Introduction
Gastric cancer is one of the most common types of
cancer globally and accounted for 989,600 ovel cases and 738,000
cancer-associated fatalities in 2008 (1). >70% of novel gastric cancer cases
and fatalities occur in the developing countries, while they have
declined in the majority of developed countries, including
countries in North America and Europe in previous decades (1). The risk factors for gastric cancer
include Helicobacter (H.) pylori infection,
history of tobacco smoking, and consumption of smoked foods, salted
meat or fish and pickled vegetables. However, intake of fresh
fruits and vegetables appears to lower the risk of gastric cancer
(1). Regional variations in
gastric cancer prevalence reflect the differences in dietary
patterns and the prevalence of H. pylori infection (2). H. pylori is an aerobic
Gram-negative bacterium found in the stomach, which causes chronic
gastritis, peptic ulcers and gastric cancer. Thus, H. pylori
infection has been classified as a class I carcinogen for gastric
cancer and gastric mucosa-associated lymphoid tissue lymphoma
(3). Lipopolysaccharide (LPS) is
the main component of Gram-negative bacterial endotoxin and elicits
marked immune responses in the host by binding to the
CD14/Toll-like receptor (TLR)4/MD2 receptor complex, which promotes
the secretion of pro-inflammatory cytokines in numerous cell types.
Of note, CD14 is a high-affinity receptor for LPS and recognizes
Gram-negative bacteria, fungi, Mycobacterium tuberculosis
and Treponema pallidum, mediating a series of inflammatory
responses in the human body (4,5).
CD14 is mainly expressed in macrophages and in
neutro-phil granulocytes. Upon H. pylori infection, LPS may
bind to CD14 and activate the immune defense system in the human
body and induce the production of cytokines to trigger inflammation
(6). Furthermore, CD14 has also
been reported to be abnormally expressed in different cancer
tissues and cells (7,8). However, it remains to be defined how
altered CD14 expression induces gastric cancer development
following H. pylori infection. Thus, in the present study,
the effects of knockdown of CD14 in the regulation of gastric
cancer cell viability, apoptosis and the inflammatory response
induced by LPS were investigated, as well as the underlying
mechanism in vitro and in nude mouse gastric cancer cell
xenografts. The present study provided novel insight regarding the
mediation of H. pylori infection via CD14 in the induction
of gastric cancer in humans.
Materials and methods
Cell lines and culture
The MGC-803 human gastric cancer cell line was
obtained from the Type Culture Collection Center of Chinese Academy
of Science (Shanghai, China) and the sh-CD14 stable CD14-knockdown
MGC-803 cell subline was constructed in our previous study
(9). The cells were stored in the
laboratory and cultured with RPMI-1640 medium supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA,
USA), 100 U/ml penicillin and 100 mg/l streptomycin (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) in a
humidified incubator with 5% CO2 at 37°C. The cells were
passaged using 0.05% trypsin-EDTA (Beyotime Institute of
Biotechnology, Haimen, China) every 2–3 days and the cells at the
logarithmic phase were used in the present study. The cells were
treated with 1 μg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA)
for 4 h and harvested for in vitro study, and treated with
10 μg/ml LPS for 16 h for the tumor xenograft assay. Cells
were divided into the following groups: Wild-type (WT) cells
(MGC-803 cells without any interference), sh-CD14 cells (MGC-803
cells subjected to stable CD14-knockdown), WT + LPS cells (MGC-803
cells treated with 1 μg/ml LPS for 4 h or 10 μg/ml
LPS for 16 h) and the LPS + sh-CD14 group (MGC-803 cells subjected
to stable CD14-knockdown treated with 1 μg/ml LPS for 4 h or
10 μg/ml LPS for 16 h). The present study was approved by
the Institutional Review Board of the People's Hospital of Tibet
Autonomous Region (Tibet, China).
Cell counting kit-8 (CCK-8) assay
To assess the cell viability, a CCK-8 assay was
performed. Briefly, the cells were detached from cell culture
dishes, counted and diluted to a concentration of 5×104
cells/ml in a single-cell suspension and then seeded into a 96-well
plate at 5.0×103 cells/well. The cells were cultured and
then treated as stated above, dependent on their assigned group. At
the end of each experiment, 10 μl CCK-8 reagent (Beyotime
Institute of Biotechnology) was added into each well and incubated
at 37°C for 1 h, and the optical density was assessed using a
microplate reader (Microplate Autoreader EL311; Bio-Tek
Instruments, Inc., Winooski, VT, USA) at 450 nm. The experiments
were performed in duplicates of five wells for each group and
repeated three times.
Nude mouse gastric cancer cell xenograft
assay
Nude mice were purchased from the Experimental
Animal Center, China Medical University (Shenyang, China). Each
group of cells was detached from the cell culture dishes, counted
and adjusted to a concentration of 2×107 cells/ml, then
0.2 ml cell suspension was injected into the axilla of each nude
mouse. The tumor formation was recorded by measuring the maximum
vertical diameters of the length (a) and width (b) every three
days. The tumor volume was calculated using the following formula:
V (mm3) = 0.5×a×b2. The growth curve of the
tumor was plotted. After four weeks, the nude mice were sacrificed
by intraperitoneal injection of 10% chloral hydrate (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China), and the final volume
and weight of the tumor xenografts were recorded. One half of each
of the xenograft tumors was fixed in 4% paraformaldehyde, embedded
in paraffin and sectioned into 5-μm tissue sections for
hematoxylin and eosin (H&E) staining (Beijing Solarbio Science
& Technology Co., Ltd.). The remaining half of the tumor was
immediately frozen in liquid nitrogen and stored at −80°C until
further use.
Flow cytometric cell apoptosis
assays
To detect changes in the levels of cell apoptosis
in vitro, the Annexin V/propidium iodide (AV/PI) apoptosis
detection kit (KeyGen Biotech, Nanjing, China) was used according
to the manufacturer's instructions. Briefly, the cells were
centrifuged at 1,000 x g for 5 min and the supernatant was
discarded. Subsequently, 500 μl binding buffer was added
into the tube to re-suspend the cells and 5 μl Annexin
V-fluorescein isothiocyanate and propidium iodide were added. The
cells were then incubated at room temperature in the dark for 10
min and subjected to flow cytometry (FACSCalibur; BD Biosciences,
Mountain View, CA, USA) at a wavelength of 488 nm and an output
power of 100 mW. The data were analyzed using the software included
in the machine package (Cell Quest; BD Biosciences).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated using TRIzol reagent
(Tiangen Biotech, Beijing, China) according to the manufacturer's
instructions and reverse transcribed into cDNA using a TianScript
cDNA first strand cDNA synthesis kit (Tiangen Biotech) according to
the manufacturer's instructions. The reaction mix consisted of 1
μg total RNA, 1 μl oligo (dT)15 and random
primer, 2 μl deoxyribunucleotide triphosphate and 2
μl double distilled (dd)H2O with a total volume
of 14.5 μl. Following denaturation at 70°C for 5 min, the
mixture was placed on ice immediately. Following a short vortex
spin, 0.5 μl RNasin (Tiangen Biotech) and 1 μl
Moloney murine leukemia virus reverse transcriptase (Bioteke
Corporation, Beijing, China) were added into the mixture for
incubation at 42°C for 50 min. To terminate the reaction, the
reaction mixture was heated at 95°C for 5 min. The primers used for
RT-PCR are shown in Table I. The
PCR amplification was performed with a 1 μl DNA template, 1
μl of each primer, 10 μl 2X Taq PCR master-mix
(Tiangen Biotech) and 8 μl ddH2O. The PCR cycles
were set at an initial 95°C for 5 min and 30 cycles of 95°C for 20
sec, 58°C for 20 sec and 72°C for 30 sec, followed by a final step
at 72°C for 5 min. The PCR products were separated by
electrophoresis on 1% agarose gels (Biowest, Madrid, Spain), images
were captured and levels of β-actin expression were used as the
internal control for gray scale analysis (Gel Image System version
4.0; Tanon Science & Technology, Shanghai, China).
 | Table IPrimers used for reverse
transcription-polymerase chain reaction. |
Table I
Primers used for reverse
transcription-polymerase chain reaction.
| Gene | Sequences | Size (bp) |
|---|
| TNF-α |
5′-GTCTCCTACCAGACCAAGGTCAAC-3′
5′-CACAGGGCAATGATCCCAAAGTAG-3′ | 221 |
| IL-1β |
5′-CCTGGACTTTCCTGTTGTCTACACC-3′
5′-TCTGTCAGGCGGGCTTTAAGTGAG-3′ | 178 |
| IL-6 |
5′-TCACCTCTTCAGAACGAATTGACA-3′
5′-AGTGCCTCTTTGCTGCTTTCACAC-3′ | 115 |
| IL-12 |
5′-AGATGGTATCACCTGGACCTTGGAC-3′
5′-ATGGCTTAGAACCTCGCCTCCTTTG-3′ | 133 |
| hBD-2 |
5′-CCTCTTCATATTCCTGATGCCTCT-3′
5′-GGTGCCAATTTGTTTATACCTTCTAG-3′ | 127 |
| TLR4 |
5′-CTTTAGACCTGTCCCTGAACC-3′
5′-CCAGAACCAAACGATGGACTT-3′ | 162 |
| hβ-actin |
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′
5′-CTGTCACCTTCACCGTTCCAGTTT-3′ | 156 |
Protein extraction and western blot
analysis
Total cellular protein was extracted with a
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and the concentration of these protein samples was
quantified using the bicinchoninic acid method (Beyotime Institute
of Biotechnology). Protein lysates with 40 μg protein were
separated by SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Millipore, Boston, MA, USA). Following
blocking with 5% fat-free milk for 1 h, the membranes were
incubated with diluted primary antibodies at 4°C overnight. Rabbit
polyclonal anti-human β-defensin 2 (hBD-2; cat. no. sc-20798) was
used at a dilution of 1:2,000, rabbit polyclonal anti-TLR4 (cat.
no. sc-10741) was used at 1:100 (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit poly-clonal anti-tumor necrosis factor-α
(TNF-α; cat. no. bs-0078R) was used at 1:500, rabbit polyclonal
anti-interleukin (IL)-1β (cat. no. bs-0812R) was used at 1:800,
rabbit polyclonal anti-IL-6 (cat. no. bs-0379R) was used at 1:800
and rabbit polyclonal IL-12 (cat. no. bs-10641R) was used at 1:500
(Bioss, Beijing, China). Following an overnight incubation, the
membranes were washed with Tris-buffered saline-Tween 20 (TBS-T;
Beijing Solarbio Science & Technology Co., Ltd.) three times
and then further incubated with a horseradish peroxidase-labeled
goat anti-rabbit secondary antibody (cat. no. A0208; Beyotime
Institute of Biotechnology) at a dilution of 1:1,000 for 1 h. The
membranes were then washed again with TBS-T and incubated with
enhanced chemiluminescence solution (Pierce Biotechnology, Inc.,
Rockford, IL, USA) for up to 5 min to develop a color reaction in
the dark against an X-ray film (Fujifilm, Shanghai, China). The
antibody on the membrane was stripped using stripping buffer
(Beyotime Institute of Biotechnology) and the membranes were
re-blotted with rabbit polyclonal anti-β-actin antibody (1:1,000
dilution; cat. no. WL0001; Wanleibio, Shenyang, China) as an
internal antibody using the same procedure.
Statistical analysis
Values are presented as the mean ± standard error of
the mean and Student's t-test was performed to compare the data
between the experimental group and the controls. P<0.05 was
considered to indicate a statistically significant difference. SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA) was used to analyze
the data.
Results
Effects of CD14 knockdown on regulation
of gastric cancer cell viability
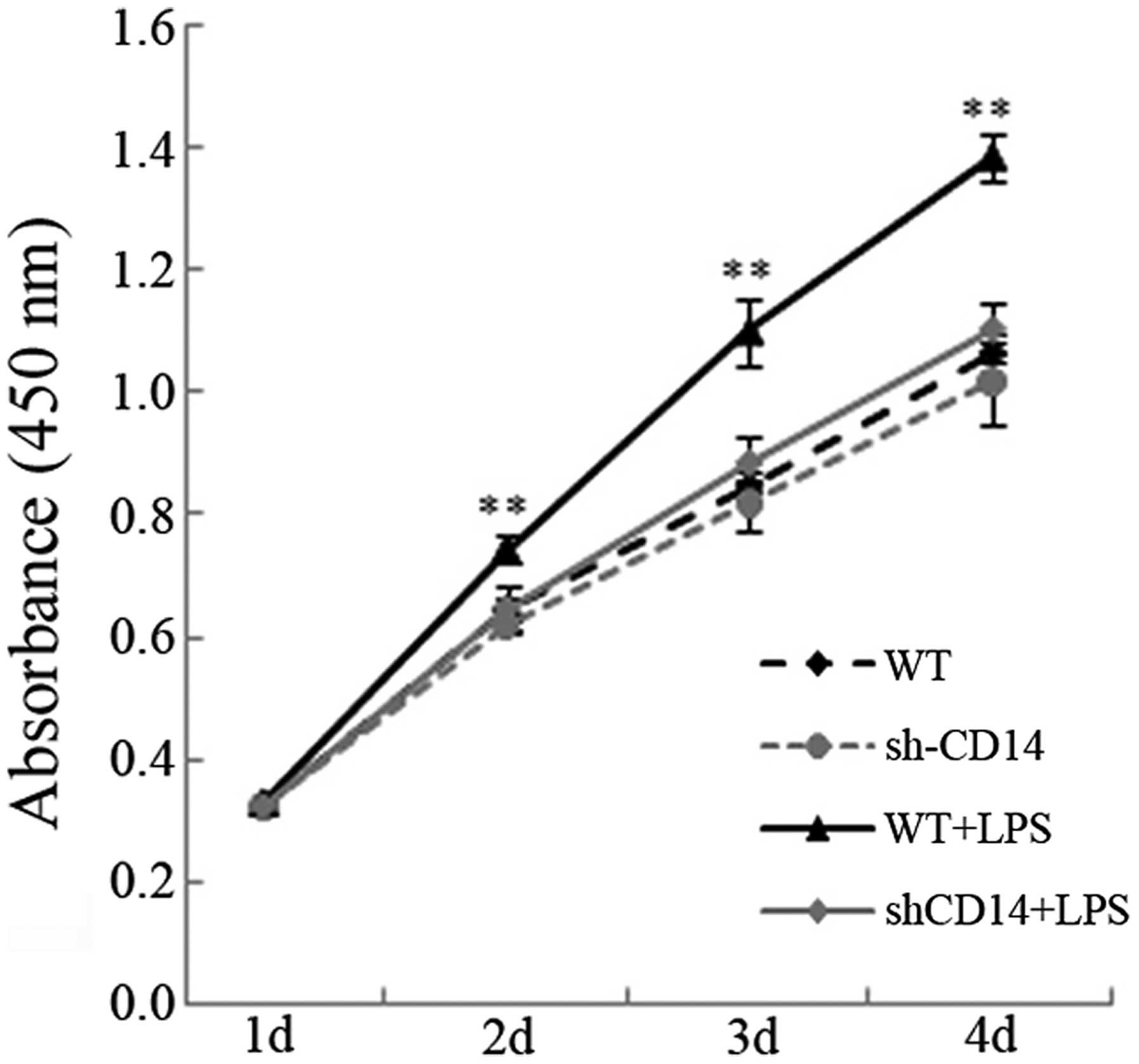
In the present study, the effects of CD14 knockdown
were initially assessed with regard to the regulation of gastric
cancer cell viability. As compared with the WT group, the cell
viability of the LPS cells was significantly increased (P<0.05),
whereas viability of the stable CD14-knockdown cells exhibited a
slight increase in viability (P<0.05), even following LPS
treatment (P>0.05; Fig. 1). The
results suggested that CD14 is important in preserving cell
viability following treatment with LPS.
Effects of CD14 knockdown on regulation
of gastric cancer cell xenograft tumor growth
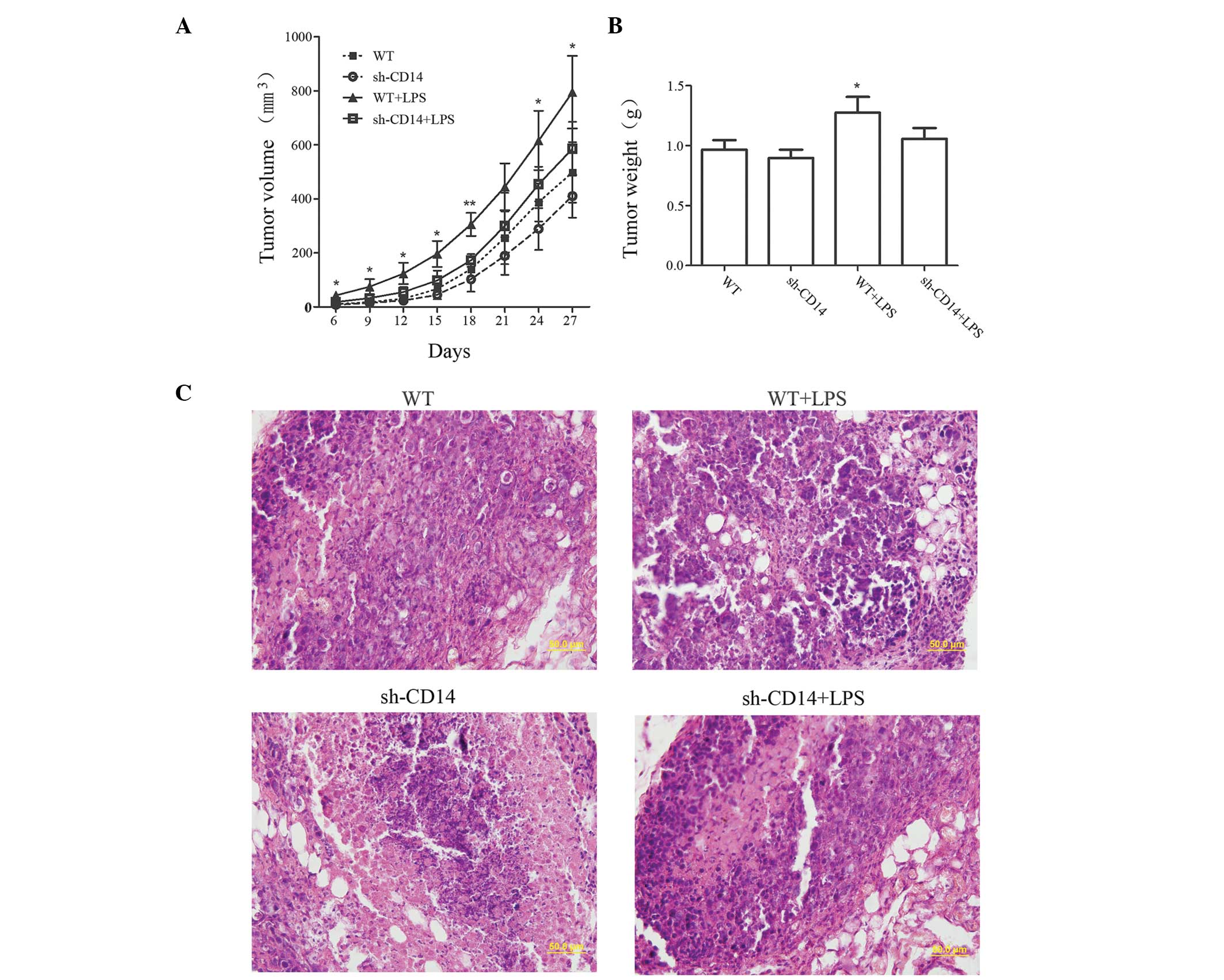
The volume of tumors was measured to examine the
effect of CD14 knockdown on the growth of tumor xenografts
(Fig. 2A). Compared with the WT
group, no significant difference was identified in the tumor volume
in the sh-CD14 group (P>0.05). By contrast, the tumor volume was
markedly increased in the LPS group. However, from day 21, the
growth of the tumors gradually slowed down in the sh-CD14 + LPS
group, while tumors remained slightly larger than in the WT group,
although no statistically significant difference was identified
(P>0.05). On day 27 of the experiment, the tumor xenograft
tissues were collected and weighed (Fig. 2B). The results revealed that the
tumor mass in the WT + LPS group was significantly higher than that
in the WT group (P<0.05). However, no statistically significant
differences were identified between the WT group and the sh-CD14
and sh-CD14 + LPS groups (P>0.05 for the two groups). H&E
staining revealed that the nucleoplasm was larger in the WT + LPS
group, exhibiting active cell division and proliferation (Fig. 2C). However, tumor xenograft
formation and growth of the sh-CD14 and sh-CD14 + LPS groups were
similar, but obviously different from those in the WT group,
indicating that LPS may promote the growth of tumor xenografts;
however, CD14 had a non-negligible role in mediating LPS signal
transduction.
Effects of CD14 knockdown on regulation
of cell apoptosis in gastric cancer cell xenograft tumors
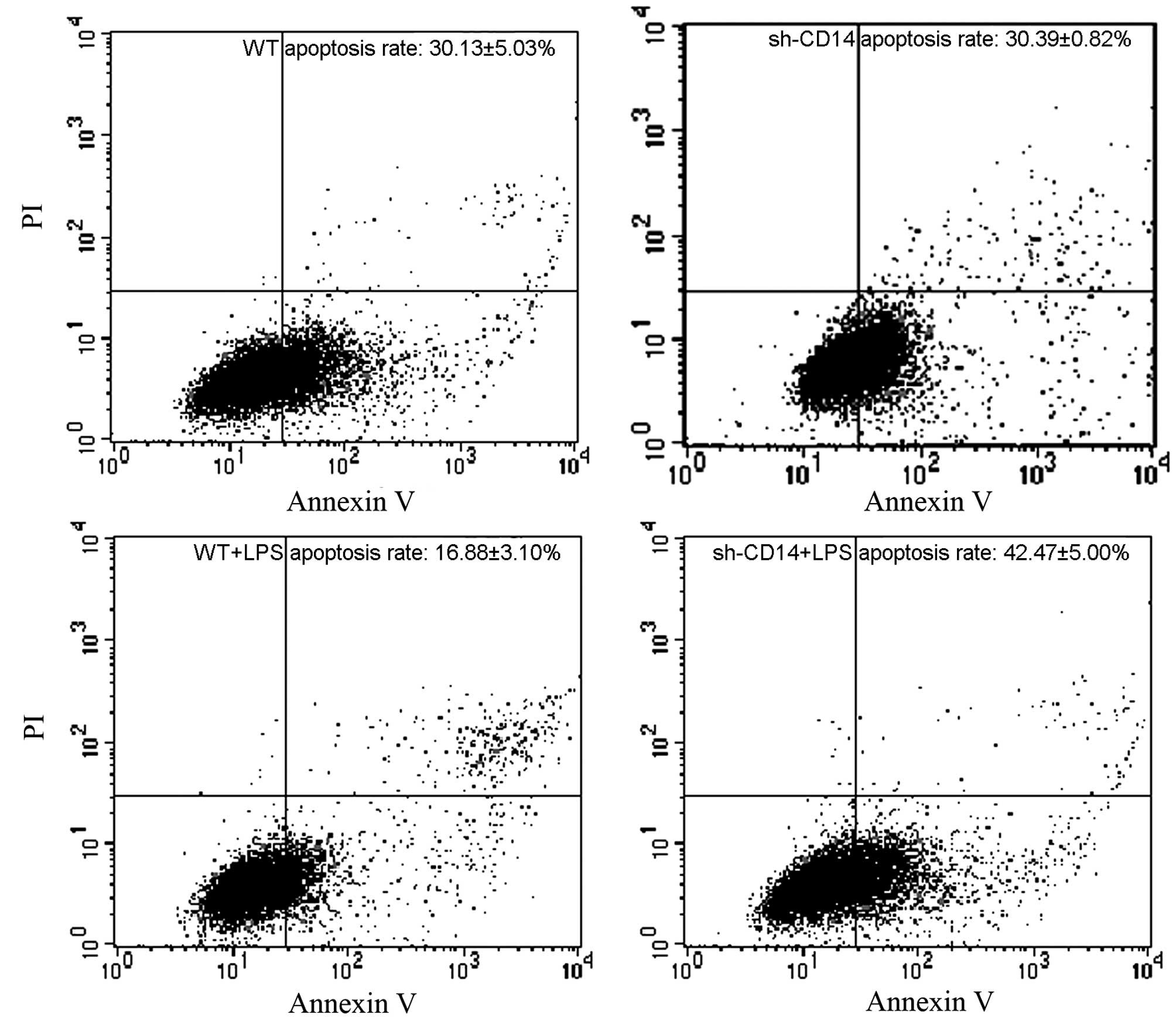
Following Annexin V/propidium iodide staining, the
apoptotic rate in each group was determined using flow cytometry
(Fig. 3). Compared with the
control group (30.13±5.03%), no signifi-cant differences were
identified in the apoptotic rate in the sh-CD14 group
(30.39±0.82%); however, the rate was markedly reduced in the LPS
group (16.88±3.10%). In contrast to that in the WT + LPS group, the
apoptotic rate was increased in the sh-CD14 + LPS group
(42.47±5.00%), but it remained lower than that in the control
group, suggesting that LPS was able to inhibit the apoptosis of
gastric cancer cells, and in addition, CD14 was important in the
process of apoptosis.
Effects of CD14 knockdown on regulation
of inflammatory gene expression in gastric cancer cell
xenografts
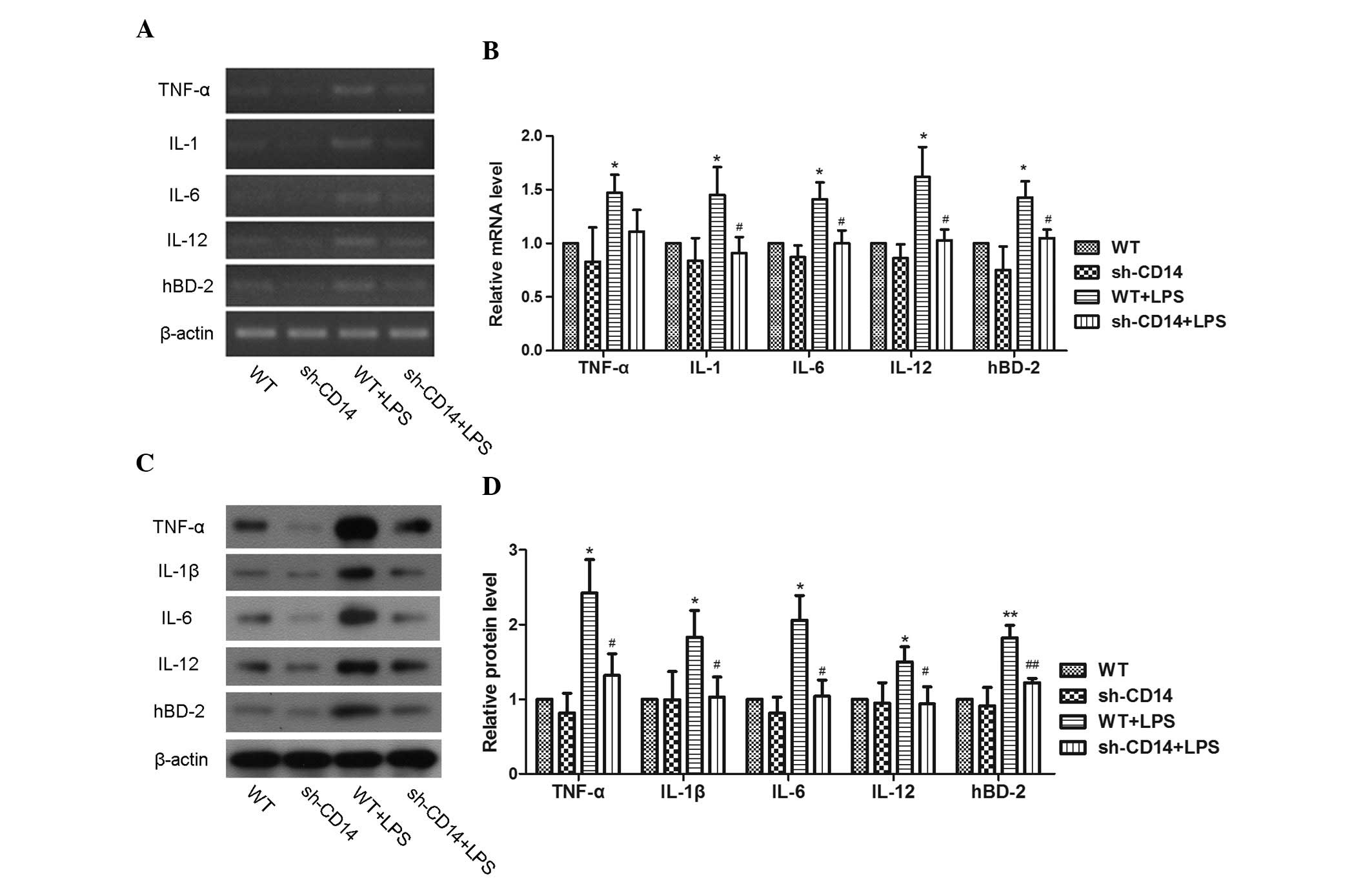
The effects of CD14 knockdown on the regulation of
inflammatory gene expression in gastric cancer cell xenografts were
assessed. The expression of TNF-α, IL-1β, IL-6, IL-12 and hBD-2
mRNA in each group is shown in Fig.
4. Compared with the WT group, the sh-CD14 group exhibited a
decrease in gene expression, but the difference was not
statistically significant (P>0.05). The expression of these
genes was induced following LPS treatment in the LPS group
(P<0.05). Compared with the LPS group, there was a decrease in
the expression of these genes in the sh-CD14 + LPS group
(P<0.05). The levels of protein expression were in accordance
with those of mRNA expression (Fig.
4).
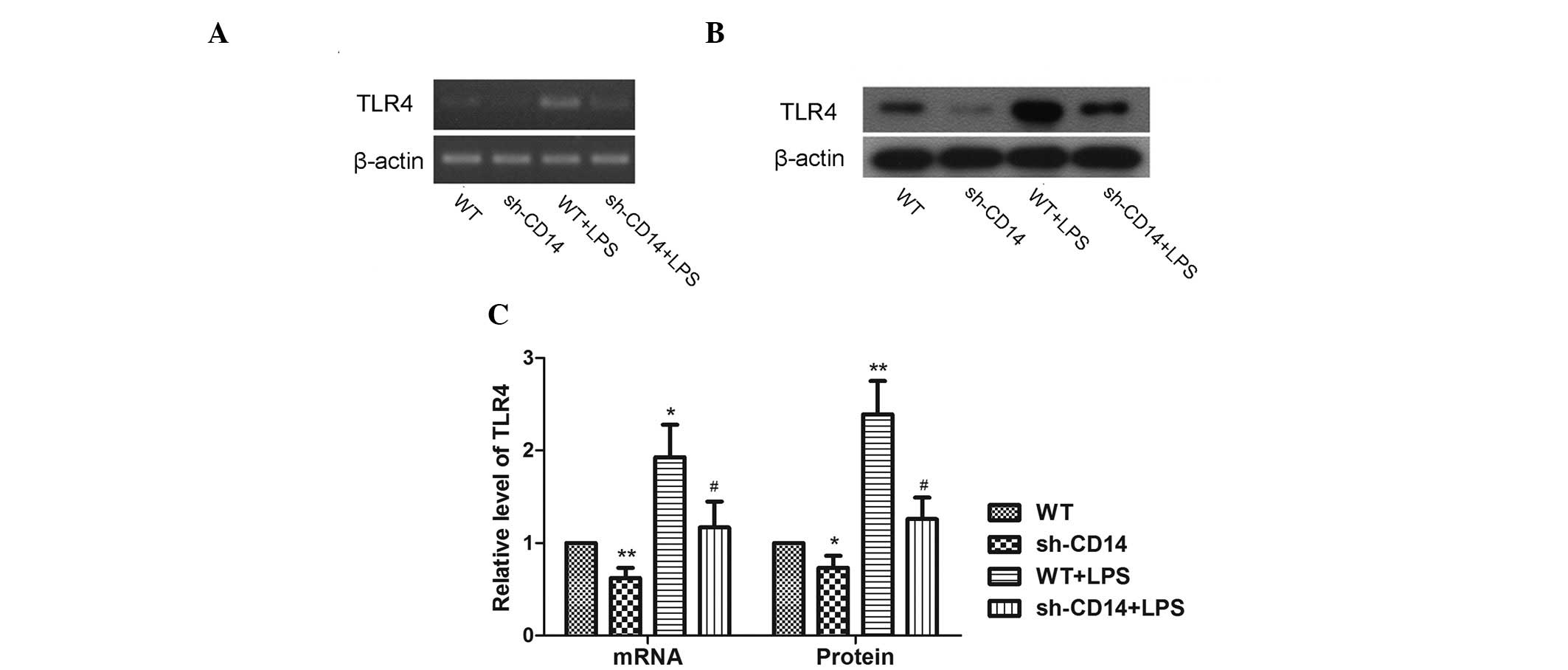
Effect of CD14 knockdown on expression of
TLR4
As CD14 binds with TLR4 to form the LPS receptor,
the TLR4 expression in the gastric cancer cell xenograft tumors was
assessed. Compared with the WT group, the expression of TLR4 mRNA
was significantly lower in the sh-CD14 group (P<0.05).
Additionally, compared with the LPS group, the expression of TLR4
mRNA was lower in the sh-CD14 + LPS group (P<0.05; Fig. 5A). The expression levels of TLR4
protein were in accordance with those of mRNA expression (Fig. 5B).
Discussion
In the present study, the effects of LPS and CD14 on
the regulation of gastric cancer cell viability, apoptosis and the
expression of inflammation-associated genes were assessed in
vitro as well as in vivo in nude mouse xenografts. The
data revealed that LPS induced gastric cancer cell proliferation,
but inhibited apoptosis and significantly increased the secretion
of TNF-α, IL-1β, IL-6, IL-12 and hBD-2 proteins and mRNA. Knockdown
of CD14 expression had no marked effect on the rate of
proliferation, apoptotic levels and expression of
inflammation-associated genes in gastric cancer cells and the
xenografts. However, in the presence of LPS, knockdown of CD14
expression markedly reduced the expression of the inflammatory
cytokines and hBD-2. These data demonstrated that LPS-promoted
growth of gastric cancer cells and tumor xenografts occurred
through CD14 expression and the associated signaling pathway.
H. pylori is a Gram-negative bacterium, which
is able to selectively infect gastric epithelial cells. It is
estimated that half of the world population is infected with H.
pylori (10,11). >80% of individuals infected with
H. pylori do not exhibit any symptoms; however, a fraction
of them do suffer from acute and chronic gastritis and peptic
ulcers, and may eventually develop gastric cancer (12). A growing number of lines of
evidence have suggested that H. pylori is able to adapt to
the local acidic environment and colonize deep in the mucus layer
of the gastric epithelium, resulting in chronic gastritis. This
host-bacterium interaction may yield persistent chronic infection
of H. pylori in the stomach and cause harm to the host
(13). Previous studies have
demonstrated that there is a high expression of CD14 and TLR4 in
tissues infected with H. pylori, particularly in gastric
cancer tissues (14,15). Another study reported that a
functional polymorphism of the CD14 promoter was able to
affect the expression of CD14 and increase the risk of gastric
cancer (16), suggesting that the
CD14 protein is important in the transduction of inflame matory
signaling and may have an impact on the outcome of the H.
pylori infection. The in vitro data from the current
study revealed that gastric cancer cell viability was increased
following LPS treatment. The knockdown of CD14 expression inhibited
LPS treatment-induced gastric cancer cell viability, indicating
that CD14 is important in mediating the effects of LPS in gastric
cancer cells. CD14 is important in mediating signaling transmission
between bacteria and the host. Of note, Grandel et al
(17) used LPS to treat A549
non-small cell lung cancer cells and observed that LPS induced
tumor cell viability in a time- and dose-dependent manner. However,
this effect was inhibited by CD14 and TLR4-neutralizing antibodies.
Following stimulation with LPS, gastric cancer cells were grafted
subcutaneously into the nude mice to establish a nude mouse
xenograft model of gastric cancer. The tumor volume was
significantly greater than that of the A549 cells without LPS
treatment and expression of the marker of proliferation, Ki-67, in
tumor tissues was also significantly increased (18). Similarly, the in vivo data
of the present study revealed that following grafting the MGC-803
gastric cancer cell line stimulated by LPS into the axilla of nude
mice to establish tumor xenografts, there was an increase in tumor
volume and weight in the LPS group, which was consistent with
findings of a previous study (17).
LPS is able to induce significant levels of
proliferation and inhibit the apoptosis of tumor cells (18). He et al (19) observed that LPS could significantly
inhibit apoptosis of gastric cancer cells and that this effect was
considered to be closely associated with the activation of TLR4 and
its downstream gene, nuclear factor-κB (NF-κB). Although CD14 is a
high-affinity receptor of LPS, the CD14 protein has neither a
transmembrane domain nor a cytoplasmic segment, and therefore, its
effect on signal transmission into the nucleus is performed in
coordination with TLR4 (20).
Baumann et al (21)
reported that the level of CD14 protein may affect TLR activity and
thus interfere with the effect of certain anti-cancer therapies or
anti-viral reactions. In the present study, following LPS
treatment, the of apoptotic rate decreased markedly; however, in
cells subjected to knockdown of CD14 expression, the inhibitory
effect of LPS on apoptosis was blocked. Accordingly, it was
hypothesized that LPS may be able to regulate the apoptosis of
gastric cancer cells via CD14-TLR4 and its downstream NF-κB
signaling pathway, with CD14 performing a critical role in this
process.
It has been widely reported that the CD14/TLR4
signaling pathway is important in cancer cell proliferation and
secretion of inflammatory factors. Wang et al (22) found that when CD14 was expressed,
LPS induced the expression of β1 inte-grin in colon cancer cells
and increased the adhesive ability of tumor cells. In addition, LPS
promoted the expression of IL-8 in melanoma cells (23). LPS treatment in ovarian cancer
cells significantly promoted the proliferation of tumor cells and
the expression of monocyte chemoattractant protein-1 and IL-6 mRNA
(24). The aforementioned studies
suggested that LPS is able to affect tumor cell viability and the
expression of cancer cell-associated inflammatory factors. hBD-2 is
a major component of the innate immune system and a major mediator
of the primary defense system of the mucosa and epithelium against
microbial invasion. In addition, it is highly expressed during
bacterial infection (25,26). In the present study, the secretion
of TNF-α, IL-1β, IL-6, IL-12 and hBD-2 in gastric cancer cells were
significantly increased, particularly following LPS treatment. This
finding is consistent with that of a previous study (27).
NF-κB is an important regulator of inflammation,
regulating the expression of numerous types of inflammatory
factors, chemokines and anti-apoptotic proteins (28). Thus, NF-κB promotes cell
proliferation and inhibits apoptosis. During this process, CD14 has
an indispensable role. The inhibition of CD14 expression blocks the
transmission of the downstream signals of LPS, offsetting its
activating effect. However, cell proliferation and apoptosis may be
regulated by numerous signaling pathways. Downstream of the TLR4
signal, the interleukin-1 receptor-associated kinase 2, protein
kinase-R, mitogen-activated protein kinases and other signaling
pathways are located. In addition, TLR2 and TLR6 exhibit
synergistic effects with CD14 via different signaling pathways to
mediate the signaling transmission of LPS (29,30).
Therefore, further studies are required to define the mechanism of
action of CD14 in the development of gastric cancer.
In conclusion, the present study reported a
significant role for CD14 in the mediation of the LPS signal.
Forced silencing of CD14 alleviated LPS-induced cell proliferation
and increased levels of apoptosis in gastric cancer cells. In
addition, upregulation of TNF-α, IL-1β, IL-6, IL-12, hBD-2 and
activation of TLR4 by LPS was also inhibited by CD14 knockdown. The
present study provides novel evidence regarding the pathogenesis of
H. pylori.
Acknowledgments
The present study was supported in part by a grant
from the National Natural Science Foundation of China (grant no.
81060165) and a grant from the National 'Twelfth Five-Year' Plan
for Science & Technology Support of China (grant no.
2013BAI05B04).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herrera V and Parsonnet J: Helicobacter
pylori and gastric adenocarcinoma. Clin Microbiol Infect.
15:971–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pugin J, Heumann ID, Tomasz A, et al: CD14
is a pattern recognition receptor. Immunity. 1:509–516. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright SD, Ramos RA, Tobias PS, Ulevitch
RJ and Mathison JC: CD14, a receptor for complexes of
lipopolysaccharide (LPS) and LPS binding protein. Science.
249:1431–1433. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li K, Dan Z, Hu X, et al: CD14
overexpression upregulates TNF-alpha-mediated inflammatory
responses and suppresses the malignancy of gastric carcinoma cells.
Mol Cell Biochem. 376:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gadducci A, Ferdeghini M, Castellani C, et
al: Serum levels of tumor necrosis factor (TNF), soluble receptors
for TNF (55- and 75-kDa sTNFr) and soluble CD14 (sCD14) in
epithelial ovarian cancer. Gynecol Oncol. 58:184–188. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanczkowski W, Tymoszuk P,
Ehrhart-Bornstein M, Wirth MP, Zacharowski K and Bornstein SR:
Abrogation of TLR4 and CD14 expression and signaling in human
adrenocortical tumors. J Clin Endocrinol Metab. 95:E421–E429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li K, Dan Z, Hu X, Gesang L, Ze Y and
Bianba Z: CD14 regulates gastric cancer cell epithelialmesenchymal
transition and invasion in vitro. Oncol Rep. 30:2725–2732.
2013.PubMed/NCBI
|
|
10
|
Peek RM Jr, Fiske C and Wilson KT: Role of
innate immunity in Helicobacter pylori-induced gastric malignancy.
Physiol Rev. 90:831–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polk DB and Peek RM Jr: Helicobacter
pylori: gastric cancer and beyond. Nat Rev Cancer. 10:403–414.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correa P and Piazuelo MB: Helicobacter
pylori infection and gastric adenocarcinoma. US Gastroenterol
Hepatol Rev. 7:59–64. 2011.PubMed/NCBI
|
|
13
|
Blaser MJ and Kirschner D: The equilibria
that allow bacterial persistence in human hosts. Nature.
449:843–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmausser B, Andrulis M, Endrich S, et
al: Expression and subcellular distribution of toll-like receptors
TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter
pylori infection. Clin Exp Immunol. 136:521–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmausser B, Andrulis M, Endrich S,
Muller-Hermelink HK and Eck M: Toll-like receptors TLR4, TLR5 and
TLR9 on gastric carcinoma cells: an implication for interaction
with Helicobacter pylori. Int J Med Microbiol. 295:179–185. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao D, Sun T, Zhang X, et al: Role of
CD14 promoter polymorphisms in Helicobacter pylori
infection-related gastric carcinoma. Clin Cancer Res. 13:2362–2368.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grandel U, Banat A and Savai R: Effect of
endotoxin on COX-2 dependent proliferation of NSCLC cells: Role of
CD14, TLRs and EGFR signaling. J Clin Oncol (Meeting Abstracts).
e221902009.
|
|
18
|
Hattar K, Savai R, Subtil FS, et al:
Endotoxin induces proliferation of NSCLC in vitro and in vivo: role
of COX-2 and EGFR activation. Cancer Immunol Immunother.
62:309–320. 2013. View Article : Google Scholar :
|
|
19
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan KL, Wong KF and Luk JM: Role of
LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis:
pathogenesis and therapeutic implications. World J Gastroenterol.
15:4745–4752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baumann CL, Aspalter IM, Sharif O, et al:
CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med.
207:2689–2701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang JH, Manning BJ, Wu QD, Blankson S,
Bouchier-Hayes D and Redmond HP: Endotoxin/lipopolysaccharide
activates NF-kappaB and enhances tumor cell adhesion and invasion
through a beta 1 integrin-dependent mechanism. J Immunol.
170:795–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Molteni M, Marabella D, Orlandi C and
Rossetti C: Melanoma cell lines are responsive in vitro to
lipopolysaccharide and express TLR-4. Cancer Lett. 235:75–83. 2006.
View Article : Google Scholar
|
|
24
|
Kelly MG, Alvero AB, Chen R, et al: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauer B, Wex T, Kuester D, Meyer T and
Malfertheiner P: Differential expression of human beta defensin 2
and 3 in gastric mucosa of Helicobacter pylori-infected
individuals. Helicobacter. 18:6–12. 2013. View Article : Google Scholar
|
|
26
|
Moran AP: The role of endotoxin in
infection: Helicobacter pylori and Campylobacter jejuni. Subcell
Biochem. 53:209–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie C, Kang J, Li Z, et al: The açaí
flavonoid velutin is a potent anti-inflammatory agent: blockade of
LPS-mediated TNF-alpha and IL-6 production through inhibiting
NF-kappaB activation and MAPK pathway. J Nutr Biochem.
23:1184–1191. 2012. View Article : Google Scholar
|
|
28
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Underhill DM and Ozinsky A: Toll-like
receptors: key mediators of microbe detection. Curr Opin Immunol.
14:103–110. 2002. View Article : Google Scholar : PubMed/NCBI
|