Introduction
The incidence of malignant melanoma is rapidly
increasing, with the estimated annual mortality rate of >9,000
in the USA (1,2). Melanoma cells often express receptors
for multiple growth factors and cytokines, which regulate their
growth, including platelet-activating factor-receptor (PAF-R), a
G-protein coupled receptor expressed in various types of cell
(3–5). The activation of PAF-R has been
demonstrated to mediate diverse biological functions in response to
various stimuli, including inflammation, immunosuppression and
tumorigenesis (6–13).
Previous studies, including ours, have demonstrated
that the activation of PAF-R positively modulates melanoma growth,
either directly (9–12) or indirectly via host
immunomodulation (13). However,
in epithelial cell types, activation of PAF-R can increase cell
death, induced by pro-oxidative stress reagents (14,15).
As melanoma is highly resistant to chemotherapy and radiation
therapy (16), identifying novel
pathways that can augment cytotoxic agent effects may offer a
promising therapeutic approach for melanoma.
Isothiocyanates (ITCs), which are present in
cruciferous vegetables, including broccoli and cabbage, possess
anti-carcinogenic properties (17,18).
Benzyl isothiocyanate (BITC), an analog of ITC suppresses the in
vitro and in vivo growth of various types of cancer
(19–22). In melanoma, BITC and other isoforms
of ITCs, including allyl and phenyl isothiocyanates and
sulforaphane, have been observed to inhibit melanoma cell growth
via different mechanisms (23–27).
Since many melanomas express functional PAF-Rs and the role of
PAF-R in the BITC-mediated suppression of melanoma cells remain to
be elucidated, the present study aimed to assess whether the
expression of PAF-R can augment the BITC-mediated cytotoxic effects
in melanoma cells.
Materials and methods
Reagents
A Qiagen RNeasy Mini kit for RNA extraction was
purchased from Qiagen Sciences (Germantown, MD, USA), and the Super
Script (R) First-Strand Synthesis system for cDNA synthesis was
purchased from Invitrogen Life Technologies, Carlsbad, CA, USA).
The PAF-R and GAPDH primers and the SYBR Green polymerase chain
reaction (PCR) reagents were purchased from SABiosciences
(Valencia, CA, USA). A caspase-3/7 activity assay kit was purchased
from Promega Corporation (Madison, WI, USA). The WEB2086 PAF-R
antagonist, was purchased from Cayman Chemicals Co. (Ann Arbor, MI,
USA). All other reagents were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cells
Murine B16 cells expressing PAF-R (B16-PAFR), empty
vector (B16-MSCV) and human SK23MEL melanoma cells were maintained
in RPMI-1640 media (Life Technologies, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (HyClone, GE Healthcare
Life Sciences, Logan, UT, USA) and 100 μg/ml mixture of
penicillin and streptomycin (Lonza, Walkersville, MD, USA). Human
SK5MEL cells were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in Eagle’s
minimum essential medium (ATCC) supplemented with 10% FBS and 100
μg/ml mixture of penicillin and streptomycin.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression of PAF-R was analyzed in the
human SK5MEL and SK23MEL cells using RT-qPCR and the expression
levels were normalized with GAPDH, as described previously
(8,13). The B16-PAF-R and B16-MSCV cells
were used as positive and negative controls. Briefly, the cells
were homogenized using an RLT buffer containing β-mercaptoethanol
(Sigma-Aldrich), in a bullet blender (Next Advance, Inc., Averill
Park, NY, USA) and carbide beads. The total RNA was extracted using
an RNAeasy kit according to the manufacturer’s instructions. The
purified RNA was quantified using a Nano Drop 2000 (Thermo Fisher
Scientific, Inc., Lafayette, CO, USA) and reverse transcribed with
a Super Script cDNA synthesis kit containing random hexamers. The
cDNA was analyzed for the PAF-R mRNA using a SYBR green-based,
quantitative fluorescent PCR method (6–8,13).
The fluorescence was detected using a Step One Real-time PCR
machine (Applied Biosystems, Foster City, CA, USA). The
quantification of each PCR product was normalized to GAPDH using
the 2−ΔΔCt method.
Cell proliferation
The B16-PAF-R and B16-MSCV cells were plated in
24-well plates (20,000 cells/well) and treated with 1 or 10 nM
1-hexadecyl-2-N-methylcarbamoyl-3-glycerophosphocholine (CPAF) and
incubated for 24, 48 or 72 h. The control cells received 0.1%
ethanol dissolved in phosphate-buffered saline (PBS;10 μl)
only. Following each time point, the cells were trypsinized,
washed, resuspended in PBS and stained using 0.4% trypan blue
according to the manufacturer’s instructions (Invitrogen Life
Technologies). The cell proliferation was assessed using a trypan
blue exclusion method and a Countess automated cell counter
(Invitrogen Life Technologies).
Cell survival
The murine or human melanoma cells were seeded into
96-well plates (5,000 cells/well) and treated with various
concentrations of BITC, as indicated in respective figures and
figure legends. The control cells received 0.1% dimethylsulfoxide
(DMSO) treatment. The cell survival rate was assessed 24 h after
treatment using a sulforhodamine-B (SRB) assay, as described
previously (19). The plates were
read at 590nm using a Bio Kinetics plate reader (EL-800; BioTek
Instruments, Inc., Winooski, VT, USA).
Reactive oxygen species (ROS)
generation
The B16-MSCV and B16-PAF-R cells (1×105
cells/well) were seeded into 6-well plates for attachment
overnight. The cells were treated with H2DCFDA dye (DFC; 5
μM) for 30 min prior to treatment with 2 μM BITC for
0, 5, 10 min or 1 h. In a separate experiment, the cells were
pre-treated with vitamin C (5 mM) for 1 h prior to treatment with 2
μM BITC for 10 min. The control cells received 0.1% DMSO
treatment. The generation of ROS was analyzed by measuring the
number of DCF-positive cells using a flow associated cell sorter
and FlowJo software version 9.7.5 (Tree Star, Inc., Ashland, OR,
USA).
Apoptosis
The B16-PAF-R and B16-MSCV cells (1×105
cells/well) were seeded into 6-well plates for attachment
overnight. The cells were then either pretreated with 5 mM vitamin
C or 10 μM WEB2086 PAF-R antagonist, for 1 h, followed by
treatment with 2 μM BITC for 24 h. The control cells
received 0.1% DMSO treatment. Apoptosis was quantified using a
luminescence caspase-3/7 glo-assay kit and normalized against the
total protein, as described previously (28). The protein content of the samples
were determined using a Bradford assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Each data set is representative of the combined
results of at least three independent experiments with similar
findings. Data were analyzed using GraphPad Prism software version
5 (GraphPad software, San Diego, CA, USA). Student’s t-test and
one-way analysis of variance with a Bonferroni Post-hoc test were
used to compare two groups or more than two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Evaluation of the expression of PAF-R and
the effect of CPAF the PAF-R agonist on melanoma cell growth
The majority of human melanoma cells express PAF-R,
however, the B16F10 murine melanoma cell line, does not (9,13).
To determine the role of the PAF-R, a B16-PAF-R cell line was
created by transduction of PAF-R-negative B16F10 cells with the
MSCV2.1 retrovirus encoding the human leukocyte PAF-R, as described
previously (13). B16-MSCV cells
were used as a control. The human melanoma cells were then screened
for the presence of functional PAF-R. RT-qPCR revealed the presence
of functional PAF-R in the human SK23MEL cells (Fig. 1A). By contrast, human melanoma
SK5MEL cells deficient in PAF-R were selected and used as a control
(Fig. 1A). To evaluate the role of
PAF-R in the progression of melanoma, the B16-PAF-R and B16-MSCV
cells were treated with the non-metabolizable PAF-R agonist, CPAF,
at different doses and time points prior to the assessment of cell
proliferation. As shown in Fig.
1B, CPAF-treatment induced the proliferation of B16-PAF-R cells
in a dose- and time-dependent manner, compared with the
vehicle-treated B16-PAF-R cells. Notably, CPAF had no effect on the
proliferation of the B16-MSCV cells at these doses, suggesting that
PAF-R was involved in melanoma cell growth.
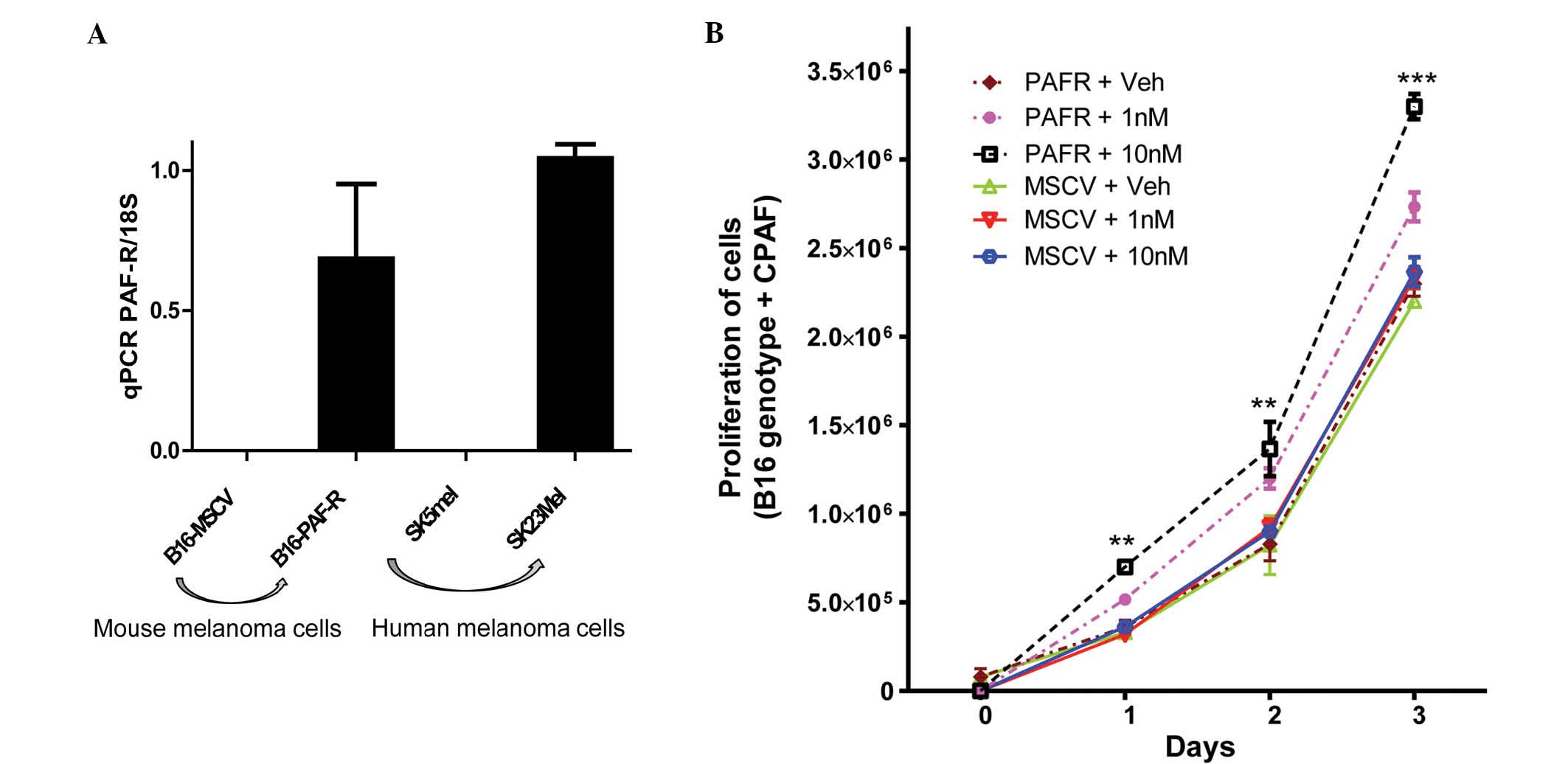 | Figure 1Effect of the PAF-R status on the
proliferation of melanoma cells. (A) Evaluation of the presence of
PAF-R status using reverse transcription-qPCR in murine and human
melanoma cells. (B) B16-PAF-R and B16-MSCV cells were seeded into
24-well plates (20,000 cells/well) and allowed to attach overnight.
The cells were treated with either vehicle or 1 or 10nM CPAF PAF-R
agonist and cultured for 1, 2 or 3 days. At each time point, the
cells were trypsinized and the number of cells were counted using a
trypan blue exclusion method. Data are expressed as the mean ±
standard deviation of the cell proliferation by CPAF over the
number of days. Statistically significant differences were observed
between the B16-MSCV and B16-PAF-R cells at 10nM CPAF on days 1, 2
and 3 (***P<0.001 and **P<0.01 vs. MSCV
+ 10 nM group). No significant differences were observed in the
baseline growth rate between the B16-MSCV and B16-PAF-R cells.
PAF-R, platelet-activating factor-receptor; MSCV, empty vector;
qPCR, quantitative polymerase chain reaction; CPAF,
1-hexadecyl-2-N-methylcarbamoyl-3-glycerophosphocholine, VEH,
vehicle. |
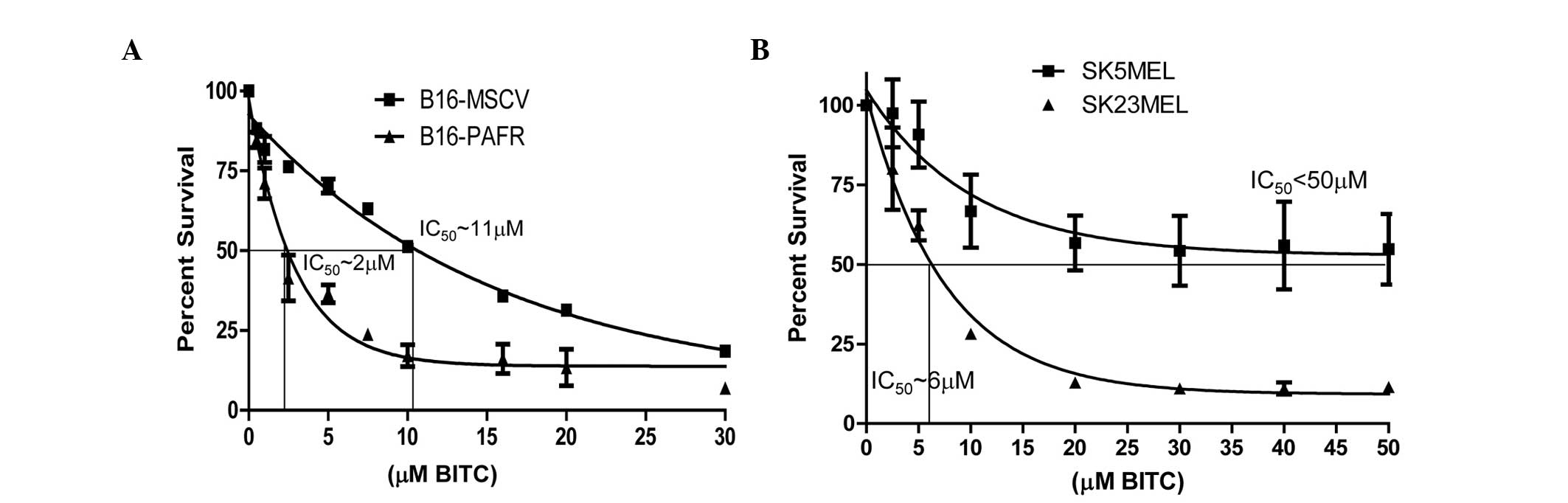
Effect of the BITC-mediated cytotoxicity
of melanoma cells with regards to the PAF-R
Previous studies have demonstrated that ITC analogs,
including BITC, exhibit decreased cytotoxicity towards melanoma
cells in vitro (half maximal inhibitory concentration 10–20
μM), indicating that higher concentrations are required to
achieve efficacy (29). The
present study aimed to determine the effect of PAF-R on the
BITC-mediated cytotoxicity of melanoma cells. The relative
cytotoxicity of BITC in the melanoma cells was compared in the
presence or absence of functional PAF-R using murine and human
melanoma cells. PAF-R positive, B16-PAF-R and SK23MEL and PAF-R
negative, B16-MSCV and SK5MEL cells were treated with different
concentrations of BITC for 24 h and cell survival was measured. As
shown in Fig. 2A and B, BITC
dose-dependently reduced the survival of these cells. However, the
expression of the PAF-R augmented the BITC-induced cytotoxicity in
the B16-PAF-R (IC50 ~2 mM) and SK23MEL (IC50
~6 mM) cells, compared with the PAF-R deficient cells. The
IC50 of BITC in PAF-R deficient murine B16-MSCV cells
was ~11 mM and in the human SK5MEL cells was >50 μM.
These findings indicated that PAF-R signaling augments the
decreased survival of the melanoma cells, which is elicited by
BITC, at a lower concentration than is required for the
PAF-R-deficient cells.
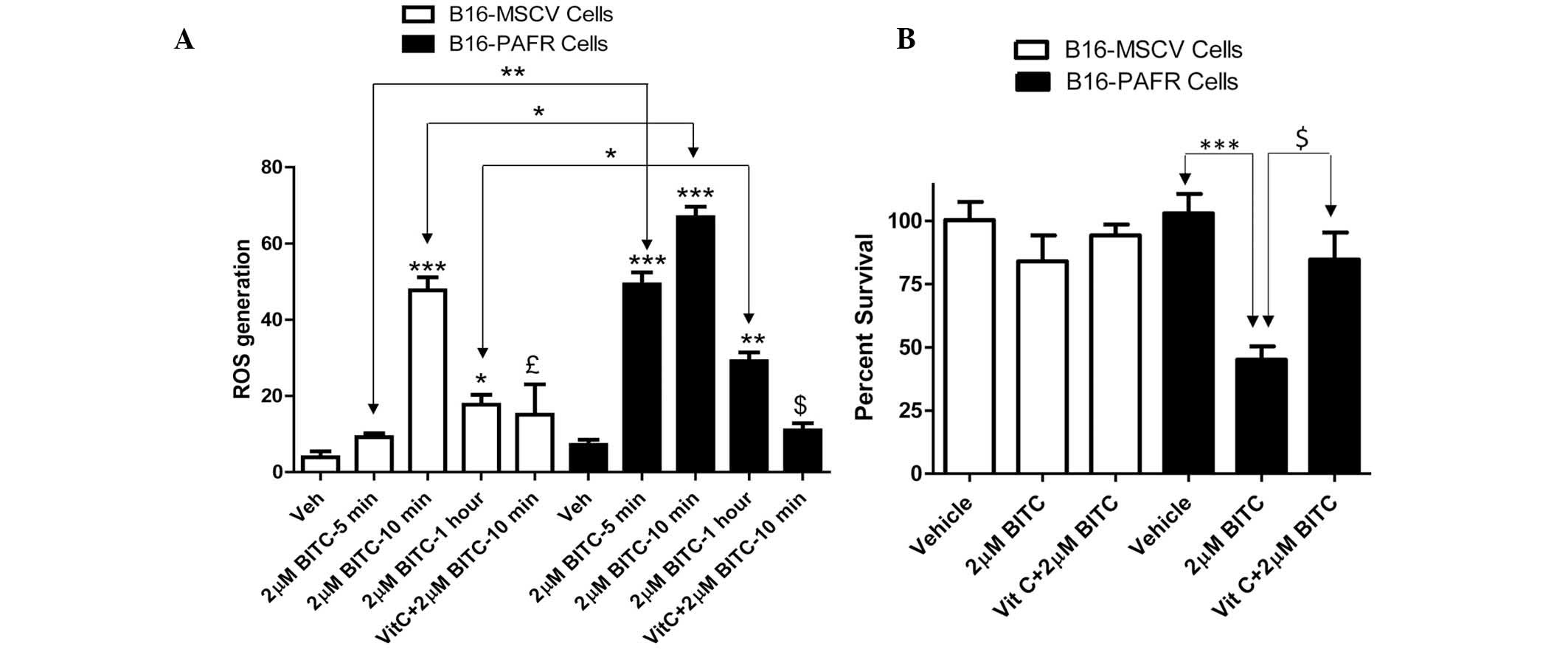
BITC treatment enhances the generation of
ROS in PAF-R-expressing melanoma cells
BITC acts as a pro-oxidative stressor, inducing the
generation of ROS as a potent mechanism of tumor cell death
(21,22,24,30–32).
By contrast, other studies have demonstrated that BITC can also
mediate potent antioxidant effects against oxidized low density
lipoprotein-induced endothelial dysfunction (33) and inflammation-mediated
carcinogenesis (34,35). To determine the mechanism
underlying the BITC-induced decreased survival rate of the PAF-R
expressing melanoma cells, the effect of BITC on ROS generation was
measured. For mechanistic studies, B16-PAF-R and B16-MSCV cells
were used as these lines were generated from the same parent
(B16F10) cells. As the IC50 of BITC in the B16-PAF-R
cells was ~2 μM, this concentration of BITC was used to
treat the B16-PAF-R and B16-MSCV cells at different time points.
The cells were pretreated with the antioxidant, vitamin C (5 mM)
for 1 h and subsequently with BITC. As shown in Fig. 3A, BITC treatment induced a
significant increase in ROS generation in each of the cell lines.
However, in the B16-PAF-R cells, ROS generation occurred as early
as 5 min after treatment and was significantly increased compared
with the B16-MSCV cells at all time points (Fig. 3A). Treatment with vitamin C
inhibited the BITC-induced ROS generation (Fig. 3A) and rescued B16-PAF-R cells
(Fig. 3B), indicating a role for
ROS in the BITC-induced suppression of the B16-PAF-R cells.
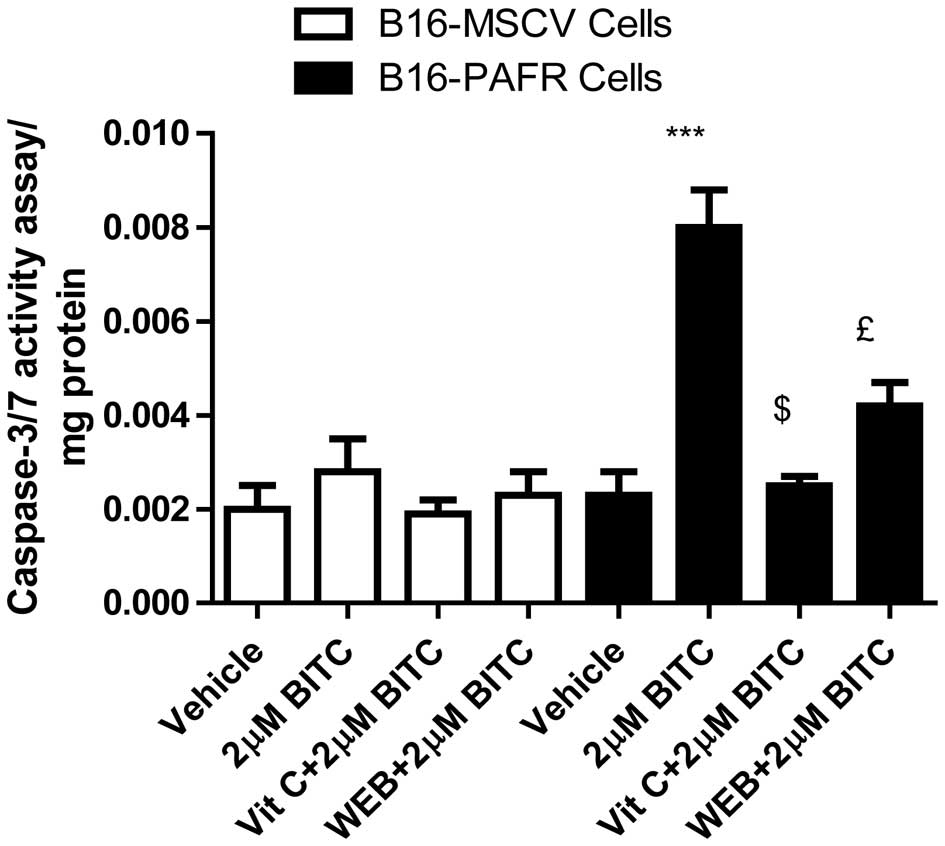
PAF-R augments BITC-induced apoptosis in
the B16-PAF-R cells
The induction of apoptosis in malignant cells has
been revealed as a major mechanism of chemopreventive/therapeutic
drug-mediated cell death (12,15,19,30–33).
To determine whether the expression of PAF-R augments the
BITC-mediated decrease in B16-PAF-R cell survival via ROS
generation, apoptosis induction was measured. The B16-PAF-R and
B16-MSCV cells were treated with 2 μM BITC for 24 h in the
presence or absence of either vitamin C or the WEB2086 PAF-R
antagonist. As shown in Fig. 4,
BITC-treatment resulted in a significant apoptotic response in the
B16-PAF-R cells compared with the B16-MSCV cells. The induction of
apoptosis in the B16-PAF-R cells was significantly attenuated
following treatment with vitamin C and WEB2086. These results
indicated the involvement of PAF-R signaling in the BITC-induced
decrease in growth of the B16-PAF-R cells mediated via increased
ROS generation and induction of apoptosis.
Discussion
The activation of PAF-R is important in diverse
biological processes, including regulating the growth of melanoma
(3–13), and the majority of types of
melanoma express PAF-R (9) and are
resistant to the currently used chemotherapeutic agents (16). Therefore, the present study
investigated the efficacy of BITC against melanoma cells, in those
either expressing PAF-R or not. Human melanoma cells, either
deficient in or expressing PAF-R, and murine melanoma B16F10 cells,
which have been extensively used in chemotherapy studies and lack
functional PAF-R expression, were genetically modified to produce
cells stably expressing PAF-R or vector-controls as suitable model
systems.
Treatment with CPAF increased the proliferation of
the PAF-R-expressing B16-PAF-R cells in a dose- and time-dependent
manner compared with the B16-MSCV cells, confirming that PAF-R
activation enhances the growth of melanoma cells. By contrast,
BITC-treatment reduced the survival rate of the murine and human
melanoma cells in a dose-dependent manner. However, the presence of
functional PAF-R potentiated the cytotoxicity of BITC in the
B16-PAF-R murine and human SK23MEL melanoma cells, requiring
relatively lower doses than were required for the PAF-R deficient
cells. This indicated that the expression of PAF-R results in
enhanced cytotoxicity of the melanoma cells by BITC.
Generation of ROS is an early event following
chemotherapy, that is important in inducing apoptosis in malignant
cells (15,30–32).
In this context, treatment with BITC at doses that substantially
reduced the survival of the B16-PAF-R cells, increased the
generation of ROS and the levels of apoptosis compared with the
B16-MSCV cells. These effects were attenuated by vitamin C,
suggesting the involvement of ROS in the BITC-induced decreased
survival of B16-PAF-R cells. These data are consistent with
previous studies, which demonstrated that the BITC-induced
suppression of malignant cells was mediated via ROS generation and
inhibited by free radical quenchers (30). The present study demonstrated that
BITC-treatment resulted in an increased level of apoptosis of the
B16-PAF-R cells via ROS and confirmed the role of the PAF-R
activation. The BITC-induced increase in apoptosis in the B16-PAF-R
cells was inhibited by the WEB2086 PAF-R antagonist, confirming the
involvement of PAF-R signaling in this process. The ability of
PAF-R signaling to promote the proliferation of the B16-PAF-R cells
and to augment BITC-mediated apoptosis was in agreement with
previous studies, which observed that CPAF-treatment induced
increased proliferation in a PAF-R-negative KB epithelial cell
line, which was genetically modified to stably express the
functional PAF-R (KBP), however this did not occur in retroviral
vector-transduced control KBM cells (36). Similarly, the expression of PAF-R
augmented ultraviolet B (UVB)-mediated apoptosis of PAF-R-positive
KBP but not PAF-R-negative KBM cells (14). This increased susceptibility of the
KBP cells towards UVB-mediated enhanced apoptosis was inhibited by
antioxidants and PAF-R antagonists. Additionally, PAF-R activation
augmented chemotherapy-induced cytotoxicity in human carcinoma cell
lines (15). These previous
studies confirm the involvement of the PAF-R signaling in mediating
pro-oxidative stressors including BITC-mediated increase in
melanoma cell apoptosis.
In conclusion, naturally occurring ITCs have been
demonstrated to possess anticarcinogenic properties, however, the
lack of specific oncogenic targets and the use of higher
concentrations to achieve optimum therapeutic efficacy have made
them unsuitable. Therefore, the selection of potent analogs, which
can target specific signaling pathways may offer more effective
agents against malignant cells. The present study demonstrated that
BITC suppressed the growth of melanoma cells and that this was
augmented by the activation of PAF-R through the ROS-mediated
pathway. Collectively, these data suggest that BITC may be used as
a novel chemotherapeutic agent against PAF-R-expressing melanoma
cells.
Acknowledgments
The present study was supported by a grant from the
American Institute for Cancer Research (no. 09A062), the American
Cancer Society-Institutional Research Grant (no. 4185607), the
Showalter Research Trust Award (no. 4485602) and the National
Institute of Health K22 (no. ES0238850). The authors would like to
thank Dr Christopher Touloukian from the Department of Surgery,
Indiana University School of Medicine (Indianapolis, USA) for the
human melanoma SK23MEL cells (37), Dr Jeffrey Travers (Department of
Dermatology, Indiana University School of Medicine, Indianapolis,
IN 46202, USA) and Dr Raymond Konger (Laboratory Medicine and
Dermatology, Indiana University School of Medicine, Indianapolis,
IN 46202, USA) for their support and critically evaluating this
manuscript. The abstract was published as abstract no. LB332 in
FASEBJ 28 (Suppl 1), 2014.
Abbreviations:
|
PAF
|
platelet-activating factor
|
|
PAF-R
|
PAF-receptor
|
|
CPAF
|
1-hexadecyl-2-N-methylcarbamoyl-3-glycerophosphocholine
|
|
BITC
|
benzyl isothiocyanate
|
|
ROS
|
reactive oxygen species
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
mRNA
|
messenger RNA
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute: SEER Stat Fact
Sheets: Melanoma of the Skin. http://seer.cancer.gov/statfacts/html/melan.html.
Accessed January 21, 2015.
|
|
3
|
Ishii S, Nagase T and Shimizu T:
Platelet-activating factor receptor. Prostaglandins Other Lipid
Mediat. 68:599–609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhuang Q, Bastien Y and Mazer BD:
Activation via multiple signaling pathways induces down-regulation
of platelet-activating factor receptors on human B lymphocytes. J
Immunol. 165:2423–2431. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travers JB, Huff JC, Rola-Pleszczynski M,
Gelfand EW, Morelli JG and Murphy RC: Identification of functional
platelet-activating factor receptors on human keratinocytes. J
Invest Dermatol. 105:816–823. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahu RP, Kozman AA, Yao Y, DaSilva SC,
Rezania S, Martel KC, Warren SJ, Travers JB and Konger RL: Loss of
the platelet activating factor receptor in mice augments
PMA-induced inflammation and cutaneous chemical carcinogenesis.
Carcinogenesis. 33:694–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahu RP, Petrache I, Van Demark MJ, Rashid
BM, Ocana JA, Tang Y, Yi Q, Turner MJ, Konger RL and Travers JB:
Cigarette smoke exposure inhibits contact hypersensitivity via the
generation of platelet-activating factor agonists. J Immunol.
190:2447–2454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hackler PC, Reuss S, Konger RL, Travers JB
and Sahu RP: Systemic platelet-activating factor receptor
activation augments experimental lung tumor growth and metastasis.
Cancer Growth Metastasis. 19:27–32. 2014.
|
|
9
|
Melnikova VO, Mourad-Zeidan AA, Lev DC and
Bar-Eli M: Platelet-activating factor mediates MMP-2 expression and
activation via phosphorylation of cAMP-response element-binding
protein and contributes to melanoma metastasis. J Biol Chem.
281:2911–2922. 2006. View Article : Google Scholar
|
|
10
|
Heon Seo K, Ko HM, Kim HA, Choi JH, Jun
Park S, Kim KJ, Lee HK and Im SY: Platelet-activating factor
induces upregulation of antiapoptotic factors in a melanoma cell
line through nuclear factor-kappaB activation. Cancer Res.
66:4681–4686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biancone L, Cantaluppi V, Del Sorbo L,
Russo S, Tjoelker LW and Camussi G: Platelet-activating factor
inactivation by local expression of platelet-activating factor
acetyl-hydrolase modifies tumor vascularization and growth. Clin
Cancer Res. 9:4214–4220. 2003.PubMed/NCBI
|
|
12
|
de Oliveira SI, Andrade LN, Onuchic AC,
Nonogaki S, Fernandes PD, Pinheiro MC, Rohde CB, Chammas R and
Jancar S: Platelet-activating factor receptor (PAF-R)-dependent
pathways control tumour growth and tumour response to chemotherapy.
BMC Cancer. 10:2002010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sahu RP, Turner MJ, DaSilva SC, Rashid BM,
Ocana JA, Perkins SM, Konger RL, Touloukian CE, Kaplan MH and
Travers JB: The environmental stressor ultraviolet B radiation
inhibits murine antitumor immunity through its ability to generate
platelet-activating factor agonists. Carcinogenesis. 33:1360–1367.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barber LA, Spandau DF, Rathman SC, Murphy
RC, Johnson CA, Kelley SW, Hurwitz SA and Travers JB: Expression of
the platelet-activating factor receptor results in enhanced
ultraviolet B radiation-induced apoptosis in a human epidermal cell
line. J Biol Chem. 273:18891–18897. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Southall MD, Yi Q, Pei Y, Lewis D,
Al-Hassani M, Spandau D and Travers JB: The epidermal
platelet-activating factor receptor augments chemotherapy-induced
apoptosis in human carcinoma cell lines. J Biol Chem.
278:16614–16621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jilaveanu LB, Aziz SA and Kluger HM:
Chemotherapy and biologic therapies for melanoma: Do they work?
Clin Dermatol. 27:614–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Block G, Patterson B and Subar A: Fruit,
vegetables and cancer prevention: A review of the epidemiological
evidence. Nutr Cancer. 18:1–29. 1992. View Article : Google Scholar
|
|
18
|
Stoner GD and Morse MA: Isothiocyanates
and plant polyphenols as inhibitors of lung and esophageal cancer.
Cancer Lett. 114:113–119. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahu RP and Srivastava SK: The role of
STAT-3 in the induction of apoptosis in pancreatic cancer cells by
benzyl isothiocyanate. J Natl Cancer Inst. 101:176–193. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warin R, Xiao D, Arlotti JA, Bommareddy A
and Singh SV: Inhibition of human breast cancer xenograft growth by
cruciferous vegetable constituent benzyl isothiocyanate. Mol
Carcinog. 49:500–507. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CL, Huang AC, Yang JS, Liao CL, Lu HF,
Chou ST, Ma CY, Hsia TC, Ko YC and Chung JG: Benzyl isothiocyanate
(BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of
reactive oxygen species causes cell cycle arrest and induces
apoptosis via activation of caspase-3, mitochondria dysfunction and
nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J
Orthop Res. 29:1199–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q
and Xu K: Isothiocyanates induce oxidative stress and suppress the
metastasis potential of human non-small cell lung cancer cells. BMC
Cancer. 10:2692010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni WY, Hsiao YP, Hsu SC, Hsueh SC, Chang
CH, Ji BC, Yang JS, Lu HF and Chung JG: Oral administration of
benzyl-isothiocyanate inhibits in vivo growth of subcutaneous
xenograft tumors of human malignant melanoma A375.S2 cells. In
Vivo. 27:623–626. 2013.PubMed/NCBI
|
|
24
|
Huang SH, Wu LW, Huang AC, Yu CC, Lien JC,
Huang YP, Yang JS, Yang JH, Hsiao YP, Wood WG, Yu CS and Chung JG:
Benzyl isothiocyanate (BITC) induces G2/M phase arrest and
apoptosis in human melanoma A375.S2 cells through reactive oxygen
species (ROS) and both mitochondria-dependent and death
receptor-mediated multiple signaling pathways. J Agric Food Chem.
60:665–675. 2012. View Article : Google Scholar
|
|
25
|
Thejass P and Kuttan G: Allyl
isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit
tumour-specific angiogenesis by downregulating nitric oxide (NO)
and tumour necrosis factor-alpha (TNF-alpha) production. Nitric
Oxide. 16:247–257. 2007. View Article : Google Scholar
|
|
26
|
Manesh C and Kuttan G: Effect of naturally
occurring allyl and phenyl isothiocyanates in the inhibition of
experimental pulmonary metastasis induced by B16F-10 melanoma
cells. Fitoterapia. 74:355–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamsa TP, Thejass P and Kuttan G:
Induction of apoptosis by sulforaphane in highly metastatic B16F-10
melanoma cells. Drug Chem Toxicol. 34:332–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahu RP, DaSilva SC, Rashid B, Martel KC,
Jernigan D, Mehta SR, Mohamed DR, Rezania S, Bradish JR, Armstrong
AB, Warren S and Konger RL: Mice lacking epidermal PPARγ exhibit a
marked augmentation in photocarcinogenesis associated with
increased UVB-induced apoptosis, inflammation and barrier
dysfunction. Int J Cancer. 131:E1055–E1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma A, Sharma AK, Madhunapantula SV,
Desai D, Huh SJ, Mosca P, Amin S and Robertson GP: Targeting Akt3
signaling in malignant melanoma using isoselenocyanates. Clin
Cancer Res. 15:1674–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sahu RP, Zhang R, Batra S, Shi Y and
Srivastava SK: Benzyl isothiocyanate-mediated generation of
reactive oxygen species causes cell cycle arrest and induces
apoptosis via activation of MAPK in human pancreatic cancer cells.
Carcinogenesis. 30:1744–1753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao D, Powolny AA and Singh SV: Benzyl
isothiocyanate targets mitochondrial respiratory chain to trigger
reactive oxygen species-dependent apoptosis in human breast cancer
cells. J Biol Chem. 283:30151–30163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyoshi N, Watanabe E, Osawa T, Okuhira M,
Murata Y, Ohshima H and Nakamura Y: ATP depletion alters the mode
of cell death induced by benzyl isothiocyanate. Biochim Biophys
Acta. 1782:566–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doroshow JH: Redox modulation of
chemotherapy-induced tumor cell killing and normal tissue toxicity.
J Natl Cancer Inst. 98:223–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang CS, Lin AH, Liu CT, Tsai CW, Chang
IS, Chen HW and Lii CK: Isothiocyanates protect against oxidized
LDL-induced endothelial dysfunction by upregulating Nrf2-dependent
antioxidation and suppressing NFκB activation. Mol Nutr Food Res.
57:1918–1930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyoshi N, Takabayashi S, Osawa T and
Nakamura Y: Benzyl isothiocyanate inhibits excessive superoxide
generation in inflammatory leukocytes: implication for prevention
against inflammation-related carcinogenesis. Carcinogenesis.
25:567–575. 2004. View Article : Google Scholar
|
|
36
|
Marques SA, Dy LC, Southall MD, Yi Q,
Smietana E, Kapur R, Marques M, Travers JB and Spandau DF: The
platelet-activating factor receptor activates the extracellular
signal-regulated kinase mitogen-activated protein kinase and
induces proliferation of epidermal cells through an epidermal
growth factor-receptor-dependent pathway. J Pharmacol Exp Ther.
300:1026–1035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brandmaier AG, Leitner WW, Ha SP, Sidney
J, Restifo NP and Touloukian CE: High-avidity autoreactive CD4+ T
cells induce host CTL, overcome T(regs) and mediate tumor
destruction. J Immunother. 32:677–688. 2009. View Article : Google Scholar : PubMed/NCBI
|