Introduction
Bisphenol A (BPA) is an environmental
endocrine-disrupting compound (EDC) that is widely used in
polycarbonate plastics, including hard plastic bottles, water
pipes, toys, metal-based food and beverage cans, medical materials,
dental sealants, and building materials (1–4).
Exposure to EDCs has become a major concern for mammalian
development, due to the common daily exposure of mammals to
BPA-contaminated food and water. Furthermore, BPA may be released
from these industrial products by various physical or chemical
processes. The released BPA may then either be inhaled as dust, or
absorbed through the skin. As determined by random sampling,
detectable BPA was shown to be present in >90% of human urine
samples (5). Due to this
prevalence, the adverse health effects of BPA have been
investigated in numerous studies (6). Estrogen has a major role in female
reproduction; however, evidence suggests that estrogen is also
involved in the development and function of male reproductive
organs (7). BPA has been detected
in maternal blood, saliva and placental tissue samples (8), and may cause the abnormal functioning
of germ cells. Prenatal exposure to BPA in mice has been
demonstrated to reduce the efficiency of sperm production and to
affect the development of reproductive organs in male pups
(9). In addition, previous studies
have demonstrated that BPA exposure results in lifelong adverse
effects on the male reproductive system in rodents, as a
consequence of neonatal exposure to potent estrogens (8–10).
In rats, reproductive capability and gonad development (11), androgen action (12), spermatogenesis efficiency and
Sertoli cell number (13), as well
as abnormalities of the reproductive tract (14), were induced by neonatal exposure to
estrogens. The disruptive effects of potent estrogens (such as
diethylstilbestrol) may also occur in adulthood following normal
pubertal development (8).
BPA may disrupt the endocrine system via various
mechanisms. Kabuto et al (14) demonstrated that exposure to BPA
during embryonic/fetal development and infancy induced tissue
oxidative stress and peroxidation, ultimately leading to
underdevelopment of the brain, kidney and testes. The function of
organs and/or systems may be modulated by exposure to environmental
chemicals that interfere with signaling molecules (15). The Wnt/β-catenin signaling pathway
has an important role in the development and regulation of cell
growth. The Wnt/β-catenin signaling pathway is predominantly
composed of Wnt, Wnt receptor proteins, β-catenin, and the T-cell
factor/lymphoid enhancer factor-1 family (Tcf/Lef) transcription
factors, along with their downstream target genes (16,17).
To date, few studies have reported the effects of BPA on the
reproductive system of male mouse pups and the Wnt/β-catenin
signaling pathway. The present study evaluated the toxicological
effects of BPA exposure on the reproductive system by observing
morphological changes in ICR male mouse pup testes. In addition,
the effects of the Wnt/β-catenin signaling pathway on the embryonic
development of BPA-treated mice were investigated in order to
clarify the molecular mechanism underlying the effects of BPA, and
in order to provide a theoretical basis for treatment
strategies.
Materials and methods
Chemicals and instruments
BPA (99% purity; Sigma-Aldrich, St Louis, MO, USA)
was dissolved in saline containing dimethyl sulfoxide (DMSO;
Shanghai Chemical Reagent Co., Ltd., Shanghai, China), with a DMSO
concentration <0.01%, in order to obtain the selected doses.
Rabbit anti-mouse β-catenin (cat. no. sc-7199), and anti-dickkopf
WNT signaling pathway inhibitor 1 (DKK-1; cat. no. sc-25516)
monoclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA), and the Secondary Antibody
Immunohistochemistry kit (biotinylated horse anti-mouse IgG; 1:200
dilution) was purchased from OriGene Technologies, Inc. (Beijing,
China). The TSJ-1A Automatic Organizations Dehydration machine was
purchased from Tianli Aviation Electrical Co., Ltd. (Tianjin,
China), and the RM2135 Leica Paraffin Slicing machine was from
Leica Microsystems (Wetzlar, Germany). The BM-II Pathological
Tissue Embedding machine was purchased from the Electric Power
Research Institute (Anhui, China), and the Nikon 80 I Biological
Microscopic Imaging and Analysis system from Nikon Corporation
(Tokyo, Japan).
Animals and experimental design
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Anhui Medical
University (Hefei, China). A total of 60 female and 30 male
9-week-old ICR mice were obtained from the Animal Center of Anhui
Province (Hefei, China) and housed at 25°C in conventional
polystyrene cages in a 12 h light/dark cycle. The mice were
provided a rodent diet and high purity water (reverse osmosis
filtered) provided in glass water bottles ad libitum. The
mice were allowed to acclimatize to the housing conditions for one
week prior to being placed in a cage (male:female, 2:1) at 9:00 pm,
and checked the following morning at 7:00 am. Mating was confirmed
by the presence of a vaginal plug, which is a well-known sign of
pregnancy. Once the vaginal plug was observed, the pregnant mice
were separated from the males and individually caged. The day the
vaginal plug was detected was defined as gestation day (GD) 1. On
GD 1, the confirmed pregnant females (40 dams in total) were
randomly divided into four treatment groups (10 dams per group): A
solvent control group, which was orally treated with DMSO, and
three groups, which were treated with 0.5, 10, or 50
µg/kg/day BPA. The mice were treated with BPA by placing a
pipette tip of their mother's milk containing the dosing solution
into the mouth once a day from GD 1 to postnatal day (PND) 42. To
maintain the BPA concentration, the dose was changed every 2–3 days
with the growth of weight. The number of offspring produced by each
pregnant mouse, the male:female ratio in each group, and the testis
coefficient were subsequently counted.
Specimens and immunohistochemistry
A total of 10 randomly chosen male pups were
assigned for staining on PND 42. The male pups were initially
weighed, prior to being anesthetized by intraperitoneal
administration of 1% 0.1–0.2 ml sodium pentobarbital
(Sigma-Aldrich) and sacrificed immediately by exsanguination, and
the abdominal cavity was then rapidly opened and testis tissue
samples were harvested. The testis tissue was subsequently weighed
and the organ coefficients were calculated (testicular organ
coefficient = weight of mice / wet weight of testis). One side
(left) of the testicular tissue sample was fixed in 10% neutral
formalin (pH 7.2–7.4) for 24 h at room temperature and rinsed in
running water overnight, prior to being embedded in paraffin. Each
testis tissue sample was cut into 5 µm sections and mounted
onto a glass slide, and every 5th section of the testis tissue
sample was developmentally evaluated by counting the number of germ
cells, which were positively stained with hematoxylin and eosin
(HE; Beyotime Institute of Biotechnology, Haimen, China). All
sections were evaluated by an observer who had no knowledge of the
treatment groups. The number of germ cells present in a subset of
sections was confirmed by another observer who also had no
knowledge of the treatment groups. The remaining sections were
immunostained as previously described (18). Immunohistochemistry was performed
on the paraffin-embedded mouse testes on PND 42, using antibodies
targeting β-catenin (1:200 dilution) and DKK-1 (1:100 dilution).
The nuclei were stained with hematoxylin. The stained sections were
then observed under a microscope (Nikon Corporation).
Immunohistochemical analysis was performed on the testes of the
male mice from each group, and the β-catenin and DKK-1-positive
cells were counted using a Biological Microscopic Imaging and
Analysis system (Nikon Corporation). The number of cells exhibiting
positive β-catenin and DKK-1 expression in each section was
recorded for statistical analysis.
Quantification of the molecules involved
in the Wnt/β-catenin pathway
The protein expression levels of β-catenin and DKK-1
were analyzed by western blotting. The remaining side (right) of
the testicular tissue samples of the male mice were removed. Under
sterile conditions, the testicular tissue samples were weighed. The
cell lysates were sonicated for 5 min on ice and centrifuged at
7,500 × g for 10 min to sediment the particulate material. The
testicular tissue samples were homogenized and the protein
concentration of the supernatant was subsequently quantified using
a bicinchoninic acid assay kit (Beyotime Institute of
Biotechnology). Following normalization to a similar protein
concentration, the samples and marker (10–230 kDa) (New England
Biolabs Inc., Ipswich, MA, USA) were concentrated, and 50 µg
protein was separated by 5 and 12% SDS-PAGE, prior to being
transferred to nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA), and incubated with the following primary antibodies:
Rabbit anti-mouse β-catenin antiserum (1:2,000 dilution),
anti-DKK-1 monoclonal antiserum (1:1,500 dilution) and anti-β-actin
(1:5,000 dilution; cat. no. sc-47778, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 1 h at room temperature, and then at 4°C
overnight. The membranes were subsequently washed three times with
tris-buffered saline containing Tween 20, and then incubated with a
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. The
immunoreactive bands were visualized using an Enhanced
Chemiluminescence Reagent Western Blot Detection system (Pierce
Biotechnology, Inc., Rockford, IL, USA). The protein expression
levels were quantified with β-actin as an internal control using
Image-Pro Plus 6.0 image analysis software (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
All statistical data were expressed as the mean ±
standard deviation. SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Statistical
analysis was performed by one-way analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of BPA on murine reproduction and
development
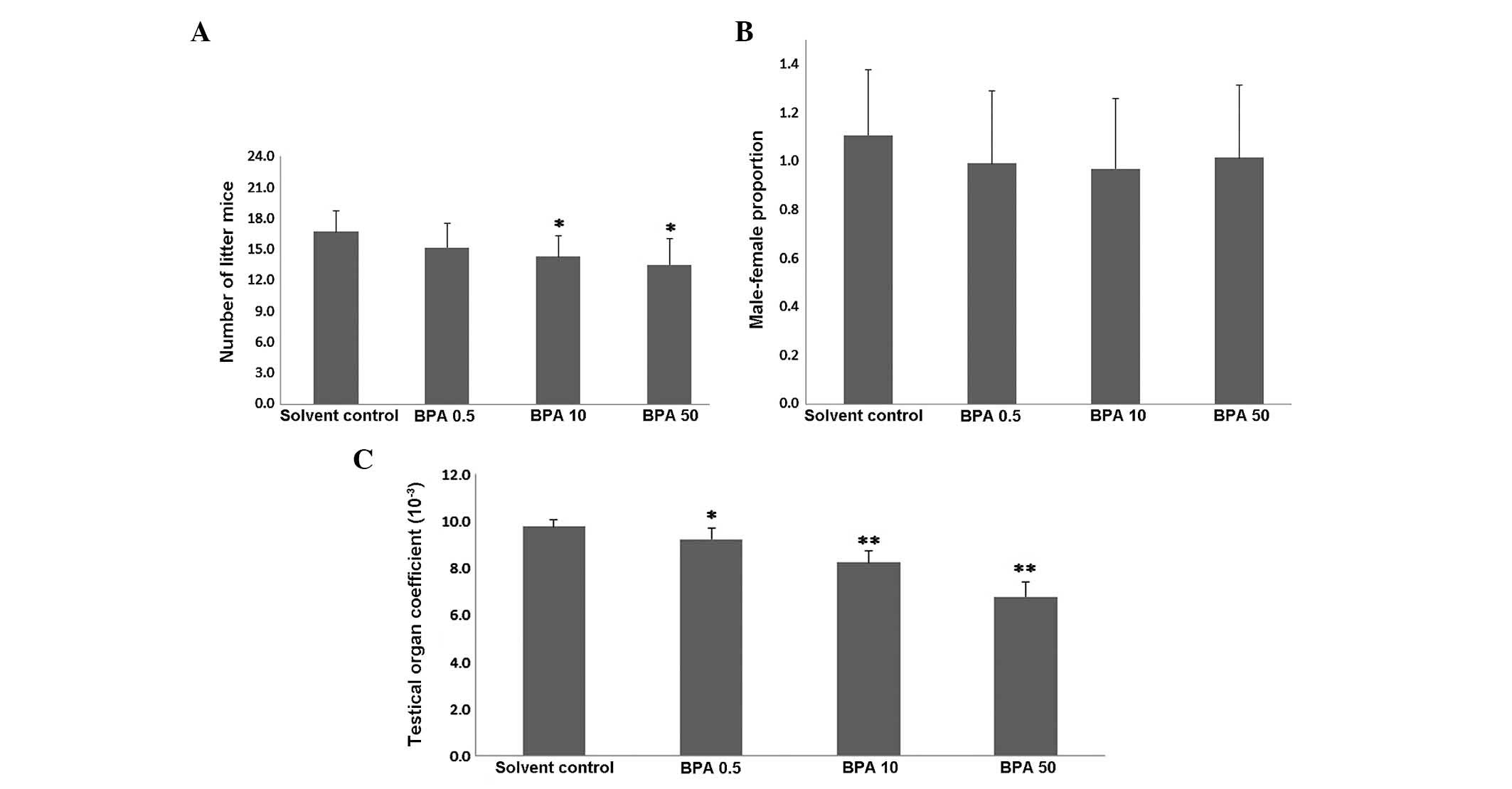
In order to determine the effects of BPA on
spermatogenesis, testis development was evaluated in the mice
treated with BPA for 42 days. The number of offspring in the ICR
pregnant mice was significantly lower (7–12/litter) in the 10 and
50 µg/kg/day BPA-treated pregnant mice, as compared with the
vehicle control group (P<0.05, Fig.
1A). However, no statistically significant difference was
observed in the male:female ratio of the BPA-exposed pregnant mice,
as compared with the vehicle control group (P>0.05, Fig. 1B). With increasing BPA dosage, the
testicular viscera coefficient of the BPA-exposed male offspring
mice declined, as compared with the control group (P<0.05 or
P<0.01, Fig. 1C).
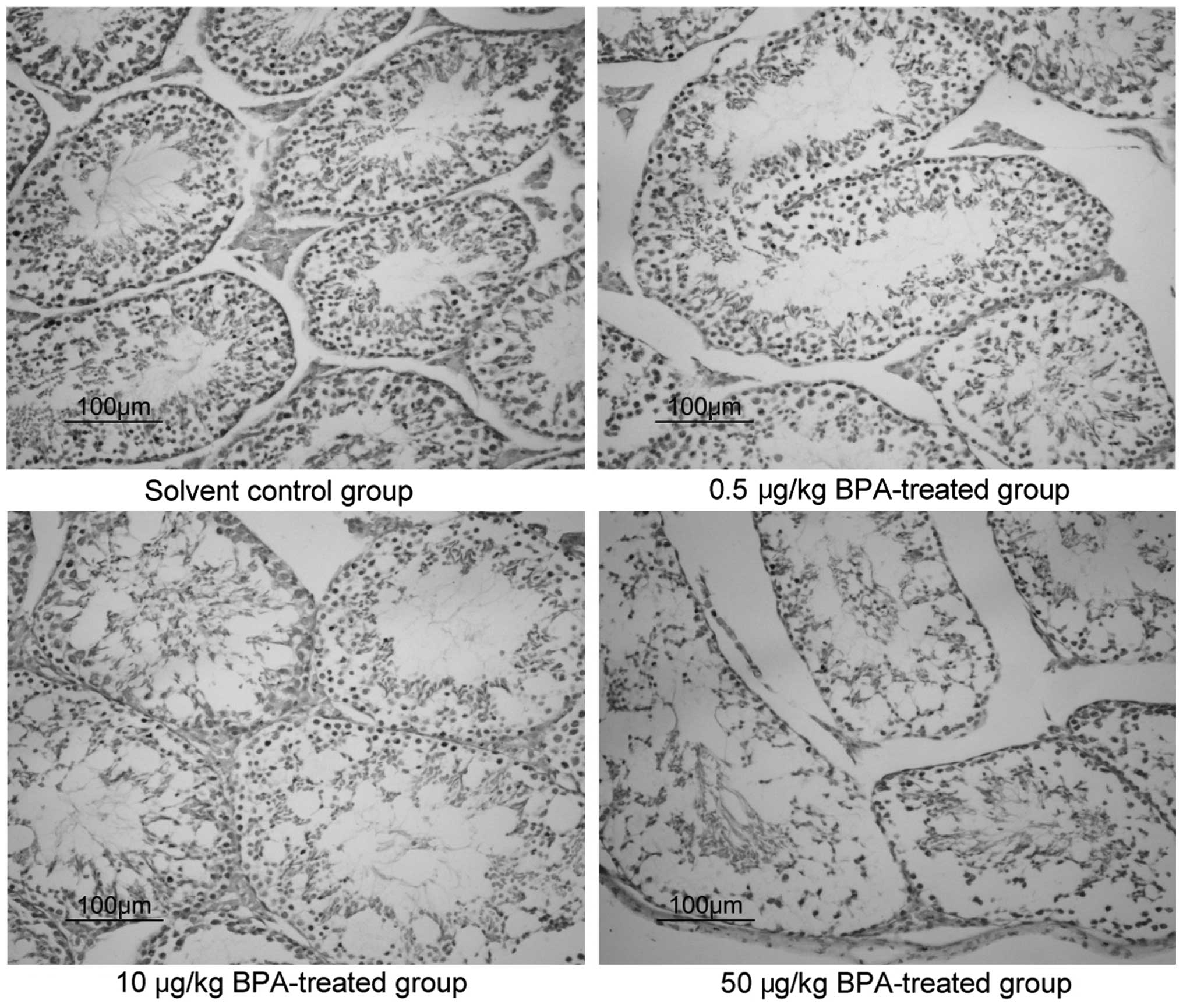
Pathological changes in the murine
testicular tissue samples, as determined by HE staining
HE staining was used to observe pathological changes
in the murine testicular tissue samples of the various groups at 6
weeks (Fig. 2). In the control
group, normal morphology of the testicular tissue was observed,
whereas in the BPA-treated groups, the seminiferous tubules of the
testicular tissue and the tube cell wall layers and cells appeared
disordered, observations that worsened in a dose-dependent
manner.
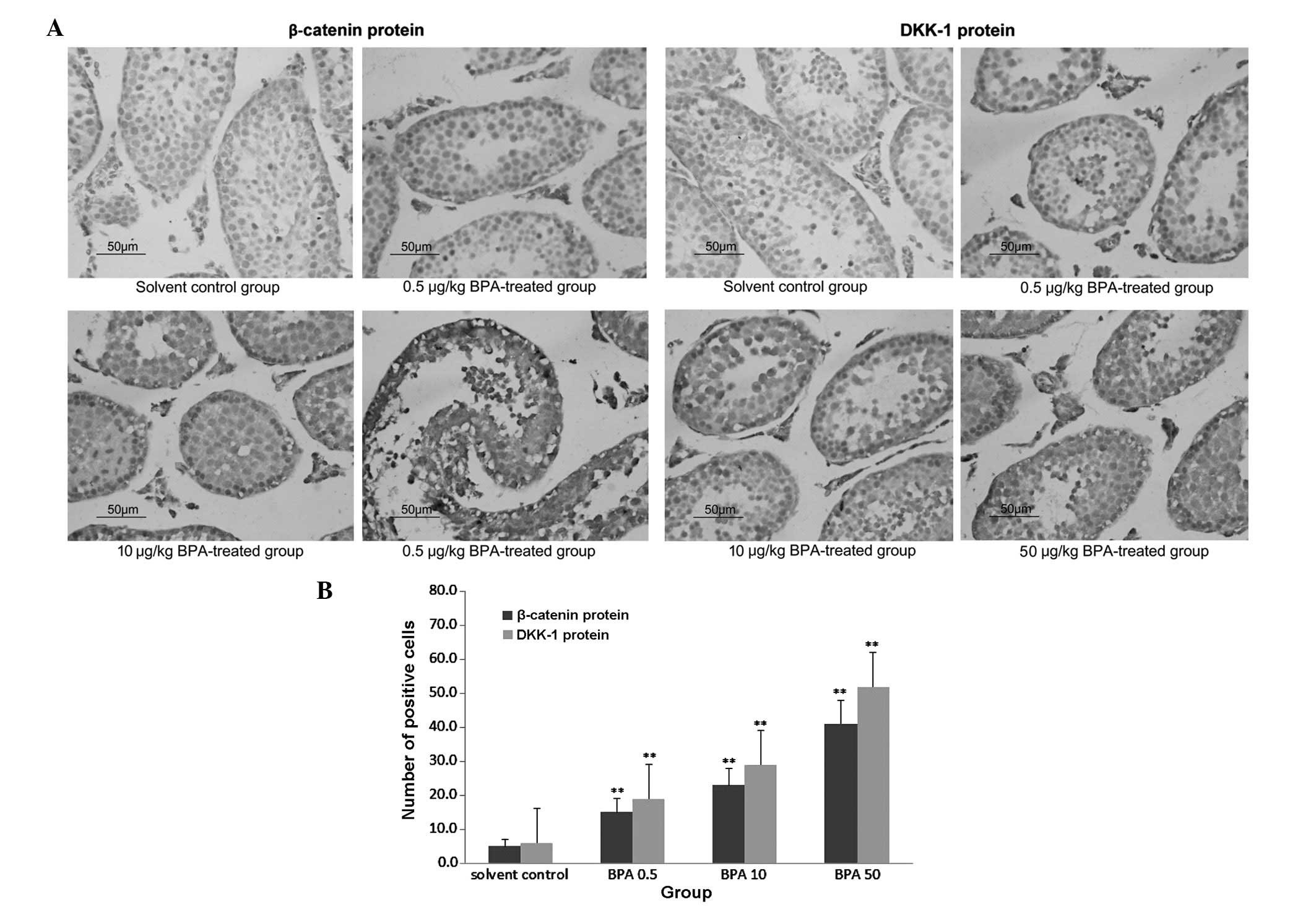
Protein expression of β-catenin and
DKK-1, as determined by immunohistochemical analysis
The protein expression of β-catenin and DKK-1 was
markedly increased in the BPA-treated groups, as compared with the
control group. The expression was increased in a dose-dependent
manner, and was predominantly observed in the spermatogenic and
Leydig cells (Fig. 3A). As shown
in Fig. 3B, the number of
β-catenin and DKK-1 positive cells significantly increased in the
BPA-treated groups (P<0.01), as compared with the solvent
control group, as determined by the Nikon 80 I Biological
Microscopic Camera and Analysis system.
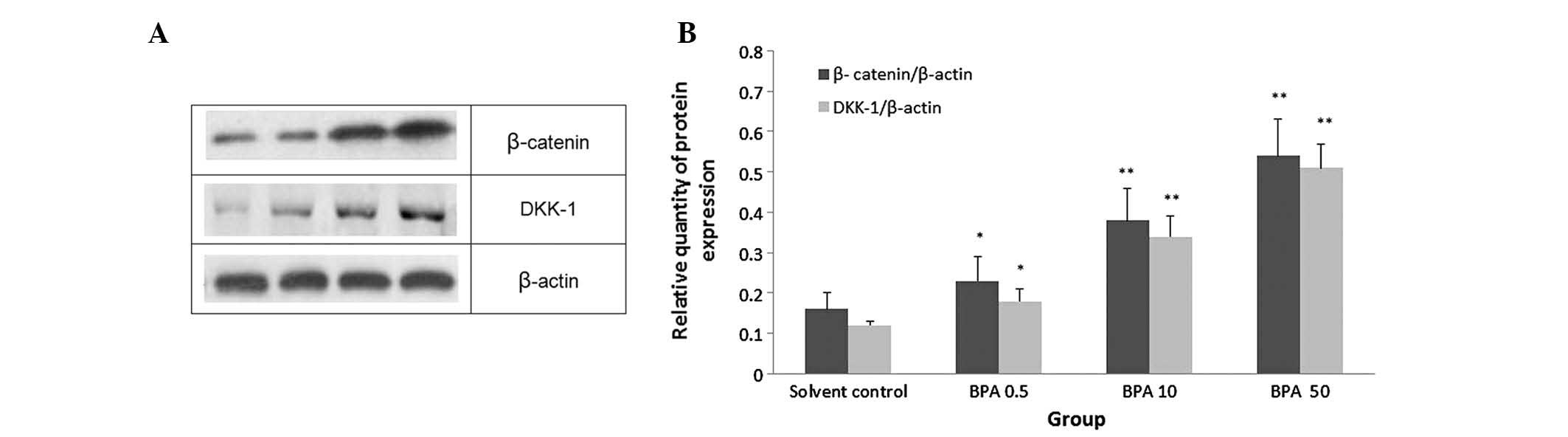
Effects of BPA on the protein expression
levels of β-catenin and DKK-1 in the testicular tissue samples
To investigate the mechanism underlying the effects
of BPA on the Wnt/β-catenin signaling pathway, the protein
expression levels of β-catenin and DKK-1 were detected in the
testicular tissue samples of the various groups by western blotting
(Fig. 4A). Higher protein
expression levels of β-catenin and DKK-1 were detected in the
BPA-treated groups, as compared with the control group, and
expression was increased in a dose-dependent manner (P<0.05, or
P<0.01; Fig. 4B).
Discussion
BPA is a monomer component of epoxy resins, which
are used in numerous food-contact plastics. Over the past decade,
numerous studies have reported that BPA acts as a potential EDC
(19,20). EDCs or xenoestrogens are able to
disrupt endocrine functions by blocking endogenous hormones
(21). At present, the hypothesis
that BPA acts as an EDC is primarily based on the results of in
vitro studies, which have seldom been confirmed in vivo.
BPA was chosen to be investigated in the present study due to its
presence in resin-based dental sealants and composites, and due to
the degradation of BPA in certain resins (1–3,22).
Human exposure to similar doses of BPA has also been reported to
occur from the consumption of foods preserved in lacquer-coated
cans (23). The BPA doses used in
the present study were selected according to previous reports on
the effects of BPA on the fertility and reproduction of mice
exposed to BPA during pregnancy (24–26).
Therefore, the long-term effects of xenoestrogens such as BPA on
human health requires further investigation in vivo. The
present study aimed to investigate the adverse effects of BPA on
the fertility and reproduction of adult male mice. The results
demonstrated that ingestion of BPA from GD 1 to PND 42 induced
adverse effects on male germ cell development. BPA was administered
at a dose of 0.5, 10 and 50 µg/kg, and no significant
effects on murine fertility were observed between the various
treatment groups. Compared with the control group, no significant
difference was identified in the number of litter mice and the
male:female ratio of the BPA-exposed pregnant mice. The testicular
coefficient of the male mouse pups was adversely affected following
ingestion of BPA xenoestrogen, and the number of pups in the
litters of the BPA-treated groups also decreased. A previous study
demonstrated that daily sperm production was significantly reduced
and the structure of sperm cells underwent significant changes in
adult mice treated with BPA (27).
The testes carry out two predominant functions: The production of
sperm during spermatogenesis, and the synthesis of testosterone and
a small amount of estrogen. The presence of normal testicular
tissue structure and function is a prerequisite for the maintenance
of reproductive capacity. BPA was shown to increase the levels of
murine sperm abnormality, and interfere with the growth and
development of the sperm by crossing the blood-testis barrier.
These effects were enhanced with an increase in exposure time
(28). The results of the present
study indicated that no significant difference was observed in the
male:female ratio of the litters. The HE staining results of the
present study contradict those of a recent study, which
demonstrated that pregnancy rates were significantly reduced in
females exposed to high doses of BPA during pregnancy (29). In addition, in the BPA-treated
groups, the seminiferous tubules of the testicular tissue and the
tube cell wall layers and cells appeared disordered, observations
that worsened in a dose-dependent manner. During cell
differentiation, BPA alters gene activity levels during sensitive
developmental periods, so that the function of BPA-exposed cells
are irreversibly disrupted. BPA acts as a long-term EDC, which
deregulates the development of testicular germ cells. Although
numerous studies have been conducted on BPA, its effects on the
reproductive system remain controversial. A previous study
demonstrated that treatment with BPA during pregnancy exerted
effects on the reproductive cells of the male offspring, effects
that increased in a dose-dependent manner (30).
The Wnt/β-catenin signaling pathway is involved in
cell growth and development, predominantly via Wnt, Wnt receptor
proteins, β-catenin, and Tcf/Lef nuclear transcription factors and
their downstream target genes (31). The standardized Wnt/β-catenin
pathway is initiated by the binding of Wnt to cell surface
molecules LRP5/6 and Frizzled (Frz), which induces the release of
cytoplasmic β-catenin from a protein complex composed of Axin1/2,
glycogen synthase kinase 3b, casein kinase 1, and adenomatous
polyposis coli. β-catenin subsequently translocates into the cell
nucleus by dephosphorylation and release. β-catenin then interacts
with the Tcf/Lef1, which activates the expression of target genes
in the nucleus (32).
Wnt is rich in cysteine residues and contains a
highly conserved glycoprotein signal. More than 19 types of Wnt
genes have been identified in the genomes of mice and humans
(33). Wnt proteins bind to the
cell membrane receptor Frz, which causes the cytoplasmic shock
protein Dishevelled to inhibit the β-catenin degradation pathway;
β-catenin then relocates from the cytoplasm into the cell nucleus,
where it acts as the downstream transcriptional activator of the
Wnt signaling pathway, via binding with Tcf/Lef nuclear sites,
which induces the activation of downstream target gene
transcription (34). Previous
studies have also shown that DKK-1 has an important role in the Wnt
signaling pathway, and is involved in embryonic development
(35,36). The present study investigated the
mechanism underlying Wnt signaling, and the association between Wnt
signaling and testis development in male mice. The results of the
present study demonstrated that the protein expression levels of
β-catenin and DKK-1 were significantly increased in the BPA-treated
groups, as compared with the control group, as determined by
immunohistochemical analyses and cell counting. These upregulated
expression levels further increased with BPA treatment, and were
predominantly observed in the spermatogenic and Leydig cells.
However, the mechanism underlying the Wnt/β-catenin signaling
pathway and its association with BPA-induced inhibition of male
mouse reproductive cell growth and development remains to be
further elucidated.
The majority of the downstream target genes of the
Wnt/β-catenin signaling pathways are involved in cell cycle
regulation, and cell proliferation, differentiation, and apoptosis,
which are important for numerous embryonic developmental events,
including the formation of the embryonic axis an abdomen, and the
establishment of cell polarity and cell fate (37). If the Wnt signaling pathway is
inhibited, embryonic development will gradually be disrupted
resulting in mutated phenotypes, such as the Wnt3 mutation that
causes head deformities, or lack of (short) limb deformities
(38). In addition, a previous
study demonstrated that induction of the downstream components of
the Wnt/β-catenin signaling pathway, including inhibitor of bone
morphology proteins and DKK-1 proteins, induces cell apoptosis,
which in turn leads to birth defects (39). The results of the present study
demonstrated that the protein expression levels of β-catenin and
DKK-1 were markedly increased following BPA treatment, in a
dose-dependent manner, as compared with the control group.
In conclusion, treatment of ICR pregnant mice with
BPA resulted in structural changes in the spermatogenic and Leydig
cells of male offspring. In addition, investigation of the
Wnt/β-catenin signaling pathway, which regulates the development of
male murine reproductive cells, suggested that BPA may pass through
the placental barrier, affecting the development of endogenous
testicular sperm cells; however, the mechanism underlying the
effects of BPA requires further research.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81373421),
and the Innovative Entrepreneurial Training of the National College
Students' Program (grant no. 201310366007). The authors are
grateful to Dr Tong Shen (Toxicology Experimental Center of the
Drug Research Office in Anhui Medical University, China) for
contributions to animal experiments.
References
|
1
|
Yamamoto T, Yasuhara A, Shiraishi H and
Nakasugi O: Bisphenol A in hazardous waste landfill leachates.
Chemosphere. 42:415–418. 2001. View Article : Google Scholar
|
|
2
|
Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y
and Taketani Y: Determination of bisphenol A concentrations in
human biological fluids reveals significant early prenatal
exposure. Hum Reprod. 17:2839–2841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vandenberg LN, Hauser R, Marcus M, Olea N
and Welshons WV: Human exposure to bisphenol A (BPA). Reprod
Toxicol. 24:139–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michałowicz J: Bisphenol A - sources,
toxicity and biotransformation. Environ Toxicol Pharmacol.
37:738–758. 2014. View Article : Google Scholar
|
|
5
|
Ouchi K and Watanabe S: Measurement of
bisphenol A in human urine using liquid chromatography with
multi-channel coulometric electrochemical detection. J Chromatogr B
Analyt Technol Biomed Life Sci. 780:365–370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pupo M, Pisano A, Lappano R, Santolla MF,
De Francesco EM, Abonante S, Rosano C and Maggiolini M: Bisphenol A
induces gene expression changes and proliferative effects through
GPER in breast cancer cells and cancer-associated fibroblasts.
Environ Health Perspect. 120:1177–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattsson A, Mura E, Brunström B, Panzica G
and Halldin K: Selective activation of estrogen receptor alpha in
Japanese quail embryos affects reproductive organ differentiation
but not the male sexual behavior or the parvocellular vasotocin
system. Gen Comp Endocrinol. 159:150–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calafat AM, Ye X, Wong LY, Reidy JA and
Needham LL: Exposure of the U.S. population to bisphenol A and
4-tertiary-octylphenol: 2003–2004. Environ Health Perspect.
116:39–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagel SC, vom Saal FS, Thayer KA, Dhar MG,
Boechler M and Welshons WV: Relative binding affinity-serum
modified access (RBA-SMA) assay predicts the relative in vivo
bioactivity of the xenoestrogens bisphenol A and octylphenol.
Environ Health Perspect. 105:70–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan SA, Ball RB and Hendry WJ III:
Effects of neonatal administration of diethylstilbestrol in male
hamsters: Disruption of reproductive function in adults after
apparently normal pubertal development. Biol Reprod. 58:137–142.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagao T, Saito Y, Usumi K, Nakagomi M,
Yoshimura S and Ono H: Disruption of the reproductive system and
reproductive performance by administration of nonylphenol to
newborn rats. Hum Exp Toxicol. 19:284–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKinnell C, Atanassova N, Williams K,
Fisher JS, Walker M, Turner KJ, Saunders TK and Sharpe RM:
Suppression of androgen action and the induction of gross
abnormalities of the reproductive tract in male rats treated
neonatally with diethylstilbestrol. J Androl. 22:323–338.
2001.PubMed/NCBI
|
|
13
|
Atanassova N, McKinnell C, Walker M,
Turner KJ, Fisher JS, Morley M, Millar MR, Groome NP and Sharpe RM:
Permanent effects of neonatal estrogen exposure in rats on
reproductive hormone levels, Sertoli cell number, and the
efficiency of spermatogenesis in adulthood. Endocrinology.
140:5364–5373. 1999.PubMed/NCBI
|
|
14
|
Kabuto H, Amakawa M and Shishibori T:
Exposure to bisphenol A during embryonic/fetal life and infancy
increases oxidative injury and causes underdevelopment of the brain
and testis in mice. Life Sci. 74:2931–2940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor JA, Richter CA, Ruhlen RL and vom
Saal FS: Estrogenic environmental chemicals and drugs: Mechanisms
for effects on the developing male urogenital system. J Steroid
Biochem Mol Biol. 127:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klapholz-Brown Z, Walmsley GG, Nusse YM,
Nusse R and Brown PO: Transcriptional program induced by Wnt
protein in human fibroblasts suggests mechanisms for cell
cooperativity in defining tissue microenvironments. PLoS One.
2:e9452007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar
|
|
18
|
Rexhepaj E, Agnarsdóttir M, Bergman J,
Edqvist PH, Bergqvist M, Uhlén M, Gallagher WM, Lundberg E and
Ponten F: A texture based pattern recognition approach to
distinguish melanoma from non-melanoma cells in histopathological
tissue microarray sections. PLoS One. 8:e620702013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrett ES and Sobolewski M: Polycystic
ovary syndrome: Do endocrine-disrupting chemicals play a role?
Semin Reprod Med. 32:166–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rochefort H: Bisphenol A and
hormone-dependent cancers: Potential risk and mechanism. Med Sci.
29:539–544. 2013.
|
|
21
|
Lee HR, Jeung EB, Cho MH, Kim TH, Leung PC
and Choi KC: Molecular mechanism(s) of endocrine-disrupting
chemicals and their potent oestrogenicity in diverse cells and
tissues that express oestrogen receptors. J Cell Mol Med. 17:1–11.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonefeld-Jørgensen EC, Ghisari M, Wielsøe
M, Bjerregaard-Olesen C, Kjeldsen LS and Long M: Biomonitoring and
hormone-disrupting effect biomarkers of persistent organic
pollutants in vitro and ex vivo. Basic Clin Pharmacol Toxicol.
115:118–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sultan C, Balaguer P, Terouanne B, Georget
V, Paris F, Jeandel C, Lumbroso S and Nicolas J: Environmental
xenoestrogens, anti-androgens and disorders of male sexual
differentiation. Mol Cell Endocrinol. 178:99–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagel SC, vom Saal FS, Thayer KA, Dhar MG,
Boechler M and Welshons WV: Relative binding affinity-serum
modified access (RBA-SMA) assay predicts the relative in vivo
bioactivity of the xenoestrogens bisphenol A and octylphenol.
Environ Health Perspect. 105:70–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
vom Saal FS, Cooke PS, Buchanan DL,
Palanza P, Thayer KA, Nagel SC, Parmigiani S and Welshons WV: A
physiologically based approach to the study of bisphenol A and
other estrogenic chemicals on the size of reproductive organs,
daily sperm production, and behavior. Toxicol Ind Health.
14:239–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta C: Reproductive malformation of the
male offspring following maternal exposure to estrogenic chemicals.
Proc Soc Exp Biol Med. 224:61–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toyama Y, Suzuki-Toyota F, Maekawa M, Ito
C and Toshimori K: Adverse effects of bisphenol A to spermiogenesis
in mice and rats. Arch Histol Cytol. 67:373–381. 2004. View Article : Google Scholar
|
|
28
|
Minamiyama Y, Ichikawa H, Takemura S,
Kusunoki H, Naito Y and Yoshikawa T: Generation of reactive oxygen
species in sperms of rats as an earlier marker for evaluating the
toxicity of endocrine-disrupting chemicals. Free Radic Res.
44:1398–1406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan PP, Pan XY, Wang HH, Li ZX, Wang XN,
Lai Q, Song WJ, Zhao HY and Dou ZH: Effects of bisphenol-A on
blastocyst development and implantation. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 36:351–356. 2014.PubMed/NCBI
|
|
30
|
Liu XL, Chen XY, Wang ZC, Shen T and Zhao
H: Effects of exposure to bisphenol A during pregnancy and
lactation on the testicular morphology and caspase-3 protein
expression of ICR pups. Biomed Rep. 1:420–424. 2013.
|
|
31
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Cai J, Cai XH and Chen L: miR-346
regulates osteogenic differentiation of human bone marrow-derived
mesenchymal stem cells by targeting the Wnt/β-catenin pathway. PLoS
One. 8:e722662013. View Article : Google Scholar
|
|
33
|
Fujimaki S, Hidaka R, Asashima M, Takemasa
T and Kuwabara T: Wnt protein-mediated satellite cell conversion in
adult and aged mice following voluntary wheel running. J Biol Chem.
289:7399–7412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Li M, Zuo K, Li D, Ye M, Ding L,
Cai H, Fu D, Fan Y and Lv Z: Upregulated miR-155 in papillary
thyroid carcinoma promotes tumor growth by targeting APC and
activating Wnt/β-catenin signaling. J Clin Endocrinol Metab.
98:E1305–E1313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye S, Wang J, Yang S, Xu W, Xie M, Han K,
Zhang B and Wu Z: Specific inhibitory protein Dkk-1 blocking
Wnt/β-catenin signaling pathway improve protective effect on the
extracellular matrix. J Huazhong Univ Sci Technolog Med Sci.
31:657–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kong XB and Zhang C: Dickkopf (Dkk) 1
promotes the differentiation of mouse embryonic stem cells toward
neuroectoderm. In Vitro Cell Dev Biol Anim. 45:185–193. 2009.
View Article : Google Scholar
|
|
37
|
Esteve P, Sandonìs A, Ibañez C, Shimono A,
Guerrero I and Bovolenta P: Secreted frizzled-related proteins are
required for Wnt/β-catenin signalling activation in the vertebrate
optic cup. Development. 138:4179–4184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Knobloch J, Schmitz I, Götz K,
Schulze-Osthoff K and Rüther U: Thalidomide induces limb anomalies
by PTEN stabilization, Akt suppression, and stimulation of
caspase-dependent cell death. Mol Cell Biol. 28:529–538. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ning B, Wang P, Pei X, Kang Y, Song J,
Wang D, Zhang W and Ma R: Dual function of β-catenin in articular
cartilage growth and degeneration at different stages of postnatal
cartilage development. Int Orthop. 36:655–664. 2012. View Article : Google Scholar :
|