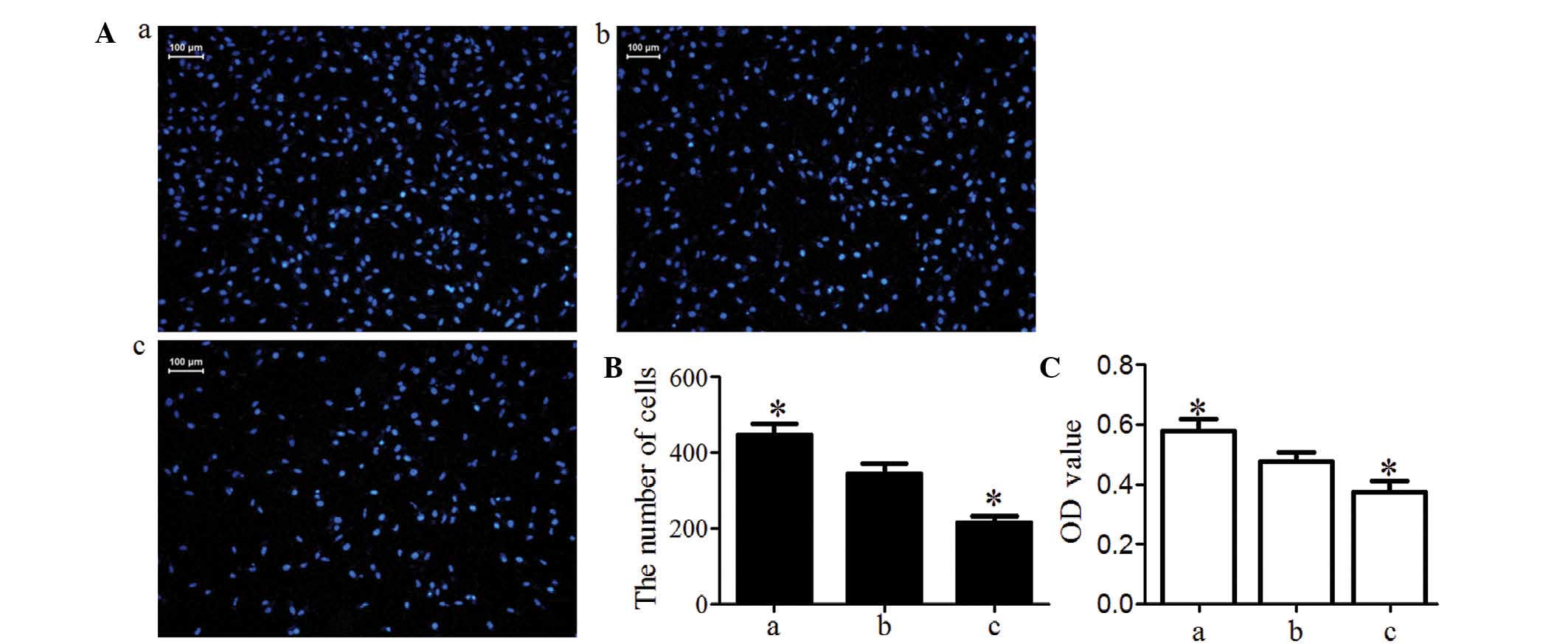
Transwell invasion chamber assay demonstrated that
the number of MG63 cells invading the Matrigel membrane was
significantly lower in the knockdown group and was significantly
higher in the overexpression group compared with that in the blank
group (P<0.05; Fig. 5A and B).
These data indicated that activin A may improve MG63-cell
invasiveness. Cells in the Transwell inserts and on the lower
surface of the inserts were washed into the bottom chamber with
medium until no cells remained on the inserts. Cells in the bottom
chamber were then incubated with MTT The OD value was proportional
to the number of viable cells. The results indicated that the
number of MG63 cells migrated into Transwell inserts on the lower
surface of the inserts and into the bottom chamber was
significantly higher in the overexpression group and was
significantly lower in the knockdown group compared with that in
the blank group (P<0.05; Fig.
5C).
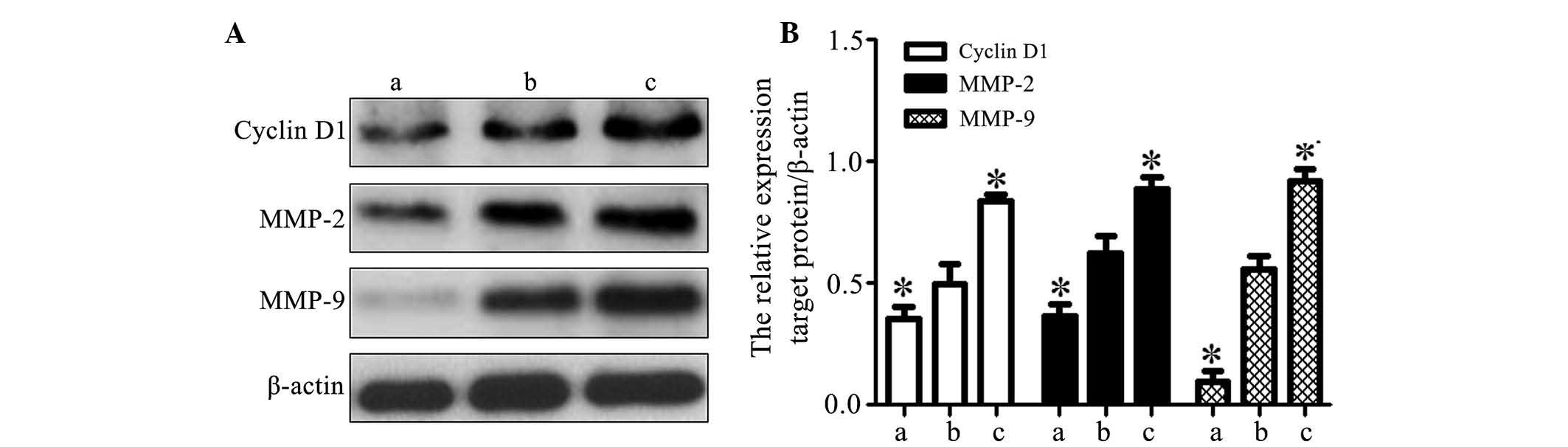
Effects of activin A on the expression of
cyclin D1, MMP-2 and MMP-9 in MG63 cells
Western blot analysis demonstrated that the
expression of cyclin D1, MMP-2 and MMP-9 was significantly
upregulated in the overexpression group and significantly
downregulated in the knockdown group as compared with that in the
blank group (P<0.05; Fig. 6).
This suggested that activin A may be associated with the
upregulation of cyclin D1, MMP-2 and MMP-9 in osteosarcoma MG63
cells.
Discussion
Activin A, belonging to the transforming growth
factor-β (TGF-β) superfamily, is a multi-functional cytokine. As
with all TGF-family members, activin A is a dimeric protein, in
this case composed of two activin βA subunits (27). Initial investigation into the
potential role of activin A focused on inflammatory processes,
metabolism, homeostasis, repair, cytoprotection, immune response
and endocrine function (9,10). Recent studies have revealed that
activin A has an important role in cell proliferation,
differentiation, apoptosis and carcinogenesis (8,11,12).
However, the roles of activin A signaling in osteosarcoma
pathogenesis remain to be elucidated.
In the present study, it was identified that activin
A was upregulated in the osteosarcoma cell lines (MG63, SaOS-2 and
U2OS) compared with the non-cancerous osteoblastic cell line
hFOB1.19. This was consistent with the results from a previous
study, which demonstrated that activin A was overexpressed in
various types of cancer (19). The
overexpression of activin A may enhance tumor formation and
progression (10). Hoda et
al (20) demonstrated that
activin A exhibited an upregulation in MPM cells. The
overexpression of activin A may be responsible for the development
of gonadal tumors (21) and was
also correlated with positive node stage, poor histological
differentiation and perineural invasion (22). Therefore, it was hypothesized that
activin A may positively regulate tumor cell proliferation,
invasion and migration. To examine the effect of activin A on
osteosarcoma cells, MG63 cells were selected and transfected with
activin A overexpression vector and activin A siRNA to enforce
activin A expression or inhibit activin A expression, respectively.
The results of the present study indicated that the expression of
activin A was effectively enforced or inhibited in MG63 cells.
The overexpression of activin A contributed to the
malignant phenotype of MPM cells (20). Inhibition of activin A in OC3 oral
carcinoma cells may attenuate cell proliferation (19). Thus, MG63 cell viability and
proliferation were examined by an MTT assay and a flow cytometric
assay, respectively. The MTT assay demonstrated that MG63 cell
viability in the activin A knockdown group was significantly lower
than that in the blank group and that MG63 cell viability in the
activin A overexpression group was significantly higher than that
in the blank group, which suggested that activin A may have a
crucial role in the improvement of MG63 cell viability. The flow
cytometric assay indicated that the proliferation index was higher
in the activin A overexpression group and was lower in the activin
A knockdown group as compared with that in the blank group, which
indicated that activin A promotes MG63 cell proliferation.
Downregulation of activin A in the conditioned media decreased cell
proliferation (28). A previous
study also identified that activin A contributed to the malignant
phenotype of MPM cells via regulation of cyclin D (20). Silencing of activin A expression by
siRNA oligonucleotides led to reduced cyclin D1 expression
(20). Therefore, it was
hypothesized that activin A improved MG63 cell proliferation, which
was possibly via regulation of cyclin D. Therefore, the expression
of cyclin D1 was determined by western blotting in the present
study. The results demonstrated that cyclin D1 was suppressed in
the knockdown group and enhanced in the overexpression group, which
may be responsible, at least partially, for the observation that
activin A improved MG63 cell proliferation. The results of the
present study also suggested that the overexpression of activin A
may be a major event in cancer pathogenesis, in part due to
improvement of its ability to upregulate cyclin D1, leading to a
failure to induce cell cycle arrest. However, further study is
required to elucidate the exact mechanism.
The overexpression of activin A was also correlated
with migration and invasion. Activin A promotes migration of
prostate cancer cells to osteoblasts (29). Yoshinaga et al (22) demonstrated that activin A enhanced
migration and invasion of esophageal squamous cell carcinoma cells
by enhancing MMP-7 activity (22).
Chang et al (19) suggested
that suppression of activin A in OC3 oral carcinoma cells may
attenuate cell migration and invasiveness. Therefore, the effect of
activin A on the invasion and migration ability of MG63 cells in
vitro was investigated in the present study. The results
revealed that the number of MG63 cells migrated into Transwell
inserts on the lower surface of the inserts and into the bottom
chamber was significantly higher in the overexpression group and
was significantly lower in the knockdown group compared with that
in the blank group, which suggested that activin A may improve the
abilities of MG63 cell invasion and migration.
Le Bras et al (30) revealed that activin A increases
cell invasion through CD44 upregulation following E-cadherin loss
and increased CD44 expression in areas of cell invasion associated
with MMP-9 In addition, activin A induced in vitro invasion
of esophageal squamous cell carcinoma cells, which was accompanied
by an increased MMP-2 and MMP-9 in esophageal squamous cell
carcinoma cells samples (28).
Incorvaia et al (31)
demonstrated that activin A, MMP-2 and MMP-9 may be regarded as
possible therapeutic targets in the treatment of metastatic bone
disease. Therefore, MMP-2 and MMP-9 were examined in the present
study. The results revealed that MMP-2 and MMP-9 exhibited
significant upregulation in the overexpression group and
significant downregulation in the knockdown group compared with
that in the blank group, which suggested that activin A may be
associated with the upregulation of MMP-2 and MMP-9 in osteosarcoma
MG63 cells. A previous study by our group revealed that miR-181a
was overexpressed in osteosarcoma cell lines and miR-181a may
facilitate proliferation and invasion (32). Neel and Lebrun (12) revealed that activin and TGF-β
regulated the expression of the miR-181 family to promote cell
migration and invasion in breast cancer cells (12). Therefore, it was hypothesized that
the overexpression of activin A in osteosarcoma may enhance the
upregulation of miR-181, facilitating the proliferation and
invasion of osteosarcoma cells. This hypothesis requires further
investigation.
In conclusion, the results of the present study
suggested that activin A may be involved in the enhancement of
proliferation, invasion and migration of MG63 cells. However,
further study is required to provide a thorough understanding of
the function and mechanism of activin A in osteosarcoma.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20131199) and
The ‘Six Talent Peaks Program’ of the Jiangsu Province of China
(grant no. 2011-ws-119).
References
|
1
|
Endo-Munoz L, Evdokiou A and Saunders NA:
The role of osteoclasts and tumour-associated macrophages in
osteosarcoma metastasis. Biochim Biophys Acta. 1826:434–442.
2012.PubMed/NCBI
|
|
2
|
Poletajew S, Fus L and Wasiutyński A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumours. Ortop Traumatol Rehabil. 13:537–545. 2011.In
English, Polish. View Article : Google Scholar
|
|
3
|
Tanzawa H, Uchiyama S and Sato K:
Statistical observation of osteosarcoma of the maxillofacial region
in Japan. Analysis of 114 Japanese cases reported between 1930 and
1989. Oral Surg Oral Med Oral Pathol. 72:444–448. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YY, Van Tassel P, Nauert C, Raymond AK
and Edeiken J: Craniofacial osteosarcomas: plain film, CT, and MR
findings in 46 cases. AJR Am J Roentgenol. 150:1397–1402. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harting MT and Blakely ML: Management of
osteosarcoma pulmonary metastases. Semin Pediatr Surg. 15:25–29.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui J, Wang W, Li Z, Zhang Z, Wu B and
Zeng L: Epigenetic changes in osteosarcoma. Bull Cancer.
98:E62–E68. 2011.PubMed/NCBI
|
|
8
|
Chen YG, Wang Q, Lin SL, Chang CD, Chuang
J and Ying SY: Activin signaling and its role in regulation of cell
proliferation, apoptosis, and carcinogenesis. Exp Biol Med
(Maywood). 231:534–544. 2006.
|
|
9
|
Sulyok S, Wankell M, Alzheimer C and
Werner S: Activin: an important regulator of wound repair,
fibrosis, and neuroprotection. Mol Cell Endocrinol. 225:127–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antsiferova M and Werner S: The bright and
the dark sides of activin in wound healing and cancer. J Cell Sci.
125:3929–3937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kypriotou M, Rivero D, Haller S, et al:
Activin A inhibits antigen-induced allergy in murine epicutaneous
sensitization. Front Immunol. 4:2462013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neel JC and Lebrun JJ: Activin and TGFβ
regulate expression of the microRNA-181 family to promote cell
migration and invasion in breast cancer cells. Cell Signal.
25:1556–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deli A, Kreidl E, Santifaller S, et al:
Activins and activin antagonists in hepatocellular carcinoma. World
J Gastroenterol. 14:1699–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuo SE, Leoni SG, Colquhoun A and
Kimura ET: Transforming growth factor-beta1 and activin A generate
anti-proliferative signaling in thyroid cancer cells. J Endocrinol.
190:141–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaneda H, Arao T, Matsumoto K, et al:
Activin A inhibits vascular endothelial cell growth and suppresses
tumour angiogenesis in gastric cancer. Br J Cancer. 105:1210–1217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burdette JE, Jeruss JS, Kurley SJ, Lee EJ
and Woodruff TK: Activin A mediates growth inhibition and cell
cycle arrest through Smads in human breast cancer cells. Cancer
Res. 65:7968–7975. 2005.PubMed/NCBI
|
|
17
|
Ramachandran A, Marshall ES, Love DR,
Baguley BC and Shelling AN: Activin is a potent growth suppressor
of epithelial ovarian cancer cells. Cancer Lett. 285:157–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ottley E and Gold E: Insensitivity to the
growth inhibitory effects of activin A: an acquired capability in
prostate cancer progression. Cytokine Growth Factor Rev.
23:119–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang KP, Kao HK, Liang Y, et al:
Overexpression of activin A in oral squamous cell carcinoma:
association with poor prognosis and tumour progression. Ann Surg
Oncol. 17:1945–1956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoda MA, Munzker J, Ghanim B, et al:
Suppression of activin A signals inhibits growth of malignant
pleural mesothelioma cells. Br J Cancer. 107:1978–1986. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marino FE, Risbridger G and Gold E: The
therapeutic potential of blocking the activin signalling pathway.
Cytokine Growth Factor Rev. 24:477–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshinaga K, Mimori K, Inoue H, et al:
Activin A enhances MMP-7 activity via the transcription factor AP-1
in an esophageal squamous cell carcinoma cell line. Int J Oncol.
33:453–459. 2008.PubMed/NCBI
|
|
23
|
Antsiferova M, Huber M, Meyer M, et al:
Activin enhances skin tumourigenesis and malignant progression by
inducing a pro-tumourigenic immune cell response. Nat Commun.
2:5762011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terpos E, Kastritis E, Christoulas D, et
al: Circulating activin A is elevated in patients with advanced
multiple myeloma and correlates with extensive bone involvement and
inferior survival; no alterations post-lenalidomide and
dexamethasone therapy. Ann Oncol. 23:2681–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Wang HJ, Ruan HJ, He XJ, et al:
MicroRNA-101 is down-regulated in gastric cancer and involved in
cell migration and invasion. Eur J Cancer. 46:2295–2303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phillips DJ, Jones KL, Clarke IJ,
Scheerlinck JP and de Kretser DM: Activin A: from sometime
reproductive factor to genuine cytokine. Vet Immunol Immunopathol.
108:23–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sobral LM, Bufalino A, Lopes MA, Graner E,
Salo T and Coletta RD: Myofibroblasts in the stroma of oral cancer
promote tumorigenesis via secretion of activin A. Oral Oncol.
47:840–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang HY, Huang HY, Hsieh CY, et al:
Activin A enhances prostate cancer cell migration through
activation of androgen receptor and is overexpressed in metastatic
prostate cancer. J Bone Miner Res. 24:1180–1193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Bras GF, Allison GL, Richards NF,
Ansari SS, Washington MK and Andl CD: CD44 upregulation in
E-cadherin-negative esophageal cancers results in cell invasion.
PloS one. 6:e270632011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Incorvaia L, Badalamenti G, Rini G, et al:
MMP-2, MMP-9 and activin A blood levels in patients with breast
cancer or prostate cancer metastatic to the bone. Anticancer Res.
27:1519–1525. 2007.PubMed/NCBI
|
|
32
|
Jianwei Z, Fan L, Xiancheng L, Enzhong B,
Shuai L and Can L: MicroRNA 181a improves proliferation and
invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol.
34:3331–3337. 2013. View Article : Google Scholar : PubMed/NCBI
|