Introduction
Endothelial dysfunction, characterized by decreased
bioavailability of nitric oxide (NO) and impaired
endothelium-dependent vasodilation, is a primary cause of vascular
complications in type 2 diabetes mellitus, and is one of the major
risk factors of cardiovascular diseases (1). The elevation of circulating free
fatty acids (FFAs), as observed in individuals with obese insulin
resistance and type 2 diabetes mellitus, is associated with
impaired endothelium-dependent vasodilation (2), suggesting a link between FFA and
endothelial dysfunction. At a molecular level, FFAs activate
nuclear factor-κB (NF-κB) via their ligation of Toll-like receptor
4 (TLR4), leading to release of pro-inflammatory cytokines,
including interleukin (IL)-6 and tumor necrosis factor-α (TNF-α),
and the formation of reactive oxygen species (ROS) (3), suggesting that the abnormal elevation
of circulating FFAs may cause endothelial dysfunction by inducing
inflammation and oxidative stress in vascular tissues. FFAs also
prevent insulin receptor substrate-1 (IRS-1) tyrosine
phosphorylation in cultured endothelial cells, which subsequently
reduces the insulin-dependent activation of endothelial NO synthase
(eNOS) and production of NO (4).
As a major component of dietary saturated fat and 20% of the total
serum FFAs (5,6), palmitic acid (PA) is often used to
induce endothelial dysfunction via NF-κB- and IRS-1-dependent
pathways in endothelial cell culture models (7).
Heme oxygenase-1 (HO-1), an inducible enzyme with
potent antioxidant and anti-inflammatory properties, catalyzes the
degradation of heme to carbon monoxide, biliverdin and free iron,
with biliverdin subsequently being metabolized to bilirubin by
biliverdin reductase (8). The
nuclear factor E2-related factor 2 (Nrf2) is important in the
transcriptional activation of HO-1 and several other genes
(9). Upon activation, Nrf2 enters
the nucleus, where it binds to AU-rich elements in the HO-1
promoter to trigger gene expression (9). Nrf2 has been observed to regulate the
induction of the expression of HO-1 in response to certain
naturally occurring compounds, including curcumin and resveratrol
(10). Several studies have
confirmed the protective role of HO-1 in several pathological
states, including endothelial dysfunction (8,9).
Resveratrol is a naturally occurring stilbene, which
is present in various types of food and beverage, and has attracted
increasing attention due to its multiple beneficial properties,
including anti-inflammatory and antioxidant activities (11). There are several naturally
occurring stilbene-like compounds, which are structurally similar
to resveratrol. Piceatannol (Pic), which was first isolated from
the seeds of Euphorbia lagascae (Euphorbiaceae), is a
naturally occurring hydroxylated analog of resveratrol and has been
also identified as a resveratrol metabolite (12). Based on its structural similarity
to resveratrol and formation through in vivo metabolism of
resveratrol (11), it has been
hypothesized that Pic may have biological activities similar to
those of resveratrol. The only difference between Pic
(3,5,4′,3′-trans-trihydroxystilbene) and resveratrol
(3,5,4′-trans-trihydroxystilbene) is the presence of an
additional hydroxyl group in one of the aromatic rings of Pic
(11). Although Pic, partly due to
this difference, has increased antioxidant activity compared with
resveratrol (11,12), whether Pic exerts other biological
effects similar to those of resveratrol remains to be
elucidated.
It has been previously demonstrated that resveratrol
prevents endothelial dysfunction in cultured human umbilical vein
endothelial cells (HUVECs) exposed to high glucose (13,14)
and in trauma-hemorrhaged animals (15). However, whether Pic, as with
resveratrol, prevents endothelial dysfunction remains to be
elucidated. The present study aimed to investigate this, using PA
as one of the predominant mediators to induce endothelial
dysfunction in HUVECs, by inducing inflammation and ROS formation
and by reducing insulin- mediated NO bioavailability. Using this
in vitro endothelial dysfunction model, the results
demonstrated that Pic induced the Nrf2-dependent expression of
HO-1, an anti-inflammatory and antioxidant, which inhibited the
PA-induced inflammatory response and formation of ROS, and
attenuated the PA-induced reduction in insulin-mediated eNOS
activation and production of NO.
Materials and methods
Materials and antibodies
The 3,3′,4,5′-tetrahydroxy-trans-stilbene
(piceatannol; Pic), hemin, 3-(4,5-Dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT), PA, bovine serum albumin
(BSA), dimethyl sulfoxide (DMSO), and tin protoporphryin-IX (SnPP)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
rabbit polyclonal Nrf2 (cat. no. sc-722; 1:500 dilution), rabbit
polyclonal Lamin B (cat. no. sc-20682; 1:1,000 dilution) and mouse
monoclonal β-actin (cat. no. sc-47778; 1:1,000 dilution) and Nrf2
small interfering (si)RNA (cat. no. sc-37030) and control siRNA
(cat. no. sc-37007) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Horseradish-peroxidase (HRP)-conjugated
secondary antibodies against rabbit (cat. no. 7074; 1:1,000
dilution) and mouse (cat. no. 7076; 1:1,000 dilution)
immunoglobulin (Ig) G and the following primary antibodies: Rabbit
monoclonal phosphorylated (p)-NF-κB p65 (cat. no. 93H1; 1:1,000
dilution), rabbit monoclonal NF-κB p65 (cat. no. C22B4; 1:1,000
dilution), rabbit monoclonal p-eNOS (cat. no. C9C3; 1:1,000
dilution) and rabbit polyclonal eNOS (cat. no. 9572; 1:1,000
dilution) were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The following rabbit polyclonal antibodies
targeting p-IRS-1 (cat. no. BS4633; 1:1,000 dilution) and IRS-1
(cat. no. E306; 1:1,000 dilution) were obtained from Bioworld
Technology (St. Louis Park, MN, USA). The PA was dissolved in
absolute ethanol at 200 mM as a stock solution and was further
diluted with medium containing 10% BSA, to obtain a concentration
of 5 mM, prior to use. All other reagents, unless otherwise stated,
were purchased from Sigma-Aldrich.
Cell culture
All of the investigations performed in the present
study were approved by the Research Ethics Committee of Wokwang
University (Iksan, South Korea). The HUVECs were purchased from
Cascade Biologics Inc. (Portland, OR, USA). The HUVECs were grown
in RPMI-1640 medium (Sigma-Aldrich), supplemented with 10% fetal
bovine serum, streptomycin (100 U/μl) and penicillin (100
U/μl) in an atmosphere of 5% CO2 and 95%
humidified air at 37°C. The medium was renewed every 2 days until
the cells had grown to confluence. The confluent cells were
detached using trypsin-EDTA (0.05% trypsin, 0.02% EDTA), and cells
between passages three and seven were used in the subsequent
experiments.
Detection of cell viability
Cell viability was determined using an MTT assay.
The cells (1×105 cells/ml) were treated with MTT at 0.5
mg/μl. The resulting purple formazan crystals were dissolved
in DMSO. The solutions were then loaded in a 96-well plate, and
analyzed on an automated microplate spectrophotometer (VersaMax;
Molecular Devices, Silicon Valley, CA, USA) at 570 nm. MTT assay
was performed in triplicate in each experiment.
Preparation of nuclear and cytosolic
extracts
To analyze Nrf2, the cells (1×105
cells/ml) were resuspended at 4°C in buffer A, containing 10 mM
HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM
dithiothreitol DTT and 0.2 mM phenylmethylsulfonyl fluoride (PMSF),
allowed to swell on ice for 10 min and then vortexed for 10 sec
using a Mini Vortexer (Thermo Fisher Scientific, Springfield
Township, NJ, USA). The samples were centrifuged at 10,000 x g for
2 min and the supernatant, containing the cytosolic fraction, was
stored at −80°C. The pellet was resuspended in cold buffer B,
containing 20 mM HEPES pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 2.5
μg/μl leupeptin and 2.5 μg/μl
aprotinin, and incubated on ice for 20 min for high salt
extraction. The cellular debris was removed by centrifugation at
13,000 x g for 10 min at 4°C and the supernatant fraction,
containing the nuclear protein extract, was stored at −80°C. The
proteins were quantified using Bradford’s reagent (Sigma-Aldrich),
according to the manufacturer’s instructions. Briefly, the
quantification of total proteins was performed by means of a
standard curve of absorbance at 595 nm obtained from solutions
containing known concentrations of BSA (0, 0.005, 0.010, 0.015,
0.020 and 0.025 mg/ml), Bradford’s reagent (0.20 ml) and sufficient
water to reach a final volume of 1 ml. The samples contained 20
μg of the nuclear extract and 200 μl Bradford’s
reagent. Following agitation, the absorbance of the samples was
measured at 595 nm using an automated microplate spectrophotometer
(VersaMax).
Western blot analysis
Equal quantities of nuclear and cytosolic extracts
(20 μg) were electroblotted onto a nitrocellulose membrane,
following separation using 8–12% sodium
dodecylsulfate-polyacrylamide gel electrophoresis. The blot was
probed using primary antibodies against HO-1, Nrf2, Lamin B, p-p65,
p65, p-eNOS, eNOS, p-IRS-1, IRS-1, and β-actin. HRP-conjugated
anti-IgG antibodies were used as the secondary antibodies to detect
the previously mentioned protein bands by enhanced
chemiluminescence (WESTSAVE-Up; AbFrontier, Seoul, Korea).
Gene silencing using Nrf2 siRNA
The siRNA against Nrf2 or the control siRNA were
introduced into the HUVECs by reverse transfection, using
Lipofectamine™ RNAiMAX reagents (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions.
In brief, the transfection mixture was applied to a 6-well plate
immediately prior to plating cells (1×105 cells/ml) in
complete medium (RPMI-1640 containing 10% FBS) without antibiotics.
The medium was replaced with fresh medium after 4 h and the cells
were incubated overnight at 37°C.
Measurement of the production of TNF-α
and IL-6
The cells, grown to confluence in 24-well plates,
were pre-incubated at 37°C for 12 h with different concentrations
of Pic, and then stimulated with PA (100 μM) for 12 h in
serum-free medium. Following collection of the medium from each
well, the levels of TNF-α and IL-6 in the supernatant were assayed
using human TNF-α and IL-6 enzyme-linked immunosorbent assay
(ELISA) kits (R&D Systems, Minneapolis, MN, USA), according to
the manufacturer’s instructions.
Measurement of the activity of heme
oxygenase
The activity of heme oxygenase was determined at the
end of each treatment, as described previously (16). Briefly, microsomes from the
harvested cells were (1×105 cells/ml) added to a
reaction mixture containing nicotinamide adenine dinucleotide
phosphate (1 mM), glucose-6-phosphate dehydrogenase (10
μg/ml), rat liver cytosol (20 μg/ml), as a source of
biliverdin reductase, and hemin (10 μM). The reaction
mixture was incubated in the dark at 37°C for 1 h and terminated by
the addition of 1 μl chloroform. Following vigorous
vortexing and centrifugation at 10,000 x g for 30 min at 4°C, the
quantity of extracted bilirubin in the chloroform layer was
determined by measuring the difference in absorbance between 464
and 530 nm using an automated microplate spectrophotometer
(VersaMax), with an extinction coefficient of 40
mmol/l−1·cm−1.
Measurement of the intracellular
formation of ROS
The intracellular formation of ROS was measured
using 2′,7′-dichlorofluorescein diacetate (DCF-DA; Molecular
Probes, Eugene, OR, USA) (7). This
nonpolar compound is converted to the membrane-impermeant polar
derivative, DCF, by esterases following uptake by the cell. DCF is
nonfluorescent, however it is rapidly oxidized to the highly
fluorescent DCF by intracellular ROS. In brief, the cells were
seeded in 96-well black plates at a concentration of
1×105 cells/μl and were pre-incubated for 12 h
with 20 μM Pic. Following the addition of DCF-DA (10
μM) at 37°C for 10 min, the cells were rinsed three times
with phosphate-buffered saline (PBS), and further incubated at 37°C
with media containing PA (100 μM) for 2 h. Following
incubation, the media was discarded, and the cells were washed with
PBS. The fluorescence intensity was determined using a fluorescence
plate reader (FLIPR; Molecular Devices) at 488 nm for excitation
and 525 nm for emission, with results presented as the percentage
of the control (treated with medium alone). For microscopic
analysis, the cells were cultured on coverslips in 6-well plates
and treated, as described above. The cells were incubated at 37°C
with DCF-DA for 10 min in the dark, and stimulated with PA for 0.5
h. The cells were then washed twice with ice-cold PBS, fixed with
2% paraformaldehyde for 3 min and washed twice again with ice-cold
PBS. The coverslips were then mounted onto slides, and analysis of
ROS production was performed using a fluorescent microscope
(Axiovert 200;Carl Zeiss Microimaging, Thornwood, NY, USA).
Measurement of the intracellular
production of NO
The cell membrane permeable probe,
4-amino-5-methylamino-2′,7′- difluorofluorescein (DAF-FM) diacetate
(Molecular Probes), was used to detect intracellular NO. Once
inside the cells, DAF-FM diacetate is deacetylated by intracellular
esterases, becoming DAF-FM, which can be detected by fluorescent
methods (7). Briefly, the cells
were seeded in 96-well black plates at a concentration of
1×105 cells/μl and were pre-incubated for 12 h
with different concentrations of Pic. The cells were stimulated
without or with PA (100 μM) for 12 h and then washed three
times with PBS. Following the addition of DAF-FM diacetate (5
μM) at 37°C for 0.5 h, the cells were rinsed three times
with PBS and further incubated with media containing insulin (100
nM; R&D Systems) at 37°C for 2 h. Following incubation, the
media was discarded and the cells were washed with PBS. The
fluorescence intensity was determined using a fluorescence plate
reader (Molecular Devices) at 495 nm for excitation and 515 nm for
emission, with results presented as the percentage of the control.
For microscopic analysis, the cells were cultured at 37°C on
coverslips in 6-well plates and treated, as described above. The
cells were incubated with DAF-FM diacetate for 0.5 h in the dark,
and stimulated with insulin for 0.5 h. The cells were then washed
twice with ice-cold PBS, fixed with 2% paraformaldehyde for 3 min
and washed twice again with ice-cold PBS. The coverslips were then
mounted onto slides, and analysis of the production of NO was
performed using a fluorescent microscope (Carl Zeiss
Microimaging).
Measurement of NF-κB p65 DNA-binding
activity
The content of NF-κB binding to DNA in nuclear
extracts was measured using a specific TransAM® NF-κB
p65 assay kit (Active motif, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Briefly, a 96-well plate was precoated
with an oligonucleotide, containing the NF-κB p65 binding consensus
site. The active form of the p65 subunit was detected using p65
antibodies, incubated for 1 h, specific for an epitope, which was
accessible only when the appropriate subunit bound to its target
DNA. An HRP-conjugated secondary antibody provided a colorimetric
readout, which was quantified using a spectrophotometer (450
nm).
Measurement of Nrf2 DNA-binding
activity
The DNA binding of Nrf2 was measured using a
specific TransAM® Nrf2 assay kit (Active Motif),
according to the manufacturer’s instructions. Briefly, the nuclear
extracts were incubated in the oligonucleotide-coated wells.
Subsequently, the wells were washed twice with PBS and incubated
with antibody against Nrf2 at 37°C. The addition of secondary
antibody conjugated to HRP provided a colorimetric readout. The
absorbance of each well was measured using a microplate reader at
450 nm (Molecular Devices).
Measurement of glucose uptake
The D-glucose analogue, 2-Deoxyglucose
(2-DG), is transported into cells and phosphorylated by the same
mechanisms as glucose. Thus, 2-DG uptake is defined as glucose
transport and intracellular phosphorylation by hexokinase.
Phosphorylation serves to trap 2-DG inside the cells as
2-deoxyglucose 6-phosphate (2-DG6P), making it possible to
determine the rates of glucose uptake over extended periods of time
(17). The insulin-stimulated
glucose uptake of the HUVECs was determined by measuring the
transport of 2-DG into the cells. Briefly, media were removed and
the cells were washed twice with Krebs-Ringer phosphate-HEPES
(KRPH) buffer, containing 2% BSA followed by stimulation with or
without 100 nM insulin for 0.5 h at 37°C. The cells were further
incubated at 37°C for 1 h KRPH buffer, containing 2% BSA and 1 mM
2-DG. The culture plates were then placed on ice, and the cells
were washed three times with PBS. The cells in six wells were
collected in 500 μl 10 mM Tris-HCl (pH 8.1) containing 1%
Triton X-100, heated at 95°C for 15 min and centrifuged at 17,800 x
g for 15 min at 4°C. A portion of the resulting supernatant was
diluted 20 times with 10 μM Tris-HCl (pH 8.1) and the 2-DG6P
content was analyzed. The 2-DG6P content was determined by
measuring the quantity of NADPH produced during 2-DG6P oxidation
according to the manufacturer’s instructions using the appropriate
commercially available ELISA kit (Abcam, Cambridge, MA, USA).
Statistical analysis
The results of all the experiments are expressed as
the mean ± standard deviation of multiple experiments (n≥3). All
statistical analyses were performed using SPSS version 10.0
software (SPSS Inc., Chicago, IL, USA). The data were compared
using one-way analysis of variance (ANOVA) with post-hoc
Bonferroni’s analysis, when applicable. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pic induces the endothelial expression of
HO-1 via the activation of Nrf2
The HUVECs were treated with different
concentrations of Pic, and an MTT assay for cell viability was
performed after 24 h incubation. A survival rate of ~95% was
observed at a Pic concentration of 20 μM, however,
significant cytotoxic signs were observed >25 μM Pic
(data not shown). Thus, for the subsequent experiments, the maximum
concentration of Pic was limited to 20 μM. Upon exposure to
the non-cytotoxic 20 μM of Pic, the HO-1 enzyme was
expressed after 6 h and increased until 12 h, following which it
reduced until 24 h (Fig. 1A),
which corresponded to the levels of HO activity (Fig. 1B). Pic increased the expression of
HO-1 (Fig. 1C) and the activity of
HO (Fig. 1D) in a
concentration-dependent manner, confirming that the upregulation of
HO-1 was accompanied by increased HO activity in the Pic-treated
cells. In addition, Pic increased the content of Nrf2 in the
nuclear fraction, in a concentration-dependent manner (Fig. 1E), and consequently enhanced the
DNA-binding activity of Nrf2 (Fig.
1F). To confirm the role of Nrf2 in Pic-induced expression of
HO-1, the present study subsequently assessed the effect of
transient transfection with Nrf2 siRNA on the Pic-induced
expression of HO-1. Silencing with Nrf2 siRNA reduced the protein
levels of Nrf2 compared with the negative controls in the total
cell lysates (data not shown). The Pic-induced expression of HO-1
(Fig. 1G) and activity of HO
(Fig. 1H) were eradicated by
transfection with Nrf2 siRNA, suggesting that Pic-induced
expression of HO-1 requires the activation of Nrf2.
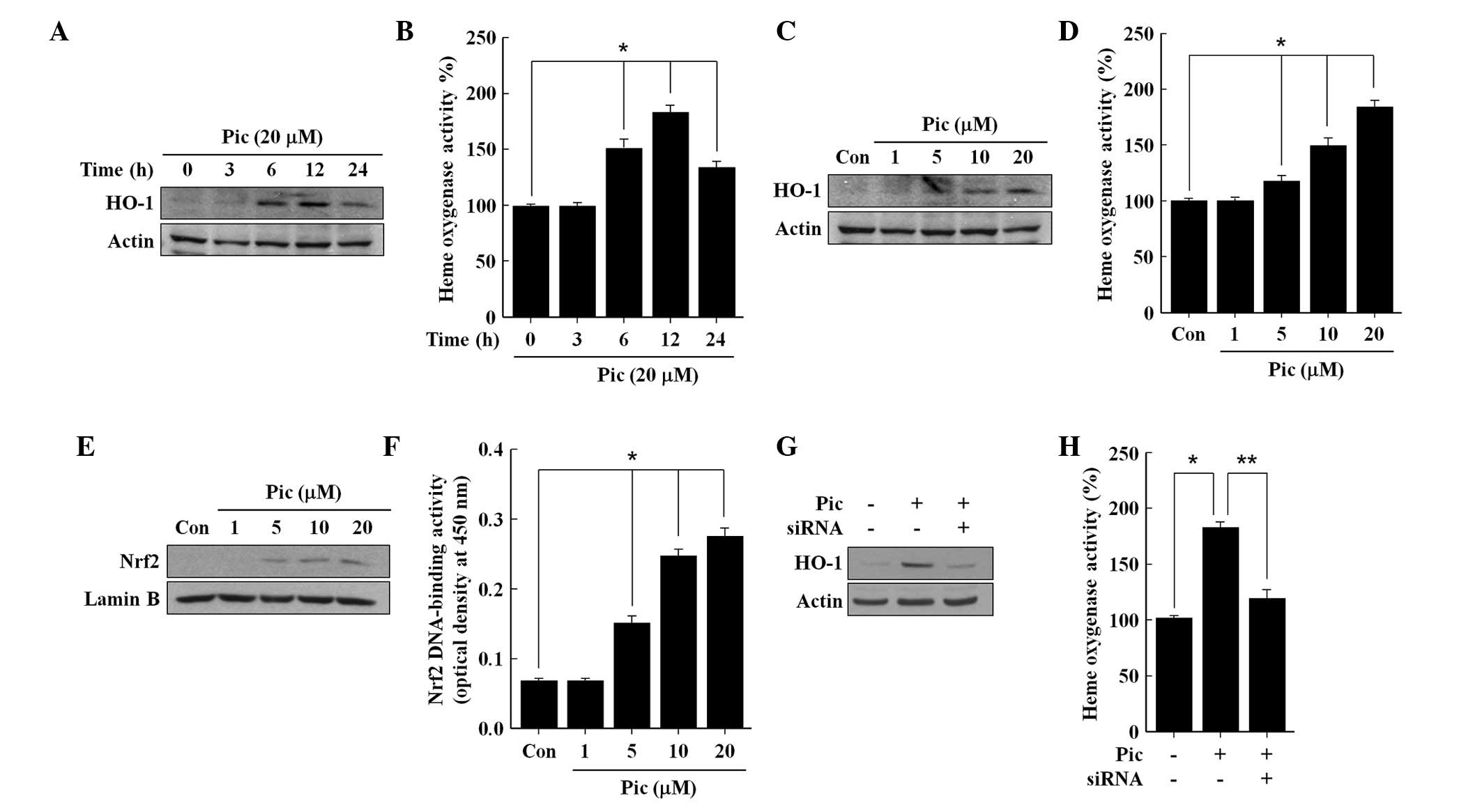 | Figure 1Effects of Pic on the expression of
HO-1, activity of HO, nuclear accumulation of Nrf2 and Nrf2
DNA-binding activity in human umbilical vein endothelial cells. The
cells were incubated with (A and B) 20 μM Pic or (C and D)
different concentrations of Pic for (A and B) different durations
or for (C and D) 12 h. The cells were incubated for 2 h with (E and
F) different concentrations of Pic, and (G and H) cells transiently
transfected with either control siRNA or Nrf2 siRNA were incubated
with 20 μM of Pic for 12 h. Western blot analysis for the
(A, C and G) expression of HO-1 and (E) nuclear accumulation of
Nrf2 nuclear was performed. Untreated cells served as controls
(Con). Representative blots, selected from three separate
experiments are shown. The (B, D and H) activity of HO and
DNA-binding activity of (F) Nrf2 were measured. All data are
expressed as the mean ± standard deviation of three independent
observations in separate cell culture wells. *P<0.01
and **P<0.05. HO, heme oxygenase; Pic, piceatannol;
siRNA, small interfering RNA; Nrf2, nuclear factor
erthyroid-2-related factor-2. |
Pic reduces the PA-induced inflammatory
response and formation of ROS
As shown in Fig. 2,
exposure of the HUVECs to the free fatty acid PA (100 μM)
markedly increased the secretions of TNF-α and IL-6, the production
of ROS, and the activation of NF-κB, which was consistent with
previously published reports (7,18).
Pretreatment with Pic for 12 h effectively inhibited the PA-induced
secretions of TNF-α and IL-6 in a concentration dependent manner
(Fig. 2A and B), and also reduced
the production of ROS (Fig. 2C and
D). Similarly, Pic decreased the PA-induced phosphorylation of
NF-κB p65 (Fig. 2E), a marker of
NF-κB activation, and therefore, reduced PA-induced NF-κB
transcriptional activity (Fig.
2F). These data demonstrated the anti-inflammatory and
antioxidant actions of Pic in endothelial cells exposed to PA.
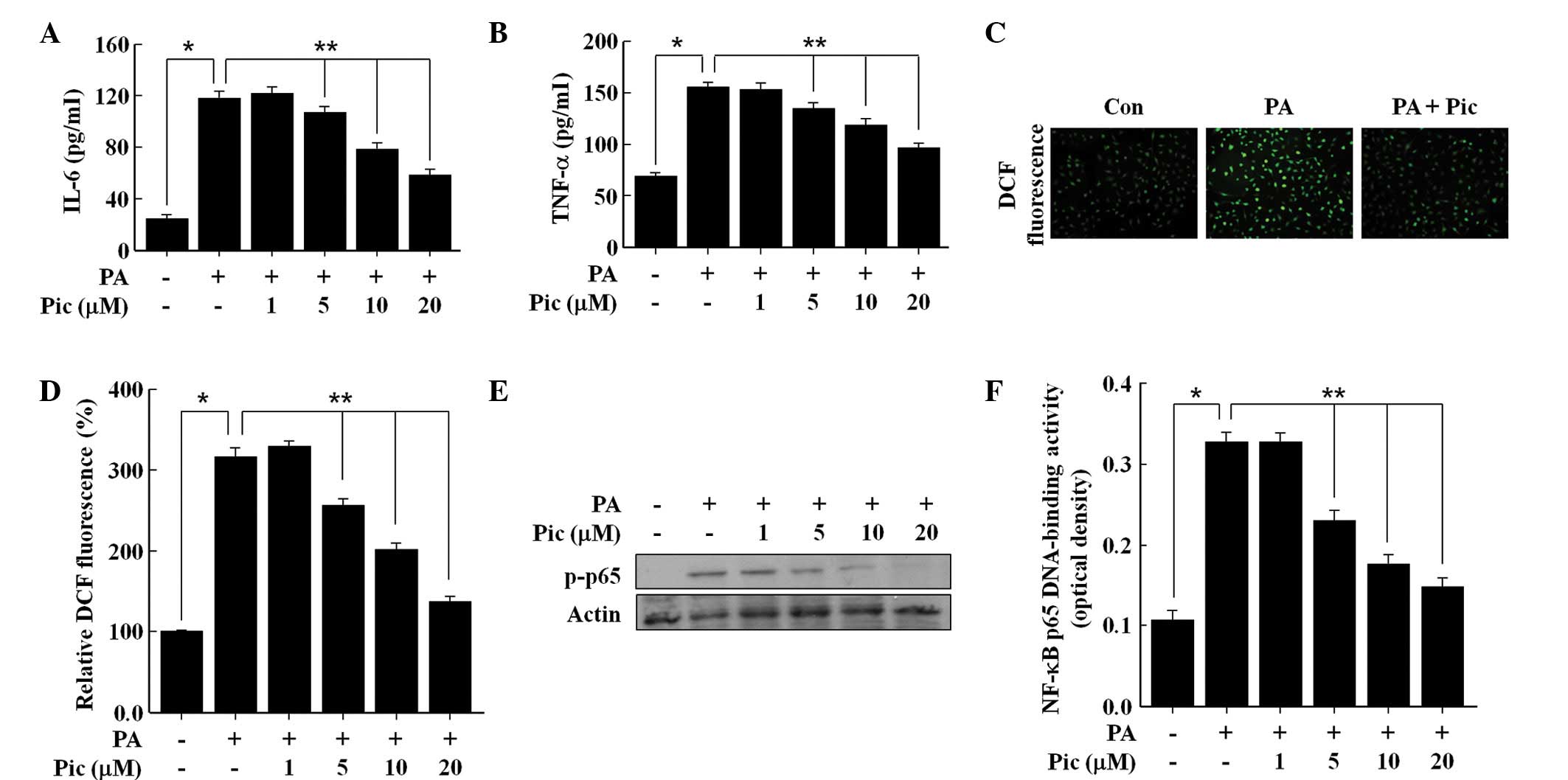 | Figure 2Effects of Pic on the secretions of
IL-6 and TNF-α, formation of ROS, phosphorylation of p65, and
DNA-binding activity of NF-κB in human umbilical vein endothelial
cells stimulated with PA. The cells were pretreated for 12 h with
different concentrations of Pic or with 20 μM of Pic, and
then exposed to 100 μM PA for (A and B) 12 h, (C) 0.5 h or
(D, E and F) 2 h. Untreated cells served as controls (Con). For
analyses, (A and B) enzyme-linked immunosorbent assays were
performed for cytokine secretion, fluorescence microscopic analysis
was performed for the (C) formation of ROS, (D) DCF fluorescence
intensity was performed for the formation of ROS, (E) western blot
analysis was performed for (E) NF-κB subunit p65 phosphorylation
and (F) NF-κB p65 DNA-binding activity were measured. (C)
Representative fluorescent images demonstrate the increase in green
fluorescence intensity of DCF produced by ROS (magnification,
×400). Representative blots or pictures, selected from three
separate experiments are shown. All data are expressed as the mean
± standard deviation of three independent observations in separate
cell culture wells. *P<0.01 and
**P<0.05. Pic, piceatannol; IL, interleukin; TNF,
tumor necrosis factor; ROS, reactive oxygen species; NF-κB, nuclear
factor-κB; p-phosphorylated; PA, palmitic acid; DCF,
2′,7′-dichlorofluorescein diacetate. |
Anti-inflammatory and antioxidant effects
of Pic are mediated via the activation of HO-1
As HO-1 is understood to suppress inflammation and
the formation of ROS in endothelial cells (8,9), the
present study investigated whether the observed anti-inflammatory
and antioxidant actions of Pic can be mediated via HO-1 enzymatic
activation. SnPP was used to suppress HO-1 enzymatic activity, and
the results revealed that the inhibition of IL-6 (Fig. 3A) and TNF-α (Fig. 3B) production by Pic pretreatment
was significantly reversed by SnPP in the PA-stimulated endothelial
cells. SnPP also eradicated the antioxidant effect of Pic.
Following inhibition of the activity of HO-1 by SnPP, Pic
pretreatment failed to prevent PA-induced ROS formation (Fig. 3C and D). Notably, SnPP
significantly impaired the inhibitory effect of Pic on the
phosphorylation of NF-κB p65 (Fig.
3E) and transcriptional activity of NF-κB (Fig. 3F) in the PA-stimulated endothelial
cells. These data demonstrated that HO-1 enzymatic activity was
essential for the anti-inflammatory and antioxidant actions of Pic
in the PA-stimulated HUVECs.
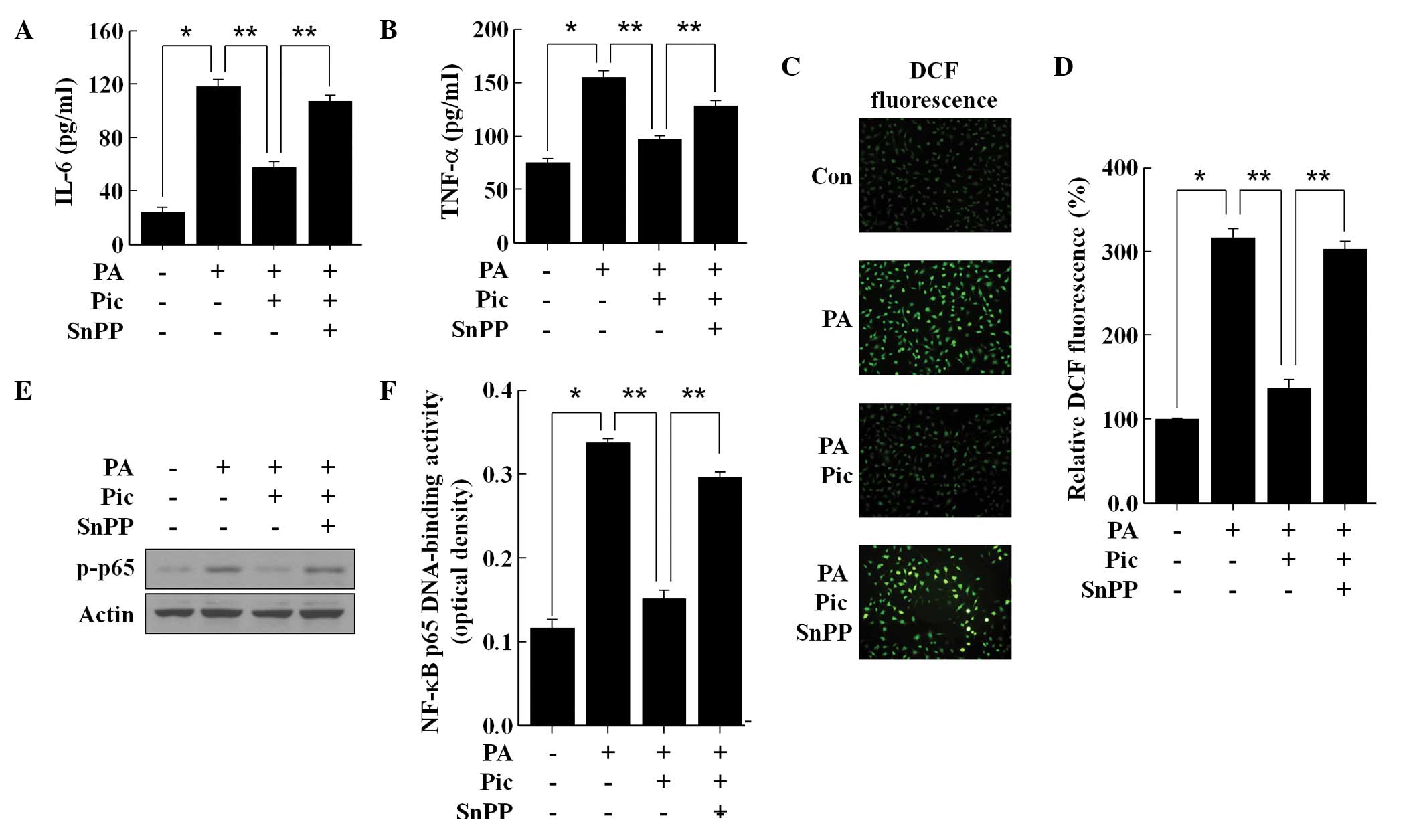 | Figure 3Involvement of the expression of HO-1
in the anti-inflammatory and anti-oxidative effects of Pic in human
umbilical vein endothelial cells stimulated with PA. The cells were
pretreated for 12 h with 20 μM of Pic and then exposed to
100 μM of PA for (A and B),12 h (C) 0.5 h or (D, E and F) 2
h in the presence or absence of 20 μM SnPP. Untreated cells
served as controls (Con). For analyses, (A and B) enzyme-linked
immunosorbent assays were performed for cytokine secretion, (C)
fluorescence microscopic analysis was performed for the formation
of RO, (D) DCF fluorescence intensity was performed for ROS
formation (E) Western blot analysis was performed for the
phosphorylation of NF-κB subunit p65 and DCF fluorescence intensity
was performed for NF-κB p65 DNA-binding activity. (C)
Representative fluorescent images demonstrate the increase in green
fluorescence intensity of DCF produced by ROS (magnification,
×400). Representative blots or pictures, selected from three
separate experiments are shown. All data are expressed as the mean
± standard deviation of three independent observations in separate
cell culture wells. *P<0.01 and
**P<0.05. Pic, piceatannol; PA, palmitic acid; snPP,
tin protoporphryin-IX; IL, interleukin; TNF, tumor necrosis factor;
ROS, reactive oxygen species; NF-κB, nuclear factor-κB;
p-phosphorylated; DCF, 2′,7′-dichlorofluorescein diacetate. |
Pic reduces PA-induced insulin resistance
and eNOS dysfunction via the activation of HO-1
In the HUVECs, PA attenuated the insulin-mediated
tyrosine phosphorylation of IRS-1 and consequently reduced glucose
uptake (Fig. 4A and B), suggesting
that PA may induce insulin resistance. PA also reduced the
insulin-mediated phosphorylation of eNOS and the subsequent
production of NO (Fig. 4C–E),
suggesting that PA may induce eNOS dysfunction. Pic effectively
prevented the inhibitory effect of PA on the insulin-mediated
tyrosine phosphorylation of IRS-1 (Fig. 4A) and uptake of glucose (Fig. 4B). Pic also restored the loss of
insulin-mediated NO production (Fig.
4C and F) by mitigating the inhibitory effect of PA on the
insulin-mediated phosphorylation of eNOS (Fig. 4D). Notably, these beneficial
effects of Pic against PA insult were significantly reversed
following inhibition of HO-1 activity by SnPP (Fig. 4), demonstrating that HO-1 enzymatic
activity was essential, at least in part, for the observed
protective effects of Pic, in endothelial cells exposed to a high
concentration of PA.
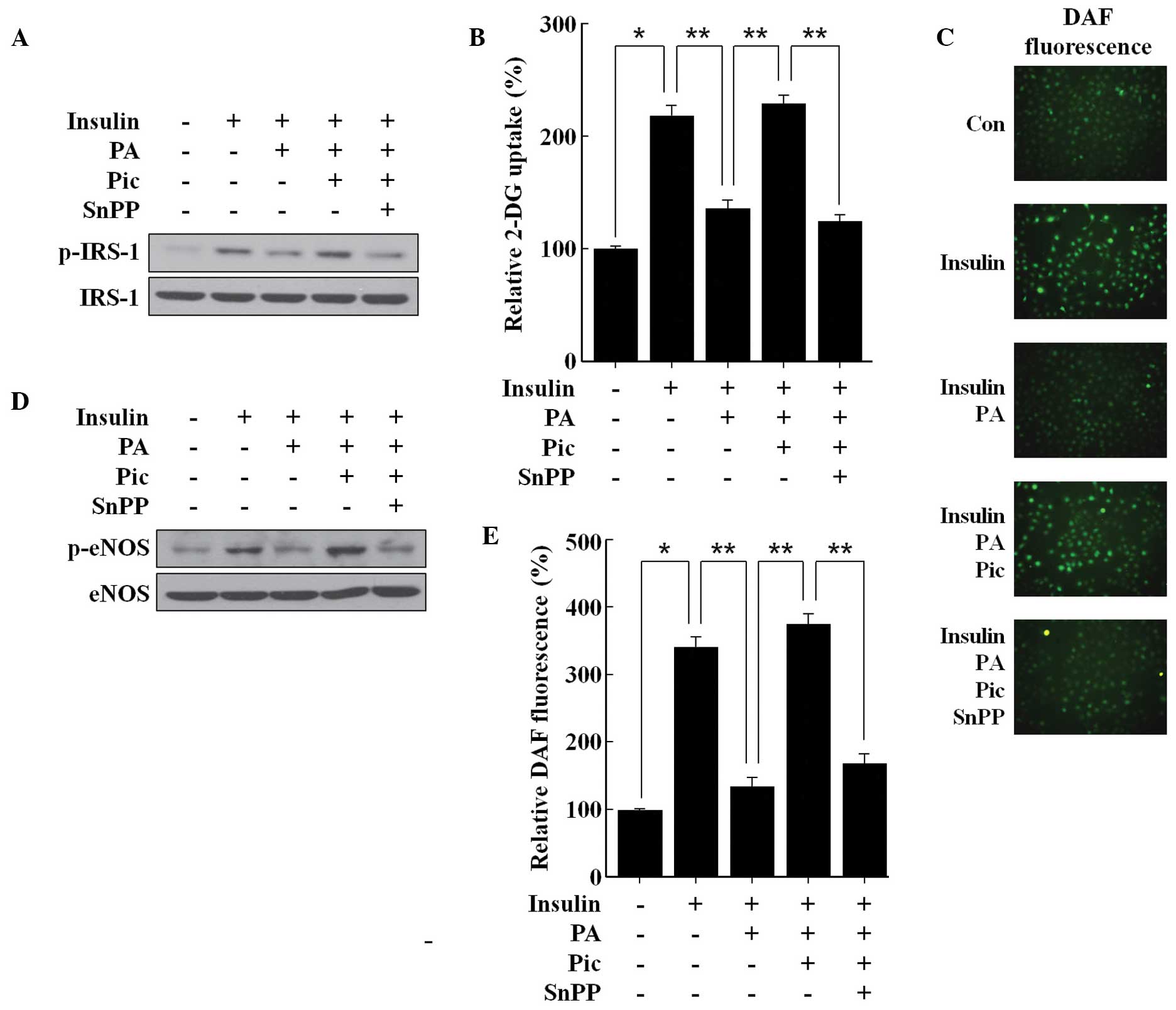 | Figure 4Effects of Pic on insulin-mediated
IRS-1 tyrosine phosphorylation, glucose uptake, activation of eNOS,
and productionof NO in human umbilical vein endothelial cells
stimulated with PA. The cells were pretreated for 12 h with 20
μM Pic and then exposed to 100 μM PA for 12 h in the
presence or absence of 20 μM of SnPP. The cells were
stimulated with 100 nM insulin for (A, B, C and D) 0.5 h or (E) 2
h. (A) Western blot analysis was performed for IRS-1 tyrosine
phosphorylation and (D) the activation of eNOS, (C) fluorescence
microscopic analysis was performed for the production of NO. (B)
Glucose uptake and the (E) DAF-FM fluorescence intensity of the
production of NO were also determined. (C) Representative
fluorescent images demonstrate the increase in green fluorescence
intensity of DAF produced by NO (magnification, ×400).
Representative blots or pictures, selected from three seperate
experiments are shown. All data are expressed as the mean ±
standard deviation of three independent observations in separate
cell culture wells. *P<0.01 and
**P<0.05. Pic, piceatannol; PA, palmitic acid; snPP,
tin protoporphryin-IX; NO, nitric oxide; eNOS, endothelial NO
synthase; p-phosphorylated; DAF,-FM,
4-amino-5-methylamino-2′,7′-difluorofluorescein. |
Discussion
PA is a circulating free fatty acid, which is often
observed at a high concentration in insulin-resistant states
(19) and has been observed to
induce inflammation and the formation of ROS, and decrease
insulin-mediated eNOS activity, which are the causes of endothelial
dysfunction, in an endothelial cell culture model (7). The present study demonstrated that,
in HUVECs stimulated with PA, pretreatment with the resveratrol
analogue, Pic, prevented inflammation and the formation of ROS, and
also attenuated the reduction of insulin-mediated eNOS activity and
production of NO. In addition, the observed protective effects of
Pic were associated with its induction of the expression of HO-1,
which is well-known to preserve endothelial function (8).
Several, but not all, naturally occurring compounds
with anti-inflammatory and antioxidant activities are known to
induce the expression of HO-1 and to exert their beneficial effects
through the HO-1-dependent pathway (10). It has been reported previously that
Pic, a naturally occurring hydroxylated analog of resveratrol, is
capable of inducing the expression of HO-1 through the activation
of Nrf2 in neuronal cells (20)
and epithelial cells (21).
Notably, Pic is a more potent inducer of HO-1 inducer compared with
resveratrol in macrophages (22),
suggesting that the existence of the additional hydroxyl group in
Pic may be critical in its induction of the expression of HO-1. A
previous study demonstrated that Pic induces the expression of HO-1
expression in HUVECs (23). The
present study further investigated the mechanism underlying the
altered expression of HO-1 following the treatment of HUVECs with
Pic, and revealed that the effect was dependent on the activation
of Nrf2. In addition to the anti-inflammatory and antioxidant
properties of endothelial HO-1 in vitro (9), it is also beneficial in vivo
in animal models of atherosclerosis and restenosis (9). In this respect, the present study
aimed to examine the potential positive effect of the expression of
HO-1 by Pic on endothelial dysfunction in HUVECs, an endothelial
cell culture model.
The elevation of circulating FFAs is considered to
be associated with to the onset and progression of endothelial
dysfunction and associated diseases (2). It has been noted that FFAs may
increase the production of pro-inflammatory cytokines and
generation of ROS via the activation of NF-κB in human endothelial
cells (3). Pro-inflammatory
cytokines and ROS have been observed to impair eNOS function and
reduce the believability of NO, possibly by disrupting the action
of insulin (19). PA, a
circulating FFA, acts as a natural dietary ligand for the
activation of TLR4 signal transduction, which activates NF-κB in
various types of cell, including endothelial cells (3), promotes the release of
pro-inflammatory cytokines, including TNF-α and IL-6, and promotes
the formation of ROS (7). The
results of the present study demonstrated that PA induced the
phosphorylation of NF-κB p65 and increased the DNA-binding activity
of NF-κB p65, resulting in increased production of TNF-α and IL-6.
PA also induced intracellular ROS formation. Notably, pretreatment
with Pic suppressed the PA-induced activation of NF-κB and
formation of ROS, and decreased the production of TNF-α and IL-6.
PA attenuated IRS-1 tyrosine phosphorylation and glucose uptake in
response to insulin, leading to impairment of downstream insulin
signaling, evidenced by reduced the phosphorylation of eNOS and
production of NO. However, these effects of PA were effectively
reversed by Pic pretreatment. Given the involvement of inflammatory
and oxidative stresses in endothelial dysfunction (8,9),
suppression of the NF-κB-dependent inflammatory response and
production of ROS by Pic may have be responsible for its
restoration of the loss of insulin-mediated phosphorylation of eNOS
and production of NO. However, the precise mechanism underlying the
anti-inflammatory and antioxidant properties of Pic remain to be
fully elucidated. Previous studies have demonstrated that Pic
induces the expression of HO-1, which can exert anti-inflammatory
and antioxidant effects (20,22)
and the present study revealed that Pic increased the expression of
HO-1 via the Nrf2 pathway in HUVECs. Therefore, the present study
investigated whether the expression of HO-1 contributed the to
anti-inflammatory and antioxidant effects of Pic, at least, under
the experimental conditions assessed. The inhibition of HO-1
activity by SnPP eradicated the anti-inflammatory and antioxidant
effects of Pic, and reversed the restored insulin-mediated
phosphorylation of eNOS and production of NO, suggesting that the
anti-inflammatory and antioxidant effects of Pic against PA insult
may be associated, at least in part, with the expression of
HO-1.
In conclusion, the present study demonstrated that
the pretreatment of HUVECs with Pic resulted in Nrf2-dependent
expression of HO-1. Furthermore, the expression of HO-1 by Pic
inhibited the PA-induced inflammatory response andformation of ROS,
and attenuated the PA-induced reduction in the activation of eNOS
and production of NO. These results indicated that Pic was
protective against PA-induced endothelial dysfunction by inducing
the Nrf2-dependent expression of HO-1, suggesting a potential
strategy of targeting the expression of HO-1 by Pic for endothelial
protection in the presence of high levels of PA, including that in
obesity, diabetes and other metabolic inflammatory diseases.
However, further investigation is required on the bioavailability
and toxicity of Pic in humans.
Acknowledgments
This study was supported by a grant from the
National Research Foundation of Korea, funded by the Korean
government, Ministry of Science, ICT and Future Planning (no.
2011-0030130).
Abbreviations:
|
2-DG
|
2-deoxyglucose
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
FFA
|
free fatty acid
|
|
HO-1
|
heme oxygenase-1
|
|
NO
|
nitric oxide
|
|
DAF-FM
|
4-amino-5-methylamino-2′,7′-difluoro
fluorescein
|
|
DCF-DA
|
2′,7′-dichlorofluorescein
diacetate
|
|
DAF-FM
|
4-amino-5-methylamino-2′
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
IL-6
|
interleukin-6
|
|
IRS-1
|
insulin receptor substrate-1
|
|
NF-κB
|
nuclear factor-κB
|
|
Nrf2
|
nuclear factor E2-related factor 2
|
|
PA
|
palmitic acid
|
|
Pic
|
piceatannol
|
|
ROS
|
reactive oxygen species
|
|
siRNA
|
small interfering RNA
|
|
SnPP
|
tin protoporphryin-IX
|
|
TLR4
|
toll-like receptor 4
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Tousoulis D, Papageorgiou N, Androulakis
E, et al: Diabetes mellitus-associated vascular impairment: novel
circulating biomarkers and therapeutic approaches. J Am Coll
Cardiol. 62:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capurso C and Capurso A: From excess
adiposity to insulin resistance: the role of free fatty acids.
Vascul Pharmacol. 57:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maloney E, Sweet IR, Hockenbery DM, et al:
Activation of NF-kappaB by palmitate in endothelial cells: a key
role for NADPH oxidase-derived superoxide in response to TLR4
activation. Arterioscler Thromb Vasc Biol. 29:1370–1375. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim F, Tysseling KA, Rice J, et al: Free
fatty acid impairment of nitric oxide production in endothelial
cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol.
25:989–994. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knopp RH, Retzlaff B, Walden C, Fish B,
Buck B and McCann B: One-year effects of increasingly
fat-restricted, carbohydrate-enriched diets on lipoprotein levels
in free-living subjects. Proc Soc Exp Biol Med. 225:191–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yli-Jama P, Meyer HE, Ringstad J and
Pedersen JI: Serum free fatty acid pattern and risk of myocardial
infarction: a case-control study. J Intern Med. 251:19–28. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu K, Zhao W, Gao X, Huang F, Kou J and
Liu B: Diosgenin ameliorates palmitate-induced endothelial
dysfunction and insulin resistance via blocking IKKβ and IRS-1
pathways. Atherosclerosis. 223:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pae HO, Son Y, Kim NH, Jeong HJ, Chang KC
and Chung HT: Role of heme oxygenase in preserving vascular
bioactive NO. Nitric Oxide. 23:251–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YM, Pae HO, Park JE, et al: Heme
oxygenase in the regulation of vascular biology: from molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
14:137–167. 2011. View Article : Google Scholar :
|
|
10
|
Pae HO, Kim EC and Chung HT: Integrative
survival response evoked by heme oxygenase-1 and heme metabolites.
J Clin Biochem Nutr. 42:197–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar
|
|
12
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann N Y Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SW, Kim CE and Kim MH: Flavonoids
inhibit high glucose-induced up-regulation of ICAM-1 via the p38
MAPK pathway in human vein endothelial cells. Biochem Biophys Res
Commun. 415:602–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Q, Hao X, Yang Q and Si L: Resveratrol
prevents hyperglycemia-induced endothelial dysfunction via
activation of adenosine monophosphate-activated protein kinase.
Biochem Biophys Res Commun. 388:389–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu HP, Hwang TL, Hwang TL, Yen CH and Lau
YT: Resveratrol prevents endothelial dysfunction and aortic
superoxide production after trauma hemorrhage through estrogen
receptor-dependent hemeoxygenase-1 pathway. Crit Care Med.
38:1147–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becker JC, Grosser N, Waltke C, et al:
Beyond gastric acid reduction: proton pump inhibitors induce heme
oxygenase-1 in gastric and endothelial cells. Biochem Biophys Res
Commun. 345:1014–1021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon B, Kwan JJ, Duddy N, Sweeney G and
Begum N: Resistin inhibits glucose uptake in L6 cells independently
of changes in insulin signaling and GLUT4 translocation. Am J
Physiol Endocrinol Metab. 285:E106–E115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo XD, Zhang DY, Gao XJ, et al: Quercetin
and quer-cetin-3-O-glucuronide are equally effective in
ameliorating endolthelial insulin resistance through inhibition of
reactive oxygen species-associated inflammation. Mol Nutr Food Res.
57:1037–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prieto D, Contreras C and Sánchez A:
Endothelial dysfunction, obesity and insulin resistance. Curr Vasc
Pharmacol. 12:412–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Son Y, Byun SJ and Pae HO: Involvement of
heme oxygenase-1 expression in neuroprotection by piceatannol, a
natural analog and a metabolite of resveratrol, against
glutamate-mediated oxidative injury in HT22 neuronal cells. Amino
Acids. 45:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HH, Park SA, Almazari I, Kim EH, Na HK
and Surh YJ: Piceatannol induces heme oxygenase-1 expression in
human mammary epithelial cells through activation of ARE-driven
Nrf2 signaling. Arch Biochem Biophys. 501:142–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Son Y, Chung HT and Pae HO: Differential
effects of resveratrol and its natural analogs, piceatannol and
3,5,4′-trans-trimethoxys-tilbene, on anti-inflammatory heme
oxigenase-1 expression in RAW264.7 macrophages. Biofactors.
40:138–145. 2014. View Article : Google Scholar
|
|
23
|
Wung BS, Hsu MC, Wu CC and Hsieh CW:
Piceatannol upregulates endothelial heme oxygenase-1 expression via
novel protein kinase C and tyrosine kinase pathways. Pharmacol Res.
53:113–122. 2006. View Article : Google Scholar
|


















