Introduction
It is well-known that aerobic organisms are exposed
to oxygen-free radicals, including reactive oxygen species (ROS),
under various conditions (1).
Thus, free radicals are produced under physiological conditions and
participate in a variety of normal cellular functions, such as the
regulation of signaling pathways, gene expression and apoptosis
(1–3). In addition, free radicals are
produced under abnormal conditions, such as in the cases of poor
diet, smoking and exposure to ionizing or ultraviolet (UV)
radiation. Excess free radicals in cells may interact and cause
damage to proteins, lipids and DNA (4). Living organisms possess a complex
endogenous defensive mechanism against free radicals that consists
of both enzymatic and non-enzymatic compounds (5–7). The
overproduction of free radicals may lead to oxidative stress, a
pathological condition in which an imbalance between the production
of free radicals and the antioxidant mechanisms is observed.
Oxidative stress has been shown to be associated with a variety of
diseases and pathological conditions, such as cancer, diabetes,
obesity and neurodegenerative and autoimmune diseases (8–12).
Aside from its endogenous mechanisms, an organism
may also acquire antioxidant components through diet (13,14).
Some of the most important antioxidants, which are found
particularly in plant foods, are polyphenols (15). These constitute a category of
products of the plant's secondary metabolism and play an important
role in a number of cellular functions (16,17).
When plant foods are consumed, the absorbed polyphenols may elicit
a variety of important bioactivities which have beneficial effects
on human health (17). Such
polyphenols can also be found in coffee, which is one of the most
popular beverages worldwide due to its pleasant taste and aroma;
the annual production of coffee is approximately 8 Mt, and the
average daily consumption is 2.3 billion cups a day (18). Traditionally, the beneficial
effects of coffee on human health were mainly attributed to its
most-investigated ingredient, caffeine; however, other components
also contribute to its valuable properties, such as its antioxidant
activity (17). The latter is
attributable mainly to its polyphenolic content, with the most
abundant polyphenols being chlorogenic acid (CGA) (19–21).
Several studies have been performed to investigate the quantity, as
well as the antioxidant and other disease-related properties, of
CGA (19,21–24).
However, although we are aware that coffee beans
undergo roasting prior to consumption, little data exist on the
effects of roasting on coffee composition, or on the differences in
antioxidant activity between green and roasted beans (25,26).
For instance, it is known that the roasting procedure (which may be
different for each variety of coffee) markedly affects CGA, leading
to their hydrolysis (27).
However, new compounds are formed from the products of this
hydrolysis, which may alter the overall antioxidant capacity of the
beans (25,28). Therefore, in the present study, we
aimed to examine the free radical scavenging activity of 13 coffee
varieties (both green and roasted coffee beans). Furthermore, 5
selected varieties were also examined for their protective activity
against free radical-induced DNA damage. Finally, C2C12 murine
myoblasts were treated with non-cytotoxic concentrations of the
most potent extract in order to examine its effects on the cellular
redox status by measuring the glutathione (GSH) and ROS levels.
Materials and methods
Coffee beans and roasting conditions
A total of 13 coffee bean varieties were used,
specifically 12 from Coffea arabica (varieties 1–5 and 7–13)
and one from the Coffea canephora robusta species (variety
6). The coffee beans were roasted to different degrees. The
roasting degrees depended on the roasting time and temperature,
with high values indicating less roasting and low values more
roasting. The roasting degrees were as follows: 110 (12 min 30 sec;
210°C) for variety 1, 110 (12 min; 210°C) for variety 2, 100 (12
min 30 sec; 211°C) for variety 3, 105 (12 min; 211°C) for variety
4, 96 (11 min; 215°C) for variety 5, 154 (12 min 30 sec; 218°C) for
variety 6, 95 (12 min; 208°C) for variety 7, 95 (12 min 30 sec;
215°C) for variety 8, 101 (12 min 30 sec; 215°C) for variety 9, 110
(12 min 30 sec; 209°C) for variety 10, 100 (12 min 30 sec; 215°C)
for variety 11, and 144 (11 min 30 sec; 194°C) for variety 12. For
variety 13, 4 different roasting times (R1: 7 min 15 sec; R2: 6 min
5 sec; R3: 5 min 32 sec; R4: 3 min 52 sec) at 215°C were used in
order to examine the effects of roasting time on the antioxidant
activity.
Preparation of extracts from coffee
beans
For each variety, 2 g of either green or roasted
beans were added to 20 ml distilled water and ground using a mortar
and pestle. Each sample was sonicated for 20 min (70% amplitude,
0.7 sec cycle), and then stirred for a further 20 min under
moderate heat (35°C). The extract was separated from solid residues
by centrifuging each sample (7,000 × g, 10 min, 25°C). Finally,
each extract was separated into aliquots that were kept at −80°C
for future use.
Assessment of the total polyphenolic
content (TPC) of the extracts
The TPC of the coffee extracts was determined using
Folin-Ciocalteu reagent, as previously described (29). A 20-µl sample of extract was
added to a tube containing 1 ml deionized water. A total of 100
µl Folin-Ciocalteu reagent was added to the reaction
mixture, followed by incubation for 3 min at room temperature.
Subsequently, 280 µl 25% w/v sodium carbonate solution and
600 µl deionized water were added to the mixture. Following
1 h of incubation at room temperature in the dark, the absorbance
was measured at 765 nm vs. a blank containing Folin-Ciocalteu
reagent and distilled water without the extract. The measurement of
absorbance was conducted on a Hitachi U-1900 radio beam
spectrophotometer (serial no. 2023–029; Hitachi, Tokyo, Japan). The
optical density of the sample (20 µl) in 25% w/v solution of
sodium carbonate (280 µl) and distilled water (1.7 ml) at
765 nm was also measured. The TPC was determined using a standard
curve of absorbance values correlated with standard concentrations
(50–1500 µg/ml) of gallic acid. The TPC is presented as
µg of gallic acid equivalents per mg of extract in
percentage form.
Assessment of CGA concentration of the
coffee extracts
A liquid chromatography (LC)-mass spectrometry (MS;
2010) system was used for the analysis of CGA. CGA (≥95%) was also
purchased from Sigma-Adrich (St. Louis, MO, USA) for making stock
solutions. Stock solutions of CGA at a concentration of 100 ppm
were prepared in methanol. The working solutions of the analytes
(0, 0.5, 1, 2.5 and 5 ppm) were prepared by further dilutions of
the stock solutions. All solutions were stored at −20°C in the
dark. To a volume of 50 µl of each sample, 950 µl of
methanol were added, following by vortexing and centrifugation at
14,000 rpm for 5 min; 20 µl of the supernatant was injected
into the LC system for analysis. The system comprised of a binary
LC pump (Shimadzu Prominence LC; Shimadzu, Kyoto, Japan), a vacuum
degasser, an autosampler, a diode array detector (SPD-M20A
Prominence; Shimadzu, Kyoto, Japan; serial no. L201545) and a
column oven. A gradient of 0.1% formic acid in water (solvent A)
and methanol (solvent B) was selected as the mobile phase, with a
flow rate of 0.7 ml/min: starting at 20% solvent B (1 min), 95%
solvent B (13 min linear ramp), and finally 20% solvent B (13.01
min). The separation of the analytes was achieved on a
Discovery® C18 HPLC column (250×4.6 mm, 5 µm)
thermostated at 30°C. A diode array detector was used for the
determination of the analytes. The maximum wavelength for CGA was
320 nm. The CGA retention time was 8.93 min.
2,2-diphenyl-1-picrylhydrazyl (DPPH)
radical scavenging assay
The free-radical scavenging capacity (RSC) of the
extracts was evaluated by DPPH radical assay, as previously
described (29). Briefly, a 1.0 ml
freshly prepared methanolic solution of DPPH radical (100
µΜ) was mixed with the tested extract solution at various
concentrations (0.5–100 µg/ml). The contents were vigorously
mixed, incubated at room temperature in the dark for 20 min, and
the absorbance was measured at 517 nm. The measurement was
conducted on a Hitachi U-1900 radio beam spectrophotometer
(Hitachi). In each experiment, the tested sample alone in methanol
was used as a blank and DPPH alone in methanol was used as the
control.
The percentage RSC of the tested extracts was
calculated using the following equation: RSC (%) =
[(Acontrol − Asample)/Acontrol]
×100, where Acontrol and Asample are the
absorbance values of the control and the test sample, respectively.
Moreover, in order to compare the radical scavenging efficiency of
the extracts, the IC50 value indicating the
concentration that caused 50% scavenging of the DPPH radical was
calculated from the graph-plotted RSC percentage against the
extract concentration. All experiments were carried out in
triplicate and on at least two separate occasions.
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
(ABTS•+) radical scavenging assay
The ABTS•+ RSC of the extract was
determined as previously described in the study by Cano et
al (31) with minor
modifications. Briefly, the reaction was carried out in 1 ml
distilled water containing ABTS•+ (1 mM), hydrogen
peroxide (H2O2) (30 µM) and
horseradish peroxidase (6 µM) in 50 mM phosphate-buffered
saline (PBS; pH 7.5). The solution was vigorously mixed followed by
incubation at room temperature in the dark for 45 min until
ABTS•+ radical formation occurred. Subsequently, 10
µl extracts, of various concentrations, were added to the
reaction mixture and the absorbance at 730 nm was read. The
measurement was conducted on a Hitachi U-1900 radio beam
spectrophotometer (Hitachi). In each experiment, the tested sample
in distilled water containing ABTS•+ and
H2O2 in 50 mM PBS-pH 7.5 was used as a blank,
and the ABTS•+ radical solution with H2O was
used as the control. The RSC percentage and the IC50
values were determined as described above for the DPPH method. All
experiments were carried out in triplicate and on at least two
separate occasions.
Hydroxyl radical-induced DNA plasmid
strand cleavage
DNA strand breakage was measured by the conversion
of supercoiled pBluescript (SK+) plasmid double-stranded DNA to the
open circular form. Hydroxyl radical-induced DNA relaxation assay
was performed according to the method described in the study by
Keum et al (32) with some
modifications. The reaction mixture (10 µl) consisted of 1
µg pBluescript (SK+) plasmid DNA, 10 mM Tris-HCl-1 mM EDTA,
the tested extract at various concentrations (600, 1,000, 1,500,
2,000, 3,300 and 6,000 µg/ml) and 40 mM
H2O2. Hydroxyl radicals (OH•) were
generated from UV photolysis of H2O2
following irradiation of the reaction mixture with a 300 W UV lamp
(OSRAM GmbH, Munich, Germany) for 3 min at a distance of 50 cm. The
reaction was terminated by the addition of 3 µl loading
buffer (0.25% bromophenol blue and 30% glycerol) and analyzed by
0.8% agarose gel electrophoresis at 80 V for 1 h. The gels were
stained with ethidium bromide (0.5 µg/ml), destained with
water, photographed and analyzed by UV transillumination using the
Alpha Innotech Multiimage (ProteinSimple, San Jose, CA, USA). In
addition, pBluescript (SK+) plasmid DNA was treated with each
extract alone, at the highest concentration used in the assay, in
order to examine the effects of the extracts on plasmid DNA
conformation. It should be noted that isolated pBluescript (SK+)
plasmid DNA contained approximately 10% open-circular DNA prior to
treatment. Each experiment was repeated at least 3 times. The
preventive activity of the tested extracts against hydroxyl
radical-induced DNA strand breakage was assessed by measuring the
inhibition of the conversion of supercoiled conformation to the
open-circular form. The percentage inhibition of radical-induced
DNA strand cleavage by the extracts was calculated using the
following formula: % inhibition = [(S −
So)/(Scontrol − So)] ×100, where
Scontrol is the percentage of supercoiled DNA in the negative
control sample (plasmid DNA alone), So is the percentage
of super-coiled plasmid DNA in the positive control sample (without
tested extracts but in the presence of the radical initiating
factor), and S is the percentage of supercoiled plasmid DNA in the
sample with the tested extracts and the radical initiating factor.
Moreover, in order to compare the percentage inhibition of the
extracts, the IC50 value indicating the concentration
that caused 50% scavenging of the DPPH radical was calculated by
comparing the graph-plotted percentage inhibition to the extract
concentration. All experiments were carried out in triplicate and
on at least two separate occasions.
Peroxyl radical-induced DNA plasmid
strand cleavage
The assay was performed using the procedure
previously described in the study by Chang et al (33). Peroxyl radicals (ROO•)
were generated from the thermal decomposition of
2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH). The reaction
mixture (10 µl) containing 1 µg pBluescript (SK+)
plasmid DNA, 2.5 mM AAPH in PBS and the tested extract at various
concentrations (50, 60, 75, 100, 150 and 300 µg/ml) was
incubated in the dark for 45 min at 37°C. Following incubation, the
reaction was terminated by the addition of 3 µl loading
buffer (0.25% bromophenol blue and 30% glycerol) and analyzed by
gel electrophoresis. Each experiment was repeated 3 times. The
preventive effects of the tested extracts against peroxyl
radical-induced DNA strand breakage were assessed as described
above for hydroxyl radical-induced DNA strand breakage.
Cell culture conditions
The C2C12 murine myoblasts were a gift from
Professor Koutsilieris (National and Kapodistrian University of
Athens, Athens, Greece). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM), containing 10% (v/v) fetal bovine
serum (FBS), 2 mM L-glutamine, 100 U/ml of penicillin and 100 U/ml
streptomycin (all from Gibco, Paisley, UK) in plastic disposable
tissue culture flasks at 37°C in an atmosphere with 5% carbon
dioxide.
XTT assay
Cell viability was assessed using an XTT assay kit
(Roche, Mannheim, Germany). Briefly, the C2C12 cells were
subcultured in a 96-well plate with 1×104 cells per well
in DMEM. Following 24 h of incubation, the cells were treated with
various concentrations of the coffee extract in serum-free DMEM for
24 h. Subsequently, 50 ml XTT test solution, which was prepared by
mixing 50 ml XTT labeling reagent with 1 ml electron coupling
reagent, were added to each well. Following 4 h of incubation,
absorbance was measured at 450 nm and also at 690 nm as a reference
wavelength in a BioTek EL×800 microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). Serum-free DMEM was used as
a negative control. In addition, the absorbance of the
grape-extract concentration alone in serum-free DMEM and XTT test
solution was tested at 450 nm. The absorbance values of the grape
extracts alone were subtracted from those derived from cell
treatment with coffee extract. Data were calculated as the
percentage of inhibition using the following formula: inhibition
(%) = [(ODcontrol − ODsample)/ODcontrol] ×100, where ODcontrol and
ODsample indicate the optical density of the negative control and
the tested compounds, respectively. All experiments were carried
out in triplicate and on two separate occasions.
Assessment of GSH and ROS levels by flow
cytometry
The intracellular GSH and ROS levels were assessed
using mercury orange and 2,7-dichlorofluorescein diacetate
(DCF-DA), respectively. The fluorescent mercury orange binds
directly to GSH, while DCF-DA within cells is deacetylated by
esterases and is further converted to fluorescent DCF by the
oxidative action of ROS. A 400-mM stock solution of mercury orange
was prepared in acetone and stored at 4°C, and a fresh 400-mM stock
solution of DCF-DA was prepared in methanol. To measure the GSH and
ROS levels, the cells were resuspended in PBS at 1×106
cells/ml and incubated in the presence of mercury orange (40
µΜ) or DCF-DA (10 µΜ) in the dark at 37°C for 30 min.
The cells were then washed, resuspended in PBS, and subjected to
flow cytometric analysis using a FACSCalibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA) with excitation and
emission wavelengths at 488 and 530 nm for ROS and at 488 and 580
nm for GSH, respectively. In addition, forward-angle and
right-angle light scattering showing the cell size and cell
internal complexity, respectively, were measured. The cells were
analyzed at a flow rate of 1,000 events/sec. Analyses were
performed on 10,000 cells per sample, and the fluorescence
intensities were measured on a logarithmic scale of 4 decades of
log of fluorescence. Data were analyzed using BD Cell Quest
software (Becton-Dickinson). Each experiment was repeated at least
3 times.
Statistical analysis
All results are expressed as the means ± standard
deviation. A Spearman's correlation analysis for examining the
results from the TPC, DPPH and ABTS•+ assays was
performed. A P-value <0.05 was considered to indicate a
statistically significant difference. In addition, one-way ANOVA
was applied, followed by Tukey's test for multiple pair-wise
comparisons using SPSS software (SPSS, Inc., Chicago, IL, USA).
Results and Discussion
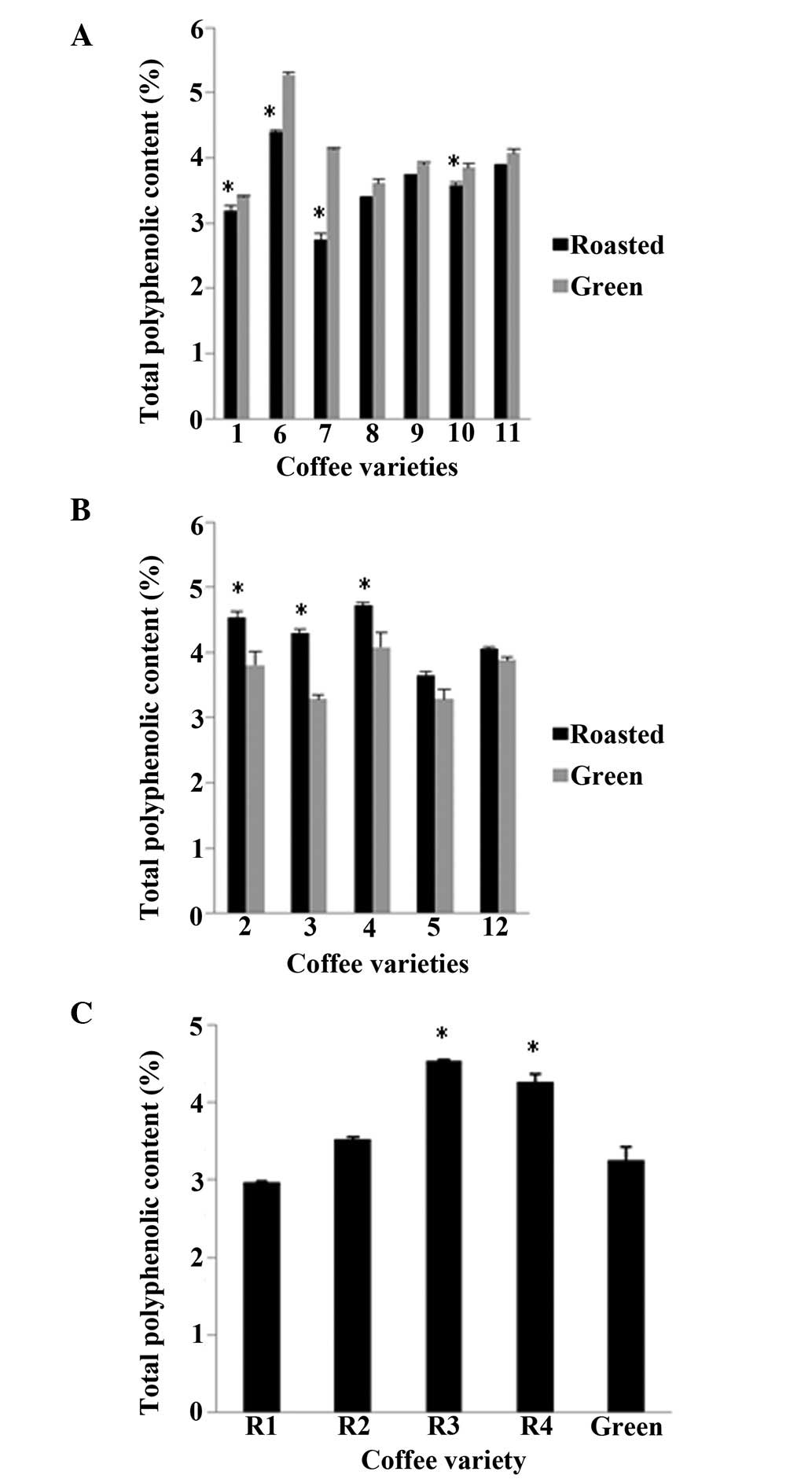
The TPC was determined in each coffee variety before
and after roasting (Fig. 1). The
TPC percentage by mass varied from 2.7 to 4.7% for the roasted
beans, with a mean value of 3.8%, whereas for the green beans, the
values varied from 3.2 to 5.2%, with a mean value of 3.8%. In 7 of
the 13 varieties, the green coffee beans had higher amounts of
polyphenols, as was expected (Fig.
1A). However, in the remaining 6, the roasted beans had more
polyphenols than their respective green beans (Fig. 1B and C). The polyphenolic
percentages obtained in the present study are in agreement with
those presented in the relevant literature, despite the fact that,
depending on the variety, large variations have been detected
(27,34,35).
Following the determination of the polyphenolic
content, the antioxidant activity of each sample was evaluated
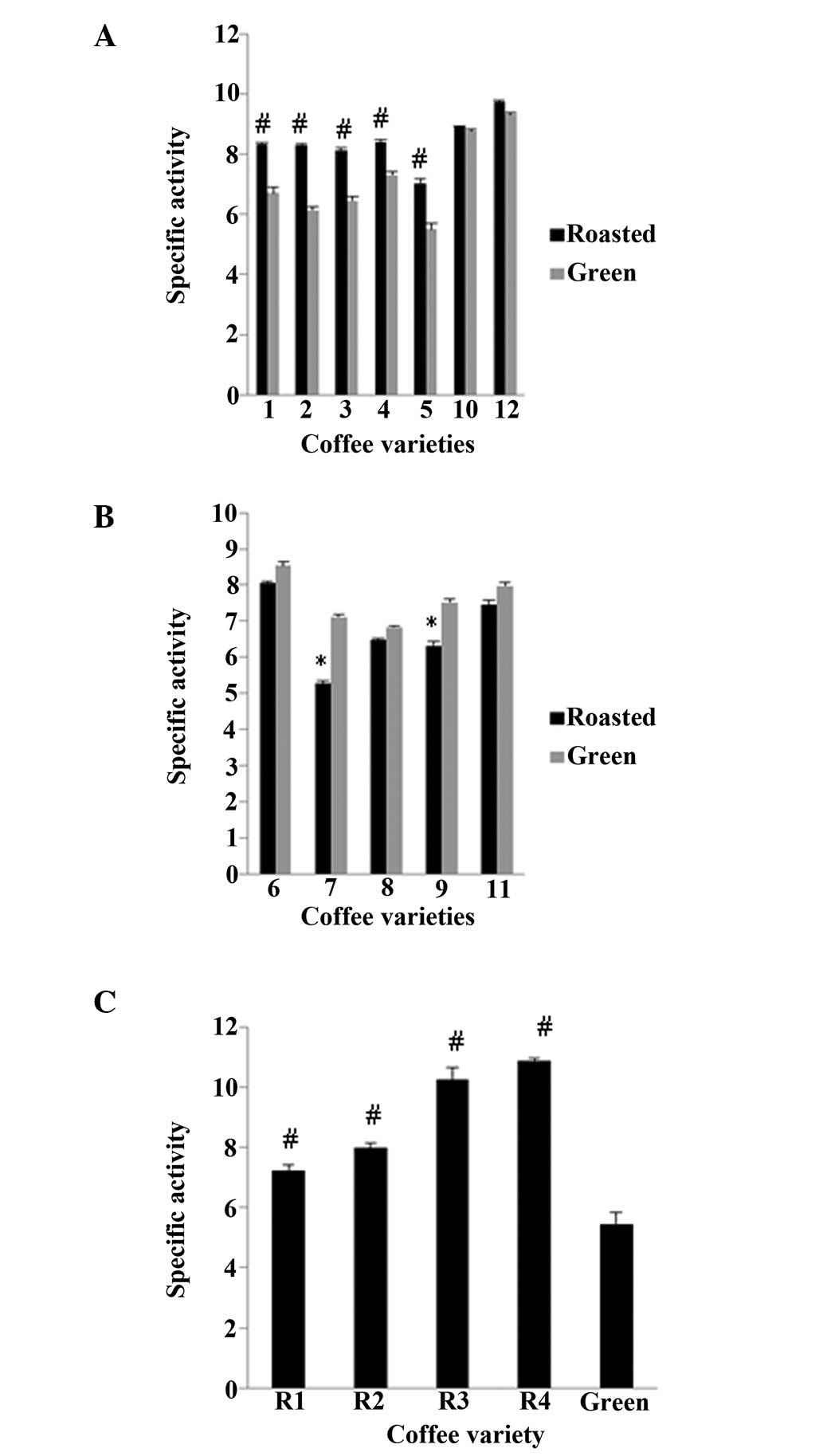
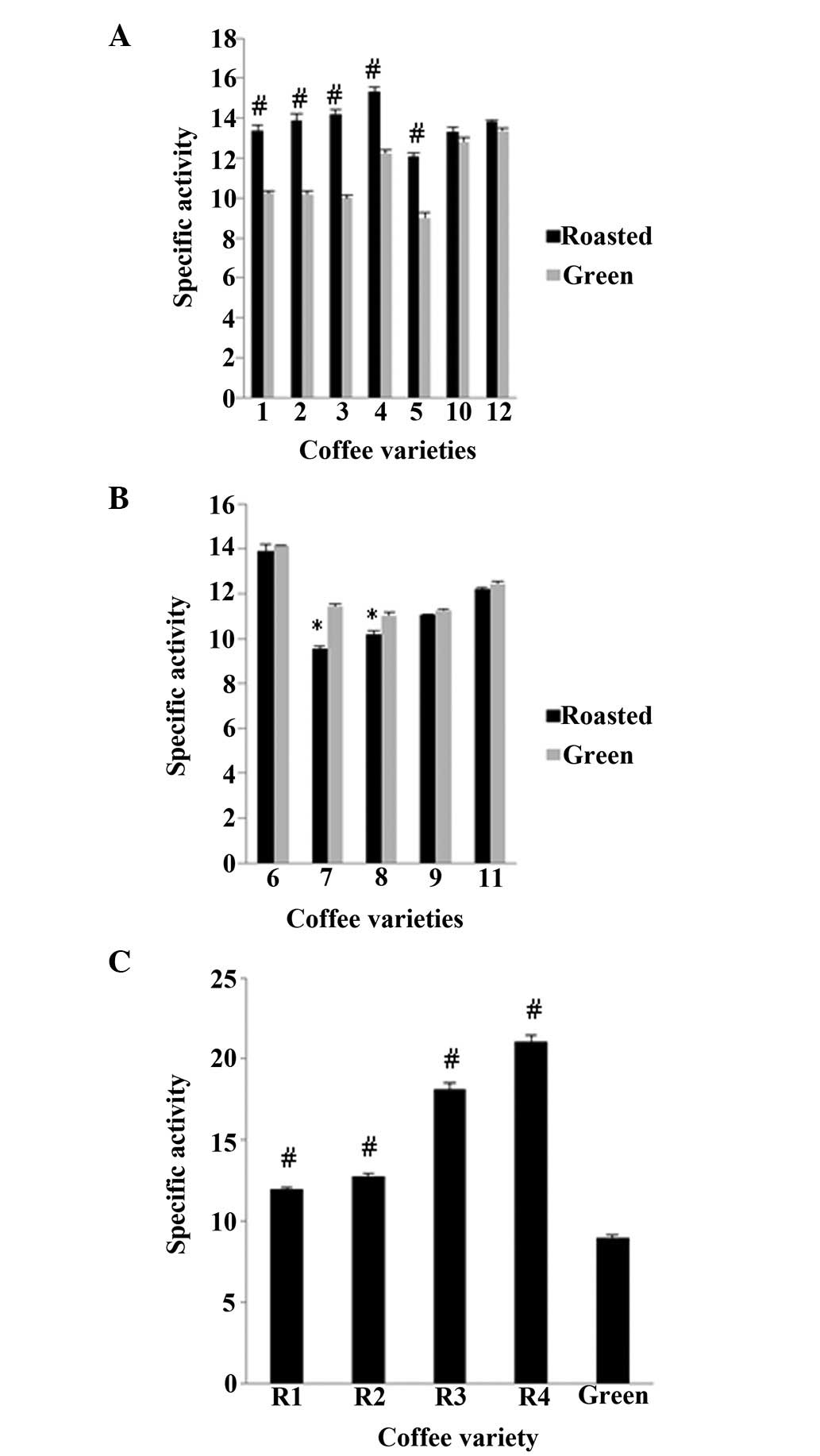
using DPPH and ABTS•+ assays (Figs. 2 and 3). In order to examine the antioxidant
potency of the polyphenols contained in each coffee sample, the
IC50 value obtained from the assays was divided by the
amount of polyphenols contained in each mg of the respective coffee
extract. According to both assays, in 8 of the 13 varieties, the
roasted beans exhibited an increased antioxidant activity compared
with their respective green beans (Fig. 1A and C and Fig. 2A and C), whereas in the remaining 5
varieties, the opposite was observed (Figs. 2B and 3B). Specifically, in varieties 1, 2, 3, 4
and 5, the roasted beans exhibited a significant (P<0.01)
increase in antioxidant activity compared to the green beans by
24.1, 35.1, 26.0, 15.2 and 27.9%, respectively, in the DPPH assay,
and in the ABTS•+ assay, this increase was 30.4, 36.2,
42.0, 25.2 and 34.1%, respectively. By contrast, in varieties 7 and
9, the antioxidant activity decreased significantly (P<0.05)
after roasting, by 26.0 and 16.0%, respectively, for the DPPH
assay, whereas in varieties 7 and 8, the decrease was 16.4 and
11.0%, respectively, in the ABTS•+ assay. The
differences between the DPPH and ABTS•+ assays may be
ascribed to the varying reactivity of these 2 radicals to the
components of each coffee sample. The fact that roasting increased
the antioxidant activity in some samples and reduced it in others
may be explained by the different phenolic composition of these
varieties. It is well known that roasting greatly affects the
chemical composition of the coffee beans due to the high
temperatures used (28,36). For example, new compounds, such as
melanoidins, are formed, which exhibit antioxidant activity,
whereas other ingredients, such as CGAs are broken down (25,37).
Moreover, we noted that the results from both DPPH
and ABTS•+ assays significantly correlated with the TPC
(Table I). Specifically, the
correlation coefficient (r) was 0.647 (P<0.01) between TPC and
DPPH, 0.766 (P<0.01) between TPC and ABTS•+, and
0.926 (P<0.01) between DPPH and ABTS•+ (Table I). The correlations between the TPC
and both the free radical scavenging assays, DPPH and
ABTS•+, indicated that there was an association between
the total amount of polyphenols and the antioxidant activity, that
is, higher amounts of polyphenols led to enhanced potency. In
addition, the correlation between DPPH and ABTS•+
suggests that the same compounds of the extracts are likely
responsible for the scavenging of both free radicals.
 | Table ICorrelation coefficient (r) between
values of TPC, DPPH and ABTS•+ assays. |
Table I
Correlation coefficient (r) between
values of TPC, DPPH and ABTS•+ assays.
| Assays | Correlation
coefficient (r) |
|---|
| TPC-DPPH | 0.647a |
|
TPC-ABTS•+ | 0.766a |
|
DPPH-ABTS•+ | 0.926a |
For variety 13, there were 4 different roasted bean
samples, and each one was roasted for a different amount of time at
215°C. The results revealed that the antioxidant activity of the
beans was dependent on the roasting time (Figs. 2C and 3C). More specifically, for all 4 samples,
the roasted beans exhibited significantly (P<0.01) higher levels
of activity than the green ones, at roasting times of 7 min 15 sec
(R1), 6 min 5 sec (R2), 5 min 32 sec (R3) and 3 min 52 sec (R4) by
32.8, 46.2, 87.9 and 99.6%, respectively for DPPH assay, and by
33.4, 42.4, 102.0 and 135.0%, respectively for ABTS•+
assay (Figs. 2C and 3C). The roasting conditions which were
used for these beans are all within the range of those typically
used when making coffee beverages. The considerable differences
observed under varying roasting conditions are in accordance with
those presented in other studies (36,38,39).
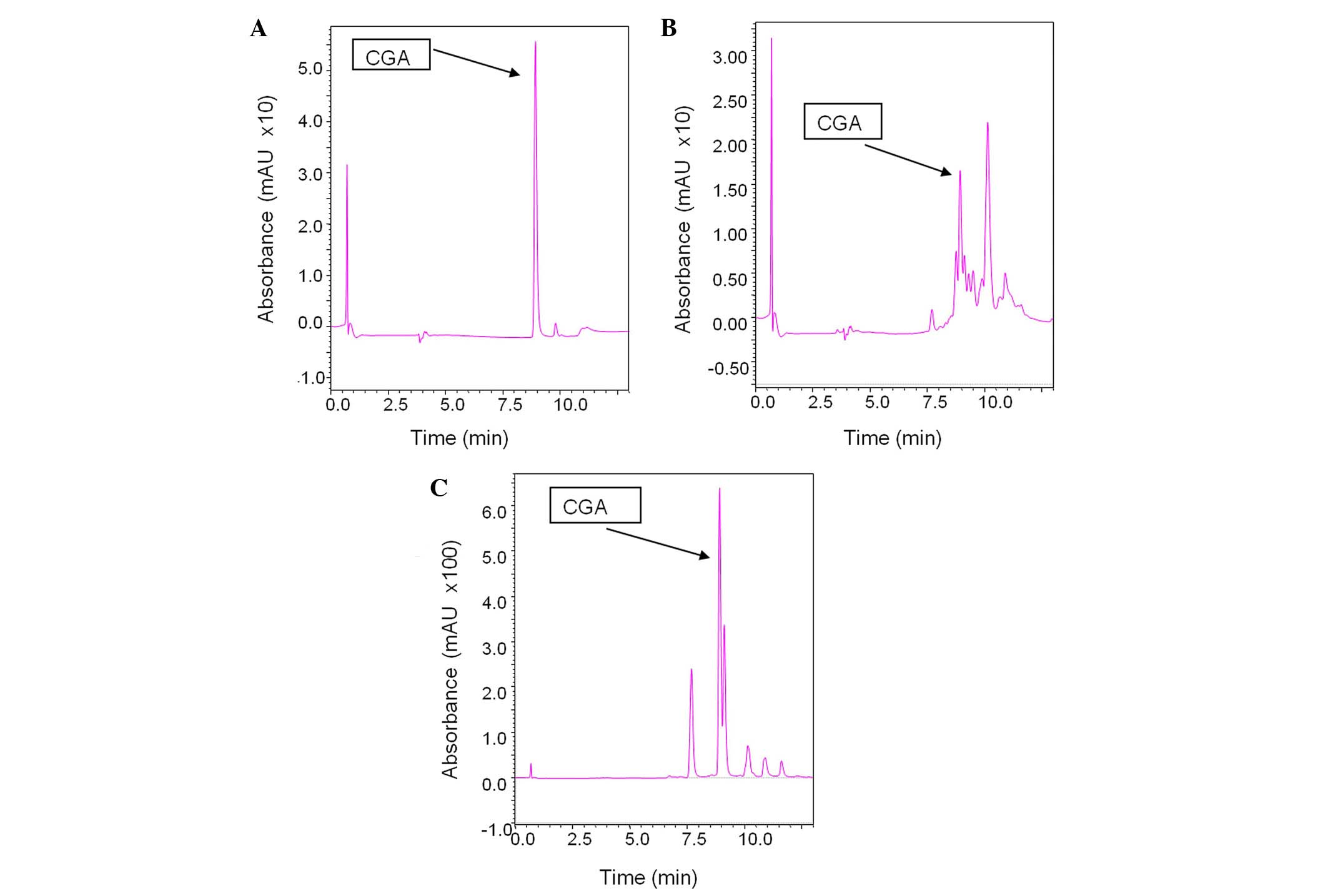
In addition, an LC-MS analysis was performed to examine the effect
of roasting on the levels of CGA. For this analysis, 2 samples from
variety 13 were used: the green extract and a sample roasted for 3
min and 52 sec (R4) at 215°C. According to the results, CGA
diminished from 2028.4 ppm in the green beans to 38.8 ppm in the
roasted sample (Fig. 4). This
difference denotes the importance of novel antioxidant substances
that are formed during the roasting procedure (e.g., melanoidins).
Specifically, although one of the most prominent antioxidant
compounds in green beans is practically non-existent in the roasted
ones, the latter exhibited higher levels of antioxidant activity.
It has previously been reported that polyphenolic compounds can be
incorporated into melanoidins during roasting, either as intact
units or following their breakdown to simpler phenols (40).
To further investigate the antioxidant capacity of
the coffee extracts, 2 assays assessing the protective effects of
the extracts against ROS-induced DNA damage were carried out. For
the ROO•- and OH•-induced DNA plasmid strand
breakage assays, 5 varieties were selected, namely 1 and 4 (those
varieties in which higher activity was observed for the roasted
beans compared to the green beans in the DPPH and ABTS•+
assays), 7 and 9 (where the green beans were more potent than the
roasted beans according to DPPH and ABTS•+ assays), and
variety 13, in which the effect of the roasting time was tested
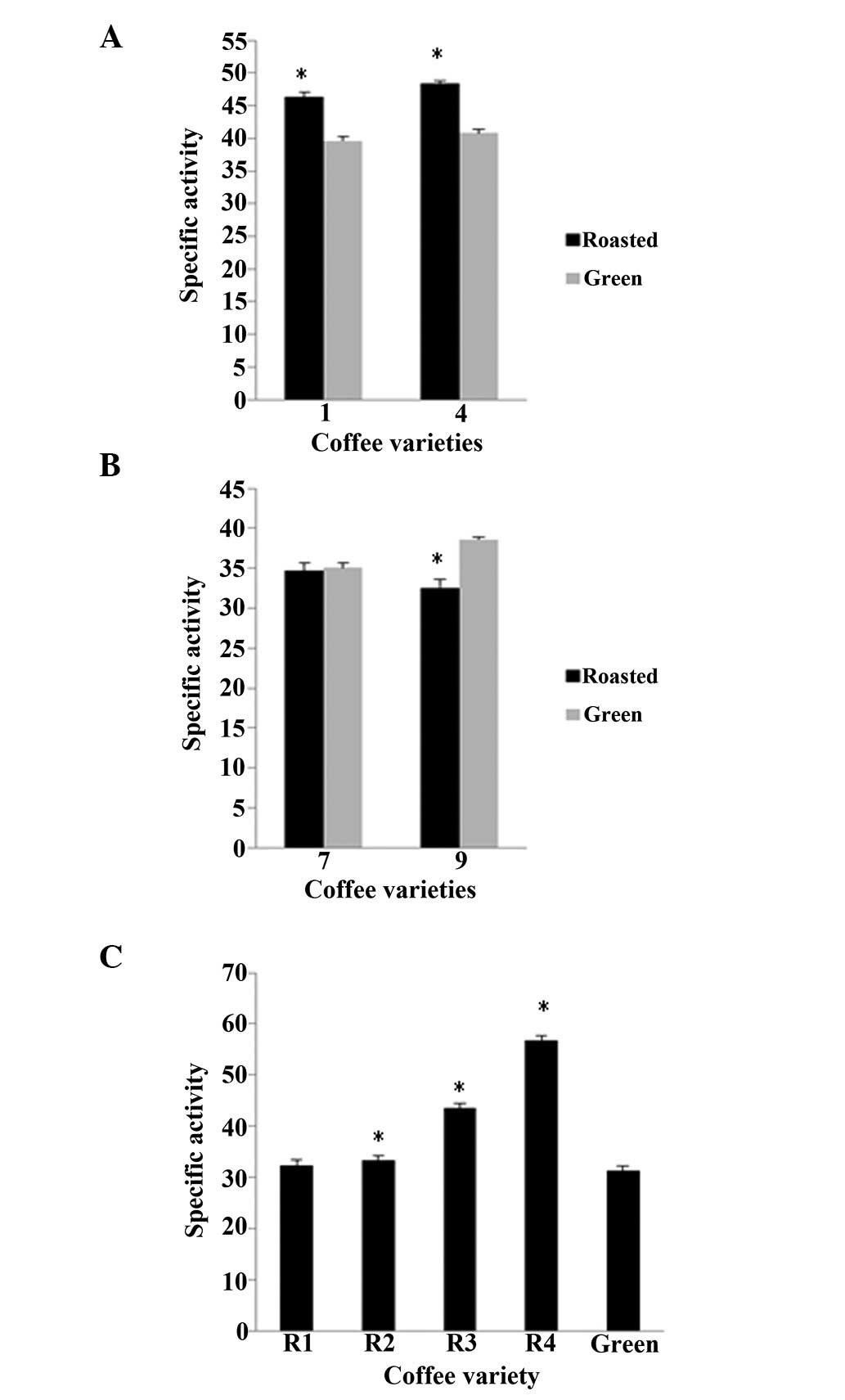
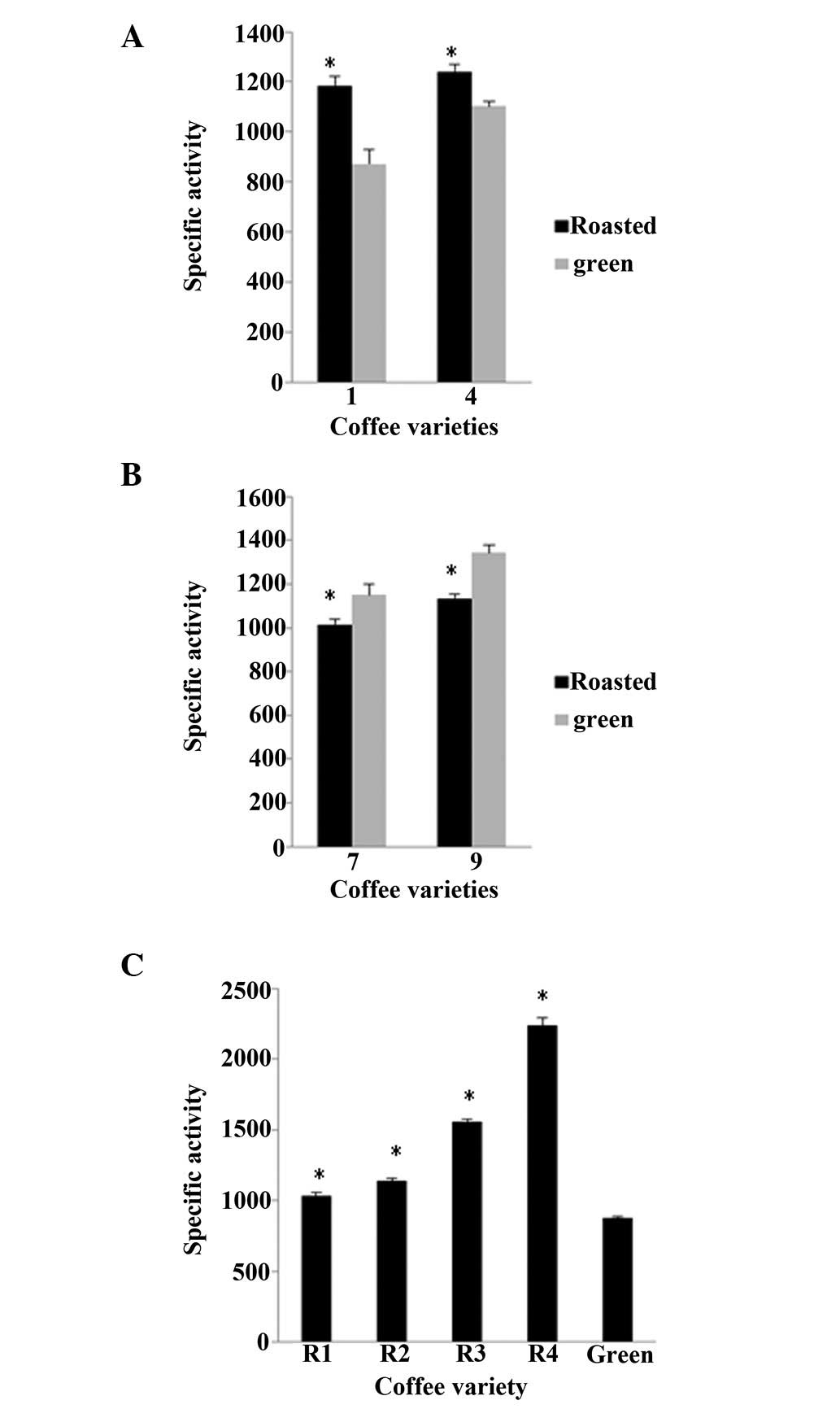
(Figs. 5 and 6). These 2 methods yielded similar
results with the DPPH and ABTS•+ assays. Specifically,
in the case of OH•, the roasted beans of varieties 1 and
4 exhibited higher specific activity by 17.0 and 19.0%,
respectively, compared to the green beans, whereas in varieties 7
and 9 the green beans had a higher activity by 4.1 and 18.5%,
respectively, than the roasted beans (Fig. 5A and B). Moreover, for variety 13,
all 4 groups of roasted beans had significantly (P<0.05) higher
activities than the green ones, by 3.2, 6.2, 39 and 81.1% for R1,
R2, R3 and R4 time points of roasting, respectively (Fig. 5C). As for the assay using
ROO•, again in varieties 1 and 4 the roasted beans
higher activities by 35.6 and 12.7%, respectively, compared to the
green beans, whereas in varieties 7 and 9, the green beans
exhibited higher levels of activity, by 13.2 and 18.4%,
respectively, than the roasted beans (Fig. 6A and B). In addition, in variety 13
the roasted beans exhibited significantly higher levels of activity
compared with the green ones, by 17.0, 29.1, 77.3 and 154.0% for
R1, R2, R3 and R4 time points of roasting, respectively (Fig. 6C).
Thus, we noted that all the tested coffee extracts
exhibited protective activity against free radical-induced DNA
damage, with the most potent being the less roasted sample from
variety 13 (R4). As shown in Figs.
5 and 6, the much lower
specific activities observed against OH•-induced DNA
strand breaks may be due to the high reaction rate of
OH• with DNA, and thus it is more difficult for
antioxidant molecules to exert their protective effects (41). To the best of our knowledge, this
is the first study to report the protective effects of coffee
extracts against DNA damage induced by OH• and
ROO• radicals. However, other studies have been
performed using different oxidants, and the oxidants in these
coffee extracts also exerted a significant protective effect
against mutagenesis (42).
Specifically, coffee inhibited
tert-butylhydroperoxide-induced mutagenicity in
Salmonella typhimurium strains TA100 and TA102. This
activity was partly attributed to cafestol and kahweol, two
diterpenes commonly found in coffee (43,44).
To summarize, all coffee extracts exhibited an
antioxidant activity similar to that observed in previous studies
by our research group on polyphenolic extracts; the antioxidant
activity of the extracts was comparable to that of grapes (45) and pomegranates (unpublished
data).
The most potent antioxidant extract [variety 13,
roasted for 3 min and 52 sec at 215°C (R4)] was selected in order
to examine its effects on the cellular redox status (specifically
in C2C12 murine myoblasts) by assessing the GSH and ROS levels by
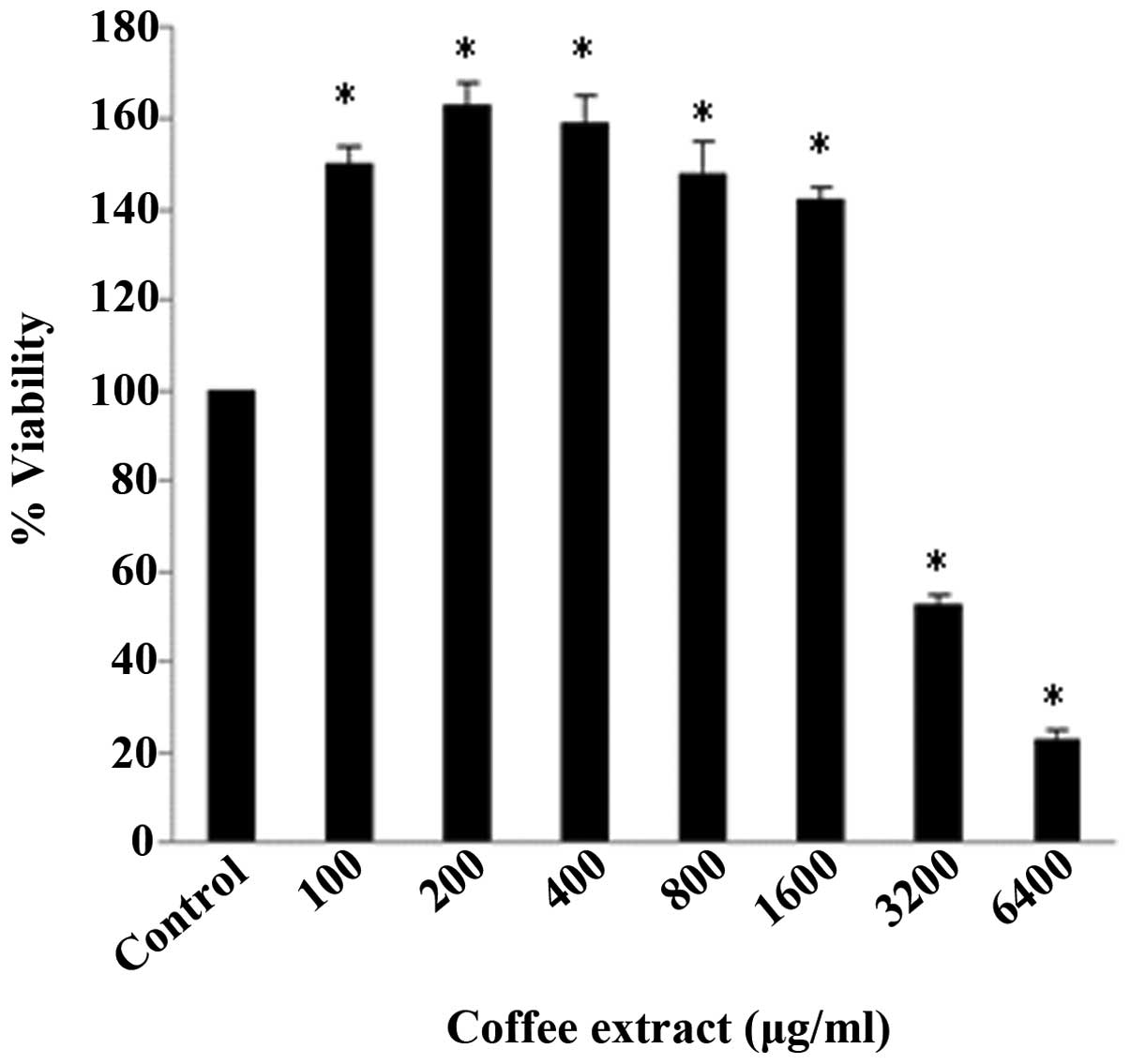
flow cytometry. Non-cytotoxic concentrations were used (according
to the results of the XTT assay) and, more specifically, the
non-cytotoxic concentrations ranged from 100–1,600 µg/ml
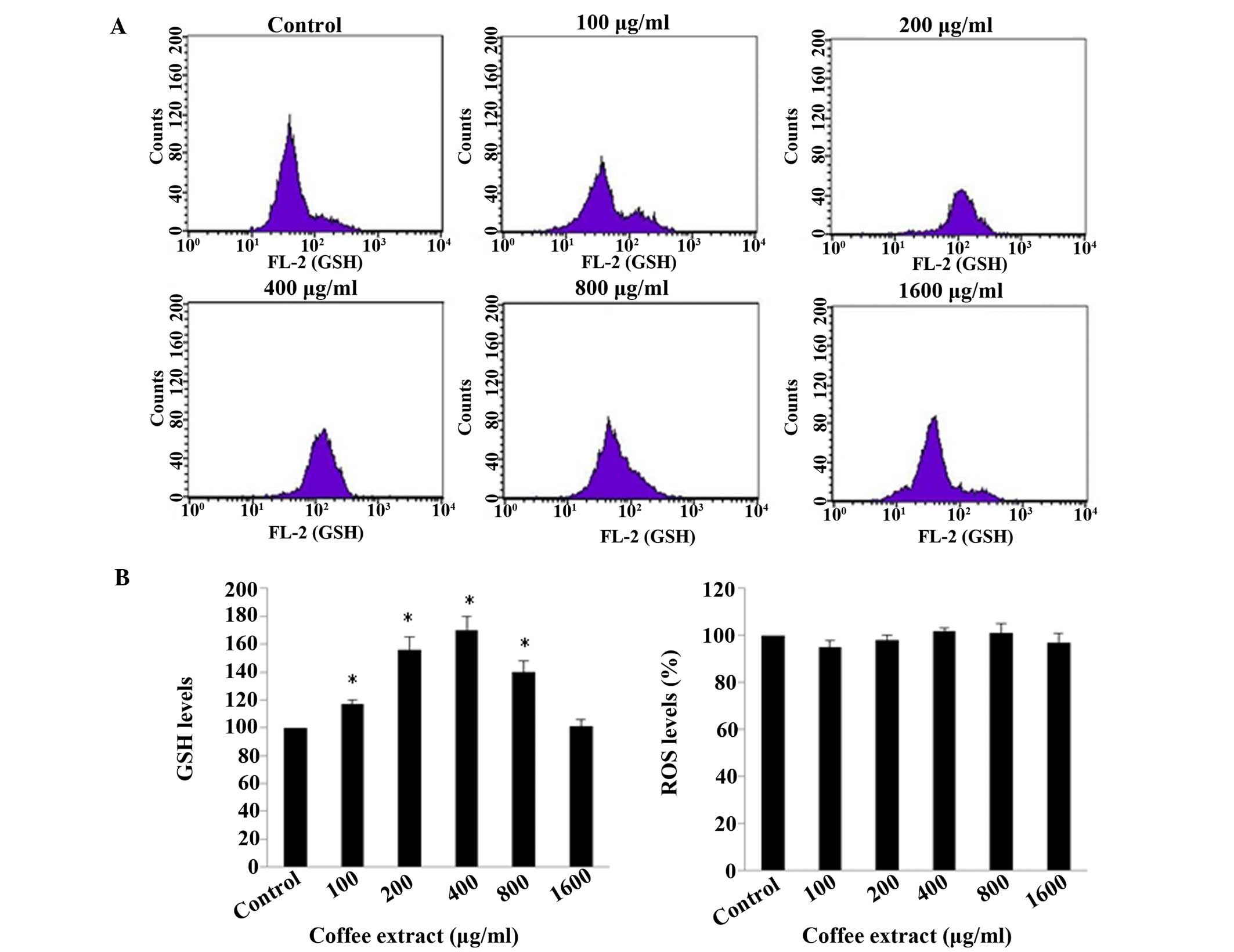
extract (Fig. 7). The results of
flow cytometric analysis revealed that the GSH levels were
increased significantly (P<0.05), by 17, 56, 70 and 40% at 100,
200, 400 and 800 µg/ml extract, respectively, compared to
the control (Fig. 8). Although the
extract increased the levels of the antioxidant molecule, GSH, the
ROS levels were not significant affected by the extract (Fig. 8). In previous studies of ours, we
also found that the ROS levels are not always accompanied by
changes in oxidative stress levels or antioxidant mechanisms
(46,47). Moreover, the increase in the GSH
levels was not linear; rather, the increase in the GSH levels
peaked at 400 µg/ml and subsequently declined, and no
increase at 1,600 µg/ml was noted, compared with the
control. The observed decline in the GSH levels may be explained by
the pro-oxidant activity of coffee extracts after reaching a
certain concentration, as has been observed in relation to other
plant polyphenolic extracts (48–51).
Indeed, 1,600 µg/ml was a crucial concentration, since it
was the highest concentration used which did not exert cytotoxic
effects (Fig. 7). Thus, our
results suggest that the tested coffee extract improved the
cellular redox status by increasing the levels of GSH, one of the
most important antioxidant molecules. Importantly, it has been
previously reported that coffee extracts lead to protein
localization of the nuclear factor (erythroid-derived 2)-like 2
(Nrf2) protein, a key transcription factor which is associated with
antioxidant systems in HT-29 cells (52,53).
Interestingly, one of the enzymes whose expression is regulated by
Nrf2 is gamma-glutamylcysteine synthetase, the first enzyme in the
biosynthetic pathway of GSH (54).
However, when tested in humans, considerable inter-individual
differences were observed in Nrf2 localization, suggesting that the
effect of coffee extracts is genotype-dependent (55,56).
However, as each coffee variety has a different chemical
composition and thus performs a different activity, determining the
potential of the coffee extracts used in our study to induce Nrf2
activity will be an intriguing task.
In conclusion, the findings of the present study
indicated that coffee extracts from green or roasted beans
exhibited potent free radical scavenging activity and also served
to protect against the DNA damage induced by free radicals.
Moreover, we noted differences in the levels of antioxidant
activity between green and roasted bean extracts derived from the
same variety. In some coffee varieties, bean roasting reduced
antioxidant activity, whereas in others the opposite was noted. It
appears that the final effect depends on the chemical composition
of the beans of each coffee variety, but this hypothesis requires
further investigation. In addition, roasting time was shown to
affect the antioxidant activity of roasted coffee beans. This
observation suggests that the roasting time should be optimized in
order to maintain the levels of antioxidant activity as high as
possible. Finally, the coffee extract with the highest antioxidant
activity (variety 13) was also shown to enhance the antioxidant
mechanisms in myoblast cells by increasing GSH levels. Currently
under way is a study in which cells treated with this extract are
used in DNA microarray analysis, and this is being undertaken in
order to examine its effects on whole genome expression, and thus
investigate in depth the molecular mechanisms responsible for its
antioxidant activity. Understanding the mechanisms through which
coffee acts as an antioxidant will lead to improvements in the
extraction and roasting processes and the ability to fully exploit
its properties.
Acknowledgments
The present study was funded by a grant (no. 5042;
'Assessment of antioxidant and anticarcinogenic activity of green
and roasted coffee varieties') awarded to Professor D.
Kouretas.
Abbreviations:
|
GSH
|
glutathione
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
H2O2
|
hydrogen peroxide
|
|
O2•−
|
superoxide radical
|
|
OH•
|
hydroxyl radical
|
|
PBS
|
phosphate-buffer saline
|
|
ROO•
|
peroxyl radical
|
|
ROS
|
reactive oxygen species
|
|
TPC
|
total polyphenolic content
|
References
|
1
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
4
|
Halliwell B: Free Radicals and Other
Reactive Species in Disease. eLS. John Wiley & Sons, Ltd;
2001
|
|
5
|
Elias RJ, Kellerby SS and Decker EA:
Antioxidant activity of proteins and peptides. Crit Rev Food Sci
Nutr. 48:430–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valko M, Leibfritz D, Moncol J, Cronin
MTD, Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
7
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elnakish MT, Hassanain HH, Janssen PM,
Angelos MG and Khan M: Emerging role of oxidative stress in
metabolic syndrome and cardiovascular diseases: important role of
Rac/NADPH oxidase. J Pathol. 231:290–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wruck CJ, Fragoulis A, Gurzynski A,
Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S,
Varoga D, Lippross S and Pufe T: Role of oxidative stress in
rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann
Rheum Dis. 70:844–850. 2011. View Article : Google Scholar
|
|
10
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: an overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
11
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Diabetes, oxidative stress and therapeutic strategies. Biochim
Biophys Acta. 1840:2709–2729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Wang W, Li L, Perry G, Lee HG and
Zhu X: Oxidative stress and mitochondrial dysfunction in
Alzheimer's disease. Biochim Biophys Acta. 1842:1240–1247. 2014.
View Article : Google Scholar :
|
|
13
|
Landete JM: Dietary intake of natural
antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr.
53:706–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang YZ, Yang S and Wu G: Free radicals,
antioxidants, and nutrition. Nutrition. 18:872–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landete JM: Updated knowledge about
polyphenols: functions, bioavailability, metabolism, and health.
Crit Rev Food Sci Nutr. 52:936–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quideau S, Deffieux D, Douat-Casassus C
and Pouysegu L: Plant polyphenols: chemical properties, biological
activities, and synthesis. Angew Chem Int Ed Engl. 50:586–621.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pandey KB and Rizvi SI: Plant polyphenols
as dietary antioxidants in human health and disease. Oxid Med Cell
Longev. 2:270–278. 2009. View Article : Google Scholar
|
|
18
|
Higdon JV and Frei B: Coffee and health: a
review of recent human research. Crit Rev Food Sci Nutr.
46:101–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henry-Vitrac C, Ibarra A, Roller M,
Mérillon JM and Vitrac X: Contribution of chlorogenic acids to the
inhibition of human hepatic glucose-6-phosphatase activity in vitro
by Svetol, a standardized decaffeinated green coffee extract. J
Agric Food Chem. 58:4141–4144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murthy PS and Naidu MM: Recovery of
phenolic antioxidants and functional compounds from coffee industry
by-products. Food Bioprocess Technol. 5:897–903. 2012. View Article : Google Scholar
|
|
21
|
Park JB: Isolation and quantification of
major chlorogenic acids in three major instant coffee brands and
their potential effects on H2O2-induced
mitochondrial membrane depolarization and apoptosis in PC-12 cells.
Food Funct. 4:1632–1638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato Y, Itagaki S, Kurokawa T, Ogura J,
Kobayashi M, Hirano T, Sugawara M and Iseki K: In vitro and in vivo
antioxidant properties of chlorogenic acid and caffeic acid. Int J
Pharm. 403:136–138. 2011. View Article : Google Scholar
|
|
23
|
Xu JG, Hu QP and Liu Y: Antioxidant and
DNA-protective activities of chlorogenic acid isomers. J Agric Food
Chem. 60:11625–11630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farah A, Monteiro M, Donangelo CM and
Lafay S: Chlorogenic acids from green coffee extract are highly
bioavailable in humans. J Nutr. 138:2309–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaiswal R, Matei MF, Golon A, Witt M and
Kuhnert N: Understanding the fate of chlorogenic acids in coffee
roasting using mass spectrometry based targeted and non-targeted
analytical strategies. Food Funct. 3:976–984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daglia M, Racchi M, Papetti A, Lanni C,
Govoni S and Gazzani G: In vitro and ex vivo antihydroxyl radical
activity of green and roasted coffee. J Agric Food Chem.
52:1700–1704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gawlik-Dziki U, Świeca M, Dziki D,
Kowalska I, Pecio Ł, Durak A and Sęczyk Ł: Lipoxygenase inhibitors
and antioxidants from green coffee - mechanism of action in the
light of potential bioaccessibility. Food Res Int. 61:48–55. 2014.
View Article : Google Scholar
|
|
28
|
Kamiyama M, Moon JK, Jang HW and Shibamoto
T: Role of degradation products of chlorogenic acid in the
antioxidant activity of roasted coffee. J Agric Food Chem.
63:1996–2005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singleton VL, Orthofer R and
Lamuela-Raventós RM: Analysis of total phenols and other oxidation
substrates and antioxidants by means of Folin-Ciocalteu reagent.
Methods in Enzymology, Oxidant and Antioxidants (Part A). Packer L:
299. Academic Press Inc; San Diego, CA: pp. 152–178. 1999,
View Article : Google Scholar
|
|
30
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
Lwt - Food Sci Technol. 28:25–30. 1995. View Article : Google Scholar
|
|
31
|
Cano A, Hernández-Ruíz J, García-Cánovas
F, Acosta M and Arnao MB: An end-point method for estimation of the
total antioxidant activity in plant material. Phytochem Anal.
9:196–202. 1998. View Article : Google Scholar
|
|
32
|
Keum YS, Park KK, Lee JM, Chun KS, Park
JH, Lee SK, Kwon H and Surh YJ: Antioxidant and anti-tumor
promoting activities of the methanol extract of heat-processed
ginseng. Cancer Lett. 150:41–48. 2000. View Article : Google Scholar
|
|
33
|
Chang ST, Wu JH, Wang SY, Kang PL, Yang NS
and Shyur LF: Antioxidant activity of extracts from Acacia confusa
bark and heartwood. J Agric Food Chem. 49:3420–3424. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodrigues NP, Salva T de JG and Bragagnolo
N: Influence of coffee genotype on bioactive compounds and the in
vitro capacity to scavenge reactive oxygen and nitrogen species. J
Agric Food Chem. 63:4815–4826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mills CE, Oruna-Concha MJ, Mottram DS,
Gibson GR and Spencer JPE: The effect of processing on chlorogenic
acid content of commercially available coffee. Food Chem.
141:3335–3340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smrke S, Opitz SEW, Vovk I and Yeretzian
C: How does roasting affect the antioxidants of a coffee brew?
Exploring the antioxidant capacity of coffee via on-line
antioxidant assays coupled with size exclusion chromatography. Food
Funct. 4:1082–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Esquivel P and Jiménez VM: Functional
properties of coffee and coffee by-products. Food Res Int.
46:488–495. 2012. View Article : Google Scholar
|
|
38
|
Nicoli MC, Anese M, Manzocco L and Lerici
CR: Antioxidant Properties of Coffee Brews in Relation to the
Roasting Degree. LWT Lebensmittel Wissenschaft & Technologie.
30:292–297. 1997. View Article : Google Scholar
|
|
39
|
Cämmerer B and Kroh L: Antioxidant
activity of coffee brews. Eur Food Res Technol. 223:469–474. 2006.
View Article : Google Scholar
|
|
40
|
Perrone D, Farah A and Donangelo CM:
Influence of coffee roasting on the incorporation of phenolic
compounds into melanoidins and their relationship with antioxidant
activity of the brew. J Agric Food Chem. 60:4265–4275. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Halliwell B: Reactive species and
antioxidants. Redox biology is a fundamental theme of aerobic life.
Plant Physiol. 141:312–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stadler RH, Turesky RJ, Müller O, Markovic
J and Leong-Morgenthaler PM: The inhibitory effects of coffee on
radical-mediated oxidation and mutagenicity. Mutat Res.
308:177–190. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cavin C, Holzhaeuser D, Scharf G,
Constable A, Huber WW and Schilter B: Cafestol and kahweol, two
coffee specific diterpenes with anticarcinogenic activity. Food
Chem Toxicol. 40:1155–1163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee KJ and Jeong HG: Protective effects of
kahweol and cafestol against hydrogen peroxide-induced oxidative
stress and DNA damage. Toxicol Lett. 173:80–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Apostolou A, Stagos D, Galitsiou E, Spyrou
A, Haroutounian S, Portesis N, Trizoglou I, Wallace Hayes A,
Tsatsakis AM and Kouretas D: Assessment of polyphenolic content,
antioxidant activity, protection against ROS-induced DNA damage and
anticancer activity of Vitis vinifera stem extracts. Food Chem
Toxicol. 61:60–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kerasioti E, Stagos D, Priftis A,
Aivazidis S, Tsatsakis AM, Hayes AW and Kouretas D: Antioxidant
effects of whey protein on muscle C2C12 cells. Food Chem.
155:271–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goutzourelas N, Stagos D, Demertzis N,
Mavridou P, Karterolioti H, Georgadakis S, Kerasioti E, Aligiannis
N, Skaltsounis L, Statiri A, et al: Effects of polyphenolic grape
extract on the oxidative status of muscle and endothelial cells.
Hum Exp Toxicol. 33:1099–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Procházková D, Boušová I and Wilhelmová N:
Antioxidant and prooxidant properties of flavonoids. Fitoterapia.
82:513–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lambert JD and Elias RJ: The antioxidant
and pro-oxidant activities of green tea polyphenols: a role in
cancer prevention. Arch Biochem Biophys. 501:65–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fukumoto LR and Mazza G: Assessing
antioxidant and prooxidant activities of phenolic compounds. J
Agric Food Chem. 48:3597–3604. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sakihama Y, Cohen MF, Grace SC and
Yamasaki H: Plant phenolic antioxidant and prooxidant activities:
phenolics-induced oxidative damage mediated by metals in plants.
Toxicology. 177:67–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boettler U, Sommerfeld K, Volz N, Pahlke
G, Teller N, Somoza V, Lang R, Hofmann T and Marko D: Coffee
constituents as modulators of Nrf2 nuclear translocation and ARE
(EpRE)-dependent gene expression. J Nutr Biochem. 22:426–440. 2011.
View Article : Google Scholar
|
|
53
|
Volz N, Boettler U, Winkler S, Teller N,
Schwarz C, Bakuradze T, Eisenbrand G, Haupt L, Griffiths LR,
Stiebitz H, et al: Effect of coffee combining green coffee bean
constituents with typical roasting products on the Nrf2/ARE pathway
in vitro and in vivo. J Agric Food Chem. 60:9631–9641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Myhrstad MCW, Carlsen H, Nordström O,
Blomhoff R and Moskaug JO: Flavonoids increase the intracellular
glutathione level by transactivation of the gamma-glutamylcysteine
synthetase catalytical subunit promoter. Free Radic Biol Med.
32:386–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Boettler U, Volz N, Teller N, Haupt LM,
Bakuradze T, Eisenbrand G, Bytof G, Lantz I, Griffiths LR and Marko
D: Induction of antioxidative Nrf2 gene transcription by coffee in
humans: depending on genotype? Mol Biol Rep. 39:7155–7162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hassmann U, Haupt LM, Smith RA, Winkler S,
Bytof G, Lantz I, Griffiths LR and Marko D: Potential antioxidant
response to coffee - A matter of genotype? Meta Gene. 2:525–539.
2014. View Article : Google Scholar
|