Introduction
Diabetic retinopathy (DR) is one of the common
complications associated with diabetic mellitus (DM) and it is the
leading cause of blindness in people over the age of 50 (1). Recent research has demonstrated that
DR is not only a microvascular disease but may be a result of
neurodegenerative processes. Glucose-induced neuron and glial cell
damage may occur in the absence of microvascular injury (2). Müller cells, the principal glial
cells of the retina, provide neurons with adenosine triphosphate
and nutrition (3), control levels
of K+, H+ and neurotransmitters in
extracellular space (4), and are
involved in signal transmission by communicate with retinal neurons
through corresponding receptors and transporters (5). Müller cells are the only cells in the
retina that contain glutamine synthetase (GS). This enzyme is
associated with the transformation of glutamate into glutamine via
synaptic pathways (6,7). In healthy retinas, Müller cells are
essential for the removal of glutamate and help to prevent the
accumulation of a toxic concentration in the retina (8). In patients with DM, Müller cells are
not able to transform glutamate to glutamine and, therefore,
glutamate concentrations are elevated in the retinas of these
individuals (9). Studies have
shown that glial fibrillary acidic protein expression is
upregulated and that the nucleus changes in Müller cells, during
the early stages of DR (3,10–14).
In addition, Müller cells secrete a number of growth factors,
cytokines (15) and inflammatory
regulators (16) during the
development of DR, and are an important source of inflammatory
factors. Therefore, the development of strategies to protect Müller
cells would be beneficial in the treatment of DR.
Agmatine is an endogenous polyamine, which is formed
by enzymatic decarboxylation of L-arginine in the mammalian brain
(17,18). Agmatine is a neurotransmitter and
neuromodulator, which is secreted from specific neuron networks
(19,20). It interacts with a number of
ligand-gated ion channels and binds with certain cellular receptors
(21). Importantly, it is capable
of inhibiting N-methyl-D-aspartic acid receptors (NMDARs) in a
voltage- and concentration-dependent manner (22,23).
Furthermore, agmatine inhibits nitric oxide synthase (NOS) isoforms
by suppressing catalytic activity (24). Previous studies have suggested that
agmatine may protect retinal ganglion cells from oxidative stress
(25,26), promote glial cell survival in
spinal cord injury (27) and
attenuate LPS-induced microglial damage (28). However, the majority of studies
have focused on the protective effects of agmatine via NMDAR
inhibition in the neurons, or via NOS inhibition in the glial cells
of the central nervous system (CNS). To the best of our knowledge,
there have been no reports of the effects of agmatine treatment on
damaged glial cells in patients with DR. NMDAR, an ionotropic
glutamate receptor that mediates Ca2+ entry when it is
activated, is present in the Müller cells of vertebrate retinas
(29). Based on the data described
above, agmatine is suggested to exert beneficial effects in DR
damaged glial cells via NMDAR inhibition. In the present study, the
protective effects of agmatine on high-concentration
glucose-induced Müller cell injury were evaluated in vitro.
The association between the protective effects of agmatine, and the
expression of NMDARs and downstream signaling proteins in Müller
cells was also investigated.
Materials and methods
Cell culture
A total of 30 male Sprague-Dawley rats, aged 4
weeks, obtained from Jilin University Laboratory Center (Jilin,
China) were used for the primary Müller cells culture. The
experimental protocols were approved by the ethics committee of
Jilin University (Jilin, China).
Isolated retinas were washed twice using
phosphate-buffered saline (PBS) and then separated using a Pasteur
pipette (Xuansheng, Shanghai, China) in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA). Cells
were filtered using a cell strainer (Nanjing Union Bio-Technology,
Nanjing, China), and then seeded and maintained using DMEM medium,
containing 10% fetal bovine serum (Gibco Life Technologies). Cells
were cultured at 37°C in humidified 5% CO2, and the
medium was replaced every 2 days. Subsequent to 5 days of
isolation, the cells were passaged every 3 days.
In order to conduct the cell survival assay, cells
from the third passage were seeded into 96-well plates and divided
into seven groups: (1) Healthy
glucose control group (control; 25 mM glucose and 30 mM mannitol);
(2) high-concentration glucose
group (HG; 55 mM glucose); (3)
high-concentration glucose, low-concentration agmatine treatment
group (HAL; 55 mM glucose and 50 μM agmatine); (4) high-concentration glucose,
medium-concentration agmatine treatment group (HAM; 55 mM glucose
and 100 μM agmatine); (5)
high-concentration glucose, high-concentration agmatine treatment
group (HAH; 55 mM glucose and 200 μM agma-tine); (6) high-concentration glucose, agmatine
treatment and NMDA group (HAN; 55 mM glucose, 100 μM
agmatine and 100 μM NMDA); and (7) high-concentration glucose and NMDA
group (HN; 55 mM glucose and 100 μM NMDA). Subsequently,
cells of passage three were seeded in to 6-well or 96-well plates
and divided into four groups, as the medium concentration of
agmatine was considered optimal: (1) Control; (2) HG; (3) HAM; and (4) HAN.
Prior to receiving treatments, cells in each group
were starved for 12 h and exposed to DMEM, containing the
corresponding treatments for 48 h.
Immunofluorescence
Müller cells were fixed on coverslips using 4%
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd., Beijing,
China) for 15 min, followed by washing in PBS for 5 min. Coverslips
were then treated with 0.1% TritonX-100 (Sigma-Aldrich, St. Louis,
MO, USA) for 30 min, at room temperature. Following a wash stage in
PBS for 5 min, blocking was achieved using goat serum (Solarbio
Science & Technology, Co., Ltd. Beijing, China) for 15 min at
room temperature. Cells were then incubated overnight with the
polyclonal NMDAR1 (1:100 dilution, catalogue no. orb99445; Biorbyt
Ltd., Cambridge, UK) or GS antibodies (1:50 dilution; catalogue no.
sc-9067; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), at 4°C.
Coverslips were washed using PBS and incubated with fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit secondary
antibody (1:100; catalogue no. A0562; Beyotime Institute of
Biotechnology, Haimen, China) for 1 h, at room temperature.
Following a wash phase using PBS, slips were then stained with DAPI
(Biosharp, Heifei, China) for 5 min. Fluorescence images were
captured using a fluorescence microscope (FV1000S-SIM/IX81, Olympus
Corporation, Tokyo, Japan).
Cell survival assay
In order to conduct a cell survival assay, 10
μl Cell Counting kit-8 solution (Beyotime Institute of
Biotechnology) was added into each well of the plate, and plates
were incubated at 37°C for 1 h. The plates were then analyzed using
an enzyme-linked immunosorbent assay (ELISA) reader (ELX-800,
BioTek Instruments, Inc., Winooski, VT, USA), at 450 nm.
Lactate dehydrogenase (LDH)
measurements
In order to conduct LDH activity measurements,
supernatants from the Müller cells were centrifuged at 1,100 × g
for 5 min. LDH activity was measured using a standard LDH kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Results were calculated using the following formula according to
the assay kit used: LDH activity (U/L) = optical density (OD) value
(sample-control)/OD value (standard-blank) × standard concentration
(2 mmol/l) × 1,000.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Cells were collected and washed with PBS. Total RNA
was extracted using RNA simple total RNA kit (Tiangen Biotech Co.,
Ltd., Beijing, China) according to the manufacturer’s instructions.
cDNA was synthesized with oligonucleotide primers, using super
Moloney Murine Leukemia Virus Reverse Transcriptase (BioTeke
Corporation, Beijing, China). RT-PCR was performed with 1 μg
cDNA using the 2 × Power Taq PCR Master Mix (BioTeke Corporation)
and SYBR Green (Solarbio Science & Technology, Co., Ltd.). PCR
reactions were performed using a PCR system (Exicycler 96, Bioneer
Corporation, Daejeon, Korea). Relative mRNA levels were normalized
against β-actin and presented as 2−ΔΔCt. Primers used
are listed in Table I.
 | Table IOligonucleotide primers for
RT-PCR. |
Table I
Oligonucleotide primers for
RT-PCR.
| Primer | Sequence
(5′-3′) |
|---|
| TNF-α-F |
TGGCGTGTTCATCCGTTCT |
| TNF-α-R |
CCACTACTTCAGCGTCTCGT |
| β-actin-F |
GGAGATTACTGCCCTGGCTCCTAGC |
| β-actin-R |
GGCCGGACTCATCGTACTCCTGCTT |
TNF-α expression analysis using
ELISA
Following treatment for 48 h, the medium was
centrifuged at 1,100 × g for 5 min and supernatants were collected
in order to conduct a TNF-α assay. TNF-α expression levels were
quantified according to the manufacturer’s instructions using a Rat
TNF-α ELISA kit specific to rats (Multisciences, Hangzhou,
China).
Flow cytometric determination of
apoptosis
Double staining using propidium iodide (PI) was
performed in order to analyze annexin V-FITC binding to membrane
phosphatidylserine and cellular DNA, according to the
manufacturer’s instructions (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China). Following treatment for 48 h, cells were harvested
and centrifuged at 88 × g for 10 min, washed twice with PBS, and
resuspended in 500 μl binding buffer (Nanjing KeyGen
Biotech. Co., Ltd.). Annexin V-FITC (5 μl) and 5 μl
PI were then added, and the samples were incubated for 15 min in
darkness at room temperature. Samples were acquired immediately on
a BD FACS Calibur flow cytometer (BD Biosciences, San Jose, CA,
USA) using CellQuest software, version 3.0 (BD Biosciences).
Annexin V-FITC and PI emissions were detected in the FL 1 and FL 2
channels. For each analysis, 100,000 counts were recorded. Data
analysis was performed using FCS Express V3.00 (DeNovo Software,
Thornhill, ON, Canada). In each plot, the lower left quadrant
represented viable cells; the upper left quadrant, necrotic cells;
the lower right quadrant, early apoptotic cells; and the upper
right quadrant, late apoptotic cells.
Hoechst staining
Apoptotic or necrotic cell death was characterized
by staining cells using Hoechst 33342. Cells were washed twice with
PBS and fixed with 5 ml fixing solution for 5 min. Subsequently,
cells were stained with 10 μg/ml Hoechst 33342 (Beyotime
Institute of Biotechnology) for 5 min at 37°C in darkness. Cells
were then washed twice with PBS and imaged using a digital camera
attached to a fluorescence microscope (AE31, Motic China Group Co.,
Ltd., Xiamen, China).
Western blot analysis
Cells were harvested and lysed by incubating with an
NP-40 lysis buffer (Beyotime Institute of Biotechnology) containing
1% phenylmethanesulfonylfluoride on ice, for 5 min. The cells were
centrifuged at 10,010 × g for 10 min at 4°C and protein
concentrations were determined using a commercial bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology).
Heat-induced dena-turation was conducted in a loading buffer [31%
SDS (w/v), 0.67% bromophenol blue (w/v) and 33.3% glycerol (v/v)]
and western blot analysis was performed, using 40 μg of
protein from each cell lysate. Samples and standards were loaded on
an SDS gel and separated at 80 V for 2.5 h. Proteins were then
transferred to polyvinylidene fluoride membranes (0.45 μm;
EMD Millipore, Billerica, MA, USA) in a transfer buffer [0.3% Tris
(w/v), 1.44% glycine (w/v) and 20% methanol (v/v)] at 70 V for 1.5
h. Following electroblotting, membranes were washed with
Tris-Buffered saline with Tween 20® (TBST; Sinopharm
Chemical Reagent Co., Ltd.) and blocked with TBST containing 5%
non-fat milk at room temperature, for 1 h. Subsequently, the
membranes were incubated in TBST containing 5% non-fat milk and
primary antibodies over night, at 4°C. The following polyclonal
primary antibodies were used: Bcl-2 (1:500; BA0412) and Bax
(1:1,000; BA0315) (Boster Biological Technology, Wuhan, China);
c-caspase-3 (cleaved-caspase-3; 1:1,000; bs-0081R; Beijing
Biosynthesis Biotechnology, Beijing, China), p-ERK (AM071) and ERK
(AM076) (1:1,000; Beyotime Institute of Biotechnology, Beijing,
China); p-JNK (WLP006) and JNK (WLN006) (1:1,000, Wanlei Life
Sciences, Shenyang, China); and p-p38 (sc-101758) and p38 (sc-7149)
(1:100 and 1:200, respectively; Santa Cruz Biotechnology, Santa
Cruz, CA, USA). Membranes were washed in TBST four times and
incubated for 45 min at 37°C with horseradish peroxidase-linked
secondary antibodies (1:5,000; Beyotime Institute of
Biotechnology). Subsequently, the membranes were washed in TBST
five times, incubated for 1 min in enhanced chemiluminescence
solution (7 Sea Pharmtech, Shanghai, China) and then exposed to a
Fuji Rx 100 X-ray film (Fuji Photo Film, Tokyo, Japan). The
membranes were then incubated in stripping buffer (Beyotime
Institute of Biotechnology) for 15 min at room temperature,
followed by incubation and detection of the reference gene, β-actin
with a polyclonal β-actin antibody (WL0001; Wanlei Life Sciences).
The density of the protein bands was normalized to the β-actin
signal.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Multiple comparisons were analyzed using one-way analysis of
variance followed by Bonferroni’s test using SPSS software, version
19 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
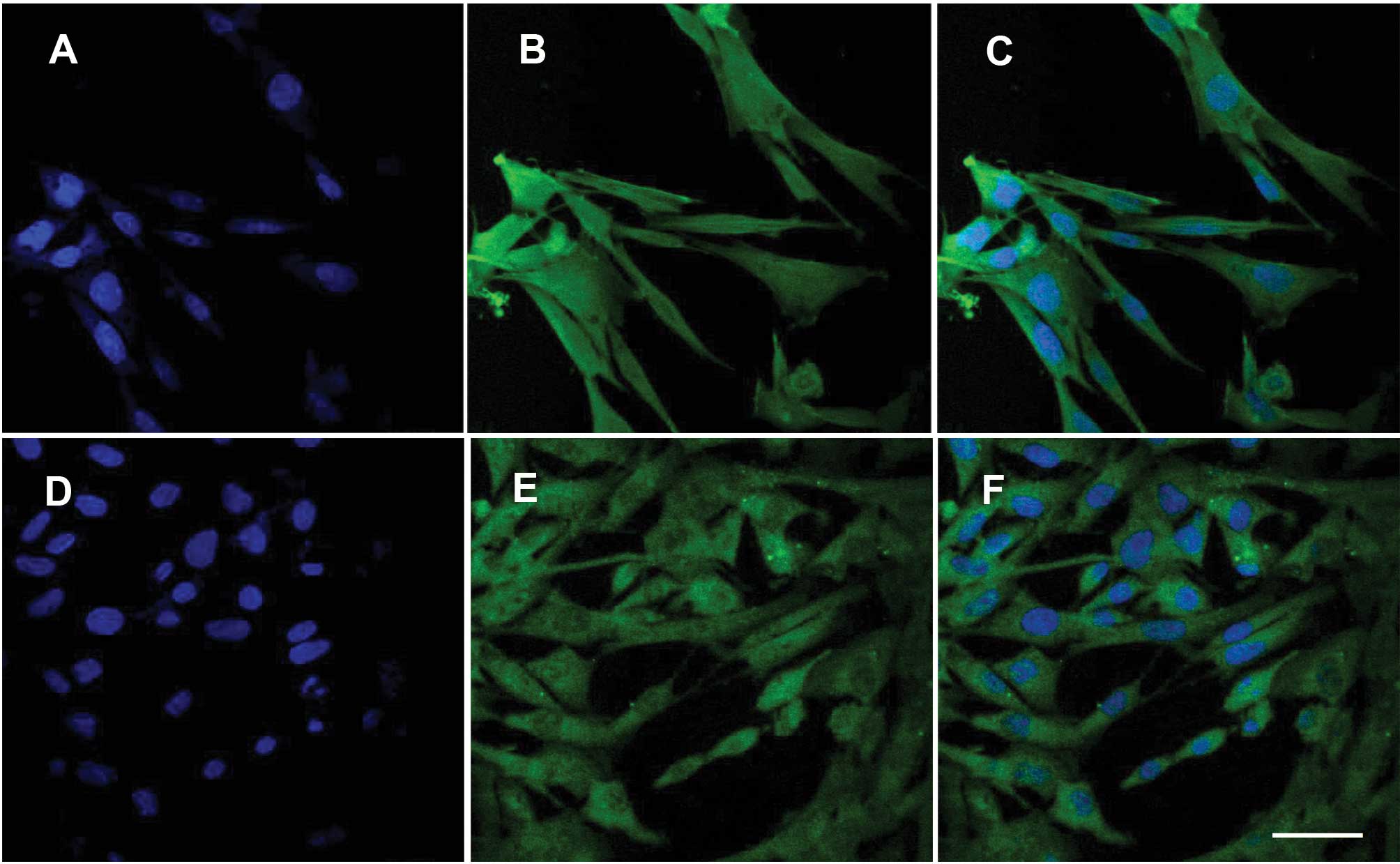
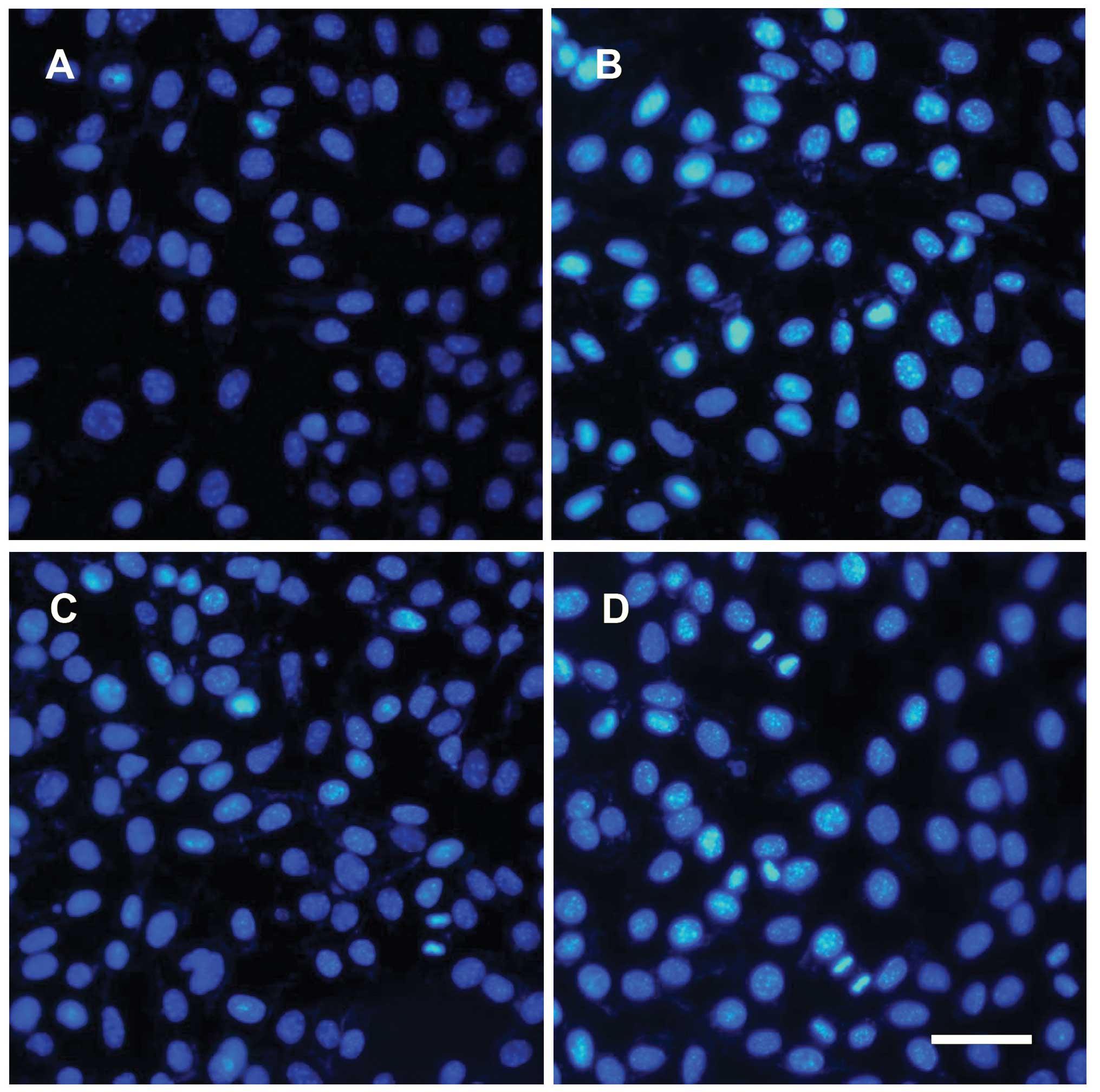
Müller cells express GS and NMDAR
In the retina, GS is only expressed in Müller cells.
Therefore, GS was used as an indicator of the presence of Müller
cells. To confirm the success of cell isolation and NMDAR
expression in the cells, isolated Müller cells were immunostained
using GS or NMDAR antibodies, and costained using DAPI. The nuclei
were positive for DAPI (Fig. 1A, C, D
and F) and the cytoplasm exhibited GS (Fig. 1B and C) or NMDAR (Fig. 1E and F) positive staining.
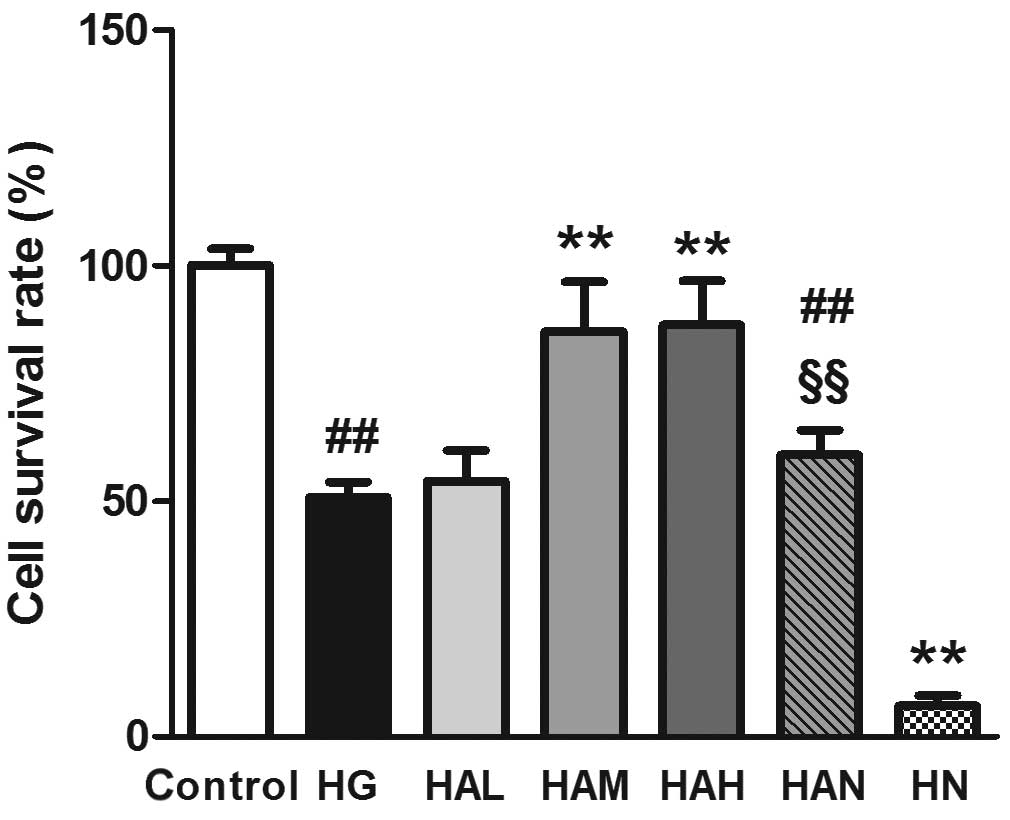
Agmatine improves Müller cell survival
rate
As shown in Fig. 2,
Müller cell survival was lower in cells in the HG group compared
with those in the control group (50.77±3.28%, compared with
100±3.56%, P<0.01). The survival rate of Müller cells was
significantly higher in cells in the HAM group compared with those
in the HG group (85.98±10.46%, P<0.01). Although the mean cell
survival rate value was lower, no significant difference was
observed between the HAM and control groups. High-concentration
agmatine treatment (HAH; 200 μM) did not lead to an increase
in cell survival rate compared with cells in the HAM group (100
μM agmatine treatment). Following treatment with NMDA and
agmatine (100 μM; HAN group), the cell survival was
significantly lower compared with cells in the HAM group
(59.84±5.22%, P<0.01). High glucose plus NMDA treatment without
agmatine (HN group) led to a significant reduction in the survival
rate of Müller cells (6.52±2.20%).
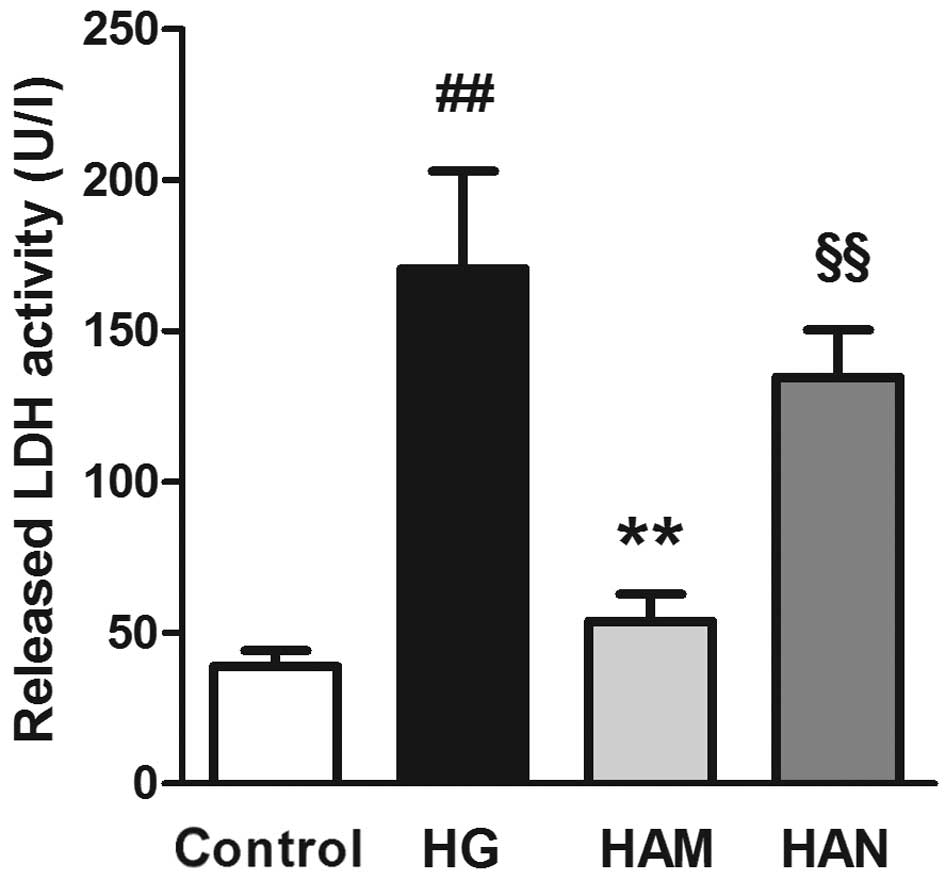
Agmatine inhibits high-concentration
glucose-induced LDH activity in Müller cells
LDH activity was measured in order to investigate
cell viability. As shown in Fig.
3, Müller cell exposure to high glucose concentrations (HG
group) led to an increase in LDH activity compared with the control
group (P<0.01), indicating a cellular toxic effect. LDH activity
was significantly lower in Müller cells in the HAM group compared
with those in the HG group (P<0.01). No significant difference
was observed between the LDH activity levels of cells in the HAM
group compared with those in the control group. LDH activity was
significantly higher in cells in the HAN group compared with those
in the HAM group.
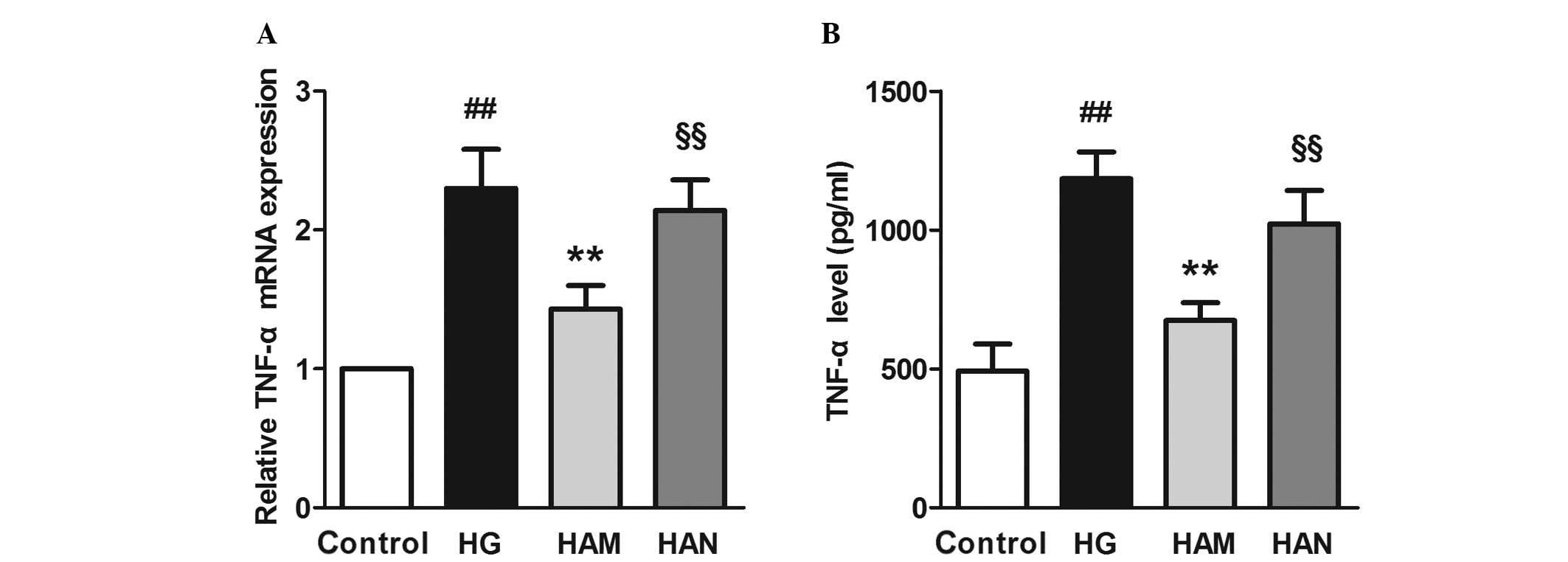
Agmatine inhibits high-concentration
glucose-induced TNF-α release and mRNA expression
TNF-α is a primary inflammatory factor that is
released during the early stages of inflammation and is an
indicator of glial cell activity. Previous studies have reported
that Müller cells release TNF-α during the development of DR
(30). In order to determine
whether TNF-α production may be regulated by agmatine treatment,
TNF-α mRNA expression and levels of TNF-α were assayed. Fig. 4 shows that TNF-α mRNA expression
and TNF-α release into the medium were significantly higher in
cells in the HG group compared with those in the control group
(P<0.01). Agmatine treatment (HAM; 100 μM agmatine) led
to a significant reduction in TNF-α levels in the medium and TNF-α
mRNA expression levels, compared with cells in the HG group
(P<0.01). NMDA treatment (HAN group) led to significantly higher
TNF-α levels in the medium and TNF-α mRNA expression levels,
compared with cells in the HAM group.
Agmatine inhibits high-concentration
glucose-induced cell apoptosis in Müller cells
A small number of cells (4.10±0.71%) were positive
for Annexin V-FITC and PI staining in the control group. Following
exposure to 55 mM glucose for 48 h, the percentage of apoptotic
significantly increased in the HG group compared with the control
group (50.07±4.30%; P<0.01; Fig.
5B). The number of apoptotic cells in the HAM group was
significantly lower compared with the HG group (20.19±1.98%;
P<0.01). The number of apoptotic cells was significantly higher
in the HAN group compared with the HAM group (35.10±2.57%;
P<0.01), suggesting that NMDA treatment suppressed the effects
of agmatine on the Müller cells.
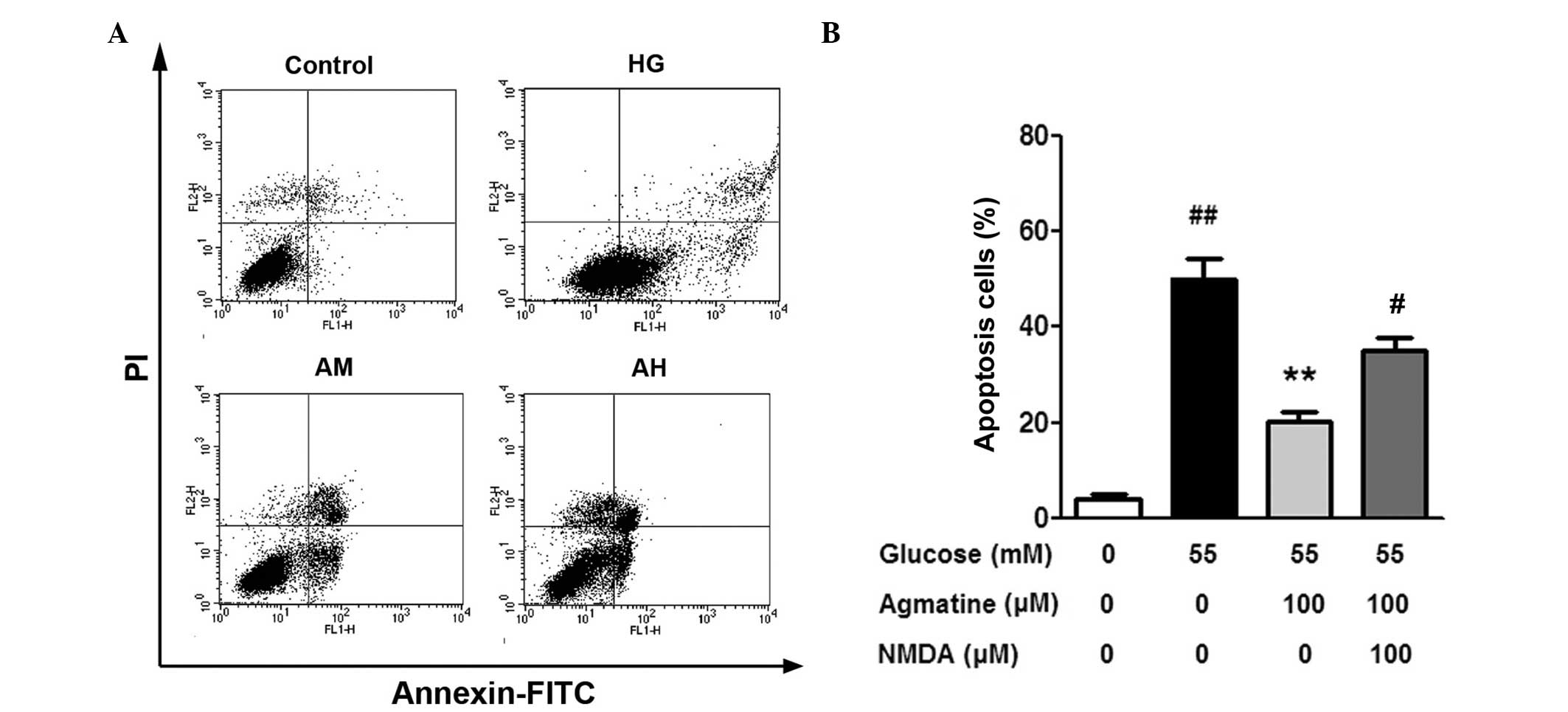 | Figure 5Effect of agmatine on glucose-induced
apoptosis in Müller cells. (A) Flow cytometric analysis
demonstrated apoptotic cells. (B) Histogram of cell apoptotic
percentage. Data are presented as the mean ± standard deviation
(n=3), ##P<0.01 compared with the control group,
**P<0.01 compared with the HG group and
#P<0.01 compared with the HAM group. HG, high
glucose; HAM, high glucose, medium agmatine concentration
treatment; NMDA, N-methyl-D-aspartic acid; HAH, high glucose, high
agmatine concentration treatment group; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Following cell apoptosis, nuclear condensation and
DNA fragmentation was detected using Hoechst 33342 staining and
fluorescence microscopy. As illustrated in Fig. 6, following incubation with glucose
for 48 h, a number of Müller cells with condensed and fragmented
nuclei were observed (Fig. 6B).
Agmatine treatment (HAM group) led to a marked decrease in the
number of apoptotic cells (Fig.
6C) and cells in the HAN group demonstrated nuclear
condensation and DNA fragmentation following NMDA treatment.
Therefore NMDA appeared to suppress the antiapoptotic effect of
agmatine (Fig. 6D).
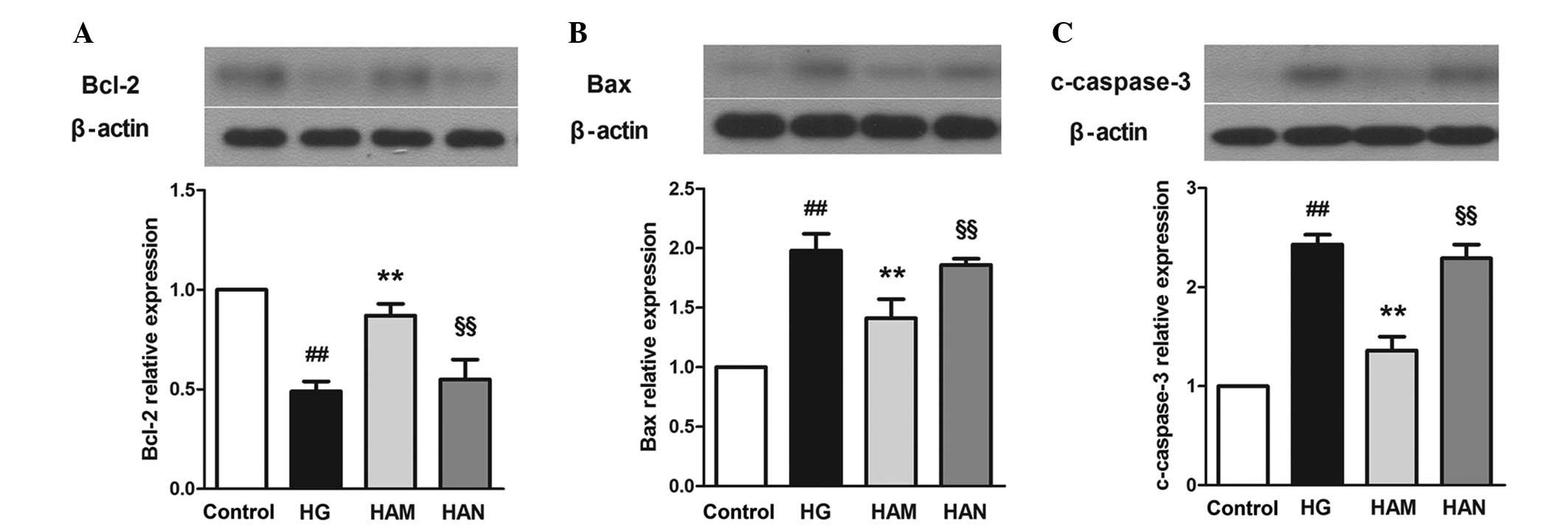
Agmatine inhibits high-concentration
glucose-induced apoptosis via regulation of apoptotic signaling
protein expression
In order to further investigate the molecular
mechanisms underlying the protective effects of agmatine on
glucose-induced apoptosis, the expression of apoptosis-associated
proteins Bax, Bcl-2 and cleaved-caspase 3 were investigated.
The results of the present study demonstrated that
glucose treatment led to a decrease in Bcl-2 expression and an
increase in Bax expression levels in Müller cells, compared with
those in the control group (Fig.
7). Bcl-2 expression levels were higher and Bax expression
levels were lower in the HAM group compared those the HG group. In
addition, caspase-3 expression, which is an important effector of
the apoptotic pathway, was higher in cells in the HG group compared
with that in the control group (P<0.01). A significant decrease
in caspase-3 expression was observed in cells in the HAM group
compared with that in cells in the HG group (P<0.01). NMDA
treatment reversed the effects of agmatine on BCl-2, Bax and
caspase-3 expression in glucose-damaged Müller cells.
Agmatine inhibits glucose-induced
mitogen-activated protein kinase (MAPK) activity
MAPKs are downstream proteins that are associated
with the NMDAR signaling pathway. Phosphorylation levels of three
MAPK-associated proteins, extracellular signal regulated kinase
(ERK), c-Jun N-terminal kinase (JNK) and p38 kinase (p38), were
detected in the present study. As shown in Fig. 8, ERK, JNK and p38 protein
phosphorylation levels were significantly higher in cells in the HG
group compared with those in the control group (P<0.01).
Following agmatine treatment (100 μM; HAM group), ERK, JNK
and p38 phosphorylation levels were significantly decreased
compared with the HG group (P<0.01). No marked changes in ERK,
JNK or p38 total protein expression levels were observed (data not
shown).
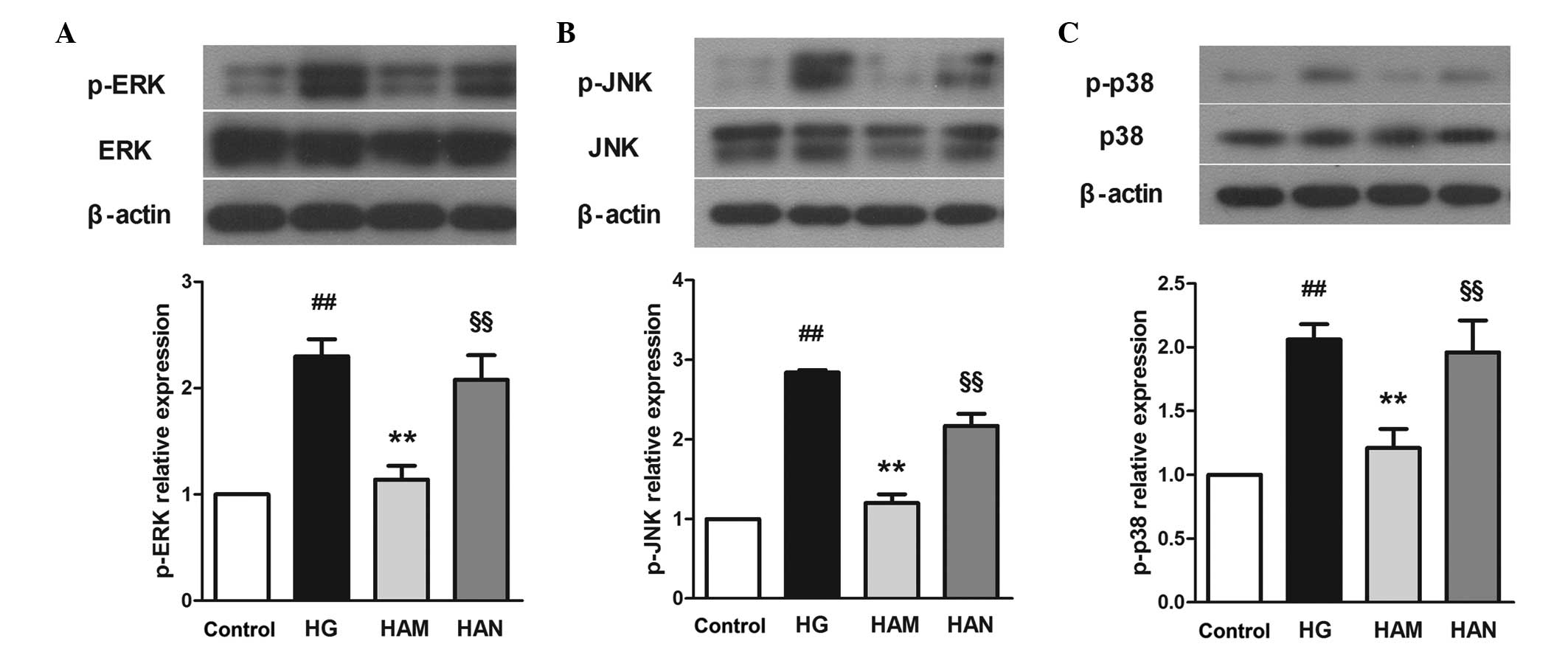 | Figure 8Effects of agmatine on
glucose-induced MAPK signaling. Agmatine treatment may protect
cells from glucose-induced cell stress by reducing ERK (A), JNK (B)
and p38 (C) phosphorylation. NMDA blocked the effects of agmatine.
Data are presented as the mean ± standard deviation (n=3),
##P<0.01 compared with the control group,
**P<0.01 compared with the HG group and
#P<0.01 compared with the HAM group. p-, phospho;
c-caspase-3, cleaved-cas-pase-3; ERK, extracellular signal
regulated kinase; JNK, c-Jun N-terminal kinase; p38, p38 kinase;
HG, high glucose; HAM, middle concentration of agmatine treatment;
NMDA, N-methyl-D-aspartic acid; MAPK, mitogen-activated protein
kinase. |
Discussion
Agmatine is associated with the CNS, it interacts
with certain receptors and neuronal pathways, and it demonstrates
neuroprotective effects. It has been investigated for use in the
treatment of CNS-associated disorders, such as spinal cord damage,
ischemia, traumatic brain injury and depression (31). Agmatine has been shown to block
NMDA currents in rat hippocampal neurons (23). Therefore, the effect of agmatine
may be mediated via NMDA receptor inhibition (32). The protective effects of agmatine
against cell damage are not restricted to the CNS; effects have
also been observed in retinal ganglion cells (26,33–35).
The results of the present study suggested that agmatine treatment
may protect Müller cells from glucose-induced cell damage.
In the present study, glucose treatment was used to
mimic DR in Müller cells. Glucose treatment induced cell death in
Müller cells, and this observation was reversed following treatment
with 100 and 200 μM agmatine. In the present study, 100
μM agmatine treatment reduced LDH activity in Müller cells
in the HAM group, compared with those in the HG group. NMDA
treatment reversed the protective effects of agmatine. It is
possible that agmatine treatment may inhibit NMDAR, which may
contribute to the protective effects of agmatine in glucose-damaged
Müller cells. However, clarification of these processes requires
further research.
Similar to astrocytes in the brain, Müller cells
release proinflammatory cytokines in response to inflammatory
responses. TNF-α is a marker of inflammation and is released during
the early stages of inflammation. The results of the present study
demonstrated that TNF-α expression was higher in Müller cells in
the HG group compared with the control group, reflecting Müller
cell inflammation. Agmatine treatment of cells in the HAM group led
to a decrease in TNF-α release from Müller cells and TNF-α mRNA
expression. These results were reversed following NMDA treatment.
These observations are in accordance with previous studies, which
showed that NMDAR treatment leads to increased TNF-α mRNA
expression and secretion in cells (36,37).
The results of the present study suggest that agmatine may reduce
glucose-induced inflammation in Müller cells via NMDAR
inhibition.
Retinal ganglion cells undergo cell apoptosis in
patients with DR (38). The
present study demonstrated that Müller cells undergo apoptosis in
response to glucose treatment. In the present study, according to
flow cytometric analysis and Hoechst staining, glucose treatment
led to increased levels of Müller cell apoptosis compared with
control cells. Agmatine treatment demonstrated antiapoptotic
effects in glucose-damaged Müller cells. In order to investigate
the mechanisms underlying the antiapoptotic effect of agmatine,
signaling protein expression levels were analyzed. The Bcl-2 family
consists of a group of important proteins that are involved in cell
apoptosis regulation. Bcl-2 is associated with tumor development
(39,40); specifically, Bcl-2 expression
inhibits the morphological changes during cell apoptosis, including
plasma membrane blebbing, DNA cleavage and nuclear condensation,
and negatively regulates cell death (41). By contrast, Bax is a proapoptotic
member of the family. A number of apoptotic signals may activate
Bax expression, followed by the formation of homo-oligomers and the
permea-bilization of the outer mitochondrial membrane, leading to
the release of mitochondrial intermembrane contents into the
cytosol (42). Bcl-2 may interact
with Bax and inhibit its oligomerization, thereby inhibiting
apoptosis (43). In general, the
ratio of Bcl-2 to Bax proteins determines the level of cell
apoptosis. Caspase-3 may be activated via extrinsic and intrinsic
apoptotic pathways. Following activation by caspase-8, caspase-3
may cleave with multiple substrates within the cell, which induces
cell apoptosis (44). The present
study demonstrated that high-concentration glucose treatment led to
a decrease in Bcl-2 expression and an increase in Bax expression,
compared with control cells. Agmatine treatment promoted the
upregulation of Bcl-2 and suppressed the expression of Bax, thereby
contributing to a reduction in c-caspase-3 expression in cells in
the HAM group. The results of the present study are consistent with
a previous study that demonstrated that agmatine treatment is
capable of suppressing the expression of apoptotic proteins in rat
retinal ganglion cells (34). The
results, therefore, suggested that Bcl-2 protein regulation may be
associated with the antiapoptotic effects of agmatine.
MAPKs consist of a family of protein kinases that
control a number of physiological processes and respond to various
stresses signals. Three kinases, ERK, JNK and p38, are considered
to be associated with stress-induced cell death. The present study
demonstrated that ERK, JNK and p38 expression levels were induced
in cells in the GS group, according to western blot analysis.
Therefore, MAPKs may be associated with high-concentration
glucose-induced Müller cell damage. In addition, MAPKs have been
shown to be activated by NMDA-induced Ca2+ influx
(45,46), and they contribute to NMDA-induced
neurotoxicity in rat retinas (47). A previous study has shown that
NMDAR1 expression was higher in mouse glomerular endothelial cells,
following glucose treatment, compared with control cells in
vitro (48). In the present
study, increased phosphorylation of MAPKs proteins was observed in
cells in the HG group compared with those in the control group.
Agmatine treatment of cells in the HAM group reduced ERK, JNK and
p38 phosphorylation levels, compared with cells in the HG group.
ERK, JNK and p38 phosphorylation levels in cells in the HAN group
were significantly lower than those in the HAM group. Therefore,
glucose may induce NMDAR expression in Müller cells, which may
activate MAPK protein expression. Agmatine treatment reduced the
phosphorylation levels of MAPKs by inhibiting NMDAR. Therefore, the
inhibition of MAPKs may partly contribute to the protective effects
of agmatine in Müller cells.
In the present study, agmatine treatment was shown
to increase cell survival rate, decrease LDH activity, reduce TNF-α
expression, regulate apoptotic-associated Bcl-2 and Bax protein
expression, and inhibit ERK, JNK and p38 protein phosphorylation,
in glucose-damaged Müller cells. Therefore, agmatine may protect
Müller cells from glucose-induced cell death via anti-inflammatory,
antiapoptotic and MAPK signaling inactivation effects. Furthermore,
protective effects of agmatine were reversed following NMDA
treatment, which indicates that agmatine protection of the Müller
cells may be associated with NMDAR inhibition. However, the present
study did not investigate whether the effects of agmatine are
associated with pathways independent of NMDAR. Furthermore, the
mechanisms underlying the effects observed in the present study
require further investigation. In conclusion, agmatine may be an
effective for treatment in DR and is a novel therapeutic candidate
for this disease.
References
|
1
|
Congdon N, O’Colmain B, Klaver CC, Klein
R, Muñoz B, Friedman DS, Kempen J, Taylor HR and Mitchell P: Eye
Diseases Prevalence Research Group: Causes and prevalence of visual
impairment among adults in the United States. Arch Ophthalmol.
122:477–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lieth E, Gardner TW, Barber AJ and
Antonetti DA: Penn State Retina Research Group: Retinal
neurodegeneration: Early pathology in diabetes. Clin Experiment
Ophthalmol. 28:3–8. 2000. View Article : Google Scholar
|
|
3
|
Schellini SA, Gregório EA, Spadella CT,
Machado JL and de-Moraes-Silva MA: Müller cells and diabetic
retinopathy. Braz J Med Biol Res. 28:977–980. 1995.PubMed/NCBI
|
|
4
|
Newman E and Reichenbach A: The Müller
cell: A functional element of the retina. Trends Neurosci.
19:307–312. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du JL, Xu LY and Yang XL: Glycine
receptors and transporters on bullfrog retinal Müller cells.
Neuroreport. 13:1653–1656. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis GP, Erickson PA, Kaska DD and Fisher
SK: An immunocytochemical comparison of Müller cells and astrocytes
in the cat retina. Exp Eye Res. 47:839–853. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linser P and Moscona AA: Induction of
glutamine synthetase in embryonic neural retina: Localization in
Müller fibers and dependence on cell interactions. Proc Natl Acad
Sci USA. 76:6476–6480. 1979. View Article : Google Scholar
|
|
8
|
Li Q and Puro DG: Diabetes-induced
dysfunction of the glutamate transporter in retinal Müller cells.
Invest Ophthalmol Vis Sci. 43:3109–3116. 2002.PubMed/NCBI
|
|
9
|
Kowluru RA, Engerman RL, Case GL and Kern
TS: Retinal glutamate in diabetes and effect of antioxidants.
Neurochem Int. 38:385–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ,
Tanase D and Strother JM: Glial reactivity and impaired glutamate
metabolism in short-term experimental diabetic retinopathy. Penn
State Retina Research Group. Diabetes. 47:815–820. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barber AJ, Antonetti DA and Gardner TW:
Altered expression of retinal occludin and glial fibrillary acidic
protein in experimental diabetes. The Penn State Retina Research
Group. Invest Ophthalmol Vis Sci. 41:3561–3568. 2000.PubMed/NCBI
|
|
12
|
Rungger-Brändle E, Dosso AA and
Leuenberger PM: Glial reactivity, an early feature of diabetic
retinopathy. Invest Ophthalmol Vis Sci. 41:1971–1980.
2000.PubMed/NCBI
|
|
13
|
Li Q, Zemel E, Miller B and Perlman I:
Early retinal damage in experimental diabetes:
Electroretinographical and morphological observations. Exp Eye Res.
74:615–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curtis TM, Hamilton R, Yong PH, McVicar
CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S and Stitt
AW: Müller glial dysfunction during diabetic retinopathy in rats is
linked to accumulation of advanced glycation end-products and
advanced lipoxidation end-products. Diabetologia. 54:690–698. 2011.
View Article : Google Scholar
|
|
15
|
Zhong Y, Li J, Chen Y, Wang JJ, Ratan R
and Zhang SX: Activation of endoplasmic reticulum stress by
hyperglycemia is essential for Müller cell-derived inflammatory
cytokine production in diabetes. Diabetes. 61:492–504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walker RJ and Steinle JJ: Role of
beta-adrenergic receptors in inflammatory marker expression in
Müller cells. Invest Ophthalmol Vis Sci. 48:5276–5281. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tabor CW and Tabor H: Polyamines. Annu Rev
Biochem. 53:749–790. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li G, Regunathan S, Barrow CJ, Eshraghi J,
Cooper R and Reis DJ: Agmatine: An endogenous clonidine-displacing
substance in the brain. Science. 263:966–969. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Otake K, Ruggiero DA, Regunathan S, Wang
H, Milner TA and Reis DJ: Regional localization of agmatine in the
rat brain: An immunocytochemical study. Brain Res. 787:1–14. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reis DJ, Yang XC and Milner TA: Agmatine
containing axon terminals in rat hippocampus form synapses on
pyramidal cells. Neurosci Lett. 250:185–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raasch W, Schäfer U, Chun J and Dominiak
P: Biological significance of agmatine, an endogenous ligand at
imidazoline binding sites. Br J Pharmacol. 133:755–780. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piletz JE, Chikkala DN and Ernsberger P:
Comparison of the properties of agmatine and endogenous
clonidine-displacing substance at imidazoline and alpha-2
adrenergic receptors. J Pharmacol Exp Ther. 272:581–587.
1995.PubMed/NCBI
|
|
23
|
Yang XC and Reis DJ: Agmatine selectively
blocks the N-methyl-D-aspartate subclass of glutamate receptor
channels in rat hippocampal neurons. J Pharmacol Exp Ther.
288:544–549. 1999.PubMed/NCBI
|
|
24
|
Satriano J, Kelly CJ and Blantz RC: An
emerging role for agmatine. Kidney Int. 56:1252–1253. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iizuka Y, Hong S, Kim CY, Kim SK and Seong
GJ: Agmatine pretreatment protects retinal ganglion cells (RGC-5
cell line) from oxidative stress in vitro. Bio cell. 32:245–250.
2008.
|
|
26
|
Iizuka Y, Hong S, Kim CY, Yang WI, Lee JE
and Seong GJ: Protective mechanism of agmatine pretreatment on
RGC-5 cells injured by oxidative stress. Braz J Med Biol Res.
43:356–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park YM, Lee WT, Bokara KK, Seo SK, Park
SH, Kim JH, Yenari MA, Park KA and Lee JE: The multifaceted effects
of agmatine on functional recovery after spinal cord injury through
Modulations of BMP-2/4/7 expressions in neurons and glial cells.
PLoS One. 8:e539112013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn SK, Hong S, Park YM, Choi JY, Lee WT,
Park KA and Lee JE: Protective effects of agmatine on
lipopolysac-charide-injured microglia and inducible nitric oxide
synthase activity. Life Sci. 91:1345–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchihori Y and Puro DG: Glutamate as a
neuron-to-glial signal for mitogenesis: Role of glial
N-methyl-D-aspartate receptors. Brain Res. 613:212–220. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mysona BA, Al-Gayyar MM, Matragoon S,
Abdelsaid MA, El-Azab MF, Saragovi HU and El-Remessy AB: Modulation
of p75(NTR) prevents diabetes- and proNGF-induced retinal
inflammation and blood-retina barrier breakdown in mice and rats.
Diabetologia. 56:2329–2339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piletz JE, Aricioglu F, Cheng JT,
Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li
J, et al: Agmatine: Clinical applications after 100 years in
translation. Drug Discov Today. 18:880–893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olmos G, DeGregorio-Rocasolano N, Paz
Regalado M, Gasull T, Assumpció Boronat M, Trullas R, Villarroel A,
Lerma J and García-Sevilla JA: Protection by imidazol(ine) drugs
and agmatine of glutamate-induced neurotoxicity in cultured
cerebellar granule cells through blockade of NMDA receptor. Br J
Pharmacol. 127:1317–1326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong S, Kim CY, Lee JE and Seong GJ:
Agmatine protects cultured retinal ganglion cells from tumor
necrosis factor-alpha-induced apoptosis. Life Sci. 84:28–32. 2009.
View Article : Google Scholar
|
|
34
|
Hong S, Lee JE, Kim CY and Seong GJ:
Agmatine protects retinal ganglion cells from hypoxia-induced
apoptosis in transformed rat retinal ganglion cell line. BMC
Neurosci. 8:812007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu MY, Wang WP and Bissette G:
Neuroprotective effects of agmatine against cell damage caused by
glucocorticoids in cultured rat hippocampal neurons. Neuroscience.
141:2019–2027. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawabata H, Setoguchi T, Yone K, Souda M,
Yoshida H, Kawahara K, Maruyama I and Komiya S: High mobility group
box 1 is upregulated after spinal cord injury and is associated
with neuronal cell apoptosis. Spine (Phila Pa 1976). 35:1109–1115.
2010.
|
|
37
|
McNearney TA, Ma Y, Chen Y, Taglialatela
G, Yin H, Zhang R and Westlund KN: A peripheral neuroimmune link:
Glutamate agonists upregulate NMDA NR1 receptor mRNA and protein,
vimentin, TNF-alpha, and RANTES in cultured human synoviocytes. Am
J Physiol Regul Integr Comp Physiol. 298:R584–R598. 2010.
View Article : Google Scholar
|
|
38
|
Kern TS and Barber AJ: Retinal ganglion
cells in diabetes. J Physiol. 586:4401–4408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
44
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ko HW, Park KY, Kim H, Han PL, Kim YU,
Gwag BJ and Choi EJ: Ca2+-mediated activation of c-Jun
N-terminal kinase and nuclear factor kappa B by NMDA in cortical
cell cultures. J Neurochem. 71:1390–1395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nicole O, Ali C, Docagne F, Plawinski L,
MacKenzie ET, Vivien D and Buisson A: Neuroprotection mediated by
glial cell line-derived neurotrophic factor: Involvement of a
reduction of NMDA-induced calcium influx by the mitogen-activated
protein kinase pathway. J Neurosci. 21:3024–3033. 2001.PubMed/NCBI
|
|
47
|
Munemasa Y, Ohtani-Kaneko R, Kitaoka Y,
Kuribayashi K, Isenoumi K, Kogo J, Yamashita K, Kumai T, Kobayashi
S, Hirata K and Ueno S: Contribution of mitogen-activated protein
kinases to NMDA-induced neurotoxicity in the rat retina. Brain Res.
1044:227–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kundu S, Pushpakumar SB, Tyagi A, Coley D
and Sen U: Hydrogen sulfide deficiency and diabetic renal
remodeling: Role of matrix metalloproteinase-9. Am J Physiol
Endocrinol Metab. 304:E1365–E1378. 2013. View Article : Google Scholar : PubMed/NCBI
|