Introduction
Lysophosphatidic acid (LPA) is a phospholipid
derivative that is synthesized by the removal of the choline group
from lysophosphatidylcholine. LPA is an intermediate in the
synthesis of phosphatidic acid and is able to act as a signaling
molecule in various cell types (1–4). In
particular, LPA acts as a potent mitogen via activation of
high-affinity G protein-coupled receptors, such as EDG2 and EDG4
(5,6). Due to its ability to stimulate cell
proliferation, aberrant LPA signaling has been linked to cancer
progression, through numerous mechanisms. Dysregulation of the LPA
receptors may lead to cellular hyperproliferation, which may in
turn contribute to oncogenesis and metastasis (7–9).
Signaling associated with LPA has also been reported to regulate
the growth of fibroblasts, vascular smooth muscle cells,
endothelial cells, and keratinocytes (10–13).
In addition, the small GTPase Rho, a downstream molecule of the LPA
receptor signal cascade, induces the formation of stress fibers and
enhances cell migration (4).
Therefore, LPA has been identified as a strong mitogenic factor.
Recently, the molecular mechanisms underlying the mitogenic effects
of LPA have been reported. Saunders et al (14) reported that LPA increases cell
growth and inhibits apoptosis via the redox-dependent activation of
extracellular signal-regulated kinases (ERK)-, Akt-, and nuclear
factor-κB-dependent signaling pathways in ovarian cancer cells
(14). Furthermore, the existence
of an association between LPA signaling and reactive oxygen species
(ROS)-mediated signaling has been suggested in other cell types
(15–17).
In addition to cancer cells, numerous studies have
reported that LPA exerts stimulatory effects on mesenchymal stem
cells (MSCs). LPA protects MSCs against hypoxia and serum
deprivation-induced apoptosis via the ERK12 and phosphoinositide
3-kinase (PI3K)/Akt signaling pathways, and induces the migration
of human lung-resident MSCs via the β-catenin pathway (18,19).
LPA also rescues MSCs from endoplasmic reticulum stress-associated
apoptosis, induced by hypoxia and serum deprivation through the p38
pathway (20). In addition, LPA
induces the osteoblastic differentiation of MSCs by increasing
cyclic adenosine mono-phosphate and Ca2+ levels
(21). LPA also affects the
paracrine activity of MSCs, and stimulates secretion of vascular
endothelial growth factor (VEGF) and stem cell-derived factor 1
(22). LPA promotes VEGF secretion
by increasing the expression of 150 kDa oxygen-related proteins in
MSCs (23). However, there are few
studies that specifically address the molecular mechanisms
underlying LPA-induced ASC proliferation and migration. Our
previous studies demonstrated that ROS generation exerts
stimulatory effects on ASC proliferation and migration (24–27);
however, the association between ROS and LPA signaling in ASCs
remains poorly understood. Therefore, the present study aimed to
investigate the stimulatory effects of LPA on ASC proliferation and
migration, and the association between ROS and LPA signaling in
ASCs, as well as the microRNA (miR) expression in ASCs following
LPA treatment.
Materials and methods
Cell culture and chemical inhibition
The sampling of human subcutaneous adipose tissue,
isolation of ASCs, and characterization of ASCs with ASC-specific
surface markers using flow cytometry were performed as previously
described (28,29). Liposuction aspirates were obtained
from a healthy female donor following the attainment of informed
consent and approval from Bundang CHA Hospital (Seongnam, Korea;
BD2011-152D). Human ASCs were cultured in α-minimum essential
medium (Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (Gibco Life Technologies) and 1%
penicillin-streptomycin (Gibco Life Technologies) at 37°C in a
humidified atmosphere containing 5% CO2.
For chemical inhibition studies, the ASCs were
co-treated with 5 mM U0126 (ERK12 inhibitor; EMD Millipore,
Billerica, MA, USA), 5 mM LY294002 (PI3KAkt inhibitor; EMD
Millipore), 0.1–1 mM Ki16425 (Sigma-Aldrich, St. Louis, MO, USA) or
oleoyl-L-α-lysophosphatidic acid sodium salt (Sigma-Aldrich). For
the inhibition of ROS generation, the ASCs were treated with 100
µM NADPH oxidase (Nox) inhibitor diphenyleneiodonium
chloride (DPI; Sigma-Aldrich).
Cell proliferation assay
ASCs were seeded at 7×103 cells/well in
48-well plates. Following incubation with the LPA (1–50 µM)
for 48 or 72 h, cell numbers were measured using the Cell Counting
kit (CCK)-8 Assay (Dojindo Molecular Technologies, Inc., Rockville,
MD, USA). Briefly, the medium in each well was replaced with medium
containing the water-soluble tetrazolium salt WST-8 (10% vv).
Following incubation for 2 h at 37°C, absorbance was measured at
450 nm using a Sunrise™ Microplate Absorbance Reader (Tecan Group
Ltd., Männedorf, Switzerland).
Cell migration assay
Cell migration was measured using a transwell
chamber. The transwell inserts with an 8 µm 3422 pore
polycarbonate membrane (Corning Life Sciences, Cambridge, MA, USA),
were coated with 0.1% gelatin (Sigma-Aldrich) in phosphate-buffered
saline (PBS) for 2 h at 37°C. The ASCs were seeded at
1×104 cells per 200 µl in the upper chamber and
incubated for 2 h at 37°C in an atmosphere containing 5%
CO2. The ASCs were allowed to migrate towards 500
µl medium containing 10 µM LPA in the lower chamber.
Migration induced by serum-free medium was used as a negative
control. Following 16 h incubation at 37°C, the transwell inserts
were fixed with ice-cold 100% methanol for 20 min and stained with
0.23% crystal violet (Sigma-Aldrich) in 2% ethanol for 30 min.
Following further washing with distilled water, the non-migratory
cells were removed by gently wiping the upper face of the transwell
inserts with a cotton swab. Images of the stained cells were
captured using a light microscope (CKX41; Olympus, Tokyo, Japan)
and were analyzed using Image J 1.47 software (National Institutes
of Health, Bethesda, MD, USA), in order to determine the number of
cells that had migrated to the lower side of membrane.
Intracellular ROS staining
Intracellular ROS production was measured by flow
cytometry using the ROS-sensitive dye 2′,7′-dichlorofluorescin
diacetate (DCF-DA; Molecular Probes Life Technologies, Carlsbad,
CA, USA), as previously described (25,26,30).
Briefly, the ASCs were seeded at 2.5×105 cells/60 mm
dish. The following day, the cells were incubated with 20 µM
DCF-DA for 10 min at 37°C, and treated with LPA for 20 min at 37°C.
Following incubation, the cells were trypsinized and resuspended in
PBS. Green fluorescence intensity was measured using a BD
FACSCalibur flow cytometer and analyzed using CellQuest Pro
software (BD Biosciences, Franklin Lakes, NJ, USA).
Polymerase chain reaction (PCR) array and
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using the RNeasy kit (Qiagen,
Inc., Valencia, CA, USA), and was reverse transcribed using a cDNA
Synthesis kit (Promega Corporation, Madison, WI, USA), according to
the manufacturer's instructions.
The synthesized cDNA was used in a PCR Array
analysis using a CFX96 Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. A PAHS-014ZD Human Signal Transduction
Pathway Finder RT2 Profiler PCR Array (Qiagen, Inc.), which was
composed of 84 transcripts representing 10 distinct signaling
pathways, was used to identify the genes associated with signaling
pathways that may be affected by LPA treatment. Differential gene
expression and statistical analysis of the data were performed
using PCR Array Data Analysis software (Qiagen, Inc.).
RT-qPCR was performed using a StepOne Plus Real-Time
PCR system (Applied Biosystems Life Technologies, Foster City, CA,
USA) using the following primers: Adrenomedullin (ADM) forward, CTT
ATT CGG CCC CAG GACAT, and reverse, ACT GGT AGA TCT GGT GTGCC;
Serpine1 forward, CCG CCT CTT CCA CAA ATCAG, and reverse, AAT GTT
GGT GAG GGC AGAGA; LPA receptor 1 (LPAR1) forward, TCA TCT GGA CTA
TGG CCATC, and reverse, CAA GTT GAA AAT GGC CCAGA; LPAR2 forward,
CAA TGC TGC TGT GTA CTCTT, and reverse, TGG GCA GAG GAT GTA TAGTG;
LPAR3 forward, CTA CAA GGA CGA GGA CATGT, and reverse, ATC CTC TAT
GTA CTG GCTGC; LPAR4 forward, TCT GCA AGA TCT CTG GAACT, and
reverse, ACA CAA TGG CAG AAT TCCTC; LPAR5 forward, TTC TCC CGT GTC
CTG ACTA, and reverse, ACA TGT ACA CGC TCA CCAC; LPAR6 forward, TTG
TAT GGG TGC ATG TTCAG, and reverse, CAA GTC TGA CAT TGC CAAGT; and
GAPDH forward, CGA GAT CCC TCC AAA ATCAA, and reverse, TGT GGT CAT
GAG TCC TTCCA. GAPDH served as a loading control. Thermal cycling
over 40 cycles consisted of an initial denaturation at 95°C for 10
min, then 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec, and
was terminated by a final extension at 60°C for 1 min.
Measurement of the expression levels of
miR-210
Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and 0.5
µg total RNA was reverse transcribed using a Mir-X miRNA
First-Strand Synthesis kit (Clontech Laboratories, Inc., Mountain
View, CA, USA) according to the manufacturer's instructions. The
expression levels of miR-210 were measured by RT-qPCR using the
miR-210 primer (CTG TGC GTG TGA CAG CGG CTGA; Genolution
Pharmaceuticals, Inc., Seoul, Korea) as previously described
(24). U6 small nuclear RNA served
as a loading control (Clontech Laboratories, Inc.).
Transfection with small interfering
(si)RNA and miR-210 inhibitor
The Stealth RNAi™ siRNA specific to human Nox4
(sense, UUA UCC AAC AAU CUC CUG GUU CUCC; and antisense, GGA GAA
CCA GGA GAU UGU UGG AUAA), the Silencer Select siRNA for human
Serpine1 (ID# s10013), and the non-targeting negative control
(scramble) siRNA (Silencer Select Negative Control#1 siRNA) were
purchased from Invitrogen Life Technologies. The miR-210 inhibitor
(hsa-miR210) and the miRNA inhibitor negative control were
purchased from Genolution Pharmaceuticals, Inc. ASCs were seeded in
60 mm dishes at a density of 2×106 with complete medium.
The following day, confluent ASCs were maintained in serum-free
medium without antibiotics for 2 h. Subsequently, the ASCs were
transfected with 20 nM of each siRNA or miR-210 inhibitor using
Lipofectamine® 2000 transfection reagent (Invitrogen
Life Technologies), according to the manufacturer's instructions in
24-well plates or 60 mm dishes.
Western blotting
Proteins of ASCs were isolated using SDS sample
buffer. The soluble protein concentration was determined using the
Quick start Bradford 1X dye reagent (Bio-Rad Laboratories, Inc.).
The western blot analysis was performed as previously described
(25) using antibodies targeting
p-ERK12 (1:2,000; cat. no. 9106; in 5% milk), ERK12 (1:4,000; cat.
no. 9102; in 5% milk), p-Akt (1:1,000; cat. no. 9271; in 5% milk),
and Akt (1:4,000; cat. no. 9272; in 5% milk). All antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish-peroxidase (HRP)-conjugated secondary mouse antibody
(1:10,000) was purchased from Vector Laboratories, Inc.
(Burlingame, CA, USA) and HRP-conjugated secondary rabbit antibody
was purchased from Cell Signaling Technology, Inc. (1:10,000; cat.
no. 7074).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis of the data was performed using
Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA).
A Student's t-test was used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
LPA increases the proliferation and
migration of ASCs
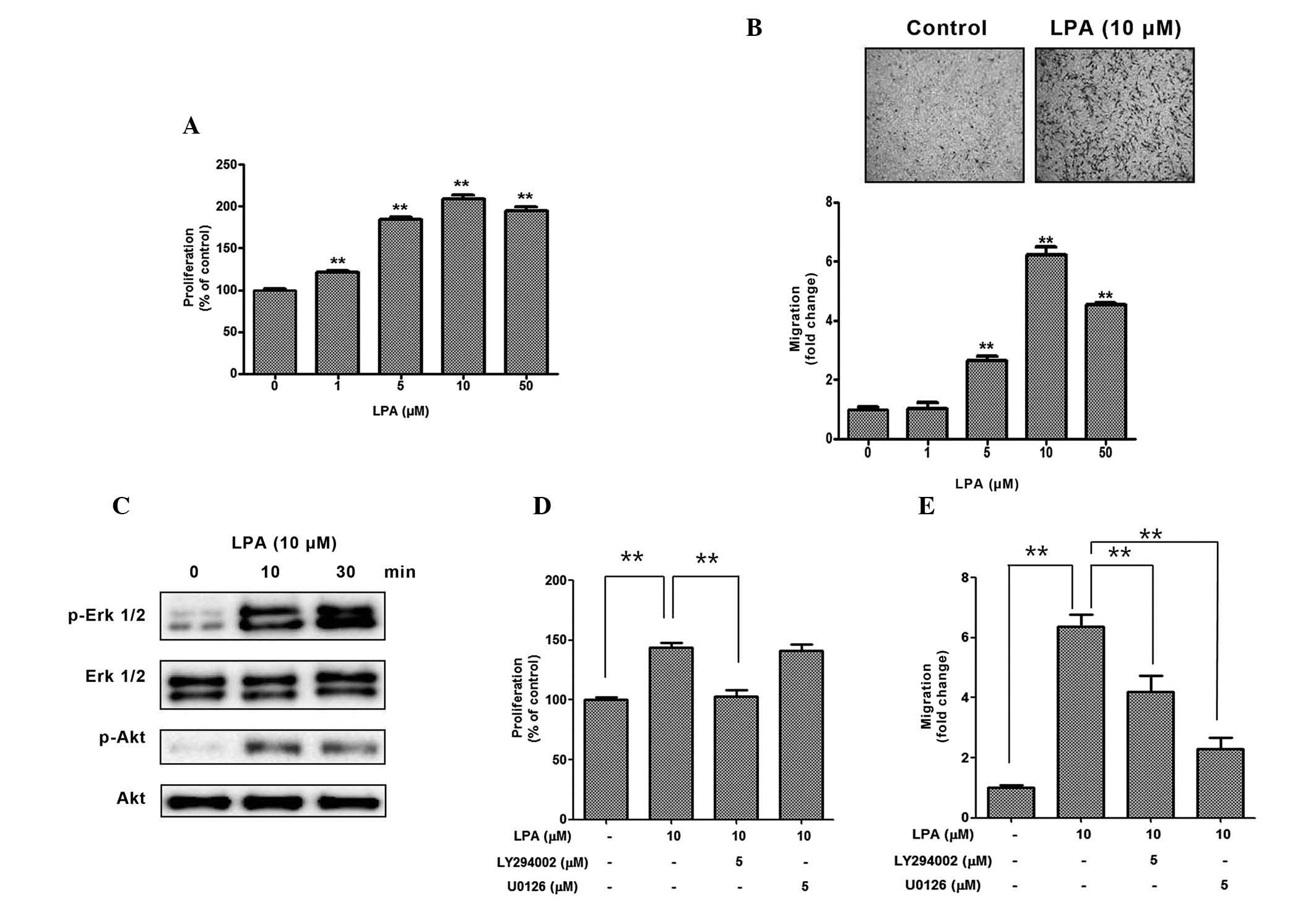
The present study initially investigated whether LPA
treatment increased the proliferation and migration of ASCs. As
expected, 1–50 mM LPA significantly increased the proliferation
(Fig. 1A) and migration (Fig. 1B) of ASCs in a dose-dependent
manner. Since PI3K/Akt and mitogen-activated protein kinase (MAPK)
signaling pathways have previously been reported to be associated
with the mitogenic effects of LPA, the phosphorylation levels of
Akt and ERK12 were measured following LPA treatment, and
phosphorylation of both proteins increased in a time-dependent
manner (Fig. 1C). In the
inhibition study, pharmacological inhibition of PI3K/Akt by
LY294002 significantly reduced the LPA-induced proliferation of
ASCs (Fig. 1D). In addition,
pharmacological inhibition of PI3K/Akt by LY294002, and of ERK by
U0126 significantly reduced the LPA-induced migration of ASCs
(Fig. 1E). These results suggest
that LPA acts as a strong mitogenic factor in ASCs.
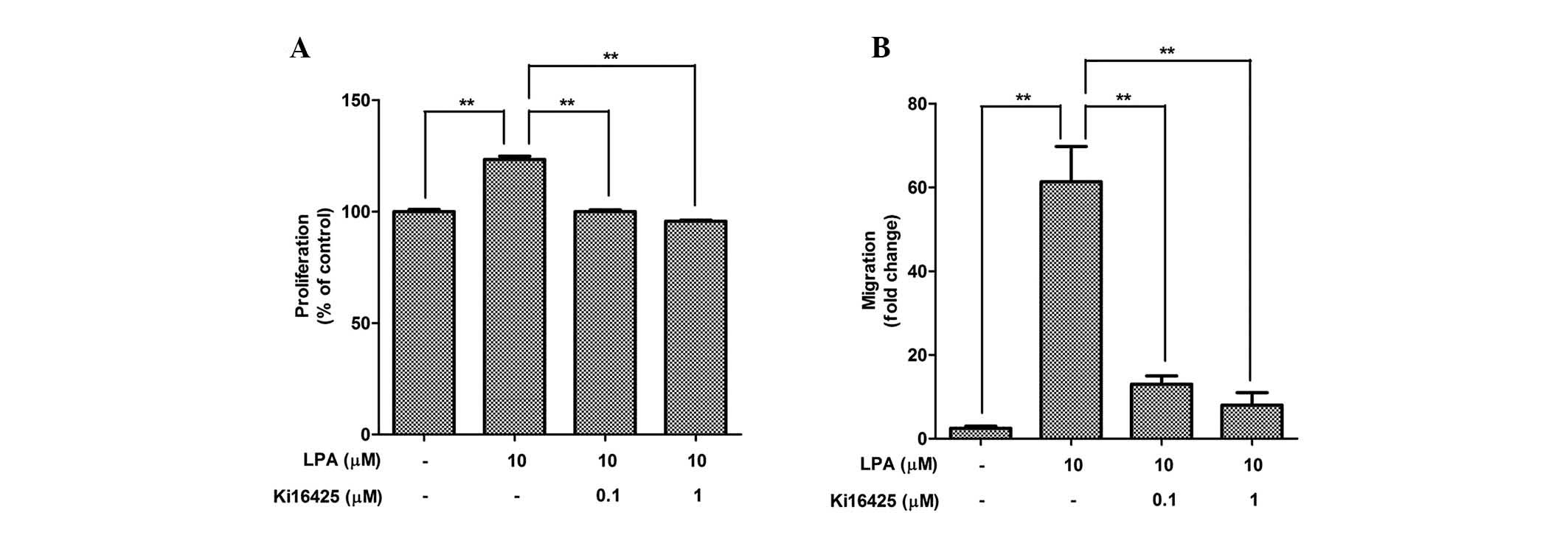
LPA mediates the mitogenic effects of
ASCs via the LPA receptor
The expression levels of LPAR were measured in the
ASCs (Table I), indicating that
LPAR1 is highly expressed in ASCs, as compared with the other
isoforms. To measure the involvement of LPAR in the mitogenic
function of LPA, pharmacological inhibition of LPAR by Ki16425 was
performed. Co-treatment with Ki16425 (0.1 and 1 mM) significantly
attenuated LPA-induced proliferation (Fig. 2A) and migration (Fig. 2B) of ASCs.
 | Table ILPAR expression in adipose-derived
stem cells. |
Table I
LPAR expression in adipose-derived
stem cells.
| Gene | Ct-value |
|---|
| LPAR1 | 24.44±0.50 |
| LPAR2 | 29.82±0.78 |
| LPAR3 | 30.85±0.94 |
| LPAR4 | 30.84±0.90 |
| LPAR5 | 31.44±0.54 |
| LPAR6 | 29.60±0.57 |
| GAPDH | 18.84±0.09 |
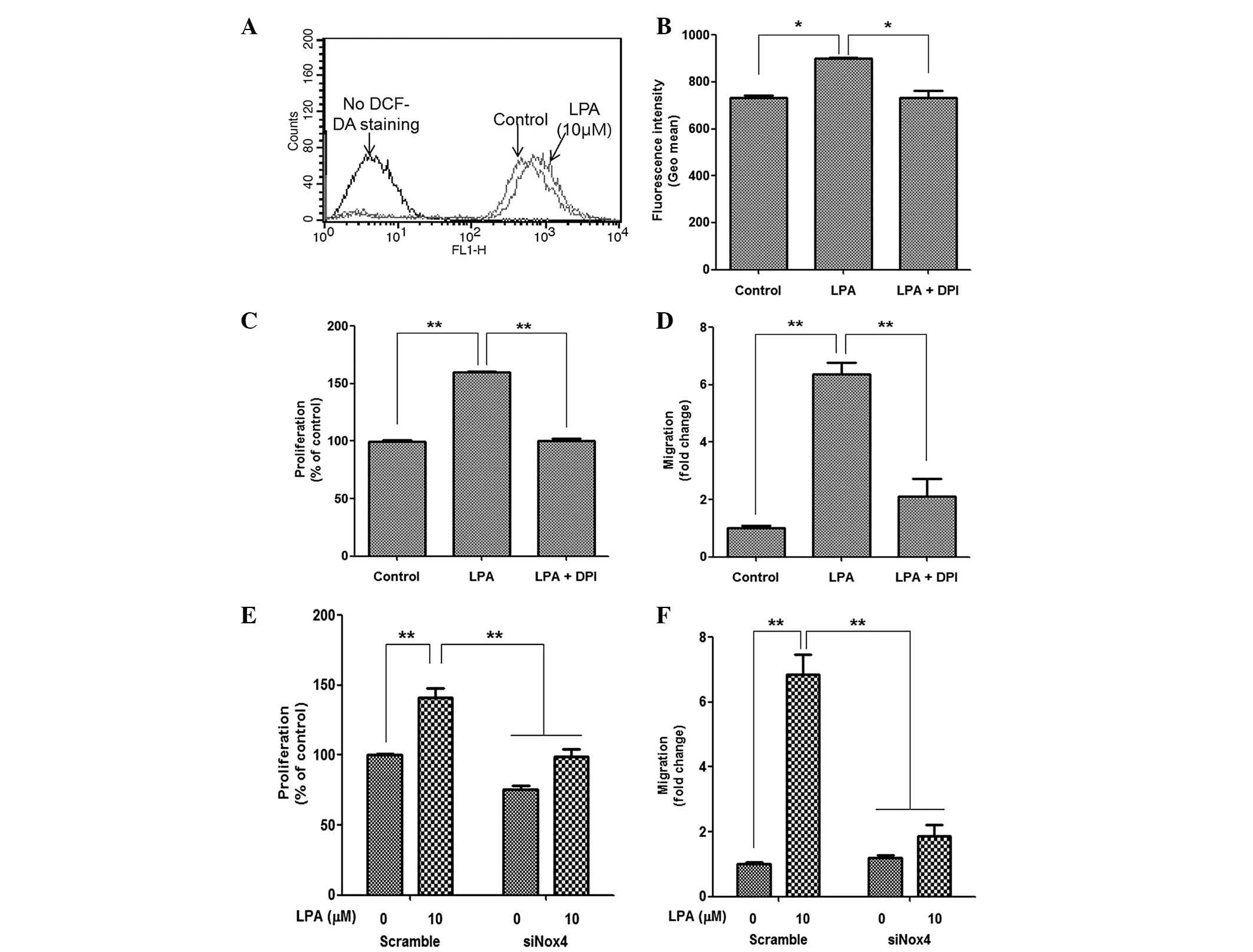
LPA treatment induces ROS generation
The present study also investigated whether the
LPA-induced proliferation and migration of ASCs was mediated by ROS
generation. As expected, LPA treatment significantly increased the
fluorescence intensity of DCF-DA, as determined by flow cytometric
analysis (Fig. 3A); and
pharmacological inhibition of Nox following treatment with DPI
significantly attenuated the fluorescence intensity of DCF-DA
(Fig. 3B). In addition, DPI
treatment significantly reduced LPA-induced proliferation (Fig. 3C) and migration (Fig. 3D) of ASCs. As Nox4 is highly
expressed in ASCs and primarily mediates ROS generation (26), downregulation of Nox4 expression in
ASCs by siRNA transfection and Nox4 silencing significantly
decreased LPA-induced cell proliferation (Fig. 3E) and migration (Fig. 3F). These results suggest that Nox4
may be associated with LPA-mediated ROS generation and downstream
mitogenic effects in ASCs.
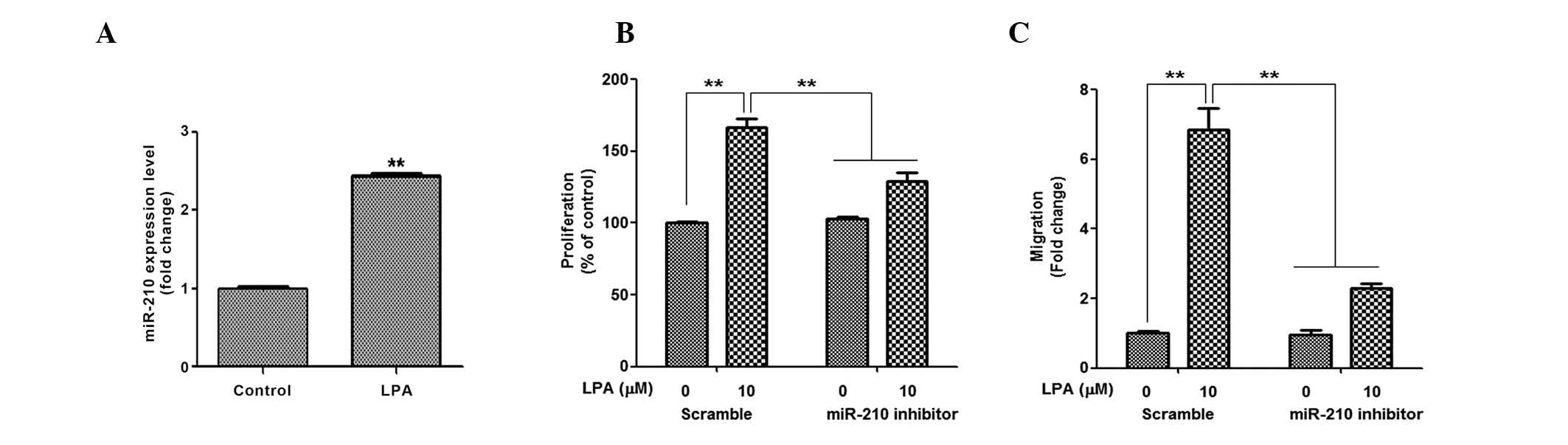
LPA increases miR-210 expression
Our previous study demonstrated that ROS generation
induced by hypoxia, or chemicals, induced miR-210 expression and
increased the proliferation and migration of ASCs (24); therefore, the present study
investigated the involvement of miR-210 in the LPA-induced
proliferation and migration of ASCs. As expected, LPA treatment
increased miR-210 expression (Fig.
4A); however, the extent of upregulation was not as great as
that induced by hypoxia and chemical ROS donors. In the inhibition
study, miR-210 inhibitors significantly attenuated LPA-induced
proliferation (Fig. 4B) and
migration (Fig. 4C) of ASCs. These
results suggest that miR-210 is involved in the LPA-induced
proliferation and migration of ASCs.
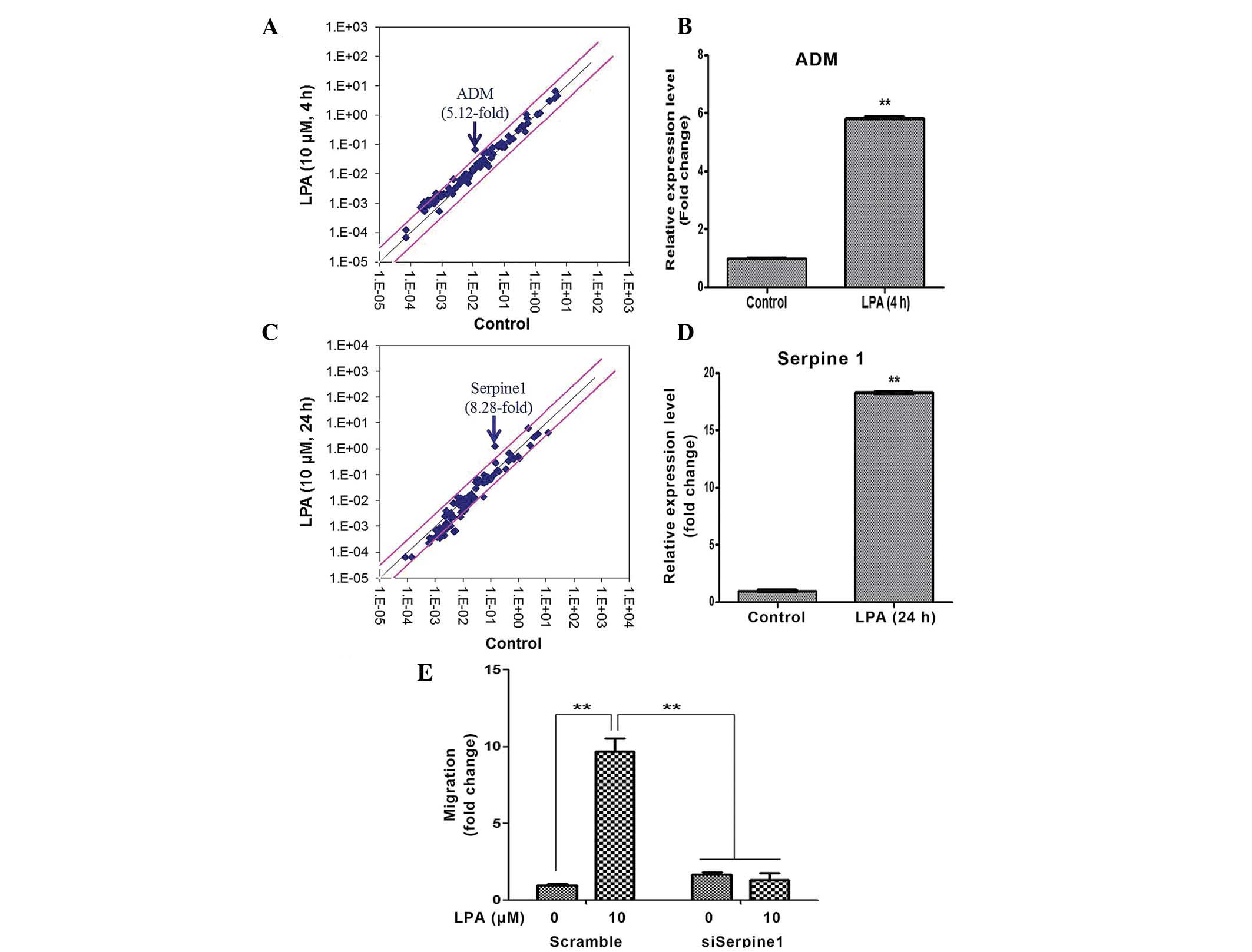
LPA upregulates the expression of ADM and
Serpine1
The human Signal Transduction Pathway Finder RT2
Profiler PCR Array was used to identify the LPA target genes that
mediated the proliferation and migration of ASCs. A total of 4 h
following LPA treatment, the expression levels of ADM increased
5.12-fold, as demonstrated by the PCR array (Fig. 5A). RT-qPCR analysis confirmed that
the expression levels of ADM were upregulated ~6-fold (Fig. 5B). A total of 24 h following LPA
treatment, the expression levels of Serpine1 increased 8.28-fold,
as determined by the PCR array (Fig.
5C), and ~18-fold, as determined by the RT-qPCR (Fig. 5D). Therefore, the present study
investigated whether ADM and/or Serpine1 mediated LPA-induced
proliferation and migration of ASCs using siRNAs specific for ADM
and Serpine1. Although silencing of ADM did not affect
proliferation or migration (data not shown), transfection with
Serpine1 siRNA significantly attenuated the LPA-induced migration
of ASCs (Fig. 5E).
Discussion
The present study investigated the signal pathways
and molecular mechanisms underlying LPA-induced proliferation and
migration of ASCs. Previous studies have demonstrated that LPA is
able to mediate the proliferation and migration of various cells,
primarily via the ERK12 and PI3K/Akt signaling pathways (19,31,32).
The results of the present study demonstrated that LPA may function
as a mitogenic signal via LPARs, and the ERK12 and PI3K/Akt
signaling pathways in ASCs. In addition to these signaling
pathways, the present study demonstrated that LPA treatment induced
ROS generation via Nox4, and that these ROS act as signaling
molecules to increase the proliferation and migration of ASCs.
Furthermore, upregulation of miR-210 expression by ROS may
contribute to the increased proliferation and migration of ASCs,
results which are concordant with those of a previous study
(24). LPA also upregulated
Serpine1 expression, which increased ASC migration.
As determined by PCR array, the expression levels of
ADM and Serpine1 increased 4 and 24 h following LPA treatment,
respectively. In addition, the results of the present study
demonstrated that Serpine1 mediated LPA-induced migration of ASCs;
however, no proliferation-associated genes were identified in the
present study. Although the involvement of ADM in ASC proliferation
was hypothesized, siRNA silencing of ADM did not inhibit the
proliferation of ASCs. A previous study also reported that ADM did
not increase, but rather inhibited, LPA-induced proliferation, and
attenuated the stimulation of the MAPK pathway by LPA treatment in
adventitial cells (33).
Therefore, the mediator(s) of LPA-induced proliferation of ASCs
have yet to be identified.
miRNAs exert their actions primarily at the
post-transcriptional level, and their expression is known to be
regulated by redox signaling (24,34–36).
In our previous study, the expression levels of miR-210 were
significantly increased via various ROS generators (hypoxia,
antimycin, rotenone, and platelet-derived growth factor subunit B),
and regulated the proliferation and migration of ASCs (24). The present study demonstrated that
LPA-induced ROS generation upregulated miR-210 expression in ASCs,
and increased the proliferation and migration of ASCs. However, the
extent of miR-210 LPA-induced upregulation was lower, as compared
with other ROS generators such as hypoxia. In addition, ROS
generation by S1P did not induce miR-210 expression in ASCs (data
not shown). Therefore, it is reasonable to assume that ROS
generation is not a prerequisite for the upregulation of miR-210
expression in ASCs.
In conclusion, the present study demonstrated that
LPA increases the proliferation and migration of ASCs via
Nox4-induced ROS generation. The present study is the first, to the
best of our knowledge, to demonstrate that miR-210 expression is
associated with LPA-induced stimulation, and that Serpine1 mediates
the LPA-induced migration of ASCs. Therefore, phospholipid
derivatives, such as LPA and S1P, may be used to stimulate ASCs
during stem cell expansion.
Acknowledgments
The present study was supported by a grant from the
Yonsei University Research Fund (grant no. 2014-12-0040).
References
|
1
|
Glazier AT, Blackmore PF, Nolan RD and
Wasilenko WJ: Attenuation of LPA-mediated calcium signaling and
inositol poly-phosphate production in rat-1 fibroblasts transformed
by the v-src oncogene. Biochem Biophys Res Commun. 245:607–612.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonkers J and Moolenaar WH: Mammary
tumorigenesis through LPA receptor signaling. Cancer Cell.
15:457–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sorensen SD, Nicole O, Peavy RD, Montoya
LM, Lee CJ, Murphy TJ, Traynelis SF and Hepler JR: Common signaling
pathways link activation of murine PAR-1, LPA, and S1P receptors to
proliferation of astrocytes. Mol Pharmacol. 64:1199–1209. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tangkijvanich P, Melton AC, Santiskulvong
C and Yee HF Jr: Rho and p38 MAP kinase signaling pathways mediate
LPA-stimulated hepatic myofibroblast migration. J Biomed Sci.
10:352–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bandoh K, Aoki J, Taira A, Tsujimoto M,
Arai H and Inoue K: Lysophosphatidic acid (LPA) receptors of the
EDG family are differentially activated by LPA species.
Structure-activity relationship of cloned LPA receptors. FEBS Lett.
478:159–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagida K, Kurikawa Y, Shimizu T and
Ishii S: Current progress in non-Edg family LPA receptor research.
Biochim Biophys Acta. 1831:33–41. 2013. View Article : Google Scholar
|
|
7
|
Hao F, Tan M, Xu X, Han J, Miller DD,
Tigyi G and Cui MZ: Lysophosphatidic acid induces prostate cancer
PC3 cell migration via activation of LPA(1), p42 and p38alpha.
Biochim Biophys Acta. 1771:883–892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sengupta S, Xiao YJ and Xu Y: A novel
laminin-induced LPA autocrine loop in the migration of ovarian
cancer cells. FASEB J. 17:1570–1572. 2003.PubMed/NCBI
|
|
9
|
Shida D, Watanabe T, Aoki J, Hama K,
Kitayama J, Sonoda H, Kishi Y, Yamaguchi H, Sasaki S, Sako A, et
al: Aberrant expression of lysophosphatidic acid (LPA) receptors in
human colorectal cancer. Lab Invest. 84:1352–1362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon YJ, Sun Y, Kim NH and Huh SO:
Phosphorylation of CREB, a cyclic AMP responsive element binding
protein, contributes partially to lysophosphatidic acid-induced
fibroblast cell proliferation. Biochem Biophys Res Commun.
380:655–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee H, Goetzl EJ and An S:
Lysophosphatidic acid and sphingosine 1-phosphate stimulate
endothelial cell wound healing. Am J Physiol Cell Physiol.
278:C612–C618. 2000.PubMed/NCBI
|
|
12
|
Sauer B, Vogler R, Zimmermann K, Fujii M,
Anzano MB, Schäfer-Korting M, Roberts AB and Kleuser B:
Lysophosphatidic acid interacts with transforming growth
factor-beta signaling to mediate keratinocyte growth arrest and
chemotaxis. J Invest Dermatol. 123:840–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokumura A, Iimori M, Nishioka Y, Kitahara
M, Sakashita M and Tanaka S: Lysophosphatidic acids induce
proliferation of cultured vascular smooth muscle cells from rat
aorta. Am J Physiol. 267:C204–C210. 1994.PubMed/NCBI
|
|
14
|
Saunders JA, Rogers LC, Klomsiri C, Poole
LB and Daniel LW: Reactive oxygen species mediate lysophosphatidic
acid induced signaling in ovarian cancer cells. Free Radic Biol
Med. 49:2058–2067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Q, Olashaw N and Wu J: Participation
of reactive oxygen species in the lysophosphatidic acid-stimulated
mitogen-activated protein kinase kinase activation pathway. J Biol
Chem. 270:28499–28502. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Idzko M, Laut M, Panther E, Sorichter S,
Dürk T, Fluhr JW, Herouy Y, Mockenhaupt M, Myrtek D, Elsner P and
Norgauer J: Lysophosphatidic acid induces chemotaxis, oxygen
radical production, CD11b up-regulation, Ca2+
mobilization, and actin reorganization in human eosinophils via
pertussis toxin-sensitive G proteins. J Immunol. 172:4480–4485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CC, Lin CE, Lin YC, Ju TK, Huang YL,
Lee MS, Chen JH and Lee H: Lysophosphatidic acid induces reactive
oxygen species generation by activating protein kinase C in PC-3
human prostate cancer cells. Biochem Biophys Res Commun.
440:564–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badri L and Lama VN: Lysophosphatidic acid
induces migration of human lung-resident mesenchymal stem cells
through the β-catenin pathway. Stem Cells. 30:2010–2019. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Baydoun AR, Xu R, Deng L, Liu X,
Zhu W, Shi L, Cong X, Hu S and Chen X: Lysophosphatidic acid
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Stem Cells. 26:135–145. 2008.
View Article : Google Scholar
|
|
20
|
Li Z, Wei H, Liu X, Hu S, Cong X and Chen
X: LPA rescues ER stress-associated apoptosis in hypoxia and serum
deprivation-stimulated mesenchymal stem cells. J Cell Biochem.
111:811–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YB, Kharode Y, Bodine PV, Yaworsky PJ,
Robinson JA and Billiard J: LPA induces osteoblast differentiation
through interplay of two receptors: LPA1 and LPA4. J Cell Biochem.
109:794–800. 2010.PubMed/NCBI
|
|
22
|
Jeon ES, Heo SC, Lee IH, Choi YJ, Park JH,
Choi KU, Park do Y, Suh DS, Yoon MS and Kim JH: Ovarian
cancer-derived lysophosphatidic acid stimulates secretion of VEGF
and stromal cell-derived factor-1 alpha from human mesenchymal stem
cells. Exp Mol Med. 42:280–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei H, Wang F, Wang X, Yang J, Li Z, Cong
X and Chen X: Lysophosphatidic acid promotes secretion of VEGF by
increasing expression of 150-kD Oxygen-regulated protein (ORP150)
in mesenchymal stem cells. Biochim Biophys Acta. 1831:1426–1434.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JH, Park SG, Song SY, Kim JK and Sung
JH: Reactive oxygen species-responsive miR-210 regulates
proliferation and migration of adipose-derived stem cells via
PTPN2. Cell Death Dis. 4:e5882013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JH, Park SH, Park SG, Choi JS, Xia Y
and Sung JH: The pivotal role of reactive oxygen species generation
in the hypoxia-induced stimulation of adipose-derived stem cells.
Stem Cells Dev. 20:1753–1761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JH, Song SY, Park SG, Song SU, Xia Y
and Sung JH: Primary involvement of NADPH oxidase 4 in
hypoxia-induced generation of reactive oxygen species in
adipose-derived stem cells. Stem Cells Dev. 21:2212–2221. 2012.
View Article : Google Scholar :
|
|
27
|
Park SG, Kim JH, Xia Y and Sung JH:
Generation of reactive oxygen species in adipose-derived stem
cells: Friend or foe? Expert Opin Ther Targets. 15:1297–1306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim WS, Park BS, Park SH, Kim HK and Sung
JH: Antiwrinkle effect of adipose-derived stem cell: Activation of
dermal fibroblast by secretory factors. J Dermatol Sci. 53:96–102.
2009. View Article : Google Scholar
|
|
29
|
Kim WS, Park BS, Sung JH, Yang JM, Park
SB, Kwak SJ and Park JS: Wound healing effect of adipose-derived
stem cells: A critical role of secretory factors on human dermal
fibroblasts. J Dermatol Sci. 48:15–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong YM, Sung YK, Kim WK, Kim JH, Kwack
MH, Yoon I, Kim DD and Sung JH: Ultraviolet B preconditioning
enhances the hair growth-promoting effects of adipose-derived stem
cells via generation of reactive oxygen species. Stem Cells Dev.
22:158–168. 2013. View Article : Google Scholar :
|
|
31
|
Kue PF, Taub JS, Harrington LB,
Polakiewicz RD, Ullrich A and Daaka Y: Lysophosphatidic
acid-regulated mitogenic ERK signaling in androgen-insensitive
prostate cancer PC-3 cells. Int J Cancer. 102:572–579. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu X, Haney N, Kropp D, Kabore AF,
Johnston JB and Gibson SB: Lysophosphatidic acid (LPA) protects
primary chronic lymphocytic leukemia cells from apoptosis through
LPA receptor activation of the anti-apoptotic protein AKT/PKB. J
Biol Chem. 280:9498–9508. 2005. View Article : Google Scholar
|
|
33
|
Yang JH, Jiang W, Pan CS, Qi YF, Wu QZ,
Pang YZ and Tang CS: Effects of adrenomedullin on cell
proliferation in rat adventitia induced by lysophosphatidic acid.
Regul Pept. 121:49–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boesch-Saadatmandi C, Wagner AE, Wolffram
S and Rimbach G: Effect of quercetin on inflammatory gene
expression in mice liver in vivo - role of redox factor 1,
miRNA-122 and miRNA-125b. Pharmacol Res. 65:523–530. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishimoto T, Sugihara H, Watanabe M,
Sawayama H, Iwatsuki M, Baba Y, Okabe H, Hidaka K, Yokoyama N,
Miyake K, et al: Macrophage-derived reactive oxygen species
suppress miR-328 targeting CD44 in cancer cells and promote redox
adaptation. Carcinogenesis. 35:1003–1011. 2014. View Article : Google Scholar
|
|
36
|
Marin T, Gongol B, Chen Z, Woo B,
Subramaniam S, Chien S and Shyy JY: Mechanosensitive microRNAs-role
in endothelial responses to shear stress and redox state. Free
Radic Biol Med. 64:61–68. 2013. View Article : Google Scholar : PubMed/NCBI
|