Introduction
Glucose metabolism is an important component of
skeletal muscle metabolism. Approximately 70–85% of glucose is
utilized by skeletal muscle. Metabolic dysfunction of skeletal
muscle is associated with a number of human diseases. One of the
pathophysiological foundations of type 2 diabetes mellitus (T2DM)
is reduced glucose utilization by skeletal muscles. High
concentrations of insulin, and free fatty acid (FFA)- and palmitic
acid-induced skeletal muscle cell insulin resistance are commonly
used experimental models of insulin resistance (1–3).
In clinical practice, glucosamine is used to treat
bone and joint diseases. Long-term glucosamine treatment has been
shown to lead to insulin resistance in patients (4). Rats fed with oral glucosamine have
been used as an animal model of insulin resistance (5,6).
Glucosamine may also be used to induce insulin resistance in liver
cells (7). However, whether
glucosamine induces insulin resistance in skeletal muscle cells
remains elusive.
Numerous factors may also lead to reduced glucose
utilization in skeletal muscles. One such factor may be the
decrease in the expression and membrane translocation of glucose
transporter 4 (GLUT4), which are regulated by the 5′-adenosine
monophosphate-activated protein kinase (AMPK) and the
phosphoinositide 3-kinase (PI3K)/phosphatase and tensin homolog
(PTEN) pathways (8). The
PI3K/protein kinase B (Akt) pathway is involved in a variety of
biological activities, such as cell apoptosis and cell
proliferation (9,10). PTEN negatively regulates
intracellular levels of phosphatidylinositol-3,4,5-trisphosphate
(PIP3) and functions as a tumor suppressor by negatively regulating
AKT/PKB signaling (11,12). PTEN is also a downstream regulator
of AMPK (13). However, the roles
of the PI3K/PTEN pathway and AMPK in the development of insulin
resistance in skeletal muscle cells remain elusive.
In the present study, a glucosamine-induced skeletal
cell model of insulin resistance was established. The expression
and translocation of GLUT4, as well as the expression of PTEN and
phosphorylated PTEN (p-TEN) and cellular apoptosis were measured in
these cells. In order to better understand the role of PTEN in
insulin-resistant skeletal muscle cells,
bisperoxopicolinatooxovanadate (BPV), a PTEN inhibitor, and
metformin, an AMPK activator, were administered to the cells.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM, low
glucose) was obtained from GE Healthcare Life Sciences (Logan, UT,
USA), fetal bovine serum was obtained from TransGen, (Beijing,
China), Glucose Oxidase Assay kit was obtained from Shanghai
Rongsheng-biotech, (Shanghai, China), the Annexin V/Propidium
Iodide (PI) Detection kit was obtained from Beijing Biosea
Biotechnology Co., Ltd. (Beijing, China), the cell lysis buffer and
the bicinchoninic acid (BCA) kits were obtained from Beyotime
Institute of Biotechnology (Shanghai, China) and the rabbit
polyclonal anti-GLUT4 (cat. no. 21619) antibody was obtained from
Signalway Antibody, LLC (College Park, MD, USA).
Cell culture
Rat primary skeletal muscle cells (L6; American Type
Culture Collection, Manassas, VA, USA) were provided by Dr Yudong
Hou from China Medical University (Shenyang, China). Cells were
cultured in DMEM supplemented with 10% fetal bovine serum in a 5%
CO2, 37°C incubator.
Establishment of the insulin-resistant
skeletal muscle cell model
In order to establish the insulin-resistant skeletal
muscle cell model, skeletal muscle cells were seeded at
1.5×104 cells/well in 96-well plates. Cells were
maintained in DMEM with a high-glucose concentration (4.5 g/l)
supplemented with 10% (v:v) fetal bovine serum and antibiotics (100
U/ml penicillin and 100 μg/ml streptomycin; Gibco Life
Technologies, Carlsbad, CA, USA) as previously described. Briefly,
cells were divided into control and glucosamine (Beyotime Institute
of Biotechnology) groups. Various concentrations of glucosamine (9,
18, 27 or 36 mmol/l) were used. Each group contained six replicates
and cells were cultured for 12 h. Glucose concentrations were
detected with a glucose detection kit-Glucose Oxidase Method using
a microplate reader (ELx808IU; Bio-Tek Instruments, Winooski, VT,
USA). Glucose concentrations were also measured at 30 min
post-insulin administration (1×10−7 mol/l, Novo Nordisk,
China). The glucose uptake rate was calculated as follows:
Difference in glucose concentration prior to and following insulin
treatment / glucose concentration prior to insulin treatment.
Glucose measurement
The glucose concentration of the cell culture medium
was measured. Glucose concentrations were initially tested before
cells were cultured. Following cell adherence, cells were divided
into four groups, namely: Blank control, glucosamine (18 mmol/l),
metformin (8 mmol/l) and BPV (38 nmol/l). Cells were treated for 12
h and glucose concentrations were measured in each well. Cells were
then further treated with insulin (1×10−7 mol/l) for 0.5
h and glucose concentrations were measured again. Glucose
concentration was determined using a glucose oxidase (glucose
detection) kit.
Detection of cell apoptosis
The cell apoptosis rate was detected using an
apoptosis kit. Following trypsinization (Gibco Life Technologies),
500 μl cells (1×106/ml) were centrifuged at 300 ×
g at 4°C for 10 min. The supernatant was discarded and the pellet
was resuspended in 200 μl binding buffer from the apoptosis
detection kit, and 10 μl Annexin V was then added. Cells
were kept in the dark at 4°C for 30 min. Binding buffer (300
μl) and 5 μl PI reagent were added and the apoptotic
rate was measured by flow cytometry(FACS LSR II; BD Biosciences,
Franklin Lakes, NJ, USA).
Western blot analysis
Cell lysates were collected using a cell lysis
buffer (Beyotime Institute of Biotechnology) and protein was
quantified using the BCA kit. Protein was then separated by
SDS-PAGE (12% separation gel and 5% stacking gel; Seebio Biotech,
Inc., Shanghai, China) for 2 h 20 min. Samples were then damp-dry
transferred for 45 min onto a polyvinylidene difluoride membrane.
The membrane was blocked with 5% bovine serum albumin overnight.
Samples were washed with Tris-buffered saline with Tween-20 (TBST;
Seebio Biotech, Inc.) and membranes were incubated for 1 h with
rabbit polyclonal primary antibodies [p-PTEN (1:1,00; cat. no.
11062), PTEN (1:1,00; cat. no. 32606) (Signalway Antibody, LLC) and
β-actin (1:1,500; cat. no. 63508; ZSBIO, Beijing, China)]. The
membrane was then washed with TBST, following which horseradish
peroxidase-labeled secondary antibodies (1:10,000, cat. nos. 12465,
21150 and 42602) were added and incubated for 40 min. Samples were
washed with TBST and the membrane was developed with enhanced
chemiluminescence (Applygen Technologies, Inc., Beijing, China).
All primary antibodies were purchased from SAB Company (College
Park, MD, USA). Secondary antibodies were purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co, Ltd. (Beijing, China).
ImageJ 1.48 software (National Institutes of Health, Bethesda, MD,
USA) was used for gray scale value analysis.
Immunofluorescent staining and
fluorescence microscopy
For immunostaining, cells were seeded onto
coverslips in six-well plates at a concentration of
1×105 cells/well. Following attachment, cells were
treated according to the group they were in. At the end of each
treatment, cells were fixed with fixation buffer (Beyotime
Institute of Biotechnology) for 10 min and blocked with blocking
buffer at 4°C overnight. Cells were then sequentially incubated
with a primary antibody to GLUT4 (diluted at 1:1,000) and a
secondary antibody (fluorescein isothiocyanate-labeled goat
anti-rabbit immunoglobulin G (Beyotime Institute of Biotechnology).
Nuclei were stained with Hoechst 33342 (Beyotime Institute of
Biotechnology). In between these steps, cells were washed with
TBST. Coverslips were then removed from the plates, which were
mounted with mounting liquid (Beyotime Institute of Biotechnology).
Images were captured with a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). ImageJ software was used for gray scale
value analysis.
Statistical analysis
Statistical analysis was performed using analysis of
variance or the χ2 test. All data are presented as the
mean ± standard deviation. Statistical analyses were conducted
using GraphPad Prism 4.0 for Mac (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of glucosamine-induced
model of insulin-resistant skeletal muscle cells
Various concentrations of glucosamine (9, 18, 27 or
36 mmol/l) were used in order to investigate the efficiency of
particular doses in inducing insulin resistance in skeletal muscle
cells. Measurement of the glucose uptake rate showed that the 18
mmol/l dose of glucosamine produced the most marked induction of
insulin resistance. Therefore, 18 mmol/l of glucosamine was
selected as for use in subsequent experiments. The original
difference in glucose concentration between the control and
glucosamine groups was not significant (P>0.05). Following
treatment with insulin for 30 min, the concentration of glucose in
the control group was significantly decreased and the glucose
uptake rate was significantly increased (32.97±2.71 vs.
5.95±4.30%). The concentration of glucose in the medium following
culture was also significantly decreased (P<0.01; 3.75±0.07 vs.
5.60±0.17; Table I). In the
glucosamine group, the glucose concentrations were also decreased,
and the difference in glucose concentrations prior to and following
insulin stimulation was also statistically significant (P<0.05;
5.70±0.19 vs. 5.44±0.07; Table I).
These results suggested that an insulin-resistance model of
skeletal muscle cells was successfully established in the present
study.
 | Table IGlucose uptake rate following 12 h
culture and insulin stimulation. |
Table I
Glucose uptake rate following 12 h
culture and insulin stimulation.
| Treatment | Group
|
|---|
| Control | Glucosamine | Glucosamine
+metformin | Glucosamine +BPV |
|---|
| 0 h (n1)
(mmol/l) | 5.60±0.17 | 5.70±0.19 | 6.39±0.33 | 5.68±0.17 |
| 12 h (n2)
(mmol/l) | 5.33±0.09 | 5.90±0.09 | 5.55±0.13 | 5.30±0.19 |
| 30 min insulin (n3)
(mmol/l) | 3.75±0.07 | 5.44±0.07 | 3.98±0.57 | 3.53±0.14 |
| 12 h glucose uptake
rate (n1–n2)/n1 (%) | 4.74±3.97 | −1.95±5.00 | 13.00±2.71 | 9.82±0.69 |
| 30 min insulin
glucose uptake rate (n1–n3)/n1 (%) | 32.97±2.71 | 5.95±4.30 | 37.53±10.71 | 39.92±0.99 |
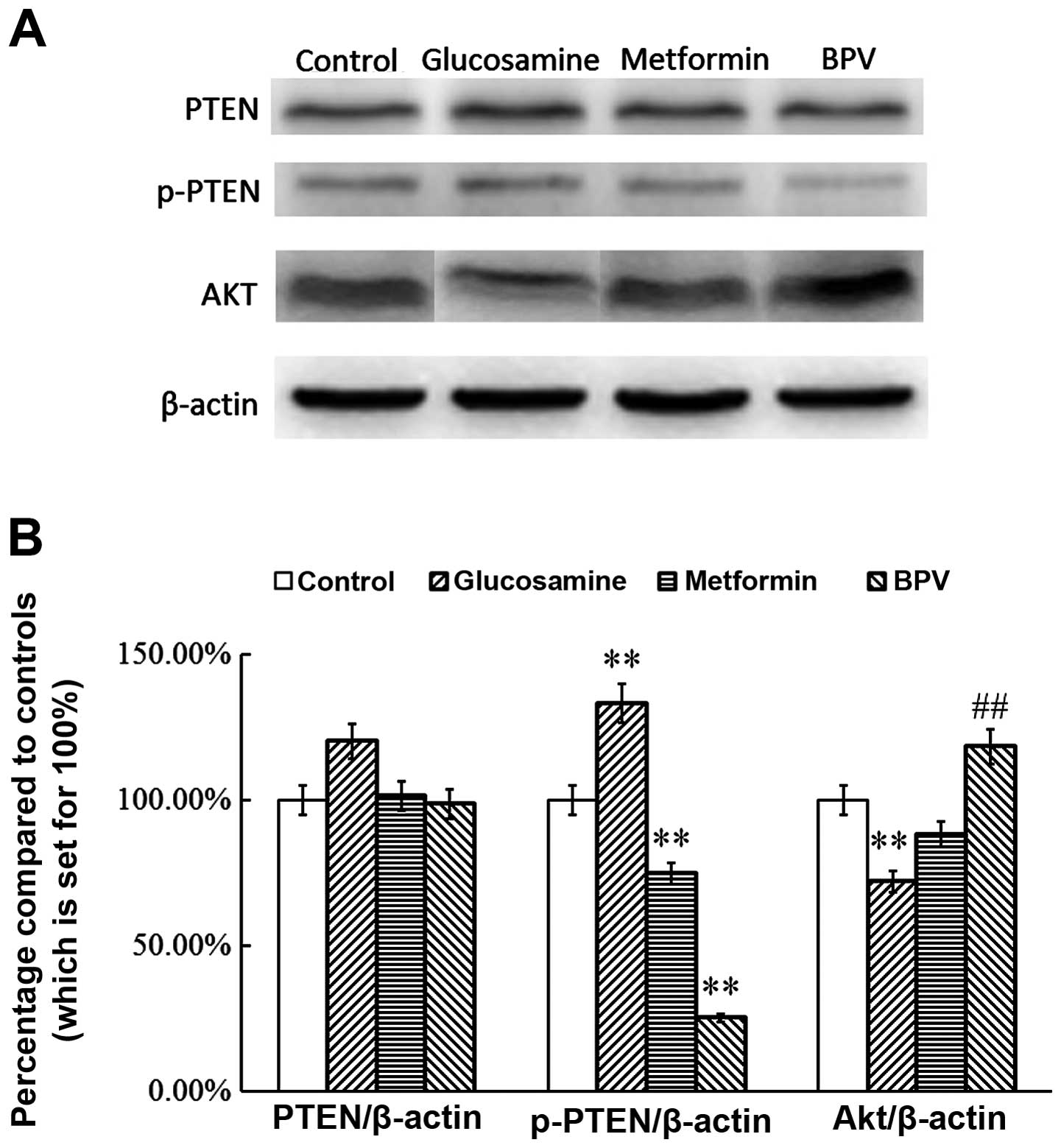
Metformin or BPV treatment inhibits the
activation of PTEN and regulates the expression of AKT in
insulin-resistant skeletal muscle cells
BPV is a known PTEN inhibitor and its effect on
C2C12 myoblast migration and differentiation has previously been
described (14). Metformin is an
AMPK inhibitor and is the most widely used anti-diabetic drug
worldwide. There is increasing evidence for the potential efficacy
of this agent as an anti-cancer drug (15). In order to confirm the effect of
inhibition of PTEN in an insulin-resistant model of skeletal muscle
cells, the effect of BPV and metformin on the activation of PTEN
was investigated. The results demonstrated that glucosamine
activates PTEN in insulin-resistant skeletal muscle cells
(P<0.01; Fig. 1). However, the
levels of p-PTEN in the metformin and BPV groups were significantly
lower than those in the control group (P<0.01). PTEN expression
was not affected by treatment with any of the agents used
(P>0.05; Fig. 1). The results
also showed that the expression of AKT was reduced in the
glucosamine group (P<0.01), and that this change was reversed by
treatment with metformin or BPV, suggesting that inhibition of PTEN
or its upstream regulator affect the expression of AKT (P<0.01;
Fig. 1). These results indicate
that metformin and BPV treatment inhibits the activation of PTEN in
insulin-resistant skeletal muscle cells.
Metformin and BPV reduce glucose uptake
in insulin-resistant skeletal muscle cells
In order to determine whether the inhibition of PTEN
and its upstream regulator reverses glucose uptake, BPV and
metformin were used to treat the insulin-resistant skeletal muscle
cells. Following treatment with insulin, the rate of glucose uptake
in the glucosamine group was significantly lower than that in the
control group (5.95±4.30 vs. 32.97±2.71; P<0.01). Metformin or
BPV treatment increased the glucose uptake rate in cells exhibiting
glucosamine-induced insulin-resistance (37.53±10.71 vs. 5.95±4.30;
and 39.92±0.99 vs. 5.95±4.30, respectively; P<0.05; Table I). Metformin or BPV treatment did
not affect the glucose uptake rate in the control group
(37.53±10.71 vs. 32.97±2.71; and 39.92±0.99 vs. 32.97±2.71,
respectively; P>0.05; Table
I).
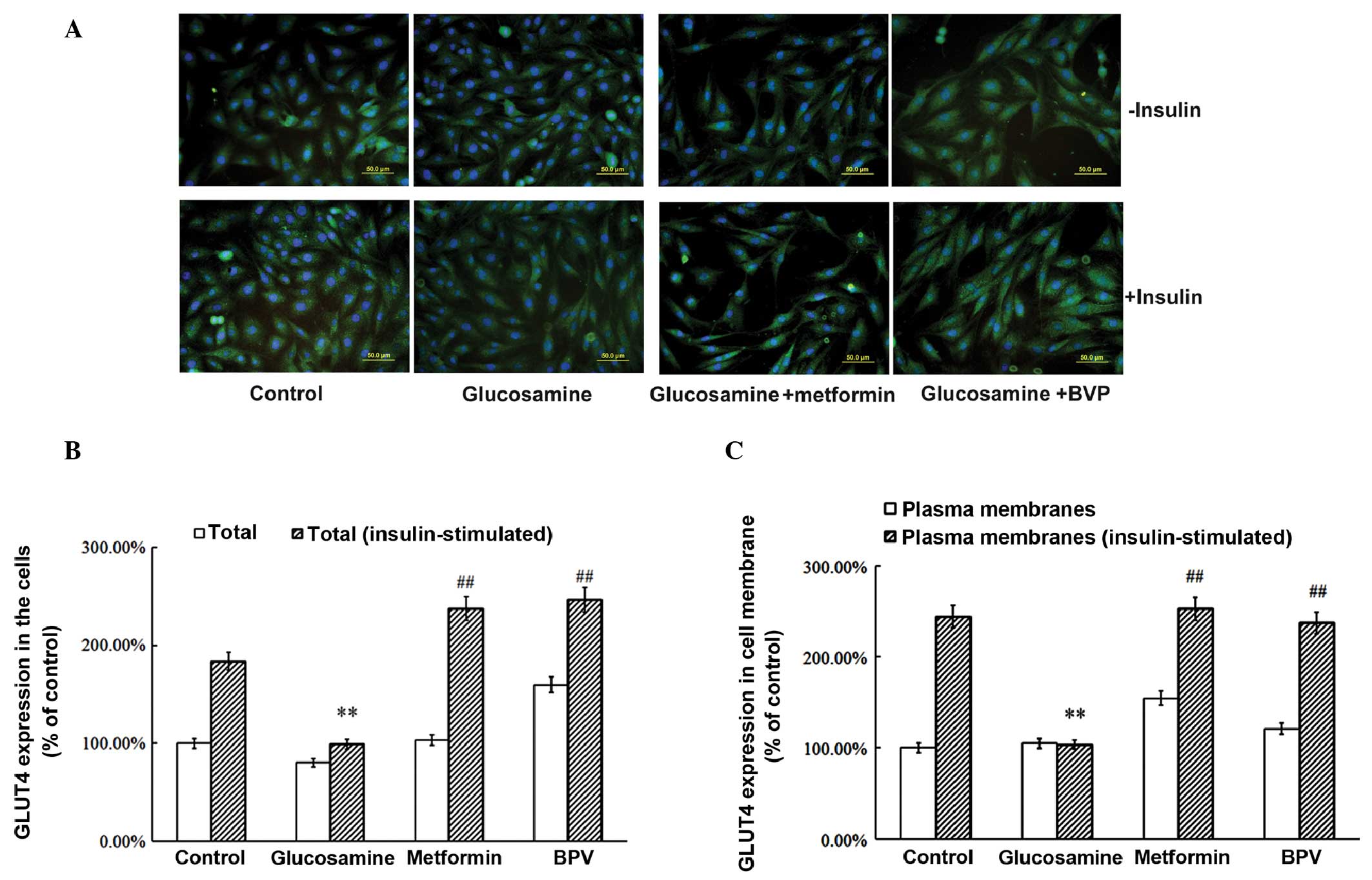
Metformin and BPV increase GLUT4
expression and translocation in insulin-resistant skeletal muscle
cells
GLUT4 is known to be important in regulating glucose
uptake. Therefore, the expression and translocation of GLUT4 were
measured in insulin-resistant skeletal muscle cells. Glucosamine
treatment was shown to significantly reduce the expression of GLUT4
compared with that in the control group (P<0.01; Fig. 2A and B). Metformin or BPV treatment
reversed this change in GLUT4 expression, which had been induced by
glucosamine (P<0.01; Fig. 2A and
B). In addition, the translocation of GLUT4 from the
intracellular membrane to the plasma membrane following insulin
stimulation was reduced by administration of glucosamine (Fig. 2A and C). By contrast, this
reduction in GLUT4 translocation was reversed by treatment with
metformin or BPV. These results suggested that the inhibition of
PTEN may reverse the reduction in expression and translocation of
GLUT4, which is induced by glucosamine.
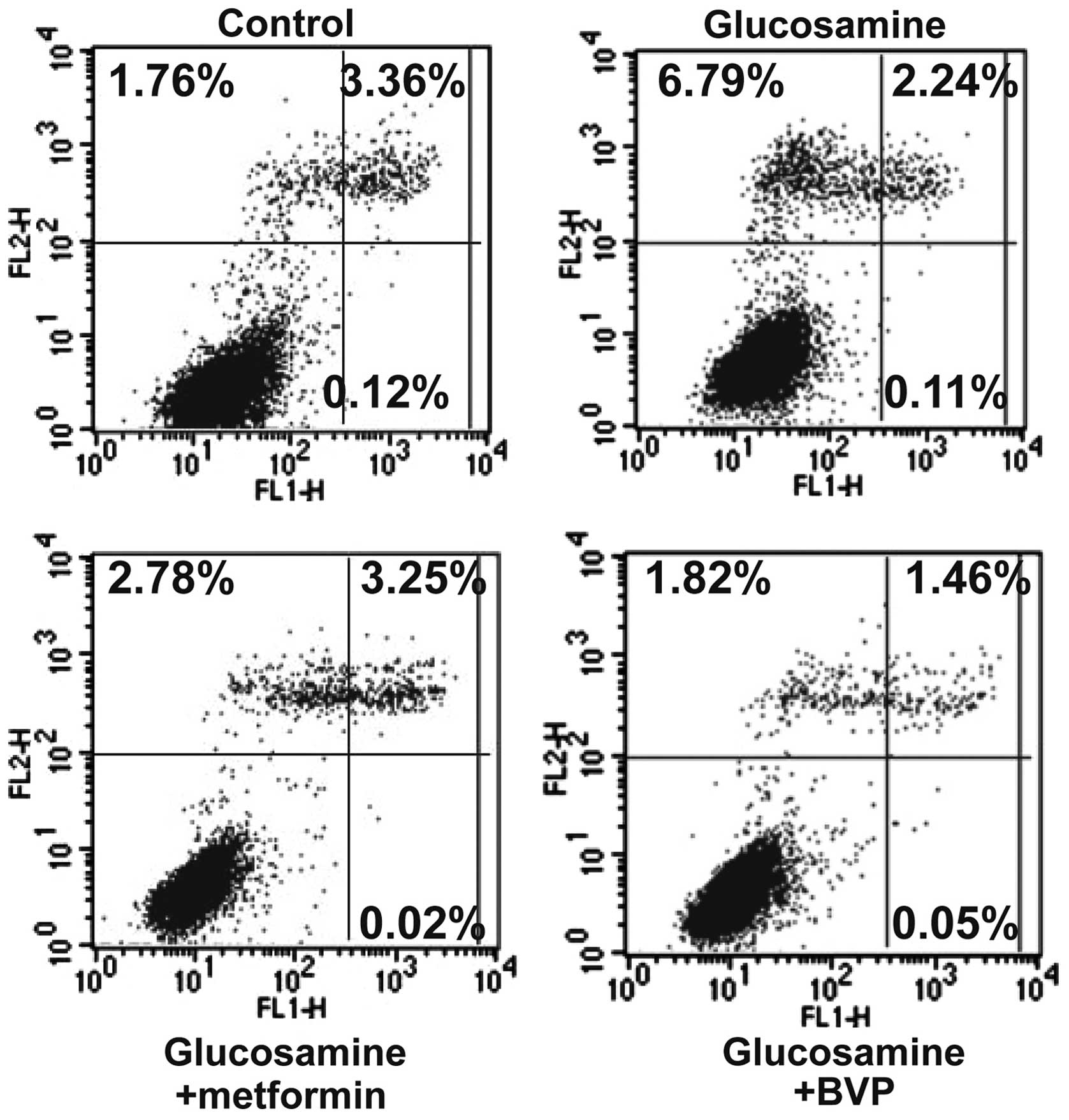
Metformin or BPV treatment reduces
glucosamine-induced cell apoptosis in insulin-resistant skeletal
muscle cells
Glucosamine administration led to a significant
increase in cell apoptosis (late apoptotic + dead cells; 5.24% vs.
9.14%, P<0.01; Fig. 3).
Metformin or BPV treatment reduced this glucosamine-induced cell
apoptosis to 6.05% and 3.33%, respectively (P<0.05 for
metformin, and P<0.01 for BPV; Fig.
3). These results suggested that PTEN inhibition protects the
cells against glucosamine-induced apoptosis.
Discussion
In the present study, a glucosamine-induced rat
model of insulin-resistance in primary skeletal muscle cells was
established. It was shown that the uptake of glucose and
insulin-stimulated GLUT4 translocation were significantly reduced
in the insulin resistance model compared with control cells. The
expression and translocation of GLUT4, and AKT expression were
reduced in skeletal muscle cells with glucosamine-induced insulin
resistance. In addition, the administration of BPV increased the
rate of glucose uptake and the expression and translocation of
GLUT4.
Within the skeletal muscle, cardiac muscle and
adipose tissue, GLUT4 functions as a transporter that conveys
glucose from the extracellular to the intracellular environment
(16). In insulin resistance,
GLUT4 activity and translocation are known to be reduced (17). Multiple cellular signal
transduction pathways regulate GLUT4 expression and translocation
(18). Among them, the PI3K/AKT
pathway is an important pathway for insulin signaling. The AKT
pathway promotes cell growth and proliferation, and stimulates
GLUT4 to translocate to the plasma membrane, thus promoting glucose
uptake (19,20). The results of the present study
suggested that the increased expression of PTEN may induce a
reduction in the expression and translocation of GLUT4 in
glucosamine-induced insulin-resistant skeletal muscle cells. This
finding is in accordance with the negative regulation of the
PI3K/AKT pathway by PTEN.
Furthermore, the administration of the AMPK agonist
metformin was shown to increase the rate of glucose uptake and of
GLUT4 expression and its translocation. In addition, the results
demonstrated that metformin increased the expression of AKT and
reduced the expression of PTEN and p-PTEN. The hypoglycemic
mechanism underlying the effects of metformin may also occur
through regulation of PTEN, which is a potential downstream
regulator of AMPK (13). The
results of the present study further support the hypothesis that
increased PTEN expression in insulin-resistant skeletal muscle
cells may contribute to reduced GLUT4 expression and impaired
translocation of this enzyme.
The apoptotic rate was shown to be increased in the
insulin-resistant skeletal muscle cells. In addition to promoting
glucose uptake and increasing the translocation and expression of
GLUT4, BPV and metformin also reduced cell apoptosis. A possible
explanation for the reversal of insulin resistance and reduction in
apoptosis induced by BPV and metformin may be that it is a result
of reducing PTEN and activating the PI3K/AKT pathway. A previous
study showed that overexpression of PTEN inhibits AKT activity and
induces apoptosis through the PI3K/AKT pathway (21).
In the present study, glucosamine induced insulin
resistance in rat skeletal muscle cells. Glucosamine was shown to
reduce the expression and translocation of GLUT4, to increase the
apoptotic rate as well as the expression of PTEN and p-PTEN, and to
decrease AKT expression. Metformin and BPV were shown to increase
glucose uptake and GLUT4 expression and translocation, which may
protect against apoptosis in glucosamine-induced insulin-resistant
skeletal muscle cells.
Acknowledgments
This study was supported by the Science and
Technology projects in Liaoning Province (grant nos. 2012225079 and
20102250002), the Science and Technology projects in Shenyang City
(grant no. F13-221-9-02) and the National Natural Science Funds
(grant no. 81100216). The authors would like to thank Dr Yifu Guan,
Dr Ying Liu, Dr Qiang Bi and Mr. Nanqi Liu of the Biochemistry
Department of China Medical University (Shenyang, China), for their
professional and technical assistance.
References
|
1
|
McCarthy AM, Spisak KO, Brozinick JT and
Elmendorf JS: Loss of cortical actin filaments in insulin-resistant
skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose
transport. Am J Physiol Cell Physiol. 291:C860–C868. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Habegger KM, Penque BA, Sealls W, Tackett
L, Bell LN, Blue EK, Gallagher PJ, Sturek M, Alloosh MA, Steinberg
HO, Considine RV and Elmendorf JS: Fat-induced membrane cholesterol
accrual provokes cortical filamentous actin destabilisation and
glucose transport dysfunction in skeletal muscle. Diabetologia.
55:457–467. 2012. View Article : Google Scholar
|
|
3
|
Rando TA and Blau HM: Primary mouse
myoblast purification, characterization and transplantation for
cell-mediated gene therapy. J Cell Biol. 125:1275–1287. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dostrovsky NR, Towheed TE, Hudson RW and
Anastassiades TP: The effect of glucosamine on glucose metabolism
in humans: a systematic review of the literature. Osteoarthritis
Cartilage. 19:375–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chien CS, Cheng SC, Wu HT, Tsao CW and
Cheng JT: Insulin resistance induced by glucosamine in fructose-fed
rats. Horm Metab Res. 41:542–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang L, Chen CH, Cheng YC, Chang CH, Lee
CT, Chang JK, Cheng JT and Chang FM: Glucosamine-induced insulin
resistance in ovariectomized rats is relevant to decreasing the
expression of glucose transport protein subtype 4 in the skeletal
muscle and in increasing the size of pancreatic islets. Menopause.
19:496–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai K and Clemmons DR: Glucosamine
induces resistance to insulin-like growth factor I (IGF-I) and
insulin in Hep G2 cell cultures: biological significance of
IGF-I/insulin hybrid receptors. Endocrinology. 144:2388–2395. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JO, Lee SK, Kim JH, Kim N, You GY,
Moon JW, Kim SJ, Park SH and Kim HS: Metformin regulates glucose
transporter 4 (GLUT4) translocation through AMP-activated protein
kinase (AMPK)-Mediated Cbl/CAP Signaling in 3T3-L1
Preadipocytecells. J Biol Chem. 287:44121–44129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meshkani R and Adeli K: Hepatic insulin
resistance, metabolic syndrome and cardiovascular disease. Clin
Biochem. 42:1331–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joshi A and Ellenson LH: Adenovirus
mediated homozygous endometrial epithelial Pten deletion results in
aggressive endometrial carcinoma. Exp Cell Res. 317:1580–1589.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nyåkern M, Tazzari PL, Finelli C, Bosi C,
Follo MY, Grafone T, Piccaluga PP, Martinelli G, Cocco L and
Martelli AM: Frequent elevation of Akt kinase phosphorylation in
blood marrow and peripheral blood mononuclear cells from high-risk
myelodysplastic syndrome patients. Leukemia. 20:230–238. 2006.
View Article : Google Scholar
|
|
13
|
Kim SA and Choi HC: Metformin inhibits
inflammatory response via AMPK-PTEN pathway in vascular smooth
muscle cells. Biochem Biophys Res Commun. 425:866–872. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimchev GA, Al-Shanti N and Stewart CE:
Phospho-tyrosine phosphatase inhibitor Bpv (Hopic) enhances C2C12
myoblast migration in vitro. Requirement of PI3K/AKT and MAPK/ERK
pathways. J Muscle Res Cell Motil. 34:125–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Takeda T, Tsuiji K, Kondo A,
Kitamura M, Wong TF and Yaegashi N: The antidiabetic drug metformin
inhibits uterine leiomyoma cell proliferation via an AMP-activated
protein kinase signaling pathway. Gynecol Endocrinol. 29:87–90.
2013. View Article : Google Scholar
|
|
16
|
Zorzano A, Fandos C and Palacin M: Role of
plasma membrane transporters in muscle metabolism. Biochem J.
349:667–688. 2000.PubMed/NCBI
|
|
17
|
Alkhateeb H, Chabowski C, Glatz JFC,
Luiken JFA and Bonen A: Two phases of palmitate-induced insulin
resistance in skeletal muscle: impaired GLUT4 translocation is
followed by a reduced GLUT4 intrinsic activity. Am J Physiol
Endocrinol Metab. 293:E783–E793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zierath JR, Krook A and
Wallberg-Henriksson H: Insulin action in skeletal muscle from
patients with NIDDM. Mol Cell Biochem. 182:153–160. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu W, Zhao L, Peng X, Yang X, Ying C, Hao
L and Sun X: Biphasic effects of chronic ethanol exposure on
insulin-stimulated glucose uptake in primary cultured rat skeletal
muscle cells: role of the Akt pathway and GLUT4. Diabetes Metab Res
Rev. 47–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou QL, Jiang Z, Mabardy AS, Del Campo
CM, Lambright DG, Holik J, Fogarty KE, Straubhaar J, Nicoloro S,
Chawla A and Czech MP: A novel pleckstrin homology
domain-containing protein enhances insulin-stimulated Akt
phosphorylation and GLUT4 translocation in adipocytes. J Biol Chem.
285:27581–27589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugimoto N, Miwa S, Ohno-Shosaku T,
Tsuchiya H, Hitomi Y, Nakamura H, Tomita K, Yachie A and Koizumi S:
Activation of tumor suppressor protein PTEN and induction of
apoptosis are involved in cAMP-mediated inhibition of cell number
in B92 glial cells. Neurosci Lett. 497:55–59. 2011. View Article : Google Scholar : PubMed/NCBI
|