Introduction
Brain tumors grow in a limited space of the cranial
cavity and easily cause damage to the central nervous system. These
types of tumor frequently incur high risks and are life
threatening. Based on the statistics, primary brain tumors account
for ~1% of all types of tumor, 2.4% of tumor-associated mortality
and 20–25% of pediatric tumors (1). Glioma is the most common primary
brain tumor, accounting for ~50% of primary brain tumors and up to
80% of malignant brain tumors (2).
The progress made in understanding glioma has been
in three predominant aspects, including molecular etiology and
pathology, diagnosis and treatment techniques, and treatment
concepts. In the ‘Chinese Guideline for the Diagnosis and Treatment
of Glioma in the Central Nervous System’ issued by the Oncology
Group of the Neurosurgery Branch of Chinese Medical Association in
2012 (3), the treatment of glioma
was suggested to comprise predominantly a combination of surgery,
radiotherapy and chemotherapy. Surgery can remove the gross tumors,
while radiotherapy and chemotherapy can destroy or inhibit the
residual tumor cells, extending survival rates. Although surgery,
as the primary choice for the treatment of a brain tumor, exhibits
the advantages of directness and thoroughness, the complex brain
structure makes the tumors inaccessible in certain patients and,
consequently, obstructs or prevents implementation of the surgical
procedures. Radiotherapy destroys tumor cells with radiation, while
limiting damage to the normal brain cells. It is the most common
measure for the treatment of a secondary brain tumor and is an
important complementary method for surgical treatment. Treatment
with chemotherapy to destroy tumor cells with compounds is
generally used in combination with surgery and radiotherapy to
increase the chances of successful treatment (4–6).
Despite significant progress in therapeutic methods, curing glioma
remains rare, with median survival rates not exceeding 2 years
(7).
Cisplatin, as a common chemotherapeutic drug, can
inhibit DNA replication and transcription in cancer cells through
DNA cross-linking, leading to tumor cell growth arrest and
apoptosis, however, tumor cells often develop clinical resistance
to chemotherapeutic drugs, resulting in treatment failure (8). Therefore, identifying the mechanisms
underlying tumor resistance and developing novel methods to reverse
drug resistance have important significance for improving the
clinical benefits for patients. Previous studies have identified
drug-resistance mechanisms of tumor cells, including reducing drug
absorption, increasing drug efflux via transporter proteins,
detoxifying antitumor drugs via the glutathione system, and causing
abnormalities in apoptotic pathways to reduce tumor cell apoptosis
(9,10).
Peroxisome proliferator-activated receptor-γ
(PPAR-γ) is a type II nuclear receptor, which is closely associated
with obesity, diabetes, atherosclerosis and other metabolic
diseases clinically, as initial studies have revealed that PPAR-γ
regulates fatty acid storage and glucose metabolism (11). Previous studies have demonstrated
the association of PPAR-γ with cancer, and a series of preclinical
studies have revealed that ligand-activated PPAR-γ is able to
arrest tumor cell growth, increase cell apoptosis and inhibit tumor
metastasis (12,13). The prevention and treatment of
glioma remains a significant clinical challenge, and the
correlation between PPAR-γ and cisplatin-resistant glioma remains
to be elucidated. The present study aimed to investigate the
expression of PPAR-γ in a cisplatin-resistant glioma cell line,
whether PPAR-γ altered the sensitivity or resistance of the
drug-resistant cells to cisplatin, and preliminarily examine the
underlying molecular mechanisms.
Materials and methods
Cells and cell culture
The glioma U87 MG cell line was purchased from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were subcultured routinely in
Dulbecco’s modified Eagle’s medium (Corning Life Sciences,
Manassas, VA, USA), containing 10% fetal bovine serum, 100 U/ml
penicillin and 100 U/ml streptomycin (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C in a saturated humidity, 5% CO2 incubator.
The U-87 MG cells were cultured in cisplatin-containing media, at
an initial concentration of 1/10 the half maximal inhibitory
concentration (IC50), and the cisplatin concentration
was increased gradually with continuous culture. Following
continuous exposure to increased concentrations of cisplatin for ~6
months, the growing tumor cells were obtained for the
cisplatin-resistant U-87 MG/CDDP cell line.
Construction of the PPAR-γ-overexpressing
U-87 MG/CDDP cell line
A PPAR-γ expression plasmid (PPARG-pCMV/hygro
plasmid; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and blank
plasmid were purchased from FITGene Tech, Inc. and transfected into
the U-87 MG/CDDP cells using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the manufacturer’s
instructions. Screening was performed in hygromycin-containing
culture media (Sigma-Aldrich), in which cells that had undergone
successful transfection with the plasmids were able to grow in the
selective culture media.
Detection of the inhibition of cell
proliferation using an MTS assay
The cells (3×103) were seeded into
96-well plates and incubated for 24 h at 37°C with 5%
CO2. Cisplatin (0, 0.1, 0.5, 1, 5, 10, 50 or 100
µM; Sigma-Aldrich) was added, and the cells were incubated
for a further 72 h. Fresh culture media, containing 20 µl
MTS (Promega Corporation, Madison, WI, USA), was added to each well
and incubated for 2 h. The absorbance was subsequently measured
witha microplate reader (Synergy 2; BioTek Instruments, Inc.,
Winooski, VT, USA) at 492 nm to detect the sensitivity of the tumor
cells to cisplatin.
Detection of the intracellular content of
rhodamine (Rh)-123, oxidative stress, expression of P-gp, cell
cycle and apoptosis by flow cytometry
The cells (3×105) were seeded into 6-well
plates and incubated for 24 h, prior to being digested with trypsin
(Sigma-Aldrich) at 37°C, centrifuged at 300 × g, washed with
cold-phosphate-buffered saline (PBS; Corning Life Sciences) three
times, and incubated in the dark at 37°C with 10 µg/ml
Rh-123 for 30 min. The cells were subsequently washed with cold-PBS
three times and analyzed by flow cytometry (FACSAria; BD
Biosciences, San Jose, CA, USA) at 488 nm to detect the
intracellular content of Rh-123 in the tumor cells.
To determine the oxidative stress, the cells
(3×105) were seeded into 6-well plates and incubated for
24 h, prior to adding 10 µM cisplatin and incubating for a
further 24 h. The cells were then digested with trypsin at 37°C,
centrifuged at 300 × g, washed with cold-PBS three times and
incubated in the dark at 37°C with 20 µl DCFH-DA for 30 min.
The cells were subsequently washed with cold-PBS three times and
analyzed by flow cytometry at 488 nm to detect the oxidative stress
of the tumor cells.
To determine the expression of P-gp, the cells
(3×105) were seeded into 6-well plates and incubated for
24 h, followed by being digested with trypsin at 37°C, centrifuged
at 300 × g for 5 min, washed with cold-PBS three times, and
incubated in the dark at 37°C with 20 µl P-glycoprotein
(gp)-phycoerythrin (PE) antibody for 15 min. The cells were then
washed with cold-PBS three times and analyzed by flow cytometry at
488 nm to detect the expression of P-gp in the tumor cells.
To examine the effects on the cell cycle, the cells
(3×105) were seeded into 6-well plates and incubated at
37°C with 5% CO2 for 24 h, followed by being digested
with trypsin at 37°C, centrifuged at 300 × g for 5 min, fixed using
cold alcohol (75%) overnight. The cells were then washed with
cold-PBS three times and incubated with 500 µl propidium
iodide (PI) staining solution with RNaseA (Beyotime Institute of
Biotechnology, Shanghai, China) for 30 min. Following staining, the
cells were washed with cold-PBS three times and analyzed by flow
cytometry at 488 nm to detect the cell cycle of the tumor
cells.
To determine the levels of apoptosis, the cells
(3×105) were seeded into 6-well plates and incubated for
24 h at 37°C with 5% CO2 prior to the addition of 10
µM cisplatin for a further 24 h at 37°C with 5%
CO2. The cells were then digested with trypsin at 37°C,
centrifuged at 300 × g for 5 min, washed with cold-PBS three times
and incubated in the dark at 37°C with 20 µl annexin
V-fluorescein isothiocyanate antibody and PI staining solution for
15 min. The cells were washed with cold-PBS three times and
analyzed by flow cytometry at 488 nm to detect the apoptosis of the
tumor cells.
Detection of the intracellular levels of
thymidylate synthase and glutathione level using a biochemical
assay
The cells (3×105) were seeded into 6-well
plates and incubated for 24 h. Following incubation, the cells were
lysed, centrifuged at 14,000 × g for 5 min at 4°C and the
supernatant was collected. The intracellular levels of thymidylate
synthase and glutathione were measured using a thymidylate synthase
and Total Glutathione Assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer’s instructions.
Detection of the production of TGF-β1
using an ELISA assay
The cells (3×105) were seeded into 6-well
plates and incubated for 24 h, prior to the addition of fresh
culture medium for 24 h and collection of the supernatant. The
levels of TGF-β1 were measured using an ELISA detection kit
(R&D Systems, Minneapolis, MN, USA), according to the
manufacturer’s instructions.
Detection of the protein expression
levels of PPAR-γ, multidrug resistance gene (M DR)1, multidrug
resistance-associated protein (MRP)1, glutathionine S-transferase
(GST)-π, survivin, B-cell lymphoma (Bcl)-2, p53, p21, caspase-3/8,
phosphorylated (p)-extracellular signal-regulated kinase (ERK) and
p-small mothers against decapentaplegic (Smad)2 by western
blotting
The cells (3×105) were seeded into 6-well
plates and incubated for 24 h at 37°C with 5% CO2.
Following incubation, the cells were lysed, centrifuged at 14,000 ×
g for 5 min at 4°C and the concentration of total protein in the
supernatant was measured using a Bradford Protein Assay Kit. The
Bradford assay involved solutions of standard or unknown protein
samples being placed in two blank tubes, one for the standard
curve, to which 30 µl H2O was added, and the
other for the unknown protein samples, to which 30 µl
protein preparation buffer was added. In addition, 1.5 ml Bradford
reagent was added to each tube and they were mixed well and
incubated at room temperature for a minimum of 5 min, but for no
more than 1 h. The absorbance could then be measured at 595 nm. The
total protein (80 µg) was separated on 12% SDS-PAGE gels
(Beyotime Institute of Biotechnology) and transferred onto a
polyvinylidene fluoride membrane (Beyotime Institute of
Biotechnology). The membrane was blocked using 5% non-fat milk for
2 h at room temperature and was subsequently incubated at 4°C
overnight with the following monoclonal IgG antibodies from
Santa-Cruz Biotechnology, Inc.: Rabbit anti-human PPAR-γ, (1:300;
sc-7196), mouse anti-human MDR1 (1:300; sc-555), rabbit anti-human
MRP1 (H-70) (1:300; sc-13960), rabbit anti-human GST-π (110–218)
(1:300; sc-33614), mouse anti-human survivin (C-6) (1:300;
sc-374616), rabbit anti-human Bcl-2 (1:300; sc-492), rabbit
anti-human p53 (FL-393) (1:300; sc-6243), rabbit anti-human p21
(C-19) (1:300; sc-397), rabbit anti-human caspase-3 (H-277) (1:200;
sc-7148), rabbit anti-human caspase-8 p18 (H-134) (1:200; sc-7890),
rabbit anti-human p-ERK (H-300) (1:200; sc-13073), rabbit
anti-human p-Smad2 (Ser 467) (1:200; sc-101801) and rabbit
anti-human β-actin (H-196) (1:5,000; sc-7210). The membrane was
washed with Tris-buffered saline (TBS), containing 0.1% Tween 20
(Beyotime Institute of Biotechnology) three times and was
subsequently incubated with horesradish peroxidase-labeled
secondary antibody (1:2,000; Sigma-Aldrich) at room temperature for
1 h. The membrane was then washed with TBS containing 0.1% Tween 20
three times and visualized using a diaminobenzidine kit (Millipore,
Billerica, MA, USA). β-actin was used as an internal loading
control.
Detection of the mRNA expression levels
of PPAR-γ, MDR1, MRP1, GST-π, survivin, Bcl-2, p53 and p21 by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
The cells (3×105) were seeded into 6-well
plates, incubated for 24 h and the total RNA was extracted using
TRIzol reagent (Life Technologies, Carlsbad, CA, USA). qPCR was
performed with 1 µg RNA, Taqman Master mix (Life
Technologies) and the following primers from [Genscript (Nanjing)
Co., Ltd., Nanjing, China]: PPAR-γ, sense
5′-CACATCTACAATGCCTACCT-3′ and antisense
5′-CTTCTCTGCCTGCCACAATGTCT-3′; MDR1, sense
5′-AAAAAGATCAACTCGTACCACTC-3′ and antisense
5′-GCACAAAATACACCAACAA-3′; MRP1, sense
5′-ACTTCCACATCTGCTTCGTCAGTG-3′ and anti-sense
5′-ATTCAGCCACAGGAGGTAGAGAGC-3′; GST-π, sense
5′-ACCTGCCTGTGACATCAT-3′ and antisense 5′-TCTCCCTTTGTGCGTTCT-3′;
survivin, sense 5′-GCATGGGTGCCCCGACGTTG-3′ and antisense:
5′-GCTCCGGCCAGAGGCCTCAA-3′; Bcl-2, sense
5′-ACGGGGTGAACTGGGGGAGGA-3′ and antisense
5′-TGTTTGGGGCAGGCATGTTGACTT-3′; p53, sense
5′-GCCCAACAACACCAGCTCC-3′ and antisense 5′-CCTGGGCATCCTTGAGTTCC-3′;
p21, sense 5′-CACTCCAAACGCCGGCTGATCTTC-3′ and antisense
5′-TGTAGAGCGGGCCTTTGAGGCCCT C-3′ and β-actin, sense
5′-TGAGCGCGGCTACAGCTT-3′ and antisense
5′-TCCTTAATGTCACGCACGATTT-3′. PCR was performed with the ABI 7500
Sequence Detection System (Applied Biosystems Life Technologies,
Foster City, CA, USA) and the reaction conditions were as follows:
94°C denaturation for 3 min, followed by 40 cycles of 95°C for 5
sec, 65°C for 35 sec and 72°C for 60 sec. β-actin was used as an
internal control, and mRNA expression levels were quantified by
comparing target gene and β-actin expression levels.
Detection of the transcriptional activity
of Twist and nuclear factor (erythroid-derived 2)-like 2 (NRF2)
using a dual luciferase reporter gene assay
The cells (3×105) were seeded into 6-well
plates and incubated for 24 h. The luciferase reporter plasmids of
Twist and NRF2 (Beyotime Institute of Biotechnology) were
transfected into the cells using Lipofectamine 2000 (Invitrogen
Life Technologies), according to the manufacturer’s instructions,
and the cells were cultured for 24 h. The fluorescence intensity
was subsequently determined using a Dual-Glo® Luciferase
assay system (Promega Corporation) to detect the transcriptional
activity of Twist and NRF2.
Statistical methods
The data are expressed as the mean ± standard
deviation and were analyzed using SPSS 11.5 software (SPSS, Inc,.
Chicago, IL, USA) by one-way analysis of variance. Each experiment
was repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
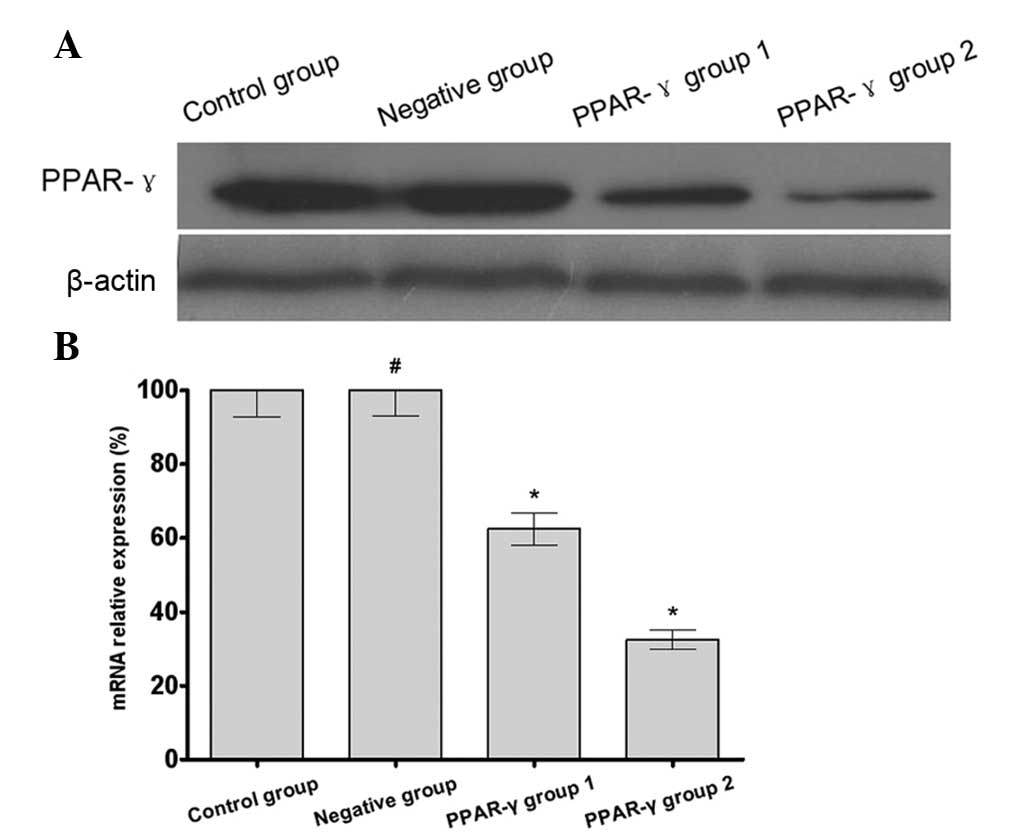
PPAR-γ increases the cisplatin
sensitivity of U-87 MG/DDP cells
The results of the western blotting and RT-qPCR
demonstrated that the expression of PPAR-γ in the U-87 MG/DDP cells
transfected with the PPARG-pCMV/hygro plasmid (PPAR-γ group 1 and
PPAR-γ group 2) was increased compared with the that in the U-87
MG/DDP cells transfected with the blank plasmid (negative group)
and the U-87 MG/DDP cells without transfection (control group). In
addition, there was increased expression of PPAR-γ in PPAR-γ group
2 compared with PPAR-γ group 1 (Fig.
1). These results indicated that the PPAR-γ overexpressing U-87
MG/DDP cell lines had been successfully established.
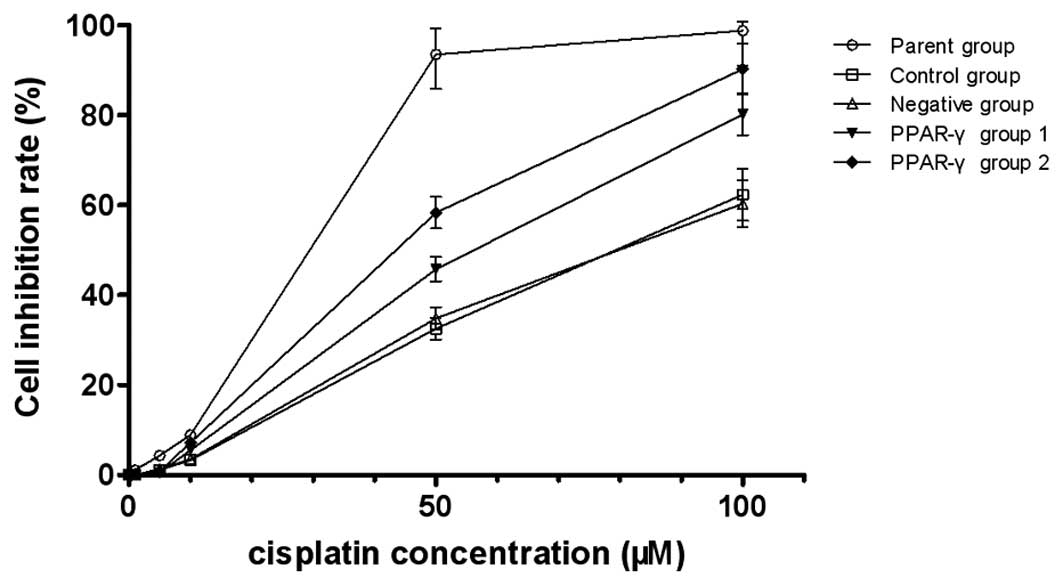
The MTS assay indicated that the there was increased
cisplatin sensitivity in the PPAR-γ-overexpressing U-87 MG/DDP
cells compared with the control and negative groups. The
IC50 of the control group, negative group, PPAR-γ group
1 and PPAR-γ group 2 was 78.3, 80.5, 53.6 and 36.4 µM,
respectively, and the reversal fold (RF) was 1.46 and 2.15,
respectively, indicating that PPAR-γ increased the sensitivity of
the U-87 MG/DDP cells to cisplatin (Fig. 2).
PPAR-γ increases intracellular Rh-123
content and oxidative stress, decreases the expression levels of
P-gp, thymidylate synthase, glutathione and TGF-β1, arrests the
cell cycle and improves the cell apoptosis of U-87 MG/DDP
cells
The results of the flow cytometry demonstrated
increased intracellular content of Rh-123 and levels of oxidative
stress in the PPAR-γ-overexpressing U-87 MG/DDP cell lines compared
with the control group, however, the expression levels of P-gp,
thymidylate synthase, glutathione and TGF-β1 were reduced in the
PPAR-γ-overexpressing U-87 MG/DDP cell lines compared with the
control group. Additionally, the G0/G1 phase rate and apoptotic
rate were increased in the PPAR-γ-overexpressing U-87 MG/DDP cell
lines compared with the control group (Fig. 3).
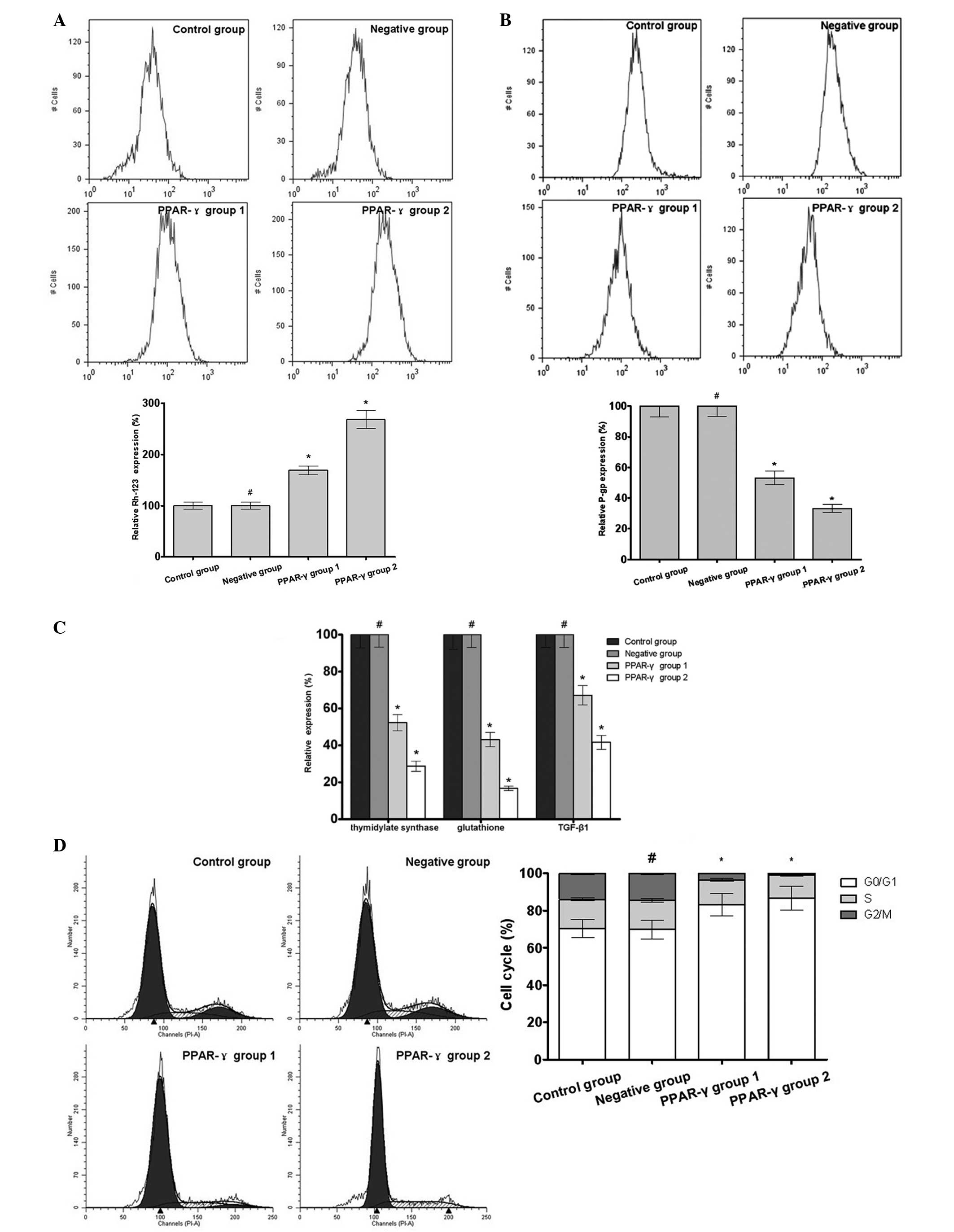 | Figure 3Effect of PPAR-γ on the intracellular
Rh-123 content, expression levels of P-gp, ROS, thymidylate
synthase, glutathione and TGF-β1, cell cycle and apoptosis of the
U-87 MG/DDP cell lines. Data are expressed as the mean ± standard
deviation. (A) Flow cytometry demonstrated an increased
intracellular content of Rh-123 in the PPAR-γ group 1 and PPAR-γ
group 2 compared with the control group (#P>0.05;
*P<0.05; n=3). (B) Flow cytometry demonstrated
decreased expression of P-gp in PPAR-γ group 1 and PPAR-γ group 2
compared with the control group (#P>0.05,
*P<0.05; n=3). (C) Expression levels of thymidylate
synthase, glutathione and TGF-β1 decreased in PPAR-γ group 1 and
PPAR-γ group 2 compared with the control group
(#P>0.05, *P<0.05; n=5). (D) Flow
cytometry demonstrated an increased G0/G1 phase rate in PPAR-γ
group 1 and PPAR-γ group 2 compared with the the control group
(#P>0.05, *P<0.05; n=3). (E) Flow
cytometry demonstrated that there was an increased expression of
ROS in the PPAR-γ group 1 and PPAR-γ group 2 compared with the
control group (#P>0.05, *P<0.05; n=3).
(F) Flow cytometry demonstrated an increased rates of apoptosis
rate in PPAR-γ group 1 and PPAR-γ group 2 compared with the control
group. Data are expressed as the mean ± standard deviation
(#P>0.05, *P<0.05; n=3). PPAR,
peroxisome proliferator-activated receptor; Rh, rhodamine; ROS,
reactive oxygen species; TGF, transforming growth factor. |
PPAR-γ regulates the expression levels of
MDR-associated genes in U-87 MG/DDP cells
The results of the western blotting demonstrated
decreased protein expression levels of MDR, MRP, GST-π, survivin
and Bcl-2 in the PPAR-γ-overexpressing U-87 MG/DDP cell lines
compared with the control and negative groups. However, the protein
expression levels of p53, p21 and caspase-3/8 were increased in the
PPAR-γ-overexpressing U-87 MG/DDP cell lines compared with the
control and negative groups. The RT-qPCR results revealed the same
trend (Fig. 4).
 | Figure 4Effect of PPAR-γ on the expression
levels of multidrug resistance-associated genes of the U-87 MG/DDP
cell lines. (A) Western blotting demonstrated decreased protein
expression levels of MDR, MRP, GST-π, survivin and Bcl-2 in PPAR-γ
group 1 and PPAR-γ group 2 compared with the control and negative
groups, however, the protein expression levels of p53, p21 and
caspase-3/8 increasedin the PPAR-γ group 1 and PPAR-γ group 2
compared with the control and negative groups. β-actin was used as
an internal loading control. (B) Reverse transcription-quantitative
polymerase chain reaction assay results demonstrated decreased mRNA
expression levels of MDR, MRP, GST-π, survivin and Bcl-2 in PPAR-γ
group 1 and PPAR-γ group 2 compared with the control group,
however, mRNA expression levels of p53 and p21 were increased in
PPAR-γ group 1 and PPAR-γ group 2 compared with the control group.
β-actin was used as an internal loading control. The data are
expressed as the mean ± standard deviation (#P>0.05,
*P<0.05; n=5). PPAR, peroxisome
proliferator-activated receptor; Bcl, B-cell lymphoma; MDR,
multidrug resistance gene; MRP, multidrug resistance-associated
protein; GST, glutathionine S-transferase. |
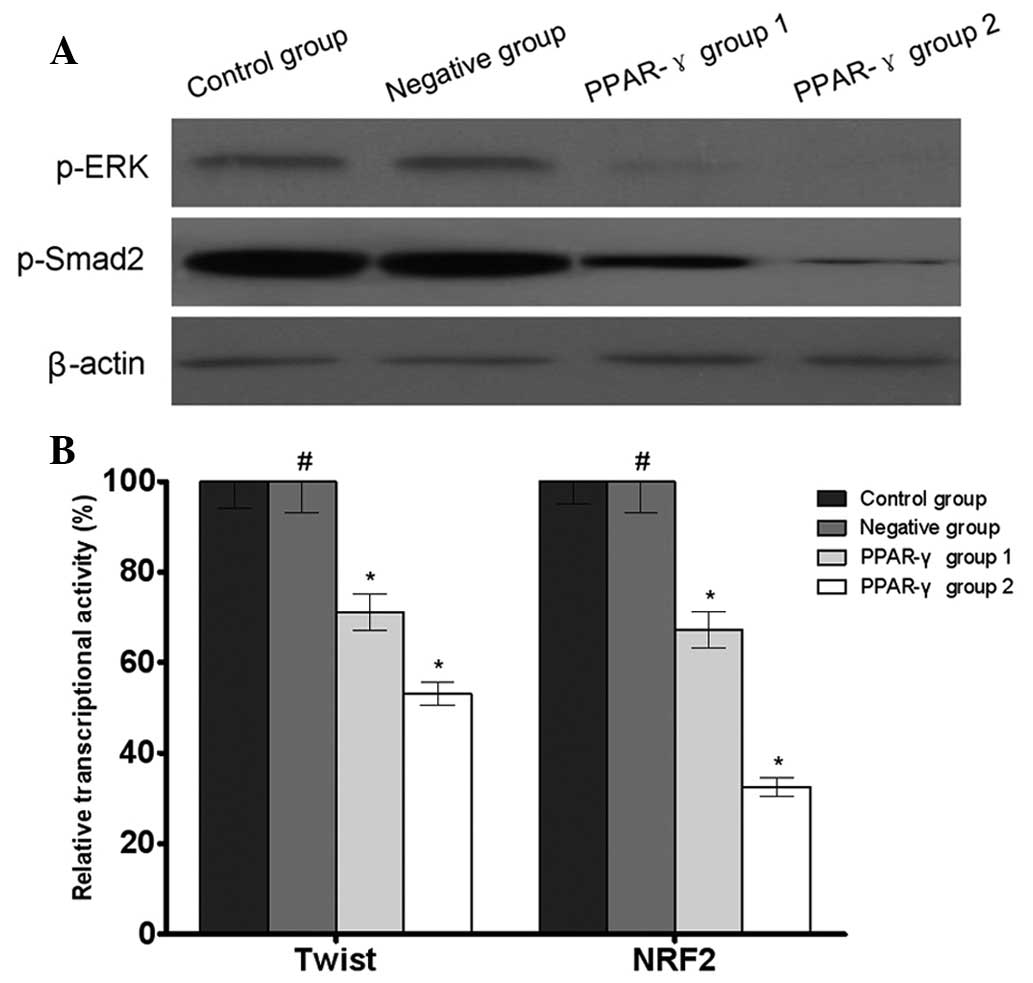
PPAR-γ regulates MDR-associated signaling
molecules in U-87 MG/DDP cells
The results of the western blotting revealed that
the phosphorylation levels of ERK and Smad2 were decreased in the
PPAR-γ-overexpressing U-87 MG/DDP cell lines compared with the
control and negative groups. In addition, the results of the
reporter gene assay demonstrated that there were decreased
transcriptional activities of Twist and NRF2 in the
PPAR-γ-overexpressing U-87 MG/DDP cell lines compared with the
control group (Fig. 5).
Discussion
Glioma is the most common type of primary malignant
brain tumor. Although surgery is the primary choice in brain tumor
treatment, a number of patients are not suitable for surgery for a
variety of reasons or surgery cannot achieve the optimal
therapeutic effect. In these cases, radiotherapy and chemotherapy
are a suitable alternative or adjuvant therapy. Platinum-containing
agents are a class of widely used chemotherapeutic drugs and
demonstrate favorable efficacy in the treatment of a variety of
malignant types of tumor. However, the tumor cells can develop drug
resistance during the therapeutic process, often leading to
treatment failure (14). This
means that searching for molecular drug targets against drug
resistance clinically is urgently required to improve the clinical
benefits for the patient.
PPAR-γ is a nuclear transcription factor, which can
bind to corresponding ligands to induce or inhibit target gene
expression. PPAR-γ is overexpressed in adipose tissue and expressed
in mammary, pulmonary, ovarian, prostate and other types of tissue.
Previous studies elucidated the close association between PPAR-γ
and cancer, for example, the ligand-induced activation of PPAR-γ
arrests the growth of ovarian cancer cells and non-small cell lung
cancer cells, and also induces tumor cell differentiation and
apoptosis. Patients with breast cancer overexpressing PPAR-γ
exhibit longer disease-free survival rates (15–17).
However, the role of PPAR-γ in cisplatin-resistant glioma remains
to be elucidated.
One of the mechanisms of tumor cell resistance to
chemotherapeutic drugs is the overexpression of MDR proteins,
including MDR1 and MRP1, leading to increased drug efflux to reduce
the intracellular drug accumulation and effective drug
concentration at the target sites (18,19).
The present study established the U-87 MG/CDDP cisplatin-resistant
cell line and used a recombinant plasmid transfection technique to
stably overexpress PPAR-γ in these drug-resistant cells. The
subsequent MTS assay revealed that the sensitivity of the U-87
MG/CDDP PPAR-γ-overexpressing cell lines to cisplatin was
increased, as evidenced by the reduced IC50. This
sensitivity was positively correlated with the expression of
PPAR-γ. The present study also used flow cytometry to detect
changes in the content of Rh-123 in tumor cells, the results of
which demonstrated that a higher level of PPAR-γ was correlated
with an increase in the intracellular content of Rh-123. Since
Rh-123 is a substrate of MDR1 and MRP1, the increase in
intracellular Rh-123 content indirectly suggested a reduced efflux
of chemotherapeutic drug molecules from tumor cells (20,21).
Furthermore, western blotting and RT-qPCR demonstrated that the
transcription and expression levels of MDR1 and MRP1 decreased,
consistent with the decreased expression of P-gp.
In addition to MDR1 and MRP1, other mechanisms may
mediate tumor cell resistance to cisplatin. GST-π, thymidylate
synthase and glutathione can act as detoxication molecules to
detoxify chemotherapeutic drug toxicity (22,23).
The present study also found that the expression levels of GST-π,
thymidylate synthase and glutathione significantly decreased
following the overexpression of PPAR-γ and there was increased
oxidative stress by cisplatin, suggesting that PPAR-γ negatively
regulated the expression levels of GST-π, thymidylate synthase and
glutathione, and consequently inhibited the tumor cell drug
resistance mediated by the latter. Therefore, PPAR-γ reversed U-87
MG/CDDP drug resistance through multiple pathways.
The flow cytometry results demonstrated that PPAR-γ
arrested the tumor cell cycle at the G0/G1 phase and increased the
levels of apoptosis. Investigation of the cell cycle- and
apoptosis-associated protein molecules revealed that PPAR-γ
negatively regulated the expression levels of durvivin and Bcl-2,
which are tumor cell apoptosis inhibitory proteins (24,25),
and positively regulated the expression levels of p21, p53 and
caspase-3/8, which are cell apoptosis inducing proteins (26–28).
Accordingly, PPAR-γ regulated tumor cell apoptosis through
coordinated regulation of multiple protein molecules. The fact that
p21/p53 can arrest cells at the G1/S checkpoint and previous
findings indicate G0/G1 transforming gene-2 as the target gene of
PPAR are consistent with the observation in the present study that
the overexpression of PPAR-γ arrested tumor cells at the G0/G1
phase (29). In addition, western
blotting demonstrated that PPAR-γ was able to downregulate the
levels of TGF-β1 and the phosphorylation of ERK and Smad2, which
are important in tumor cell growth (30). It was also observed that PPAR-γ was
able to downregulate the transcriptional activity of Twist and NRF2
in the U-87 MG/CDDP tumor cells. These transcription factors are
all important in the carcinogenesis and progression of tumor cells
(31–33).
In conclusion, the present study demonstrated that
the overexpression of PPAR-γ was able to reverse drug resistance in
the U-87 MG/CDDP cisplatin-resistant glioma cell line. The
mechanisms of action were found to include: Downregulation of MDR1
and MRP1, increased intracellular drug accumulation, increased
tumor cell sensitivity to drugs, regulation of multiple proteins
associated with the cell cycle and cell apoptosis, inhibition of
tumor cell growth and increased apoptosis through the
TGF-β1/ERK/Smad2 pathway.
References
|
1
|
Shete S, Lau CC, Houlston RS, et al:
Genome-wide high-density SNP linkage search for glioma
susceptibility loci: results from the Gliogene consortium. Cancer
Res. 71:7568–7575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabo B: Primary malignant brain tumours,
psychosocial distress and the intimate partner experience: what do
we know? Can J Neurosci Nurs. 3:9–15. 2014.
|
|
3
|
Zhou LF: The first step in a
long-run-celebrating the announcement of ‘Guideline for the
management of CNS gliomas in China’. Zhonghua Yi Xue Za Zhi.
93:24172013.In Chinese.
|
|
4
|
Yang P, Wang Y, Peng X, et al: Management
and survival rates in patients with glioma in China (2004-2010): a
retrospective study from a single-institution. J Neurooncol.
113:259–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu JS, Zhang J, Zhuang DX, et al: Current
status of cerebral glioma surgery in China. Chin Med J (Engl).
124:2569–2577. 2011.
|
|
6
|
Wang J, Liu X, Ba YM, et al: Effect of
sonographically guided cerebral glioma surgery on survival time. J
Ultrasound Med. 31:757–762. 2012.PubMed/NCBI
|
|
7
|
Diaz-Miqueli A and Martinez GS:
Nimotuzumab as a radiosen-sitizing agent in the treatment of high
grade glioma: challenges and opportunities. Onco Targets Ther.
6:931–942. 2013. View Article : Google Scholar
|
|
8
|
Wu Z, Chan CL, Eastman A and Bresnick E:
Expression of human O6-methylguanine-DNA methyltransferase in
Chinese hamster ovary cells and restoration of cellular resistance
to certain N-nitroso compounds. Mol Carcinog. 4:482–488. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Liu L, Liu X, et al: Shugoshin1
enhances multidrug resistance of gastric cancer cells by regulating
MRP1, Bcl-2 and Bax genes. Tumour Biol. 34:2205–2214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung KK, Chan JY and Fung KP:
Antiproliferative effect of pheophorbide a-mediated photodynamic
therapy and its synergistic effect with doxorubicin on multiple
drug-resistant uterine sarcoma cell MES-SA/Dx5. Drug Chem Toxicol.
36:474–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ching J, Amiridis S, Stylli SS, et al: A
novel treatment strategy for glioblastoma multiforme and glioma
associated seizures: increasing glutamate uptake with PPARγ
agonists. J Clin Neurosci. 1:21–28. 2015. View Article : Google Scholar
|
|
12
|
Abbott BD: Review of the expression of
peroxisome prolif-erator-activated receptors alpha (PPAR alpha),
beta (PPAR beta) and gamma (PPAR gamma) in rodent and human
development. Reprod Toxicol. 27:246–257. 2009. View Article : Google Scholar
|
|
13
|
Khoo NK, Hebbar S, Zhao W, et al:
Differential activation of catalase expression and activity by PPAR
agonists: implications for astrocyte protection in anti-glioma
therapy. Redox Biol. 1:70–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai Y, Liao H, Liu T, et al: MiR-296-3p
regulates cell growth and multi-drug resistance of human
glioblastoma by targeting ether-à-go-go (EAG1). Eur J Cancer.
49:710–724. 2013. View Article : Google Scholar
|
|
15
|
Reka AK, Goswami MT, Krishnapuram R, et
al: Molecular cross-regulation between PPAR-γ and other signaling
pathways: implications for lung cancer therapy. Lung Cancer.
72:154–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawai M and Rosen CJ: PPARγ: a circadian
transcription factor in adipogenesis and osteogenesis. Nat Rev
Endocrinol. 6:629–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siersbaek R, Nielsen R and Mandrup S:
PPARgamma in adipocyte differentiation and metabolism-novel
insights from genome-wide studies. FEBS Lett. 584:3242–3249. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martín V, Sanchez-Sanchez AM, Herrera F,
et al: Melatonin-induced methylation of the ABCG2/BCRP promoter as
a novel mechanism to overcome multidrug resistance in brain tumour
stem cells. Br J Cancer. 108:2005–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quezada C, Peigñan L, Segura R, et al:
Study of resistance to chemotherapy mediated by ABC transporters in
biopsies of glioblastoma multiforme. Rev Med Chil. 139:415–424.
2011.In Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garrido W, Muñoz M, San Martín R and
Quezada C: FK506 confers chemosensitivity to anticancer drugs in
glioblastoma multiforme cells by decreasing the expression of the
multiple resistance-associated protein-1. Biochem Biophys Res
Commun. 411:62–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shityakov S and Förster C: Multidrug
resistance protein P-gp interaction with nanoparticles (fullerenes
and carbon nanotube) to assess their drug delivery potential: a
theoretical molecular docking study. Int J Comput Biol Drug Des.
6:343–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu FS, Chen XM, Huang ZG, et al:
Rofecoxib augments anticancer effects by reversing intrinsic
multidrug resistance gene expression in BGC-823 gastric cancer
cells. J Dig Dis. 11:34–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou YT, Li K and Tian H: Effects of
vinorelbine on cisplatin resistance reversal in human lung cancer
A549/DDP cells. Asian Pac J Cancer Prev. 14:4635–4639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryan BM, O’Donovan N and Duffy MJ:
Survivin: a new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horowitz JC, Ajayi IO, Kulasekaran P, et
al: Survivin expression induced by endothelin-1 promotes
myofibroblast resistance to apoptosis. Int J Biochem Cell Biol.
44:158–169. 2012. View Article : Google Scholar
|
|
26
|
Zhu Y, Liu XJ, Yang P, et al:
Alkylglyceronephosphate synthase (AGPS) alters the lipids signaling
pathways and support the chemotherapy resistance of glioma and
hepatic carcinoma cell lines. Asian Pac J Cancer Prev.
15:3219–3226. 2014. View Article : Google Scholar
|
|
27
|
Bharatham N, Chi SW and Yoon HS: Molecular
basis of Bcl-X(L)-p53 interaction: insights from molecular dynamics
simulations. PLoS One. 6:e260142011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zandbergen F, Mandard S, Escher P, et al:
The G0/G1 switch gene 2 is a novel PPAR target gene. 392:313–324.
2005.
|
|
29
|
Yuan L, Zhang Y, Xia J, et al: Resveratrol
induces cell cycle arrest via a p53-independent pathway in A549
cells. Mol Med Rep. 4:2459–2464. 2015.
|
|
30
|
Liu LC, Tsao TC, Hsu SR, et al: EGCG
inhibits transforming growth factor-β-mediated
epithelial-to-mesenchymal transition via the inhibition of Smad2
and Erk1/2 signaling pathways in nonsmall cell lung cancer cells. J
Agric Food Chem. 60:9863–9873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilmore TD: Introduction to NF-kappaB:
players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura T, Toita H, Yoshimoto A, et al:
Potential involvement of Twist2 and Erk in the regulation of
osteoblastogenesis by HB-EGF-EGFR signaling. Cell Struct Funct.
35:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|