Introduction
The thymus is essential for a properly functioning
immune response. The three-dimensional structure of the thymic
meshwork comprises the cortex and medulla, which are predominantly
composed of distinct developing T-cell subsets and thymic stromal
cells (TSCs). TSCs are a diverse group of cells that includes
cortical thymic epithelial cells (cTECs), medullary (m)TECs,
mesenchymal cells, fibroblasts, pericytes, endothelial cells, and
adipocytes (1). TECs provide
essential microenvironments for the maturation of T cells. In
addition, cTECs are responsible for the attraction of T-cell
precursors, commitment of cells to the T-cell lineage, expansion of
immature double-negative (DN) thymocytes, and positive selection of
double-positive (DP) thymocytes (2). mTECs consist of a heterogeneous
population of epithelial cells, which generate a suitable
microenvironment for recently positively selected CD4 and CD8
single-positive thymocytes (3).
It is currently unclear whether thymic involution is
initiated early on in life; however, thymic involution has severe
negative effects on immune responsiveness with progressive age
(4). Age-related thymic involution
has been shown to be associated with reduced immune surveillance,
increased risk of infection and cancer, vaccination failure, and
delayed T-cell reconstitution in patients undergoing hematopoietic
stem cell transplantations (5–7). The
progressive loss of thymic function results in a decrease in
adaptive immunity. The predominant causes of age-related thymic
involution include: Reduced numbers of hematopoietic stem cells
(HSCs), accompanied with intrinsic defects (8,9);
reduced TECs and deterioration of the stromal microenvironment
(10,11); and extrinsic circulating factors
that affect the aged microenvironment, including alterations in
hormones, growth factors and cytokines (12). Microenvironmental alterations in
the aged thymus are considered to be the determining cause for
defective T-cell development (13,14).
During aging of the immune system, the gradual reduction in the
number of naïve T cells is associated with a marked destruction of
the epithelial network, including reduced TECs, increased
fibroblasts, disrupted thymic perivascular space, and the
increasing presence of adipocytes (10,15,16).
Thymocytes are the major components of the thymic microenvironment
in young individuals; however, adipocytes constitute the bulk of
the aged thymic cellular space (15,16).
As age increases, the thymus undergoes marked fibrotic and fatty
alterations, which result in its transformation into adipose
tissue.
Despite studies regarding thymic senescence, the
molecular mechanism underlying thymic ageing remains to be
elucidated. The formation of an appropriate thymic microenvironment
is dependent on interactions between developing thymocytes and
TECs, which is known as thymic crosstalk (17,18).
Understanding the signaling mechanisms that regulate tissue
development and maintenance of TECs may result in the
identification of the process of thymic involution. Previous
studies have demonstrated that maintenance and functional integrity
of the thymic stroma requires stimulation via the Notch, bone
morphogenetic protein, and Wnt signaling pathways (19–21).
Numerous studies have reported the importance of Wnt signaling and
Wnt proteins in the maintenance of various stem cell lineages. For
example, Wnt3A deficiency has been shown to result in decreased
numbers of HSCs in fetal liver, and decreased self-renewal capacity
(22). In addition, the Wnt
signaling pathway has been implicated in lineage-commitment and
cell-fate regulation during development and aging (23,24).
The majority of the members of the Wnt glycoprotein family, which
currently consists of 19 molecules, have been implicated in the
development of the embryonic thymus, and in the maintenance of
adult thymic epithelium (25).
Wnt protein expression has previously been detected
in thymocytes and TECs, and their receptors and associated
regulatory molecules. In addition, TEC cell lines and primary TECs
are both capable of responding to Wnt proteins in vitro
(21,25–27).
Canonical and non-canonical signaling pathways have been
identified, which are initiated by various combinations of the
Wnt/Frizzled complex. The most studied signaling pathway is the
canonical Wnt pathway, which results in stabilization of β-catenin
via inactivation of the 'destruction complex', which consists of
adenomatous polyposis coli, axin, and glycogen synthase kinase 3
(GSK-3) (28). In the absence of
Wnt signaling, GSK-3 phosphorylates axin, which culminates in the
degradation of β-catenin (28). In
the presence of Wnt signaling, GSK-3 activity is inhibited,
resulting in the hypophosphorylation of axin, which protects
β-catenin from degradation and allows it to accumulate (29). Stabilized β-catenin may then
translocate to the nucleus where it targets the lymphoid enhancer
factor (LEF)-1, as well as T-cell factors (TCF)-1, TCF-3 and TCF-4.
The binding of β-catenin to LEF or TCF initiates the transcription
of numerous genes, including forkhead box (FoxN1), B-cell
lymphoma-extra large (Bcl-xL), axin, cyclin D1 and c-Myc (30). The thymic degeneration observed in
response to transgenic Dickkopf Wnt signaling pathway inhibitor 1
expression is caused by a loss of TEC stem/progenitor cell
maintenance or proliferation of an immature TEC subset (3). In addition, loss of Wnt signaling
within TECs results in a decrease in the K5K8DP TEC subset, which
is localized at the corticomedullary junction, and a reduction in
the number of cycling TECs primarily within immature subsets
(3). Downregulation of Wnt4 has
previously been identified as a trigger for epithelial-mesenchymal
transition and pre-adipocyte-differentiation during thymic
involution (31).
The aim of the present study was to evaluate the
role of the specific Wnt protein, Wnt4, in thymic involution. Wnt4
is one of the most abundant Wnt molecules present in the thymus,
and it is produced by both thymocytes and TSCs (25). Previous studies have demonstrated
that Wnt4 is responsible for the direct upregulation of FoxN1,
which is associated with the differentiation of TECs and the
subsequent maintenance of thymic epithelial identity (25). FoxN1 is an essential transcription
factor that is required for adequate epithelial morphogenesis, and
the capacity of TECs to attract lymphoid precursors from the bone
marrow (32). Apoptosis inhibiting
factor Bcl-xL is highly expressed in normal mouse thymocytes,
whereas in Tcf-1 knockout mice Wnt4 signaling is suppressed, which
leads to decreased expression of Bcl-xL and increased apoptosis in
thymocytes (33). Exposure to
glucocorticoids, similar to physiological aging, results in
significant thymic senescence (34). Dexamethasone (Dex) treatment has
been shown to trigger Wnt4 and FoxN1 downregulation, leading to the
increased expression of preadipocyte-type differentiation markers
lamina-associated polypeptide 2 and peroxisome
proliferator-activated receptor γ (31,34).
Furthermore, in a Wnt4-overexpressing TEP1 cell line (35), Wnt4 overexpression effectively
suppressed Dex-induced increases in adipocyte markers, and
protected thymic epithelium-derived cells against Dex-induced
senescence at the molecular level (36). Considering the vital role Wnt4 has
in Dex-induced senescence, it was hypothesized that Wnt4 may
regulate proliferation and apoptosis within the thymus, and
contribute to age-related thymic involution. Using in vitro
and in vivo approaches, the present study provided evidence
that a decreased expression of Wnt4 in the aging thymus may be one
of the molecular triggers underlying the process of age-related
thymic senescence.
Materials and methods
Murine model
C57BL/6 mice were purchased from Charles River
Laboratories, Inc. (Wilmington, MA, USA). The mice were 1 month old
at the time of purchase, and were housed in the Laboratory Center
Animal Ministry of Shengjing Hospital (China Medical University,
Shenyang, China) until they had reached 5, 10, 15, or 20 months
old. All of the mice were maintained in a specific, pathogen-free
environment. There were six mice in each group, (male:female, 1:1)
and they were maintained at 21~27°C with 40~70% humidity and a
12/12 h light/dark cycle. Animal procedures were performed
according to the protocol approved by the Experiment Animal Center
of China Medical University, in accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (37).
Separation of thymocytes and TSCs
The mice were sacrificed with ether gas anaesthesia,
then the pleura was cut open using eye scissors, the thymus was
exposed and then it was removed with curved eye tweezers. Thymic
lobes were rinsed in sterilized phosphate-buffered saline (PBS) a
number of times. The lobes were enzymatically digested using 1.5
mg/ml collagenase II (cat. no. 17101; Gibco Life Technologies,
Grand Island, NY, USA), 1 mg/ml trypsin (cat. no. 25200-056; Gibco
Life Technologies) and 0.2 mg/ml DNase I (cat. no. D5025;
Sigma-Aldrich, St. Louis, MO, USA), and were incubated at 37°C for
30 min with intermittent agitation. The resulting single cell
suspension was resuspended in buffer (2 mM EDTA in PBS with 0.5%
bovine serum albumin; Gibco Life Technologies) and passed through a
100 µm strainer (cat. no. 130-041-407; Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany) to remove any remaining undigested
tissue. The cells were then purified using magnetic cell sorting
(MACS) LS separation columns (Miltenyi Biotec GmbH). Cell
suspensions were labeled with anti-CD45-fluorescein isothiocyanate
(FITC) and washed with MACS buffer (Miltenyi Biotec GmbH), followed
by incubation with anti-FITC microbeads (Miltenyi Biotec GmbH).
Thymocytes (positively separated) and TSCs (negatively separated)
were subsequently used for total RNA isolation, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis, and flow cytometric analysis.
Proliferation analysis
The mice were intraperitoneally injected with 10
mg/kg 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen Life Technologies)
in PBS. EdU is a nucleoside analog to thymidine, which is
incorporated into DNA during active DNA synthesis. Detection is
based on a click reaction, also known as a copper catalyzed
covalent reaction, between an azide and an alkyne. After 24 h, the
thymi of the mice were harvested and single-cell suspensions were
purified using MACS, as mentioned above. Thymocytes were stained
with CD4-allophycocyanin (APC) (cat. no. 100515; BioLegend, San
Diego, CA, USA) and CD8-PerCP (cat. no. 100731; BioLegend)
antibodies, whereas TSCs were labeled with EpCAM1-APC (clone G8.8)
antibodies (cat. no. 118213; BioLegend). The cells were then
prepared for EdU detection, according to the manufacturer's
instructions (cat. no. C10425; Invitrogen Life Technologies,
Carlsbad, CA, USA). The proliferation and apoptosis of the cells
was analyzed using a flow cytometer (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA).
Apoptosis analysis
As described above, thymocytes and TSCs were
separated using MACS. TSCs were labeled with EpCAM1-APC, whereas
thymocytes were stained with CD4-APC and CD8-PerCP. For the early
thymocyte progenitors (ETP), freshly isolated thymocytes were
stained with phycoerythrin (PE)-conjugated lineage markers, as well
as CD25-PE (cat. no. 101903) and CD127-PE (cat. no. 135009),
CD117-APC (cat. no. 105811) and CD44-FITC (cat. no. 103021)
(BioLegend). For the Annexin V apoptosis assay, the cells were
stained with Annexin V-FITC (cat. no. KGA107; Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). Propidium iodide was used to
exclude dead cells from the apoptosis assays.
RT-qPCR analysis
Total RNA was extracted from the separated
thymocytes and TSCs using TRIzol® reagent (Invitrogen
Life Technologies), and reverse transcribed using PrimeScript RT
reagent kit (cat. no. RR047A; Takara Bio Inc., Otsu, Japan). For
qPCR analysis, ABI 7500 system (Applied Biosystems Life
Technologies, Foster City, CA, USA) and SYBR® Premix
Ex Taq™ (cat. no. RR820A; Takara Bio Inc.) were used, with 2
µl cDNA. The threshold cycles (CT) for three replicate
reactions were determined using Sequence Detection System software
(ABI 7500 software, version 2.3; Applied Biosystems Life
Technologies), and relative transcription abundance was calculated
following normalization with β-actin. The primer pairs used were:
FoxN1, forward 5′-TTCCTCAAGGGCAACCAC-3′, reverse
5′-CCCATGTCCACAGGGATC-3′; Wnt4, forward 5′-CTCAAAGGCCTGATCCAGAG-3′,
reverse 5′-TCACAGCCACACTTCTCCAG-3′; Bcl-xL, forward
5′-GCGTGGAAAGCGTAGACAAGGAGATG-3′, reverse
5′-ACTGAAGAGTGAGCCCAGCAGAACC-3′; and β-actin, forward
5′-GCTGTCCCTGTATGCCTCT-3′, and reverse 5′-ATGTCACGC ACGATTTCC-3′
(Takara Bio Inc.). The cycling conditions were as follows: Initial
denaturation at 95°C for 30 sec, 45 cycles of 95°C for 5 sec and
60°C for 20 sec.
MTEC1 cell stimulation
The mouse thymus epithelial cell 1 (MTEC1) cell line
originated from a mouse thymic medulla cell line established by the
Department of Immunology, Peking University Health Science Center
(China). The cells were cultured in medium (Gibco Life
Technologies) supplemented with 10% fetal bovine serum (Gibco Life
Technologies) and plated in 24-well cultures to ensure that total
cell density (5×106) was uniform between the compared
samples. The cell lines were separately treated with 30 mM LiCl
(cat. no. 213233; Sigma-Aldrich), 10−5 M Dex (cat. no.
D4902; Sigma-Aldrich) or 500 ng/ml mouse recombinant Wnt4 protein
(cat. no. 475-WN; R&D Systems, Inc., Minneapolis, MN USA). For
some experiments, the cells were co-cultured in 10−5 M
Dex and 500 ng/ml recombinant Wnt4 protein. The control groups of
all of the experiments were cultured in common cell medium. After
24 h, the cells underwent total RNA isolation and flow
cytometry.
MTEC1 cell investigation
Following the treatments previously described, the
MTEC1 cell line was investigated. For proliferation analysis, EdU
was added to the cell culture medium at a final concentration of 50
µM, and incubated for 2 h at 37°C. The cells were then
harvested and EdU detection was conducted according to the
manufacturer's instructions (cat. no. C10425; Invitrogen Life
Technologies). For apoptosis analysis, MTEC1 cells were stained
with Annexin V-FITC (cat. no. KGA107; Nanjing KeyGen Biotech Co.,
Ltd.). Propidium iodide (PI) was used to exclude dead cells from
the apoptosis assays. To determine the mRNA expression levels of
FoxN1 and Bcl-xL, RT-qPCR was performed as described above.
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences between various experimental groups were compared
using a Student's t-test with GraphPad Prism software, version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Significant downregulation of Wnt4
signaling is accompanied by age-related thymic involution
Given that the early stages of T-cell development
are highly dependent on the availability of stromal niches
(38), and since the number of
TECs has been suggested to be the limiting factor in determining
thymic size (39), the present
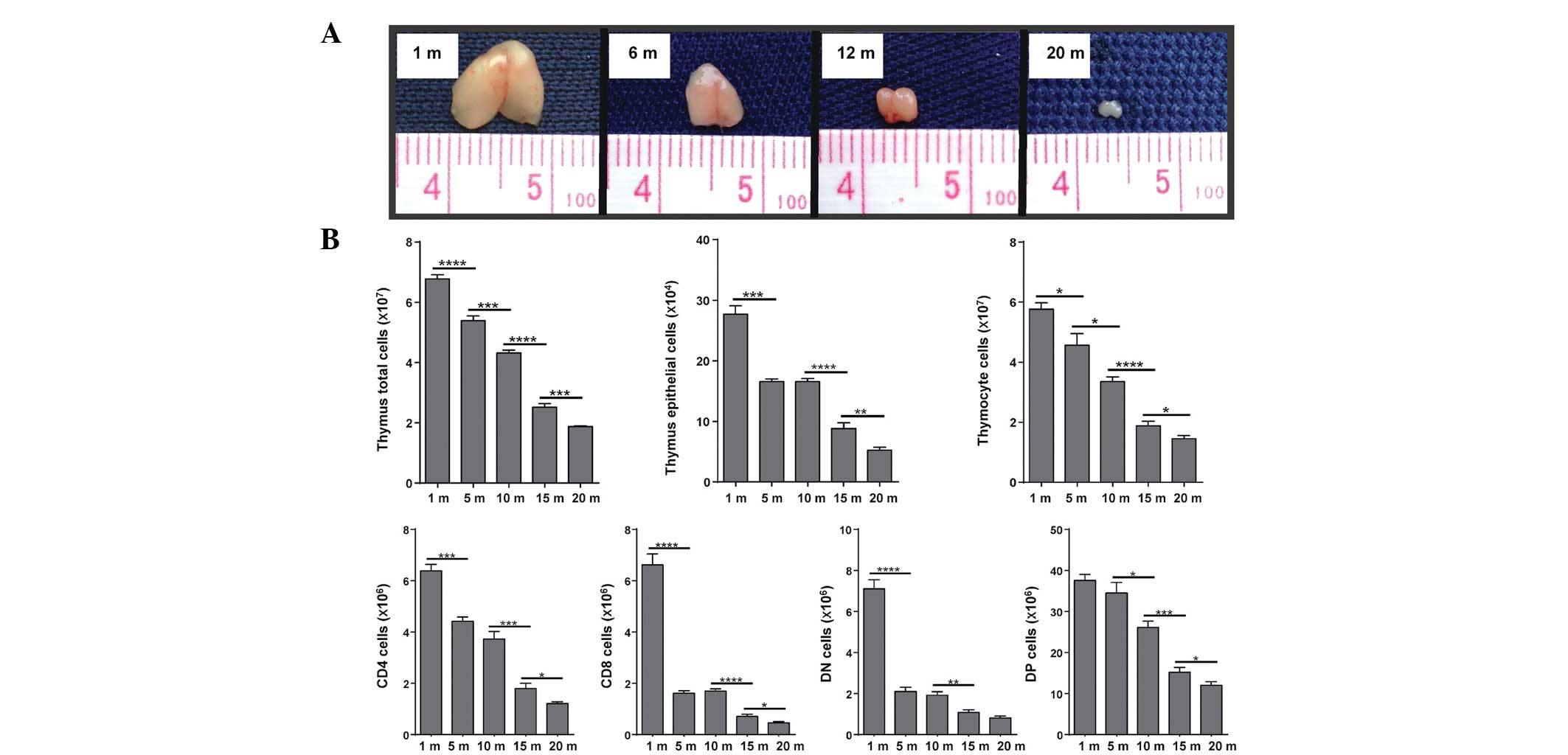
study measured the thymic size and the absolute cell numbers of the
C57BL/6 mice at various ages (Fig.
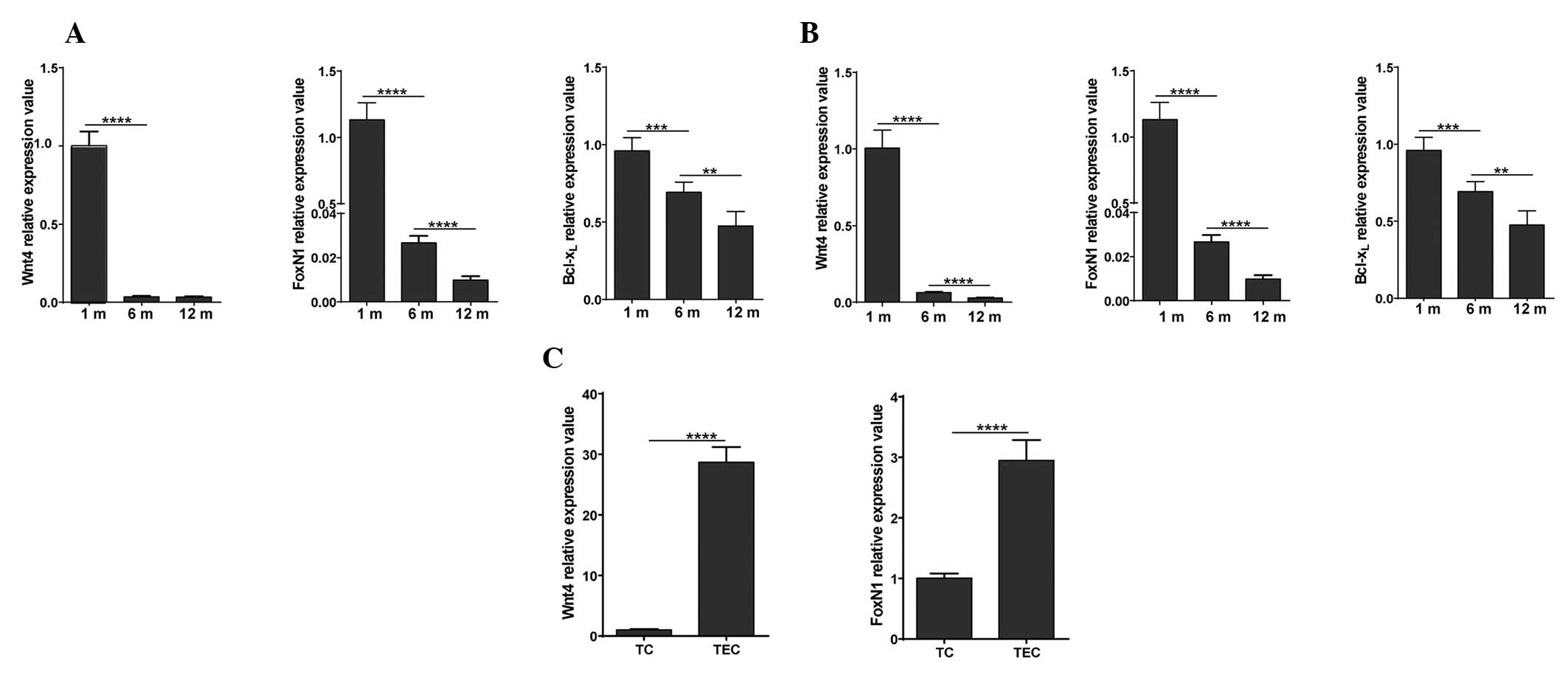
1). To detect gene expression alterations, thymocytes and TECs
were purified from 1 month, 6 month and 1-year old C57BL/6 mice
based on CD45 expression, and RT-qPCR analysis was performed. Wnt4
signal-related genes, including Wnt4, FoxN1 and
Bcl-xL were detected. In addition, Wnt4 and FoxN1 mRNA
expression was compared by RT-qPCR between the thymocytes and TECs
of 1-month old C57BL/6 mice (Fig.
2).
Thymic size (Fig.
1A) and the absolute numbers of thymocytes and TECs (Fig. 1B) were significantly decreased as
the age of the mice increased. Concurrently, the mRNA expression
levels of Wnt4, FoxN1 and Bcl-xL decreased in the same manner
(Fig. 2A and B). Highly decreased
levels of FoxN1 may be caused by the strong downregulation of Wnt4,
which occurred by the time the mice reached 1 year of age.
Furthermore, Wnt4 expression was almost 30 times higher in the
TECs, as compared with in the thymocytes. Concordantly, the mRNA
expression levels of FoxN1 were also more abundant in TECs, as
compared with in thymocytes. These results indicate that Wnt4 is
predominantly expressed in TECs, and changes in thymic size and
absolute numbers of thymic cells are accompanied by a decrease in
the transcriptional levels of Wnt4, as well as the expression
levels of FoxN1 and Bcl-xL, which are two target genes of the Wnt
signaling pathway.
Decreased proliferation and increased
apoptosis of thymic cells with age
To analyze the cause of the reduction in thymic cell
number, the present study evaluated the proliferation and apoptosis
of thymic cells at various ages. TSC composition, as well as
organization, is severely disrupted with advancing age. In
addition, naïve T-cell production is highest in young individuals
and is reduced as thymic involution progresses with age. These
processes lead to a significant decrease in the absolute numbers of
thymocytes and TECs. With advancing age, the thymus undergoes
marked fibrotic and fatty changes, which culminate in its
transformation into adipose tissue (40). A balance between cell proliferation
and apoptosis can maintain a normal number of cells; therefore, the
present study aimed to examine thymic cell proliferation and
apoptosis at various ages.
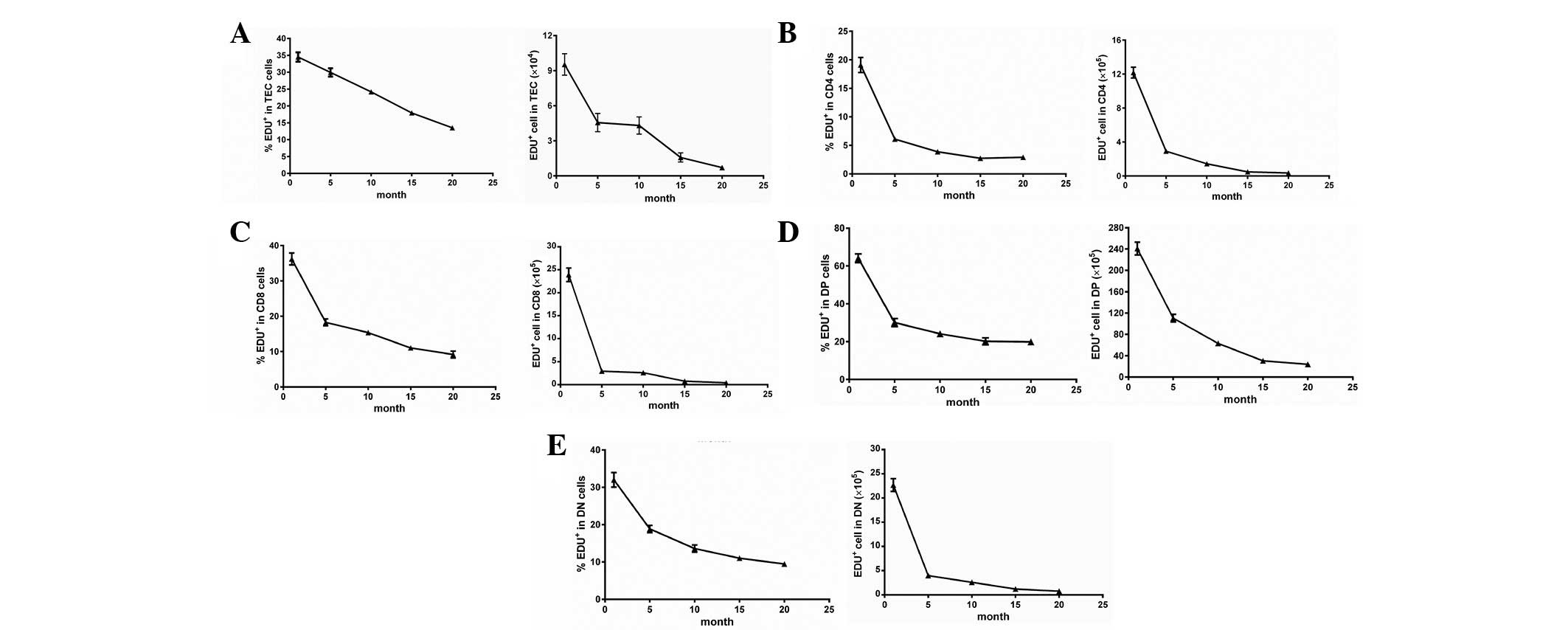
Thymocytes and TSCs were separated from 1-, 5-, 10-,
15- and 20-month old C57BL/6 mice, based on CD45 expression. Based
on the expression of CD4 and CD8, the proliferation of distinct
developing T-cell subsets was determined by EdU-flow cytometric
analysis. The proliferation of thymocytes and TECs continuously
declined with advancing age (Fig.
3). In particular, the percentage of proliferation was markedly
decreased between months 1 and 5, which may provide evidence
suggesting that thymic involution is initiated early on in life.
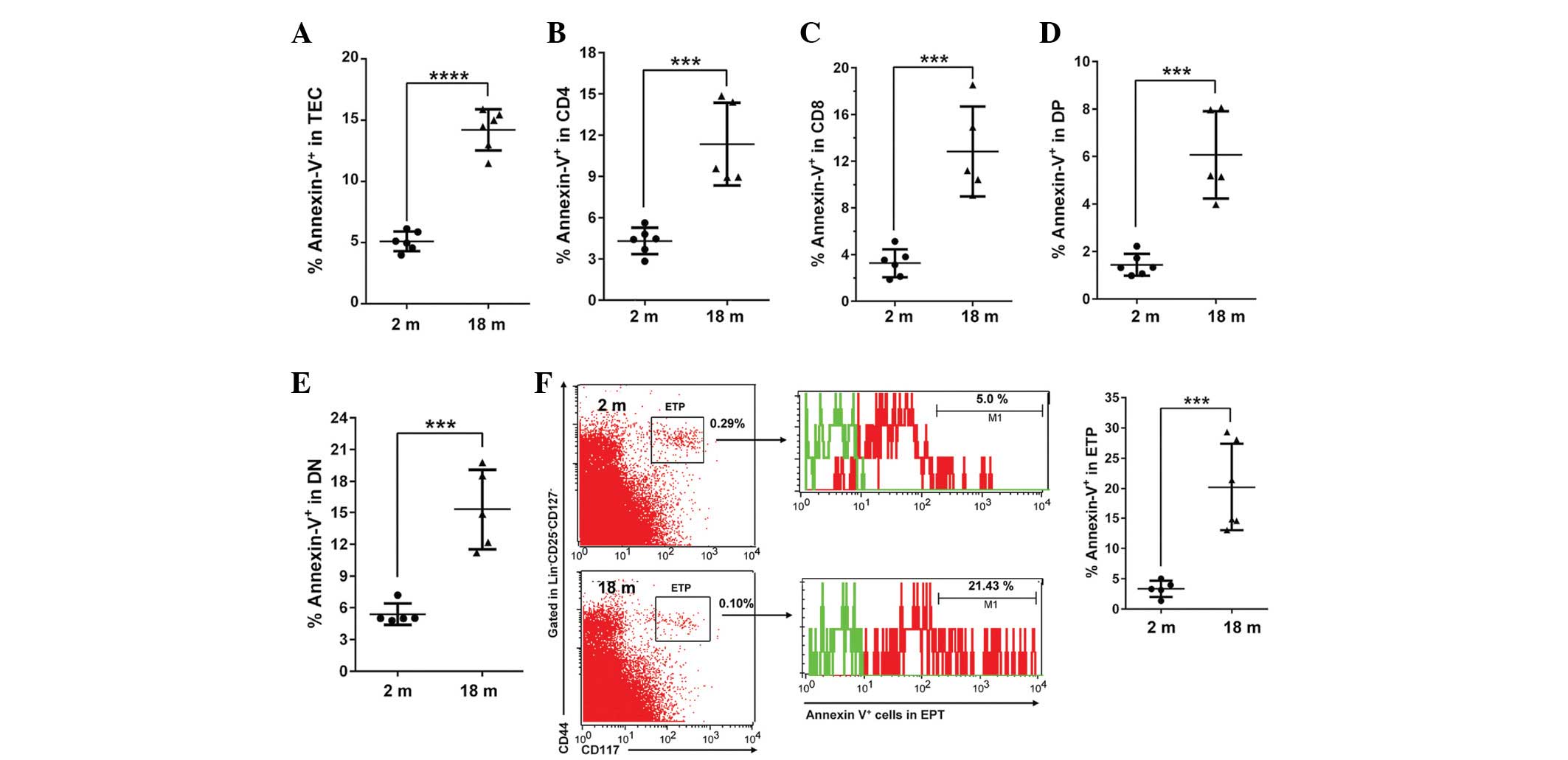
Furthermore, Annexin V analysis demonstrated that the apoptosis of
TECs and TCs, including CD4, CD8, DP, DN and ETPs, was
significantly increased in the aged thymi (Fig. 4).
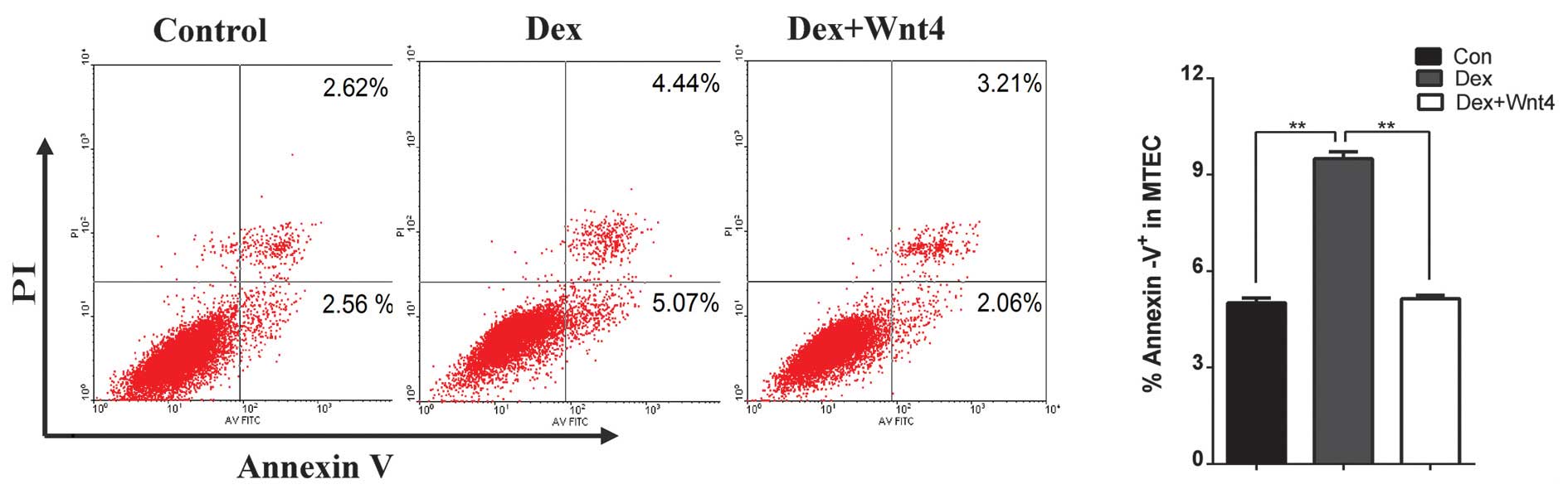
Wnt4 signaling modulation alleviates
Dex-induced MTEC1 cell apoptosis
Glucocorticoids are particularly effective immune
suppressants, as they induce rapid peripheral T cell and thymocyte
apoptosis, resulting in impaired T cell-dependent immune responses.
Primary TECs express glucocorticoid receptors, and high-dosage Dex
induces degeneration of the thymic epithelium within 24 h of
treatment (37). The present study
aimed to determine how exogenous glucocorticoids affect the thymic
epithelial network, and if Wnt4 has an important role in thymic
senescence. The MTEC1 cells were treated with 10−5 M
Dex, or co-cultured in 10−5 M Dex and 500 ng/ml
recombinant Wnt4. After 48 h, it was determined whether high
glucose altered the rate of apoptosis of MTEC1 cells. As compared
with the control group, Dex significantly promoted cell apoptosis,
whereas recombinant Wnt4 attenuated Dex-induced cell apoptosis
(Fig. 5).
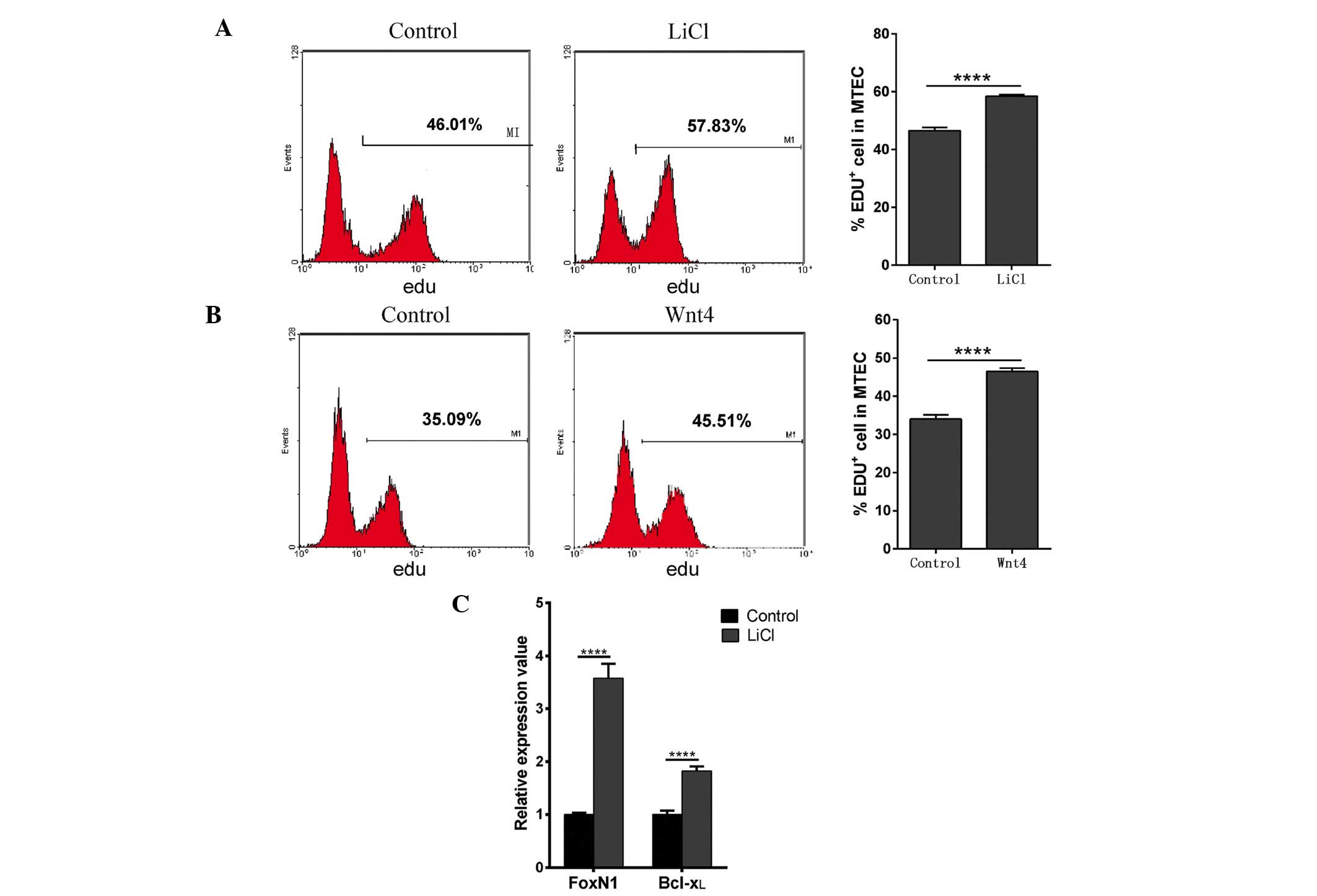
Activation of the Wnt4 signaling pathway
promotes MTEC1 cell proliferation
The biological role of Wnt4 in Dex-mediated MTEC1
cell apoptosis was detected in the present study. To determine
whether activation of the Wnt4 signaling pathway has a role in cell
proliferation, the effects of LiCl and exogenous Wnt4 were
determined. LiCl is an inhibitor of GSK-3β (41), and thus prevents phosphorylation
and degradation of β-catenin. MTEC1 cells were cultured in medium
containing 30 mM LiCl for 24 h, cell proliferation was then
examined using EdU, and the mRNA expression levels of Bcl-xL and
FoxN1 were determined by RT-qPCR. Concurrently, to determine
whether exogenous Wnt4 resulted in increased cell proliferation,
MTEC1 cells were examined following incubation with recombinant
Wnt4-supplemented media for 16 h. MTEC1 cells cultured in LiCl and
exogenous Wnt4 exhibited ~10% increased proliferation, as compared
with the control group (Fig. 6A and
B). As expected, LiCl treatment alone resulted in an increase
in FoxN1 and Bcl-xL mRNA expression levels (Fig. 6C). Notably, cell culture with
medium supplemented with LiCl also resulted in increased
proliferation, suggesting that activation of Wnt signaling is a
critical step during MTEC1 proliferation, and is not solely limited
to the expression of Wnt4.
Discussion
It has been demonstrated that high-dosage Dex
induces degeneration of thymic epithelium within 24 h of treatment.
In addition, the overexpression of Wnt4 can prevent the
upregulation of adipose differentiation-related aging markers, thus
suggesting an important role of Wnt4 in thymic senescence (37). The present study hypothesized that
during age-associated thymic involution Wnt4 signaling is
associated with thymic proliferation and apoptosis. The results of
the present study suggested that enhancing the canonical Wnt4
signal transduction pathway alleviates Dex-mediated MTEC1 cell
apoptosis and promotes MTEC1 cell proliferation.
Thymic involution is believed to be initiated early
on in life. In the present study, a progressive reduction in thymic
size occurred from month 6, and almost complete thymic involution
had occurred by month 20. TECs provide growth factors and signals
that promote the migration and T lineage commitment of developing
lymphocytes. To maintain normal function, the key thymic
microenvironment is dependent on proficient cell numbers and normal
cell function. By contrast, an imbalance between cell proliferation
and apoptosis results in a marked reduction of thymic cell numbers.
The present study demonstrated that the aged thymus is
characterized by markedly decreased TEC numbers, as well as
decreased levels of proliferation, and increased apoptosis of TECs.
Notably, the proliferation rate of TECs plummeted from month 5,
suggesting that a dysfunctional microenvironment contributes to
abnormal thymic function. The lack of a normal thymic
microenvironment also results in defective thymic lymphopoiesis in
aged thymi. As compared with normal thymic lymphopoiesis in the
young thymus, the number of thymocyte sub-populations, especially
CD8 and DN, in the elderly mice was markedly reduced after 5
months, alongside decreased proliferation and increased apoptosis.
These results indicated that T-cell development or proliferation is
defective in the aged thymus. With advancing age, normal functional
thymocytes and TECs in the thymus are reduced, and adipocytes
constitute the bulk of the aged thymic cellular space; the thymus
undergoes striking fibrotic and fatty alterations that culminate in
its transformation into adipose tissue.
It has been suggested that the absence of Wnt4 may
suppress fetal and early postnatal thymic expansion, resulting in
decreased TEC numbers, an alteration of the medullary-to-cortical
TEC ratio, and a disproportionate loss of immature thymic
precursors (42). The present
study demonstrated that changes in thymic cell numbers and function
are accompanied by a decrease in the transcription levels of Wnt4,
as well as the downregulation of FoxN1 and Bcl-xL, which are two
target genes of the Wnt signaling pathway. In addition, Wnt4 and
FoxN1 were shown to be more abundant in TECs, as compared with
thymocytes. A marked decrease of FoxN1 mRNA expression levels was
detected in the aged thymus. FOXN1 is a key transcription factor
that is responsible for the differentiation of TECs, and its
decreased expression may be one reason underlying the reduced
proliferation of TECs in elderly mice. Stabilization of β-catenin,
which is a critical coactivator of TCF, enhances DP thymocyte
survival via the upregulation of Bcl-xL. Spontaneous or
glucocorticoid-induced thymocyte apoptosis has previously been
shown to be associated with reduced levels of β-catenin and Bcl-xL
(43). The results of the present
study suggested that downregulation of Bcl-xL may result in
increased apoptosis of thymocytes and TECs in elderly mice.
Concurrently, activation of Wnt4 signaling was shown to result in
increased FoxN1 and Bcl-xL mRNA expression levels. In order to
further study the role of Wnt4 signaling in cell proliferation and
apoptosis, in vitro studies were conducted. The results
indicated that activation of the Wnt4 signaling pathway may promote
MTEC1 cell proliferation, and alleviate Dex-mediated MTEC1 cell
apoptosis. These findings suggested that normal expression level of
Wnt4 have a critical role in maintaining the balance between cell
proliferation and apoptosis.
In conclusion, based on the present study, it may be
suggested that continuously decreasing proliferation and increasing
apoptosis occurs during age-related thymic involution. In addition,
an age-associated downregulation of Wnt4 may be responsible for the
disruption between the balance of cell proliferation and apoptosis.
Wnt4 and its downstream signaling pathways may therefore represent
interesting candidates to improve thymic function in patients with
thymic atrophy.
Abbreviations:
|
TSCs
|
thymic stromal cells
|
|
TECs
|
thymic epithelial cells
|
|
cTECs
|
cortical thymic epithelial cells
|
|
mTECs
|
medullary thymic epithelial cells
|
|
DN
|
double negative
|
|
DP
|
double positive
|
|
GSK-3
|
glycogen synthase kinase 3
|
|
LEF
|
lymphoid enhancer factor
|
|
TCF
|
T-cell factors
|
|
Dex
|
dexamethasone
|
|
MACS
|
magnetic cell sorting
|
|
PCR
|
polymerase chain reaction
|
|
PI
|
propidium iodide
|
|
CT
|
threshold cycles
|
|
MTEC1
|
mouse thymus epithelial cell 1
|
|
SD
|
standard deviation
|
|
ETP
|
early thymocyte progenitors
|
|
LiCl
|
lithium chloride
|
Acknowledgments
The authors of the present study would like to thank
Professor Yu Zhang (Department of Immunology, Peking University
Health Science Center) for providing the MTEC1 cells. The present
study was supported by the National Natural Scientific Foundation
of China (grant nos. 30872715 and 81270430), the Special Research
Fund for Doctoral Program of Education Department of China (grant
no. 20112104110011) and the Free Research Program Fund of Shengjing
Hospital (grant no. 200805) to X.Z.
References
|
1
|
Petrie HT and Zúñiga-Pflücker JC: Zoned
out: Functional mapping of stromal signaling microenvironments in
the thymus. Annu Rev Immunol. 25:649–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savage PA and Davis MM: A kinetic window
constricts the T cell receptor repertoire in the thymus. Immunity.
14:243–252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osada M, Jardine L, Misir R, Andl T,
Millar SE and Pezzano M: DKK1 mediated inhibition of Wnt signaling
in postnatal mice leads to loss of TEC progenitors and thymic
degeneration. PLoS One. 5:e90622010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dixit VD: Adipose-immune interactions
during obesity and caloric restriction: Reciprocal mechanisms
regulating immunity and health span. J Leukoc Biol. 84:882–892.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McElhaney JE and Effros RB:
Immunosenescence: what does it mean to health outcomes in older
adults? Curr Opin Immunol. 21:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch HE, Goldberg GL, Chidgey A, Van den
Brink MR, Boyd R and Sempowski GD: Thymic involution and immune
reconstitution. Trends Immunol. 30:366–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holland AM and van den Brink MR:
Rejuvenation of the aging T cell compartment. Curr Opin Immunol.
21:454–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min H, Montecino-Rodriguez E and Dorshkind
K: Reduction in the developmental potential of intrathymic T cell
progenitors with age. J Immunol. 173:245–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zediak VP, Maillard I and Bhandoola A:
Multiple prethymic defects underlie age-related loss of T
progenitor competence. Blood. 110:1161–1167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gray DH, Seach N, Ueno T, Milton MK,
Liston A, Lew AM, Goodnow CC and Boyd RL: Developmental kinetics,
turnover, and stimulatory capacity of thymic epithelial cells.
Blood. 108:3777–3785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Xiao S and Manley NR: Foxn1 is
required to maintain the postnatal thymic microenvironment in a
dosage-sensitive manner. Blood. 113:567–574. 2009. View Article : Google Scholar :
|
|
12
|
Nobori S, Shimizu A, Okumi M,
Samelson-Jones E, Griesemer A, Hirakata A, Sachs DH and Yamada K:
Thymic rejuvenation and the induction of tolerance by adult thymic
grafts. Proc Natl Acad Sci USA. 103:19081–19086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF
and Su DM: The aged thymus shows normal recruitment of
lymphohemato-poietic progenitors but has defects in thymic
epithelial cells. Int Immunol. 19:1201–1211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF
and Su DM: Lymphohematopoietic progenitors do not have a
synchronized defect with age-related thymic involution. Aging Cell.
6:663–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flores KG, Li J, Sempowski GD, Haynes BF
and Hale LP: Analysis of the human thymic perivascular space during
aging. J Clin Invest. 104:1031–1039. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Youm YH, Sun Y, Rim JS, Galbán CJ,
Vandanmagsar B and Dixit VD: Axin expression in thymic stromal
cells contributes to age-related increase in thymic adiposity and
associated with reduced thymopoiesis independently of ghrelin
signaling. J Leukoc Biol. 85:928–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Ewijk W, Holländer G, Terhorst C and
Wang B: Stepwise development of thymic microenvironments in vivo is
regulated by thymocyte subsets. Development. 127:1583–1591.
2000.PubMed/NCBI
|
|
18
|
van Ewijk W, Wang B, Hollander G, Kawamoto
H, Spanopoulou E, Itoi M, Amagai T, Jiang YF, Germeraad WT, Chen WF
and Katsura Y: Thymic microenvironments, 3-D versus 2-D? Semin
Immunol. 11:57–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleul C and Boehm T: BMP signaling is
required for normal thymus development. J Immunol. 175:5213–5221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson G, Pongracz J, Parnell S and
Jenkinson EJ: Notch ligand-bearing thymic epithelial cells initiate
and sustain Notch signaling in thymocytes independently of T cell
receptor signaling. Eur J Immunol. 31:3349–3354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Osada M, Ito E, Fermin HA, Vasquez-Cintron
E, Venkatesh T, Friedel RH and Pezzano M: The Wnt signaling
antagonist Kremen1 is required for development of thymic
architecture. Clin Dev Immunol. 13:299–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luis TC, Weerkamp F, Naber BA, Baert MR,
de Haas EF, Nikolic T, Heuvelmans S, De Krjiger RR, van Dongen JJ
and Staal FJ: Wnt3a deficiency irreversibly impairs hematopoietic
stem cell self-renewal and leads to defects in progenitor cell
differentiation. Blood. 113:546–554. 2009. View Article : Google Scholar
|
|
23
|
Manolagas SC and Almeida M: Gone with the
Wnts: Beta-catenin, T-cell factor, forkhead box O, and oxidative
stress in age-dependent diseases of bone, lipid, and glucose
metabolism. Mol Endocrinol. 21:2605–2614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kléber M and Sommer L: Wnt signaling and
the regulation of stem cell function. Curr Opin Cell Biol.
16:681–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balciunaite G, Keller MP, Balciunaite E,
Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ and
Holländer GA: Wnt glycoproteins regulate the expression of FoxN1,
the gene defective in nude mice. Nat Immunol. 3:1102–1108. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pongracz J, Hare K, Harman B, Anderson G
and Jenkinson EJ: Thymic epithelial cells provide WNT signals to
developing thymocytes. Eur J Immunol. 33:1949–1956. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weerkamp F, Baert MR, Naber BA, Koster EE,
de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA and Staal FJ:
Wnt signaling in the thymus is regulated by differential expression
of intracellular signaling molecules. Proc Natl Acad Sci USA.
103:3322–3326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cadigan KM and Liu YI: Wnt signaling:
Complexity at the surface. J Cell Sci. 119:395–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller JR: The Wnts. Genome Biol.
3:REVIEWS30012002.PubMed/NCBI
|
|
31
|
Kvell K, Varecza Z, Bartis D, Hesse S,
Parnell S, Anderson G, Jenkinson EJ and Pongracz JE: Wnt4 and
LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One.
5:e107012010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirokawa K, Utsuyama M, Kasai M, Kurashima
C, Ishijima S and Zeng YX: Understanding the mechanism of the
age-change of thymic function to promote T cell differentiation.
Immunol Lett. 40:269–277. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ioannidis V, Beermann F, Clevers H and
Held W: The beta-catenin - TCF-1 pathway ensures CD4(+)CD8(+)
thymocyte survival. Nat Immunol. 2:691–697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Youm YH, Yang H, Sun Y, Smith RG, Manley
NR, Vandanmagsar B and Dixit VD: Deficient ghrelin
receptor-mediated signaling compromises thymic stromal cell
microenvironment by accelerating thymic adiposity. J Biol Chem.
284:7068–7077. 2009. View Article : Google Scholar :
|
|
35
|
Beardsley TR, Pierschbacher M, Wetzel GD
and Hays EF: Induction of T-cell maturation by a cloned line of
thymic epithelium (TEPI). Proc Natl Acad Sci USA. 80:6005–6009.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press; Washington, DC:
2011
|
|
37
|
Talaber G, Kvell K, Varecza Z, Boldizsar
F, Parnell SM, Jenkinson EJ, Anderson G, Berki T and Pongracz JE:
Wnt-4 protects thymic epithelial cells against
dexamethasone-induced senescence. Rejuvenation Res. 14:241–248.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prockop SE and Petrie HT: Regulation of
thymus size by competition for stromal niches among early T cell
progenitors. J Immunol. 173:1604–1611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jenkinson WE, Bacon A, White AJ, Anderson
G and Jenkinson EJ: An epithelial progenitor pool regulates thymus
growth. J Immunol. 181:6101–6108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dixit VD: Thymic fatness and approaches to
enhance thymopoietic fitness in aging. Curr Opin Immunol.
22:521–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hocevar BA, Mou F, Rennolds JL, Morris SM,
Cooper JA and Howe PH: Regulation of the Wnt signaling pathway by
disabled-2 (Dab2). EMBO J. 22:3084–3094. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heinonen KM, Vanegas JR, Brochu S, Shan J,
Vainio SJ and Perrault C: Wnt4 regulates thymic cellularity through
the expansion of thymic epithelial cells and early thymic
progenitors. Blood. 118:5163–5173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie H, Huang Z, Sadim MS and Sun Z:
Stabilized beta-catenin extends thymocyte survival by up-regulating
Bcl-xL. J Immunol. 175:7981–7988. 2005. View Article : Google Scholar : PubMed/NCBI
|