Introduction
The body and organ size of a mammal is determined by
the number and size of cells; however, the total cell mass is not
always adjusted toward the normal size if it is experimentally or
accidentally perturbed (1).
Previous genetic screens of Drosophila have revealed that
the Hippo signalling pathway is critical in restricting organ size
by controlling cell cycle exit and cell death (2,3). The
Hippo pathway restricts cell growth and proliferation and promotes
apoptosis by regulating the nuclear localisation of Yes-associated
protein (YAP) and TEA domain family member (TEAD) in mammals
(4). This regulation is achieved
by the transcriptional activation of the Hippo pathway target
genes, including cyclin E, diap1, and bantam microRNA (5–7).
YAP, a 65-kDa protein, is a transcriptional co-activator of several
transcription factors via its own WW-domain. It is also a potent
growth promoter, which has been identified as an oncogenic protein
in mammalian cells (8–10). The TEAD family of transcription
factors is considered to be a major partner of YAP and TAZ in the
Hippo pathway (11). Substantial
evidence has revealed that TAED1 and YAP share a substantial number
of target genes (12–14). In support of this evidence, TEAD1
and TEAD2 double-knockout-mice have been observed to exhibit
phenotypes similar to those of YAP-knockout mice (15). Furthermore, ablation of the
expression of TAED decreases the ability of YAP/TAZ to promote
anchorage independent growth (12,16).
Despite its conservation and close association with cancer, the
Hippo pathway has not been systematically investigated in mammalian
cells.
Prostate cancer (PCa) is a malignant carcinoma with
one of the highest morbidity rates worldwide, primarily endangering
the health of aging males (17),
particularly castration-resistant prostate cancer (CRPC). The
treatment options for patients with CRPC remain limited. Although
the mechanisms involved in the occurrence and development of CRPC
remain to be elucidated, it has been observed that dysregulation of
the Hippo signalling pathway is important in the proliferation of
tumour cells, and the activation of YAP gives rise to carcinoma
(5,18). YAP is considered to be the key
component downstream of the Hippo signalling pathway, and the
importance of Hippo signalling in controlling mammalian organ size
has been investigated extensively in the liver, where transgenic
overexpression of YAP leads to hepatomegaly (19). The overexpression of YAP has also
been observed in gastric cancer (20) and in PCa (21,22).
However, the function of YAP in CRPC cells remains to be
elucidated.
Studies have revealed that the Hippo signalling
pathway is involved in cell cycle regulation (23). The dysregulation of this pathway,
which leads to YAP activation, induces oncogenic transformation in
cooperation with distinct transcription factors, including TEAD
family members (24). In the
present study, PCa specimens were obtained to perform analyses of
the correlation between YAP, and the staging and grading of the
clinical pathology, Gleason score and level of prostate specific
antigen (PSA) in the PCa cells. In addition, the PC-3 CRPC cell
line was selected to further investigate YAP in vitro. The
pMagic7.1-RNA interference-YAP-1, 2, 3 plasmid was used to inhibit
YAP, to determine the effect of YAP inhibition on the proliferation
and apoptosis of the PC-3 cells. The differences in the expression
of YAP and TEAD1 following transfection were also analysed to
determine the role of the Hippo signalling pathway in PC-3
cells.
Materials and methods
Specimen collection
Tissue specimens used in the present study were
acquired from the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) between March 2009 and July 2012. The
study was approved by the Ethics Committee of Chongqing Medical
University, and informed, written consent regarding the use of
tissues was obtained from all patients. There were 62 male patients
in total, with an age range from 53–81 years old. A total of 32 PCa
samples were obtained during surgery. Certain specimens were
collected from patients with dysuria following drug and surgical
castration. Furthermore, 15 samples of benign prostatic hyperplasia
(BPH) tissue from transurethral resection of the prostate were
obtained as a control, and 15 samples of para-prostate carcinoma
tissue (para-PCa) were also obtained during surgery. All specimens
were confirmed pathologically and stored at −80°C. However, PCa and
CRPC cannot be distinguished by pathological diagnosis. The ages of
the patients with PCa ranged between 53 and 81 years (mean, 67).
According to Gleason's grading system (25), there were eight cases with scores
of ≤6, 11 cases with scores of 7 and 13 cases with scores >8.
The tumour-node-metastasis (TNM) staging (26) indicated that there were 15 cases of
T1-T2 and 17 cases of T3-T4. In addition, nine cases had
metastases.
Immunohistochemistry (IHC)
The specimens were fixed with 10% formaldehyde
(ZSGB-Bio, Beijing, China), cut into sections (5 µm) and
stained according to the streptomycin anti-biotin-peroxidase
two-step method (27). Briefly,
following gradient alcohol dehydration, the sections were incubated
in 10% hydrogen peroxide for 10 min and antigen retrieval was
performed using a microwave vacuum histo-processor (RHS-1;
Milestone, Sorisole, Italy) at 121°C for 15 min. Following blocking
with goat serum (Gibco-BRL, Carlsbad, CA, USA), the goat
anti-rabbit monoclonal primary antibody (1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and goat anti-rabbit IgG
horseradish peroxidase (HRP)-labeled secondary antibody (ZSGB-Bio)
were added for 1 h at 37°C. Following 3,3′-diaminobenzidine and
hematoxylin (ZSGB-Bio) treatment, the sections were sealed with
neutral resin (ZSGB-Bio) and observed under an inverted microscope
(LW300-38LF; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
A semiquantitative scoring criterion for YAP in the
tissue specimens was used. Tissue sections were observed under an
inverted microscope, and staining intensity and positive areas were
recorded. The staining intensity was evaluated on a scale between 0
and 3 (0, negative; 1, weak; 2, moderate; 3, strong). The
percentage of positive areas was scored using a four-tier system
(0, 0%; 1, ≤25%; 2, ≤50%; 3, ≤100%). The intensity of the
immunoreaction was calculated from the score of the staining
intensity and the percentage of positive areas. An intensity of 0–1
was negative, 2–4 was weak positive, 5–6 was medium positive and
7–8 was strong positive.
Cell culture
The PC-3 cells, obtained from osseous metastasis of
the prostate cancer of an elderly man, were purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China), and was a CRPC cell line (28). The cells were cultured in a mixture
of Dulbecco's modified Eagle's medium (DMEM) and F12 (1:1; HyClone
Laboratories, Inc., South Logan, UT, USA), containing 10% fetal
bovine serum (FBS; Gibco-BRL), in an incubator at 37°C with 5%
CO2.
Structure of YAP-RNAi
Using the sequence of the YAP gene (NM_006106) from
GenBank (http://www.ncbi.nlm.nih.gov/gene/?term=YAP+NM-006106),
the Shanghai SBO Medical Biotechnology Co., Ltd. website and online
RNA screening technology (http://www.sbo-bio.com.cn), the interference sequences
were designed as follows: RNAi-1, forward
5′-CCGGGCTCATTCCTCTCCAGCTTCTCAAGAGAAAGCTGGAGAGGAATGAGCTTTTTTG-3′
and reverse
5′-AATTCAAAAAAGCTCATTCCTCTCCAGCTTTCTCTTGAGAAGCTGGAGAGGAATGAGC-3′;
RNAi-2, forward
5′-CCGGCTTAACAGTGGCACCTATTTCAAGAGAATAGGTGCCACTGTTAAGGTTTTTTG-3′ and
reverse
5′-AATTCAAAAAACCTTAACAGTGGCACCTATTCTCTTGAAATAGGTGCCACTGTTAAGG-3′;
RNAi-3, forward
5′-CCGGCCGTTTCCCAGACTACCTTCTCAAGAGAAAGGTAGTCTGGGAAACGGTTTTTTG-3′
and reverse
5′-AATTCAAAAAACCGTTTCCCAGACTACCTTTCTCTTGAGAAGGTAGTCTGGGAAACGG-3′;
negative control, forward
5′-FCCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3′
and 5′-reverse
AATTCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAA-3′.
The forward sequences were compared using BLAST
(http://www.ncbi.nlm.nih.gov/BLAST) to
the published Expressed Sequence Tags database, and the three pairs
of specific sequences were confirmed in addition to the human YAP
genes. No genetic homology with other genes was found. The three
pairs of RNAi oligonucleotides targeting human YAP mRNA and the
control sequence were synthesised at SBO-Bio (Shanghai, China)
(29).
A total of three pairs of RNAi plasmid vectors,
pMagic7.1-Puro/green fluorescent protein (GFP)-RNAi-YAP 1, 2, and
3, and a negative control (NC) vector were constructed (Beijing
ComWin Biotech Co., Ltd, Beijing, China). Three pairs of plasmids
and the NC vector were transfected into the PC-3 prostate cancer
cells using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). Non-transfected (NT) PC-3 cells were used as a
control.
Stable YAP-inhibition in PC-3 cells
On the day prior to transfection, PC-3 cells in the
logarithmic growth stage were transferred into 6-well plates
(2×105 cells/well), and 2 ml DMEM/F12 containing 10% FBS
was added. The plates were incubated overnight at 37°C to ensure
that the cells occupied ~90% of the well. Transfection was
performed using Lipofectamine 2000, according to the manufacturer's
instructions. Green fluorescence was observed using an inverted
fluorescence microscope (LW300-38LF; Bio-Rad Laboratories, Inc.) 48
h after transfection. Puromycin (Invitrogen Life Technologies) was
added (1 mg/l per well) to the plates for screening of stable cell
lines for 2 days at 37°C, during which several cells underwent
apoptosis, and the medium was replaced without puromycin. The cell
clusters exhibiting green fluorescence were selected and inoculated
into flasks. The NT group acted as a control. The medium was
replaced every other day. After 2 weeks, the clusters of cells were
observed under an inverted fluorescence microscope and, after 1
month, cells were obtained, which were observed to be stably
expressing YAP inhibition (29).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each group of cells
using RNAiso reagent (Tiangen Biotech Co., Ltd., Beijing, China).
The total RNA was quantified using a UV spectrophotometer (UV2800;
Bio-Rad Laboratories, Inc.), and 1 µg RNA was used for the
RT reaction to obtain cDNA. The qPCR reactions were performed with
0.5 µg DNA to amplify the YAP gene, with GAPDH as the
internal reference using the ABI 2720/2700 Cycler (Applied
Biosystems Life Technologies, Foster City, CA, USA). The cycling
conditions were as follows: 94°C for 30 sec, 55°C for 30 sec and
72°C and 45 sec (30 cycles). The primer sequences for the YAP mRNA
obtained from Beyotime Institute of Biotechnology were as follows:
Forward 5-TGAACAAACGTCCAGCAAGATAC-3 and reverse
5-CAGCCCCCAAAATGAACAGTAG-3. The target fragment was 165 bp.
Primer sequences for the TEAD1 mRNA were as follows: Forward
5-TGAATCAGTGGACATTCGTCA-3 and reverse
5-GCCATTCTCAAACCTTGCATA-3. The target fragment was 280 bp.
Primer sequences for GAPDH mRNA were as follows: Forward
5-ACCACCATGGAGAAGGCTGG-3 and reverse
5-CTCAGTGTAGCCCAGGATGC-3. The target fragment was 500 bp.
The PCR products were analysed using agarose gel electrophoresis
(Sino-American Biotechnology Co., Ltd., Luoyang, China) with 100 V
and then 300 mA and then were visualised under UV light. A Bio-Rad
gel formatter (Bio-Rad Laboratories, Inc.) was used to analyse the
original band (30).
Western blot analysis
The cells of different prostatic tissues, three
stable transfection experimental groups, the NC group, and the NT
group were collected. Cell lysates were prepared using a mixture of
phenylmethylsulfonyl fluoride and protease inhibitor (1:99;
Beyotime Institute of Biotechnology, Shanghai, China). The proteins
were separated by gel electrophoresis and then transferred onto
0.45 µm polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology). The mouse anti-rabbit primary antibody
(1:200) and HRP-labeled secondary antibody (1:5,000) were added for
1 h at room temperature. The membrane was placed in a Vilber
Lourmat (Bio-Rad Laboratories, Inc.) for enhanced chemiluminescence
development. The density values were determined against the target
protein, β-actin.
Proliferation assay
An MTT assay was performed to determine the rates of
proliferation. As the RNAi-YAP-1 transfection group exhibited the
most efficient inhibition of YAP-1, cells stably expressing
pMagic7.1-Puro/GFP-RNAi-YAP-1 were selected to assess
proliferation, and the NC group was used as the control group. Each
group was seeded at a low density (2×103 cells/200
µl). Blank medium was used to obtain a zero setting. The
cells were incubated for 7 days at 37°C, and the cells were
randomly removed each day and the cell numbers were determined
using a microplate reader (M450; Bio-Rad Laboratories, Inc.). A
proliferation curve was generated, and the doubling time of the
cells was calculated using the Patterson formula: Td = T × lg2/lg
(Nt / N0), where Td indicates the doubling time, T indicates the
number of days, Nt indicates the number of cells on the final day
and N0 indicates the number of cells on the first day.
Cell cycle assay
The PC-3 cells transfected with RNAi-YAP-1, which
yielded the highest inhibition ratio, and the NC group were
cultured with DMEM/F12 containing 10% FBS at 4°C for 12 h. The
cells were then harvested and fixed with 70% ethanol overnight at
4°C. The fixed cells were stained with 50 µg/µl
propidium iodide (Bioscience, Shanghai, China) and 100
µg/µl RNase (Sigma-Aldrich, St. Louis, MO, USA). The
cell cycle profiles of the two groups were measured using flow
cytometry (FCM; FC 500 Series Flow Cytometry System; Beckman
Coulter, Inc., Brea, CA, USA), and the resulting data were
analysed.
Apoptosis assay
The cells of the RNAi-YAP-1-transfected group and
the NC group were collected and cultured, as above. The cells were
harvested and fixed with 70% ethanol at 4°C overnight. The fixed
cells were stained with 50 µg/µl propidium iodide at
4°C for 30 min. The apoptotic profile was determined, following
analysis of the cells by FCM, using a 630 nm argon ion laser.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. A χ2 test was used to compare
the prostatic tissues. Student's two-tailed t-test was used to
compare between groups. Regression analysis was used to perform
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Protein expression of YAP in different
prostatic tissues
No positivity for YAP protein (intensity score ≤1)
were observed in the BPH tissue (0/15; Fig. 1A). In the PCa tissue, 78.13%
(25/32) of the samples (intensity score >5) exhibited positive
expression, and the positive areas were primarily located in the
cytoplasm and nuclei of the PCa glandular epithelium (Fig. 1B). In the para-PCa tissue, the
frequency of samples (intensity score 2–4) was 26.67% (4/15), and
the positive areas were predominantly in the cytoplasm, with a
small quantity in the nuclei (Fig.
1C). The percentage of YAP protein expressed in the PCa tissue
was significantly higher, compared with the BPH tissue (P=0.002)
and para-PCa tissue (P=0.007; Table
I). Based on the results of the western blot analysis, the
expression of YAP was high in the PCa tissue, but low in the BPH
tissue and para-PCa tissue (Fig.
1D).
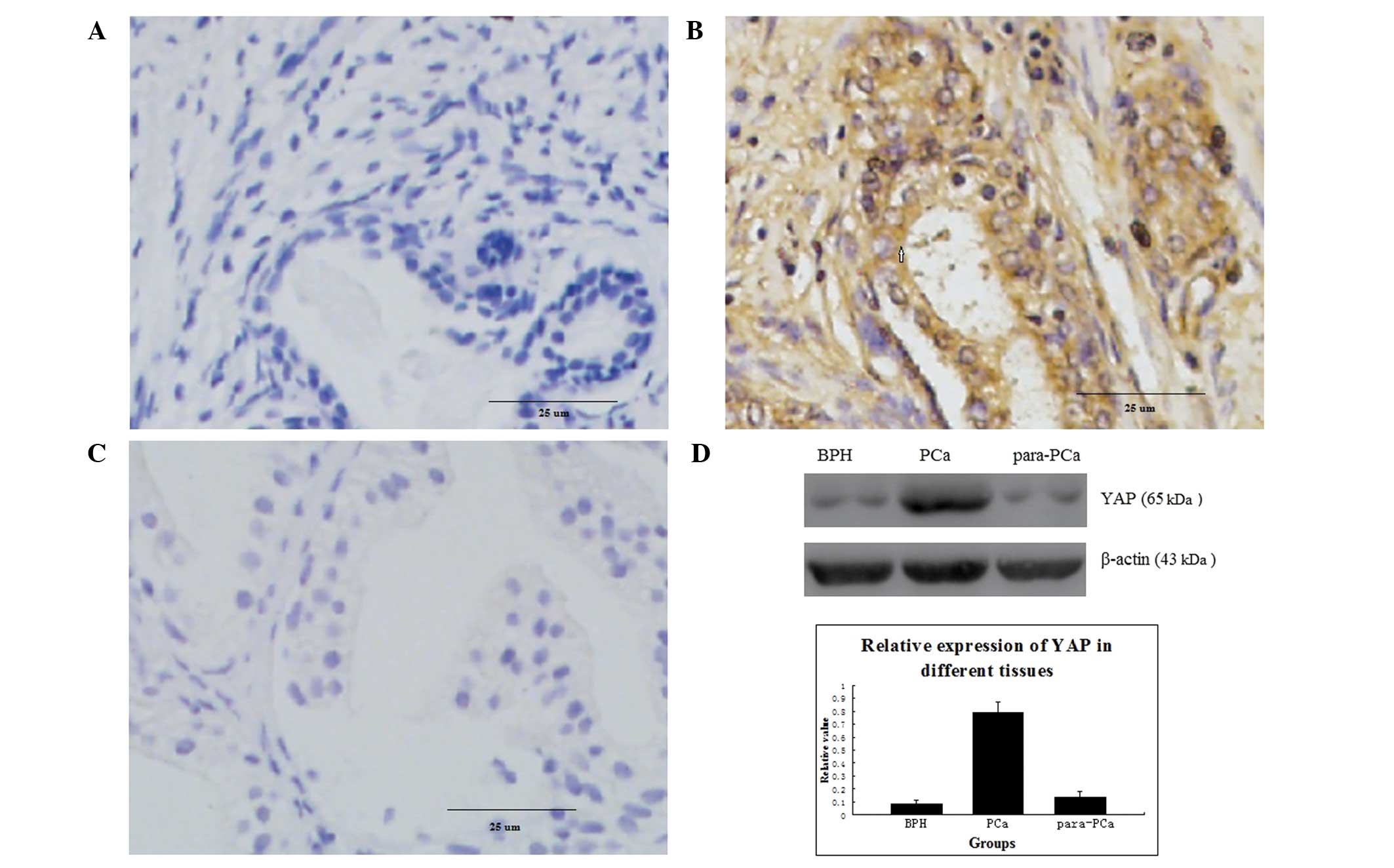 | Figure 1Immunohistochemical analysis of
protein expression in 32 samples of PCa tissue, 15 samples of BPH
tissue and 15 samples of para-PCa tissue. (A) Protein expression of
YAP in BPH (magnification, ×400); (B) Overexpression of YAP protein
in PCa (magnification, ×400); (C) Protein expression of YAP in
para-PCa tissue (magnification, ×400); The difference in the
expression of YAP was significant between PCa and BPH (P=0.007),
and between PCa and para-PCa (P=0.002); (D) Expression of YAP in
different prostatic cells, analysed using western blotting, in all
tissues. Data are expressed as the mean ± standard deviation. PCa,
prostate cancer; YAP, Yes-associated protein; BPH, benign prostatic
hyperplasia. |
 | Table IExpression of YAP in different
tissues. |
Table I
Expression of YAP in different
tissues.
| Tissue | Cases (n) | Score of YAP
intensity
| Positive (%) | P-value |
|---|
| 0–1 | 2 | 3–4 | 5–6 |
|---|
| Para-PCa | 15 | 11 | 3 | 1 | 0 | 26.67 | 0.007 |
| BPH | 15 | 15 | 0 | 0 | 0 | 0 | 0.002 |
| PCa | 32 | 7 | 2 | 7 | 16 | 78.13 | |
| Total | 62 | 33 | 5 | 8 | 16 | 46.77 | |
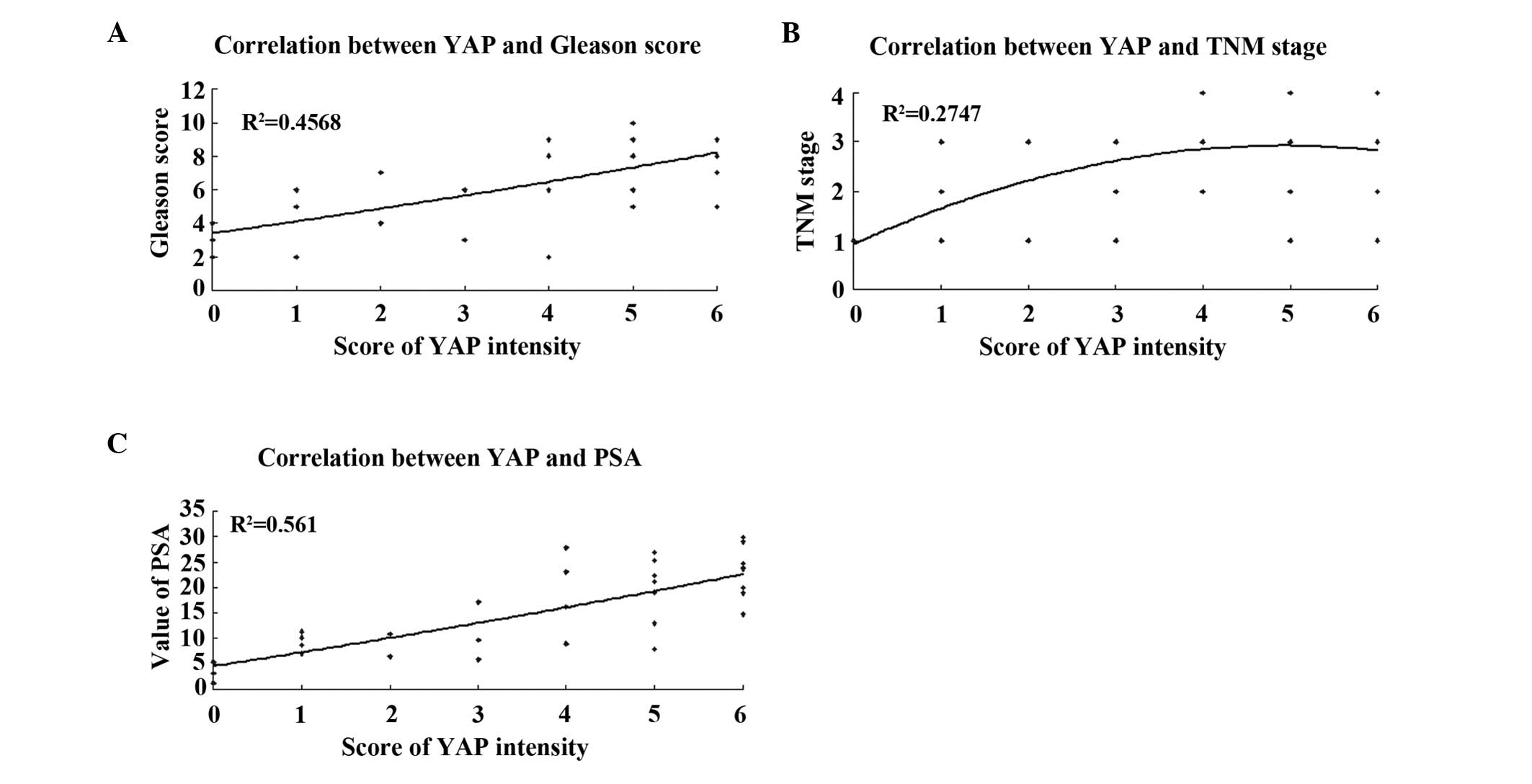
Correlation between the expression of YAP
and the clinicopathological grading and staging of PCa
The frequencies of samples with positive expression
of YAP among tissues with Gleason scores of <7, 7, and ≥8 were
50% (4/8), 72.7% (8/11), and 100% (13/13), respectively, revealing
a positive correlation (P=0.008; Fig.
2A). Based on the TNM staging, the samples in which YAP was
present exhibited an increasing trend between T1 and T4. For T1+T2
and T3+T4 stages, the frequencies were 60% (9/15) and 94.1%
(16/17), respectively (P=0.033; Fig.
2B). The expression of YAP increased significantly with
increasing PSA levels (<10 ng/ml vs. ≥10 ng/ml, P=0.0032;
Table II, Fig. 2C).
 | Table IIAssociation of YAP with the
clinicopathologic features of prostate cancer. |
Table II
Association of YAP with the
clinicopathologic features of prostate cancer.
| Feature | Cases (n) | Score of YAP
intensity
| Positive (%) | P-value |
|---|
| 0–1 | 2 | 3–4 | 5–6 |
|---|
| Gleason
grading | | | | | | | 0.008 |
| <7 | 8 | 4 | 1 | 3 | 0 | 50 | |
| 7 | 11 | 3 | 1 | 2 | 5 | 72.7 | |
| 8–10 | 13 | 0 | 0 | 2 | 11 | 100 | |
| TNM stage | | | | | | | 0.033 |
| T1+T2 | 15 | 6 | 1 | 3 | 5 | 60 | |
| T3+T4 | 17 | 1 | 1 | 4 | 11 | 94.1 | |
| PSA level
(ng/ml) | | | | | | | 0.032 |
| <10 | 11 | 5 | 1 | 3 | 2 | 54.5 | |
| ≥10 | 21 | 2 | 1 | 4 | 14 | 90.5 | |
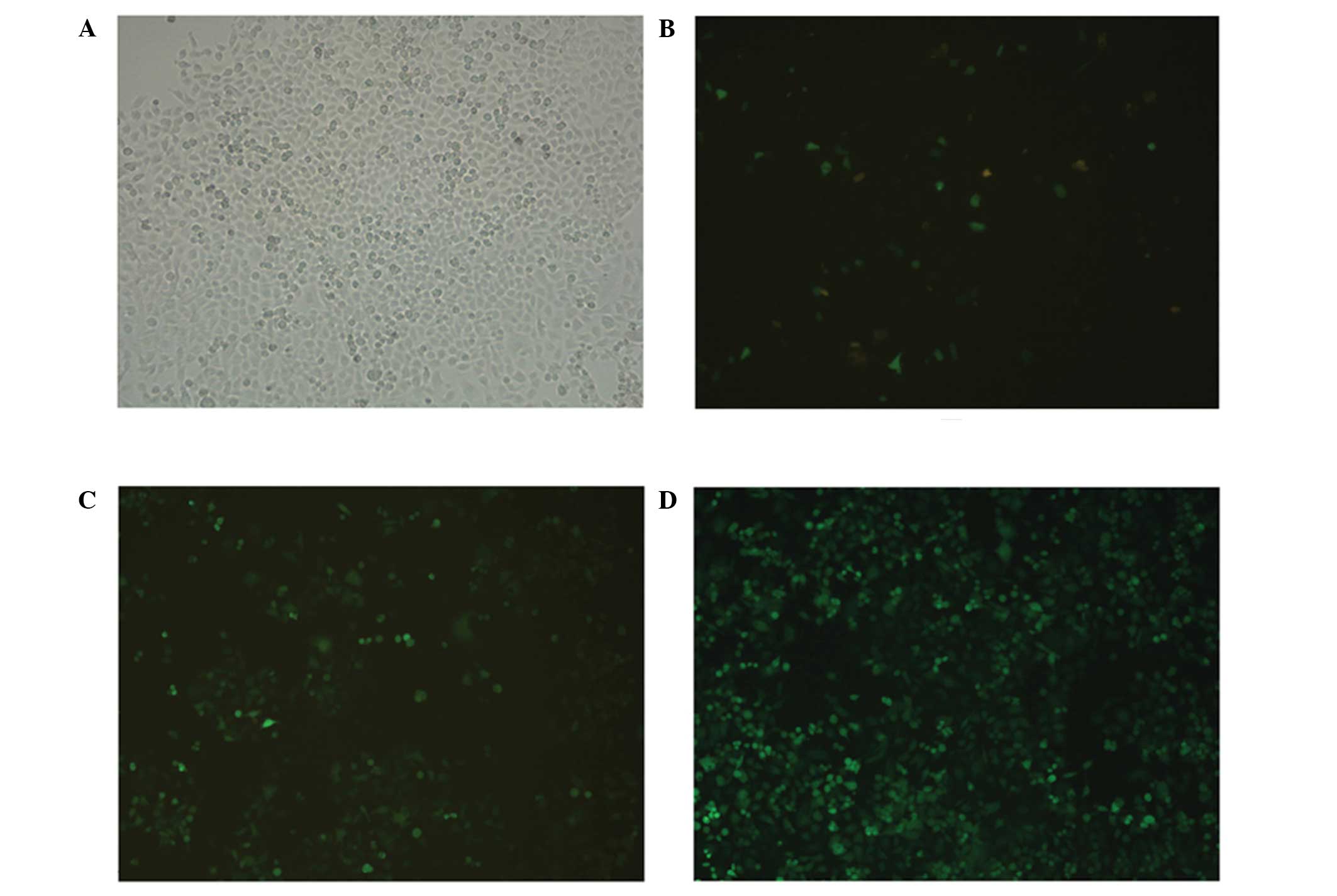
Plasmid-transfected PC-3 cells
GFP was expressed in the PC-3 cells 48 h after
transfection with the pMagic7.1-Puro/GFP-RNAi-1, 2, and 3 plasmids,
which suggested that each group was transfected successfully
(Fig. 3A and B). After 2 weeks of
selection with 1 mg/l puromycin, small clusters of green
fluorescent cells were observed (Fig.
3C). Subsequently, three groups of stable YAP interference cell
lines, as well as one non-targeted knockdown control cell line,
were established from the PC-3 cells (Fig. 3D).
mRNA and protein expression of YAP
following transfection
The results of the RT-qPCR analysis demonstrated
that the mRNA expression level of YAP in the RNAi transfected cells
was markedly, compared with those in the NT and NC groups (Fig. 4A). Decreased protein expression of
YAP was also confirmed by western blot analysis (Fig. 4B). The lowest expression level of
YAP was observed in the cells transfected with the RNAi-YAP-1
plasmid, which indicated that the RNAi-YAP-1 plasmid had the
highest inhibition ratio of the three plasmids and, therefore, the
RNAi-YAP-1 plasmid was selected for subsequent investigation.
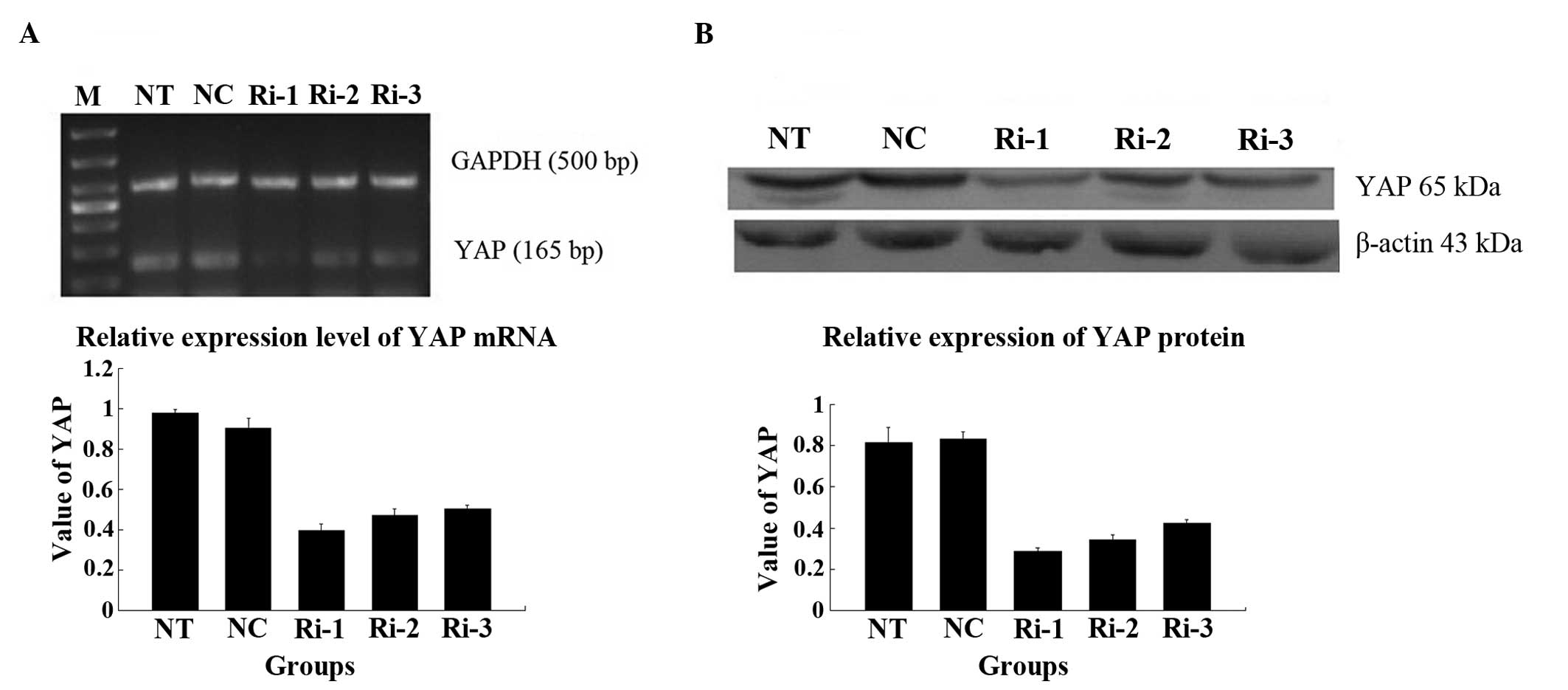 | Figure 4Different expression levels of the
YAP in the stable cells following RNA-YAP interference-1, 2, 3
plasmid transfection. (A) mRNA expression of YAP in the cells of
different groups following transfection with RNAi-YAP-1, 2 and 3;
(B) Protein expression of YAP in the cells of different groups
following transfection with RNAi-YAP-1, 2 and 3. RNAi-YAP-1 had the
most marked effect on YAP inhibition, and RNAi-YAP-2 and RNAi-YAP-3
exhibited weaker effects. Therefore, the RNAi-YAP-1 plasmid was
used for the subsequent investigations. Data are expressed as the
mean ± standard deviation; *P<0.05,
**P<0.01 vs. control. YAP, yes-associated protein;
NC, negative control; NT, non-transfected; Ri, RNA-YAP. |
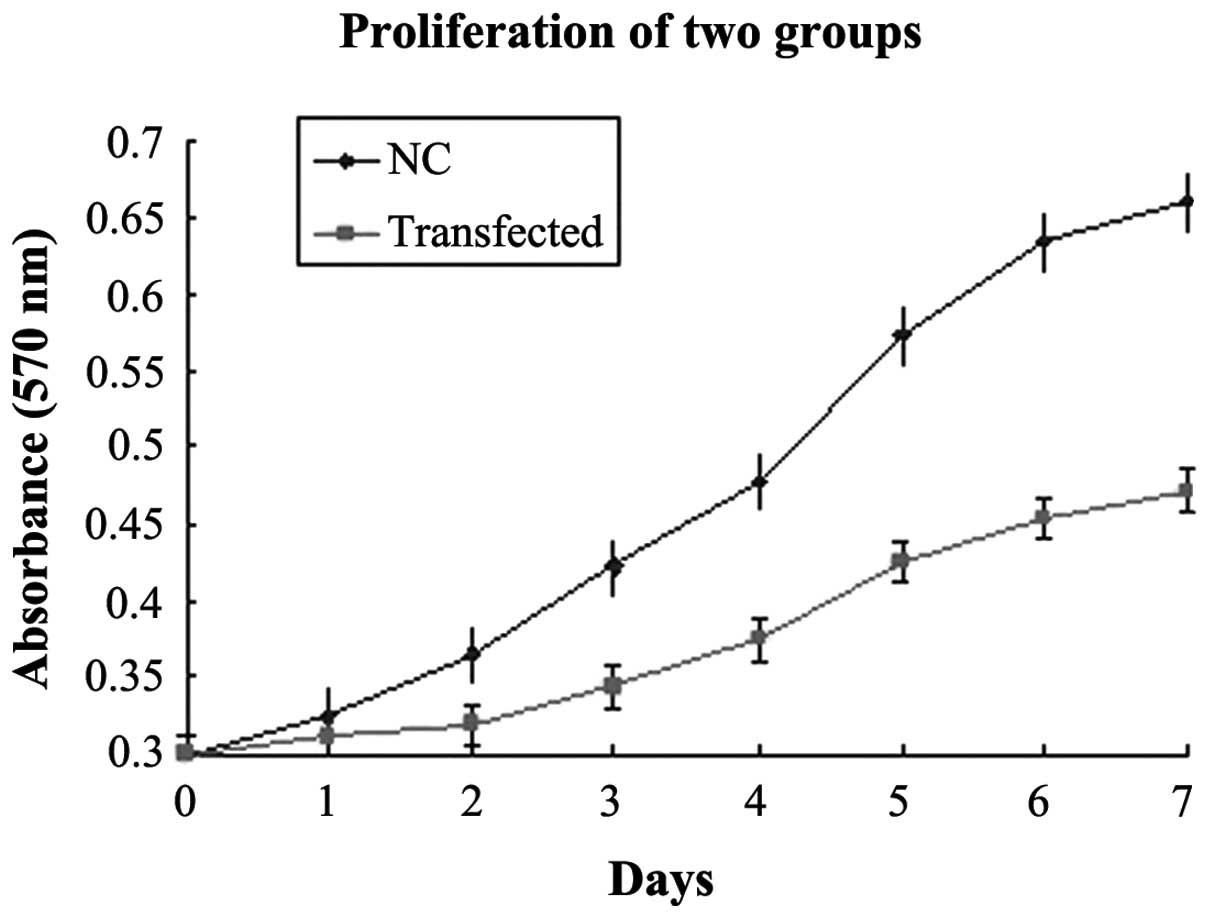
Interference with RNAi-YAP-1 suppresses
PC-3 cell growth
To understand the physiological role of YAP, the
expression of endogenous YAP was inhibited in the PC-3 cells.
RT-qPCR and western blot analysis revealed that high expression
levels of YAP in the PC-3 cells. To determine the effect of the
expression of YAP on PC-3 cell growth, proliferation curves of the
stable RNAi-YAP-1 interference clones were determined and compared
with the corresponding NC (Fig.
5). The two-factor analysis of variance revealed that the
difference between the two groups was statistically significant
(P=0.022), based on the optical density value from the third day.
The doubling times of the two groups were also significantly
different, in which the transfected group exhibited a doubling time
of 57.8 h, and the NC group exhibited a doubling time of 23.2 h
(P=0.028).
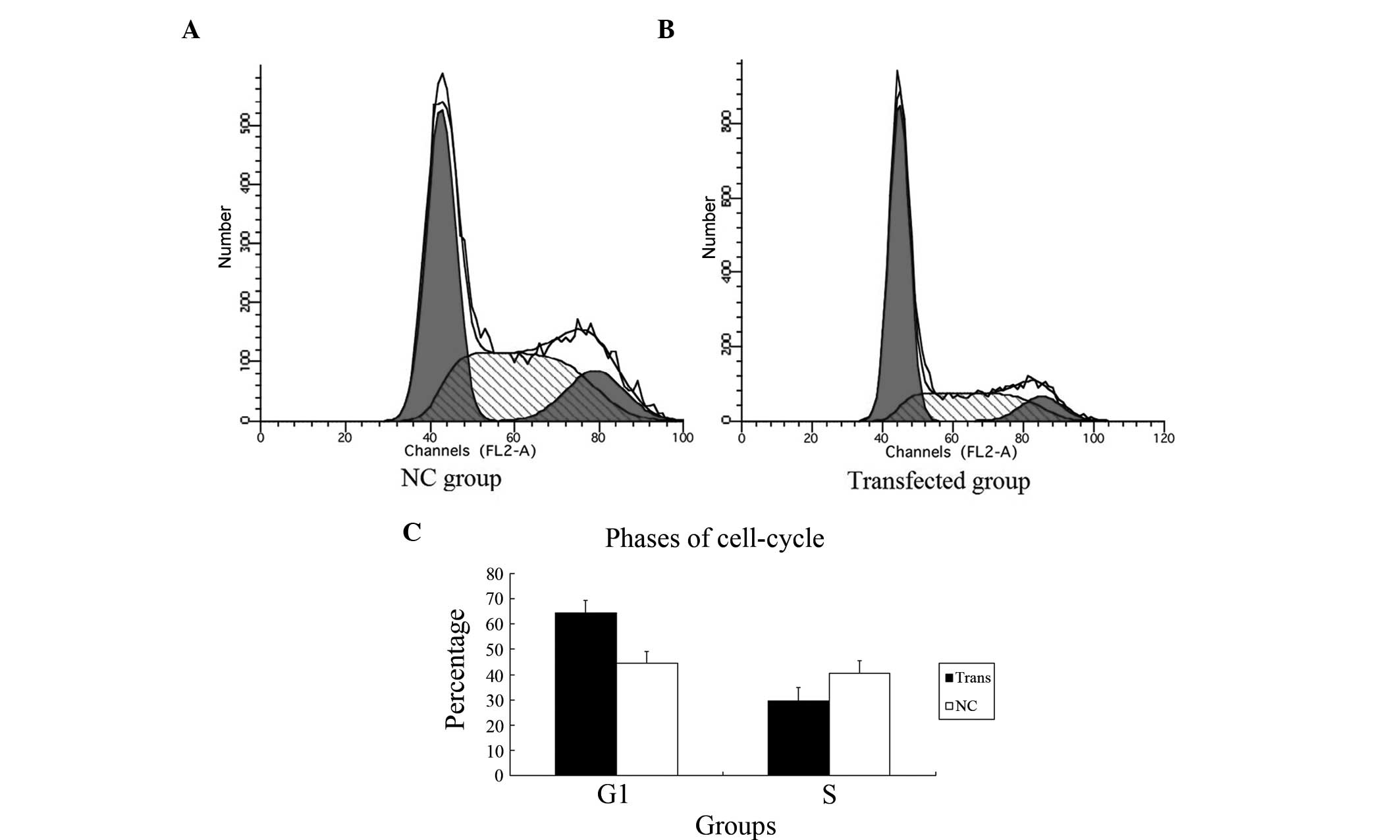
Inhibiting YAP dysregulates the
cell-cycle of PC-3 cells
To determine whether the knockdown of YAP was
sufficient to induce G1/S cell cycle arrest in the PC-3 cells, cell
cycle analysis was performed. As shown in Fig. 6, in the transfected group, FCM
revealed more cells in the G1 phase (61.4%) and fewer cells in the
S phase (29.4%), compared with the NC group (G1 phase, 44.8%; S
phase 40.7%; P=0.009). These findings indicated that a dysregulated
cell cycle in PC-3 cells subjected to YAP-knockdown, led to arrest
of cells in the G1 phase.
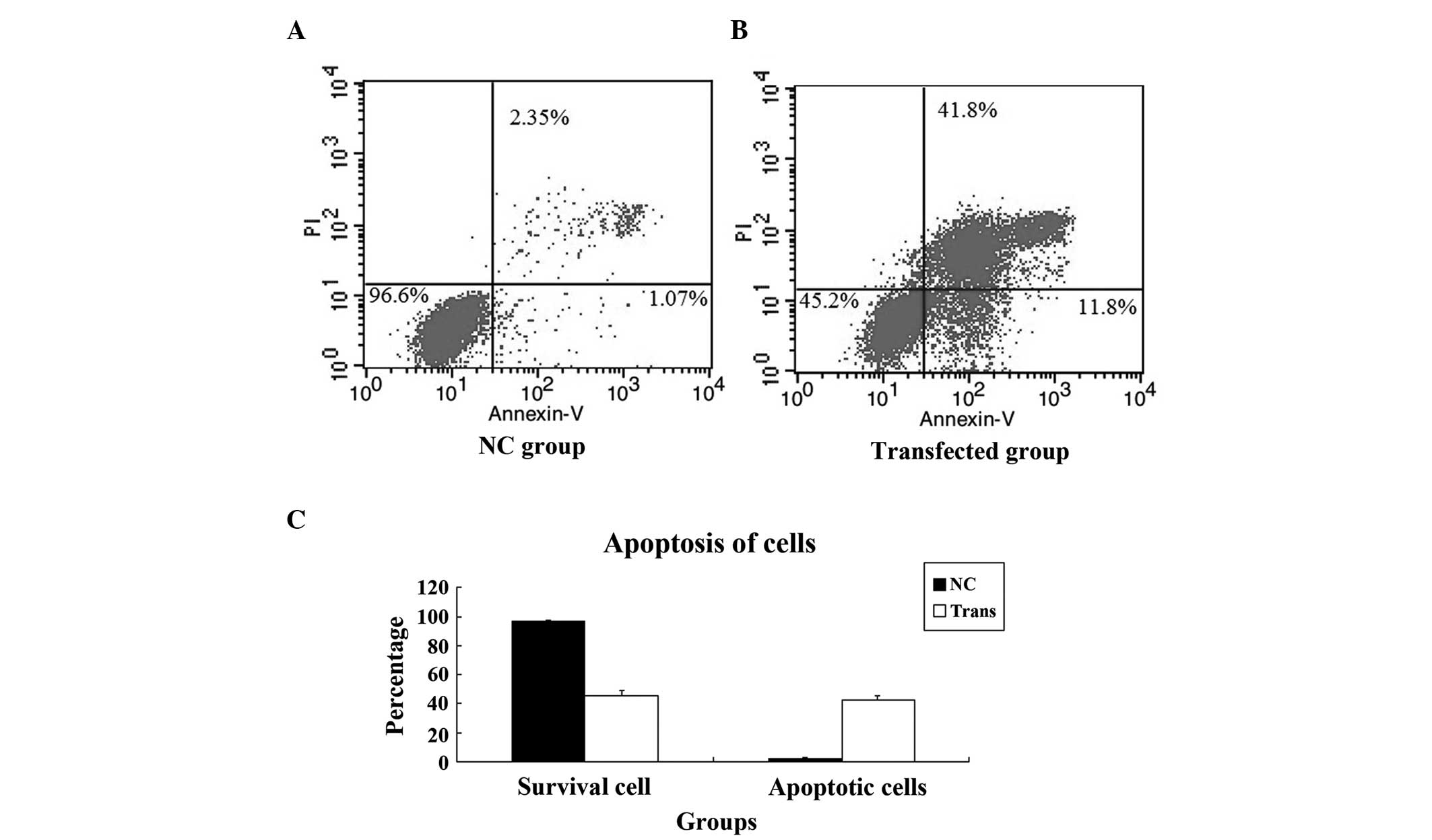
Knockdown of YAP induces apoptosis of
PC-3 cells
In order to understand the reason for the growth
suppression observed in the transfected PC-3 cells, an apoptosis
assay was performed. Apoptosis of the cells in the NC group and
transfected group was detected using FCM, which revealed that the
apoptosis of PC-3 cells was induced by YAP knockdown. The
difference in the apoptotic rate between the NC and transfected
cells was statistically significant (P=0.002; Fig. 7).
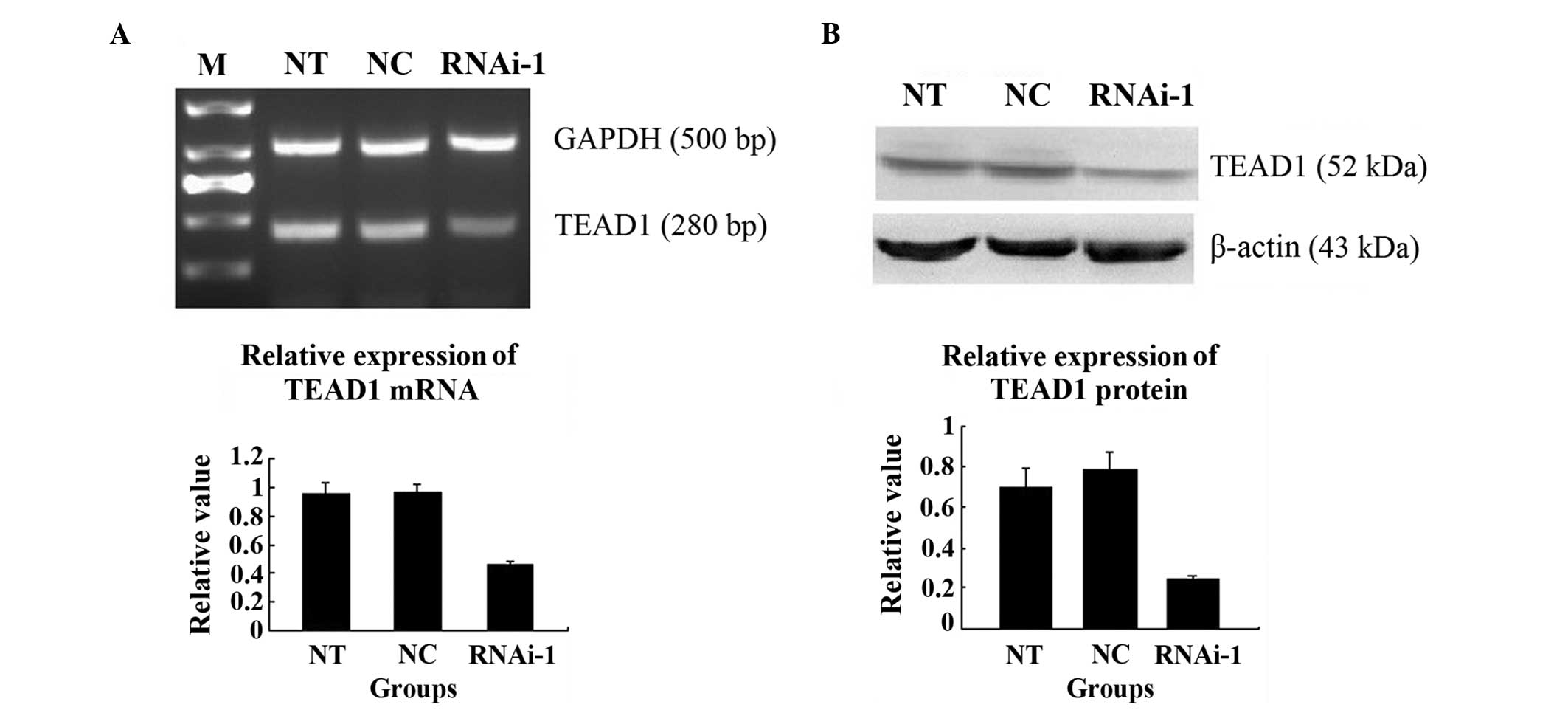
Knockdown of YAP inhibits the mRNA and
protein expression of TEAD1
TEAD1 is a downstream gene of YAP in the Hippo
signalling pathway (5,11). It has been hypothesized that the
activity of the TEAD1 gene is regulated by the YAP protein, and
binds with the TEAD1 combining domain to form a compound, which
generates TEAD1 protein (12). In
the present study, the PC-3 cells transfected with RNAi-YAP-1, the
mRNA expression was significantly lower, compared with those in the
NT and NC groups (P=0.001; Fig.
8A). A western blot assay was also performed, which
demonstrated that the protein expression of TEAD1 was also lower in
the cells transfected with RNAi-YAP-1 (P=0.005; Fig. 8B). By contrast, YAP was inhibited
successfully following TEAD1 inhibition.
Discussion
Carcinoma results from the loss of normal cell
communication, which causes unregulated cell proliferation,
migration and the inhibition of apoptosis (31). The Hippo pathway is important in
the regulation of tumour cells and tissue growth (32). The overexpression of YAP, which is
the core component in the Hippo-YAP pathway, increased organ size
and caused cancer in transgenic mice (33). The expression of YAP has been
significantly associated with tumour metastasis, grade and stage
(34,35). YAP depletion suppresses the
expression of cell cycle-promoting genes in tumour cells and
regulates tumour growth and metastasis (32,36).
There is also evidence that YAP is a valid molecular target
(37). In the present study, it
was demonstrated that YAP closely correlates with PCa, and that the
knockdown of YAP is essential in growth suppression and cell cycle
dysregulation in CRPC cells.
Initially, the present study demonstrated that the
protein level of YAP protein was correlated with YAP biological
activities, with certain traits that have been associated with PCa
staging and grading. Para-PCa and BPH tissues had low frequencies
of YAP-positive cells, whereas PCa tissue had high frequencies (P=
0.008). In addition, the frequency of YAP-positive cells increased
significantly between low-grade PCa and high-grade PCa (P=0.033).
It was suggested that the YAP activity was closely-associated with
progression between well-differentiated and high-grade tumours. YAP
activity is a clinically relevant tool to predict an increasing
proclivity to develop metastases and higher PSA levels (P=0.0032).
Correlation analysis demonstrated that the protein expression of
YAP was significantly correlated with the mRNA expression,
indicating that the YAP gene transcription and protein expression
were coordinated. In the earlier stages of PCa, high levels of
expression of YAP were observed, and increased expression of YAP
was accompanied by increased stages of malignancy. The YAP gene is
a tumour-specific gene, which may be involved in the occurrence and
development of PCa.
It is known that current treatments, including
docetaxel only provide a modest increase in survival rates of
patients with CRPC, and the majority of patients eventually
progress due to drug resistance (38). Therefore, the present study
investigated PC-3 cells to determine whether there an association
exists between YAP and CRPC, and the results demonstrated that YAP
was expressed in PC-3 cells enabling further investigations.
The potency of the Hippo pathway in driving tissue
growth appears to reside in its ability to coordinately stimulate
cell proliferation and suppress apoptosis (39). A key goal is to understand how this
coordinated control is achieved. The results of the present study
demonstrated that pMagic7.1-Puro/GFP-RNAi can target YAP in PC-3
cells. Specific RNAi vectors targeting human YAP were transfected
into PC-3 cells with liposomes, and cell lines that stably
expressrf pMagic7.1-Puro/GFP-RNAi-YAP were obtained, as indicated
by the expression of GFP. Using RT-qPCR and western blotting, the
present study demonstrated that the mRNA and protein expression of
YAP were inhibited effectively in these cells. Using FCM and an MTT
colorimetric assay, it was observed that the proliferation of the
PC-3 cells decreased significantly, and cell-cycle was arrested at
the G1 stage in vitro when the YAP gene was suppressed. This
result was consistent with previous findings associated with YAP in
the majority of other tumour types (40). The mRNA and protein expression of
TEAD1 was also inhibited following the inhibited YAP.
In conclusion, the present study demonstrated the
efficacy of potent YAP knockdown in inhibiting the growth and
inducing apoptosis of CRPC cells in vitro. The inhibitory
effect induced by the deactivation of YAP may be explained, in
part, by inactivation of the Hippo-YAP pathway in the CRPC cells.
An important direction for future investigations is to elucidate
how YAP is involved in the development and progression of CRPC and
how it regulates the proliferation and cell-cycle of CRPC cells.
This may be facilitated by investigating the mechanism underlying
the Hippo-YAP pathway in CRPC. The results obtained in the present
study demonstrate the importance of YAP activity in CRPC cell
biology and indicate that further analysis of the molecular basis
for its tumour suppressive role is warranted. Understanding these
mechanisms may contribute to the development of preventive and
therapeutic strategies for the treatment of CRPC.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (grant no. 30972999). The contents of
the present study are solely the responsibility of the authors and
do not necessarily represent the official views of the Natural
Science Foundation of China.
References
|
1
|
Conlon I and Raff M: Size control in
animal development. Cell. 96:235–244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu S, Huang J, Dong J and Pan D: Hippo
encodes a Ste-20 family protein kinase that restricts cell
proliferation and promotes apoptosis in conjunction with Salvador
and warts. Cell. 114:445–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Chan SW, Zhang X, Walsh M, Lim CJ,
Hong W and Song H: Structural basis of YAP recognition by TEAD4 in
the hippo pathway. Genes Dev. 24:290–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating yorkie, the Drosophila
Homolog of YAP. Cell. 122:421–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nolo R, Morrison CM, Tao C, Zhang X and
Halder G: The bantam microRNA is a target of the hippo
tumor-suppressor pathway. Curr Biol. 16:1895–1904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson BJ and Cohen SM: The Hippo
pathway regulates the bantam microRNA to control cell proliferation
and apoptosis in Drosophila. Cell. 126:767–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertini E, Oka T, Sudol M, Strano S and
Blandino G: YAP: At the crossroad between transformation and tumor
suppression. Cell Cycle. 8:49–57. 2009. View Article : Google Scholar
|
|
9
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawada A, Kiyonari H, Ukita K, Nishioka N,
Imuta Y and Sasaki H: Redundant roles of Tead1 and Tead2 in
notochord development and the regulation of cell proliferation and
survival. Mol Cell Biol. 28:3177–3189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Ren F, Zhang Q, Chen Y, Wang B
and Jiang J: The TEAD/TEF family of transcription factor Scalloped
mediates hippo signaling in organ size control. Dev Cell.
14:377–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ota M and Sasaki H: Mammalian Tead
proteins regulate cell proliferation and contact inhibition as
transcriptional mediators of hippo signaling. Development.
135:4059–4069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan SW, Lim CJ, Loo LS, Chong YF, Huang C
and Hong W: TEADs mediate nuclear retention of TAZ to promote
oncogenic transformation. J Bio Chem. 284:14347–14358. 2009.
View Article : Google Scholar
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. Ca Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katoh M: Function and cancer genomics of
FAT family genes (review). Int J Oncol. 41:1913–1918.
2012.PubMed/NCBI
|
|
19
|
Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu
Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al: Hippo signaling is
a potent in vivo growth and tumor suppressor pathway in the
mammalian liver. Proc Natl Acad Sci USA. 107:1437–1442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Zhu JS, Xu ZP and Zhang Q:
Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits
growth and induces aoptosis in the SGC7901 gastric cancer cell
line. Mol Med Rep. 4:1075–1082. 2011.PubMed/NCBI
|
|
21
|
Li W, Wang L, Katoh H, Liu R, Zheng P and
Liu Y: Identification of a tumor suppressor relay between the FOXP3
and the Hippo pathways in breast and prostate cancers. Cancer Res.
71:2162–2171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zagurovskaya M, Shareef MM, Das A, Reeves
A, Gupta S, Sudol M, Bedford MT, Prichard J, Mohiuddin M and Ahmed
MM: EGR-1 forms a complex with YAP-1 and up regulates Bax
expression in irradiated prostate carcinoma cells. Oncogene.
28:1121–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tapon N, Harvey KF, Bell DW, Wahrer DC,
Schiripo TA, Haber D and Hariharan IK: Salvador promotes both cell
cycle exit and apoptosis in Drosophila and is mutated in human
cancer cell lines. Cell. 110:467–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishioka N, Inoue K, Adachi K, Kiyonari H,
Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N,
et al: The Hippo signaling pathway components Lats and Yap pattern
Tead4 activity to distinguish mouse trophectoderm from inner cell
mass. Dev Cell. 16:398–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berney DM: Low Gleason score prostatic
adenocarcinomas are no longer viable entities. Histopathology.
50:683–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Na Y, Ye Z and Sun G: Diagnosis and
Treatment Guideline of Chinese Urology Diseases. People's Medical
Publishing House; Beijing: pp. 52–53. 2011, In Chinese.
|
|
27
|
Hudson TS, Perkins SN, Hursting SD, Young
HA, Kim YS, Wang TC and Wang TT: Inhibition of androgen-responsive
LNCaP prostate cancer cell tumor xenograft growth by dietary
phenethyl isothiocyanate correlates with decreased angiogenesis and
inhibition of cell attachment. Int J Oncol. 40:1113–1121.
2012.PubMed/NCBI
|
|
28
|
Sheng X, Li Z, Wang DL, Li WB, Luo Z, Chen
KH, Cao JJ, Yu C and Liu WJ: Isolation and enrichment of PC-3
prostate cancer stem-like cells using MACS and serum-free medium.
Oncol Lett. 5:787–792. 2013.PubMed/NCBI
|
|
29
|
Wang DL, Lan JH, Chen L, Huang B, Li Z,
Zhao XM, Ma Q, Sheng X, Li WB and Tang WX: Integrin-linked kinase
functions as a tumor promoter in bladder transitional cell
carcinoma. Asian Pac J Cancer Prev. 13:2799–2806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okamoto Y, Ohkubo T, Ikebe T and Yamazaki
J: Blockade of TRPM8 activity reduces the invasion potential of
oral squamous carcinoma cell lines. Int J Oncol. 40:1431–1440.
2012.PubMed/NCBI
|
|
31
|
De Ganck A, De Corte V, Bruyneel E, Bracke
M, Vandekerckhove J and Gettemans J: Down-regulation of myopodin
expression reduces invasion and motility of PC-3 prostate cancer
cells. Int J Oncol. 34:1403–1409. 2009.PubMed/NCBI
|
|
32
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao B, Li L, Wang L, Wang CY, Yu J and
Guan KL: Cell detachment activates the Hippo pathway via
cytoskeleton reorganization to induce anoikis. Genes Dev. 26:54–68.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamazaki H, Nishiyama K, Tanaka E, Maeda
O, Meguro N, Kinouchi T, Usami M, Kakimoto K, Ono Y and Nishimura
T: Reduction of irradiation volume and toxicities with 3-D
radiotherapy planning over conventional radiotherapy for prostate
cancer treated with long-term hormonal therapy. Anticancer Res.
28:3913–3920. 2008.
|
|
36
|
Mizuno T, Murakami H, Fujii M, Ishiguro F,
Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H, et al:
YAP induces malignant mesothelioma cell proliferation by up
regulating transcription of cell cycle-promoting genes. Oncogene.
31:5117–5122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rick FG, Schally AV, Szalontay L, Block
NL, Szepeshazi K, Nadji M, Zarandi M, Hohla F, Buchholz S and Seitz
S: Antagonists of growth hormone-releasing hormone inhibit growth
of androgen-independent prostate cancer through inactivation of ERK
and Akt kinases. Proc Natl Acad Sci USA. 109:1655–1660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Grusche FA and Harvey KF: Control
of tissue growth and cell transformation by the
Salvador/Warts/Hippo pathway. PLoS One. 7:e319942012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|