Introduction
Prostate cancer (Pca) has one of the highest
mortality rates for malignant cancers worldwide. It is the most
frequently diagnosed type of cancer and the second leading cause of
mortality in males in the USA (1).
The majority of Pca-associated mortality is due to metastatic
castration-resistant Pca (CRPC). While novel treatments, such as
docetaxel, enzalutamide, abiraterone, sipuleucel-T, cabazitaxel and
radium-223, have been demonstrated to improve survival for patients
with metastatic CRPC, the disease remains incurable (2). To determine more efficacious
therapeutic methods, the underlying mechanism of Pca onset, and
transition from castration-sensitive Pca to CRPC, must be
established.
Molecular changes in Pca have been extensively
investigated, and numerous genes have been observed to be
aberrantly expressed during Pca onset and development (3). PAK6, a serine threonine kinase that
belongs to the PAK family (4), is
an androgen receptor-interacting protein (5). PAK6 was recently indicated to be
overexpressed in primary and metastatic Pca (6). Furthermore, increased PAK6 expression
has been observed in the LAPC4, PC-3 and DU-145 cell lines
(7). PAK6 overexpression in tumors
may promote cell proliferation and inhibit apoptosis. Previous
studies demonstrate that PAK6 knockdown in prostate cell lines
inhibits cell growth, and enhances docetaxel chemosensitivity and
radio-sensitivity (8,9). However, the mechanism of aberrant
PAK6 expression remains to be fully elucidated.
Recent research demonstrates that
post-transcriptional events perform significant functions in
aberrant gene expression. MicroRNAs (miRs) are important molecules
involved in post-transcriptional events (10). miRs are small and endogenously
produced non-coding RNAs of 19–25 nucleotides in length that
negatively regulate target gene expression by binding complementary
sequences in the 3′-untranslated region (UTR) of mRNAs, which
results in translational repression or direct mRNA cleavage
(11). Various miRs have been
demonstrated to contribute to Pca by affecting cell development,
proliferation, differentiation and apoptosis. miR-143, for example,
inhibits Pca cell proliferation and migration, and enhances cell
sensitivity toward docetaxel via KRAS down-regulation (12).
Considering these previous findings, it is
hypothesized that specific miRs participate in PAK6 regulation.
Thus, the aim of the present study was to identify an miR that
targets PAK6 and determine the function of this miR in Pca.
Materials and methods
Human Pca specimens
All of the specimens used in the present study were
collected from the Affiliated Zhongda Hospital of Southeast
University (Nanjing, China) between March 2012 and April 2014.
Ethical approval was obtained from the relevant ethics committee at
the Affiliated Zhongda Hospital of Southeast University. All of the
samples were collected upon receipt of written informed consent
from patients. Benign prostate hyperplasia (BPH) tissues were
obtained from 10 patients who had undergone transurethral prostatic
resection (TURP). Pca tissues were obtained from 30
androgen-dependent Pca (ADPC) patients who had undergone radical
prostatectomy. All patients were divided into three groups based on
a Gleason score of <7, 7 or >7 (13). Nine patients were diagnosed with
CRPC, as their serum prostate-specific antigen levels continued to
increase despite maximum androgen deprivation therapy. All patients
with CRPC were in stage T4 (distant metastasis) and presented with
a Gleason score of >7 (13).
These patients had undergone TURP due to urinary retention.
Histological diagnosis was conducted on freshly frozen sections
following hematoxylin and eosin staining (Beyotime Institute of
Biotechnology, Shanghai, China). Ten ADPC samples with Gleason
score >7 and >60% tumor content and nine patients with CRPC
were included in the present study for quantitative reverse
transcription-polymerase chain reaction (qRT-PCR). The specimens
used for miR qRT-PCR were snap-frozen in liquid nitrogen (Nanjing
University Physics Refrigeration Laboratory, Nanjing, China).
Immunohistochemistry
All surgical samples were fixed in 10% buffered
formaldehyde solution (BioSharp, Hefei, China) and embedded in
paraffin (Sigma-Aldrich, St. Louis, MO, USA). Paraffin sections
(4-µm thick) were reacted with polyclonal antibodies against
PAK6 (1:50; cat. no. 13539-1-AP; ProteinTech Group, Inc., Chicago,
IL, USA). Phosphate-buffered saline (PBS; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) served as a negative
control (NC).
qRT-PCR
Total RNA was extracted from specimens using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA synthesis
was performed using PrimeScript® 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China).
qRT-PCR reactions were performed using the SYBR Green PCR Master
mix from the Hairpin-it™ miRs RT-PCR Quantitation kit (GenePharma
Co., Ltd., Shanghai, China) according to the manufacturer's
instructions. PCR conditions were used to detect miRs as follows:
95°C for 3 min, 40 cycles at 95°C for 12 sec and 62°C for 40 sec.
The miR-328 expression relative to U6 was calculated using the
2−ΔΔCt method.
Western blot analysis
Specimens and cells were lysed with
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology). Protein concentrations were determined by the
bicinchoninic acid method (Beyotime Institute of Biotechnology).
Equal amounts of proteins (30 µg) were separated by 10%
SDS-PAGE (Beyotime Institute of Biotechnology) at 80 V for 30 min
then 100 V for 1.5 h. Electrophoresed proteins were transferred to
a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) and subsequently blocked with 5% skimmed milk (BioSharp) at
room temperature for 1 h. The membranes were incubated with rabbit
anti-human PAK6 polyclonal antibody (1:400; cat. no 13539-1-AP;
ProteinTech Group, Inc.), rabbit anti-human cleaved caspase-3
antibody (1:500; cat. no. 9654; Cell Signaling Technology, Danvers,
MA, USA), mouse anti-human caspase-9 monoclonal antibody (1:500;
cat. no. 9492; Cell Signaling Technology), rabbit anti-human bcl-2
polyclonal antibody (1:500; cat. no. 12789-1-AP; ProteinTech Group,
Inc.), or rabbit anti-human GAPDH polyclonal antibody (1:1,000;
cat. no. sc-25778; Santa Cruz Biotechnology Inc., Dallas, TX, USA)
in 5% skimmed milk overnight at 4°C. The blots were washed with
Tris-buffered saline with Tween 20, incubated with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (1:3,000;
cat. no. ZB-2301; Zhongshan Golden Bridge Biotechnology Co., Ltd.)
at 37°C for 1 h, and visualized using Immobilon Western Chemilum
HRP Substrate (EMD Millipore). Protein levels were determined by
normalization against GAPDH.
Plasmid construction and luciferase
reporter assay
A PAK6 3′-UTR-luciferase reporter was created by
ligating the PAK6 3′-UTR PCR product into the XhoI and
NotI restriction sites of the psiCHECK-2™ Vector (Promega
Corp., Madison, WI, USA). Deletion of the binding site for miR-328
generated the mutant reporter. Following a 48-h cotransfection,
luciferase activity was evaluated using a dual-luciferase reporter
assay system (Promega Corp.).
Cell culture
The PC-3 and DU-145 human Pca cell lines, and
HEK-293 cells were purchased from the Chinese Academy of Sciences
Cell Bank (Shanghai, China). HEK-293 and DU-145 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences, Logan, UT, USA), and PC-3 was cultured in DMEM F12 (GE
Healthcare Life Sciences) supplemented with 100 U/ml penicillin (GE
Healthcare Life Sciences), 100 mg/ml streptomycin (GE Healthcare
Life Sciences), and 10% fetal bovine serum (GE Healthcare Life
Sciences). All cell cultures were incubated at 37°C in an
atmosphere of 5% CO2.
Oligonucleotides and cell
transfection
miR mimic oligonucleotide duplexes were chemically
synthesized by GenePharma, Co., Ltd. based on the following
sequences: Sense, 5′-CUGGCCCUCUCUGCCCUUCCGU-3′ and antisense,
5′-GGAAGGGCAGAGAGGGCCAGUU-3′ for hsa-miR-328 mimic; and sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′ for the NC. For cell transfection,
DU-145 and PC-3 cells were seeded in 6-well plates and transfected
at 60–70% confluence using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer's instructions.
Cell proliferation and cytotoxicity
assays
DU-145 and PC-3 cells were seeded in 6-well plates,
cultured at 37°C in an atmosphere containing 5% CO2
overnight, transfected with oligonucleotides, and cultured for a
further 48 h. Subsequently, the cells were trypsinized (Gibco;
Thermo Fisher Scientific Inc., Waltham, MA, USA) and seeded at a
density of 3,000 cells/well (200 ml/well) in 96-well plates.
Following overnight incubation at 37°C in an atmosphere containing
5% CO2, the cells were treated with various docetaxel
concentrations. Cell proliferation was evaluated using a Cell
Counting Kit-8 (CCK-8) assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Absorbance was detected at 450 nm using an automatic multi-well MK3
spectrophotometer (Thermo Fisher Scientific, Inc.). Five wells were
analyzed for cell viability in each treatment group.
Cell cycle and apoptosis assays
Approximately 48 h post-transfection, cells were
harvested and stained with propidium iodide [PI; MultiSciences
(Lianke) Biotech Co., Ltd., Hangzhou, China] for cell cycle assay
and by Annexin V-fluorescein isothiocyanate and PI (Ubio Biological
Technology PVT Ltd., Jinan, China) for the apoptosis assay,
according to the manufacturer's instructions. Treated cells were
analyzed by flow cytometry (FACS101; BD Biosciences, Franklin
Lakes, NJ, USA).
Tumorigenicity assays in a nude mouse
model
Six 4-week-old immunodeficient BALB/c-nu/nu male
mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) for injection of PC-3 cells. The mice were
maintained under specific pathogen-free conditions under a 12 h
light/12 h dark cycle at 26–28°C and 50–65% humidity. Cell
suspensions (100 µl; 5×106 cells) were
subcutaneously injected into the dorsal scapular region of each
mouse. Tumor volume was measured using a caliper every three days
and the following formula was used: Volume (mm3) =
(length × width2)/2. When the tumor volume reached 40–50
mm3, the mice were randomly divided into two groups with
three mice per group. The mice were treated with 200 pmol NC or
hsa-miR-328 mimics in 10 µl RNA free water by local
injection of the xenograft tumor at multiple sites. This treatment
was performed once every five days for 15 days, and tumors were
harvested one week later. Animal experiments were undertaken in
accordance with National Institute of Health Guide for the Care and
Use of Laboratory Animals guidelines and were approved by the
ethics committee of the Affiliated Zhongda Hospital of Southeast
University.
Statistical analysis
Data from at least three independent experiments are
presented as means ± standard error of the mean. Differences
between groups were calculated by Student's t-test or one-way
analysis of variance using the SPSS 16.0 software package (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
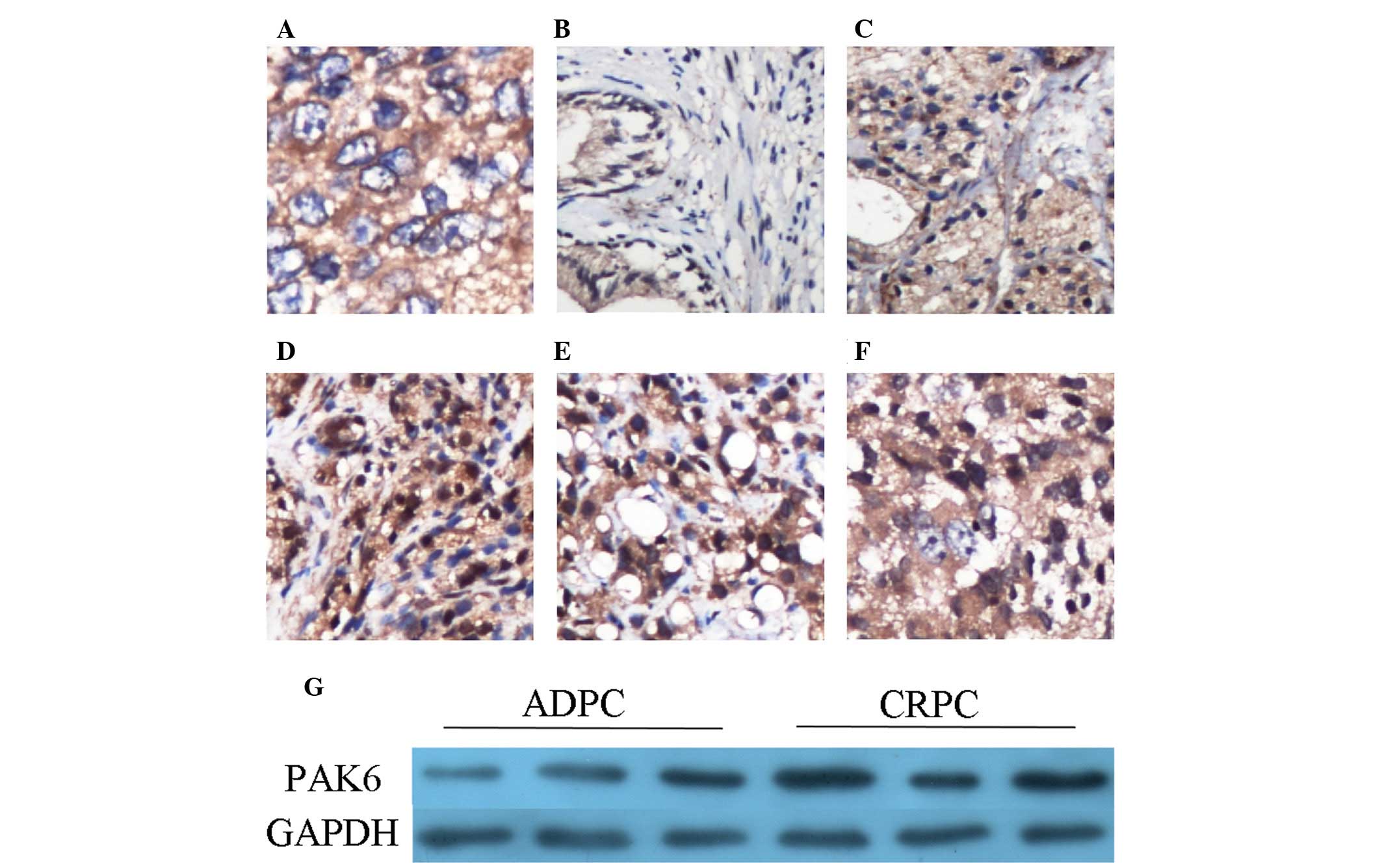
PAK6 protein expression in tissues
PAK6 expression levels were detected using
immunohistochemistry. Cytoplasmic staining was observed, although
no nuclear expression was demonstrated in the Pca tissue samples
(Fig. 1A). In the BPH tissue
samples, PAK6 was predominantly expressed in the epithelium;
however, the staining was weak in the majority of BPH tissues
(Fig. 1B). In the Pca samples, the
staining intensity appears to be greater in groups with Gleason
scores of 7 and >7, when compared with the group with Gleason
score of <7 (Fig. 1C–E). The
staining intensity was notably increased in the CRPC tissue samples
(Fig. 1F). The results of
immunostaining are summarized in Table
I. PAK6 expression levels were detected by western blot
analysis in three pairs of ADPC and CRPC tissues, and
overexpression of PAK6 was observed in the CRPC tissue samples
(Fig. 1G). These results are
consistent with those of a previous study (6).
 | Table Ip21-activated kinase 6 immunostaining
in BPH, Pca and CRPC samples. |
Table I
p21-activated kinase 6 immunostaining
in BPH, Pca and CRPC samples.
| Sample | Total | Negative | Weak | Intense |
|---|
| BPH | 10 | 4 | 6 | 0 |
| Pca | | | | |
| GS <7 | 10 | 1 | 5 | 4 |
| GS 7 | 10 | 0 | 7 | 3 |
| GS >7 | 10 | 1 | 5 | 4 |
| CRPC | 9 | 0 | 1 | 8 |
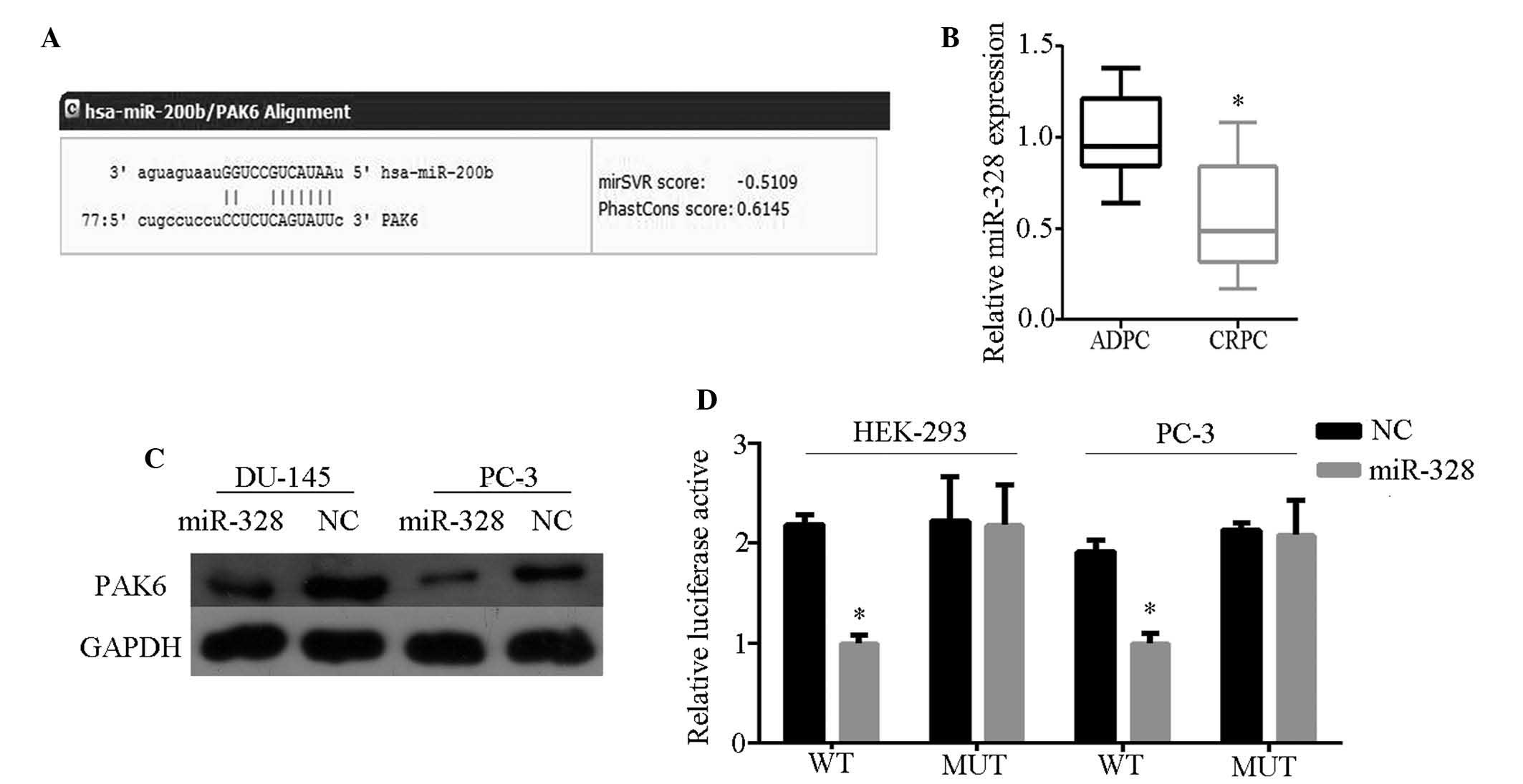
PAK6 is directly targeted by miR-328
To determine which miR affects PAK6 expression,
bioinformatics analysis was performed using TargetScan Human 6.2
(http://www.targetscan.org/) and miRanda
(http://www.microrna.org/microrna/home.do). Of the miRs
identified, miR-328 was identified to be bound to the 3′-UTR of
PAK6 mRNA with high scores (Fig.
2A). miR-328 expression was subsequently detected in 10 ADPC
and nine CRPC tissue samples by qRT-PCR, and weak expression was
observed in CRPC tissues (Fig.
2B). In addition, PAK6 protein expression was inhibited in
cells transfected with miR-328 mimics (Fig. 2C). These results indicate that PAK6
is the potential target of miR-328. To determine direct miR-target
interactions, a luciferase reporter assay was conducted by
constructing wild- and mutant-type cells with the luciferase
vector. A significant decrease was observed in the luciferase
activities of HEK-293 and PC-3 cells when compared with those of
the control and mutant-type cells (Fig. 2D). These characteristics indicate
that miR-328 directly targets PAK6.
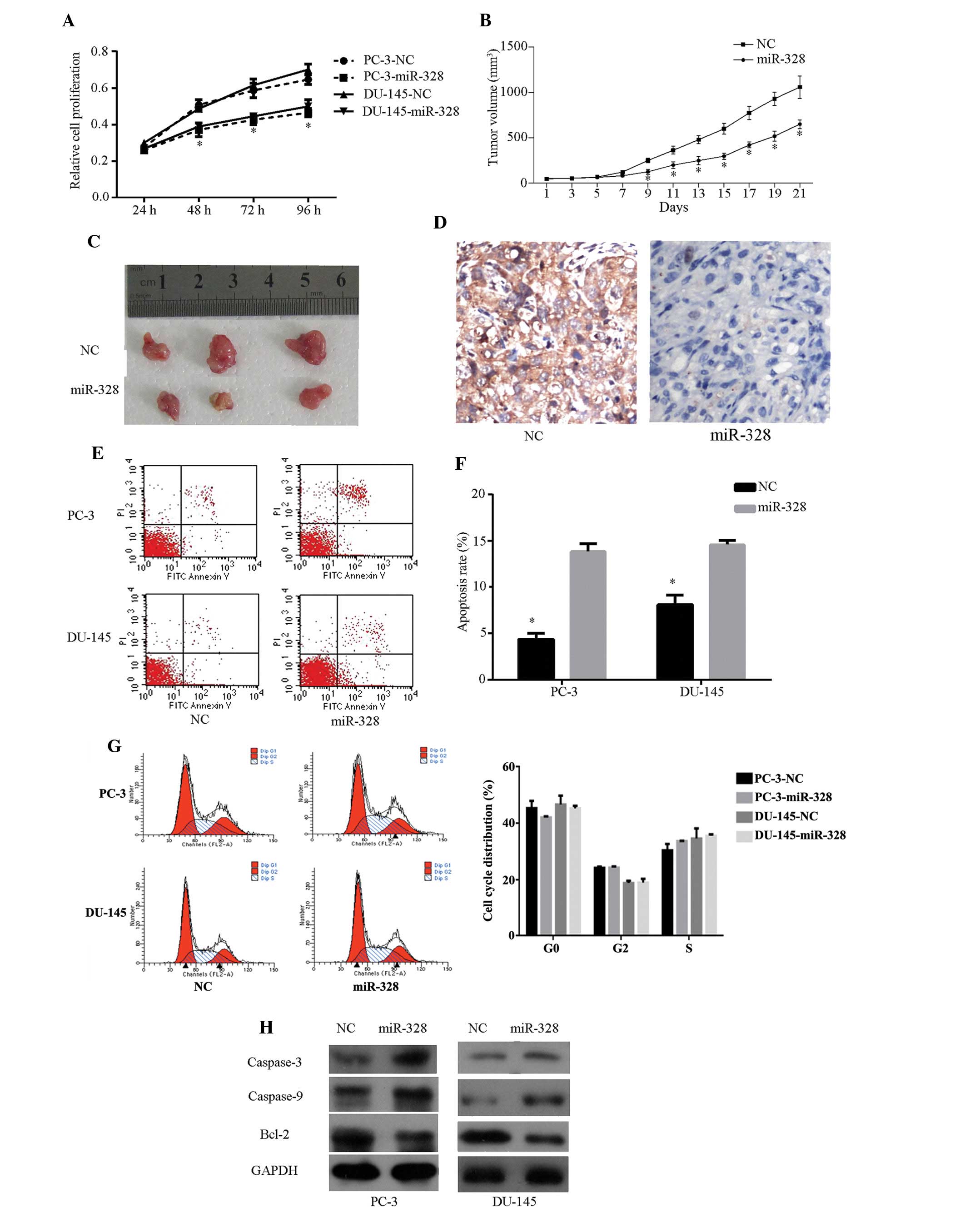
miR-328 inhibits cell growth and promotes
cell apoptosis
To investigate the functional roles of miR-328 in
Pca progression, miR-328 or NC mimics were transfected into PC-3
and DU-145 CRPC cell lines. NC mimic-incorporated green fluorescent
protein demonstrated high transfection efficiency (Fig. 3G). CCK-8 assay and nude mouse
transplantation tumor experiments demonstrated that miR-328
overexpression inhibits PC-3 cell proliferation in vivo and
in vitro (Fig. 3A–3C). In addition, reduced staining was
observed in miR-328-treated xenografts (Fig. 3D). The proapoptotic effect of
miR-328 was observed in PC-3 and DU-145 cells by flow cytometry
assay (Fig. 3E and F). The same
assay, however, indicated that miR-328 does not affect cell cycle
progression (data not shown). The viability of caspase-3, -9 and
bcl-2 was examined in cells following transfection. Compared with
cells transfected with NC mimics, increased cleavage of caspase-3
and -9 and decreased bcl-2 were observed in cells transfected with
miR-328 (Fig. 3H). These results
indicate that miR-328 may inhibit cell growth and promote cell
apoptosis.
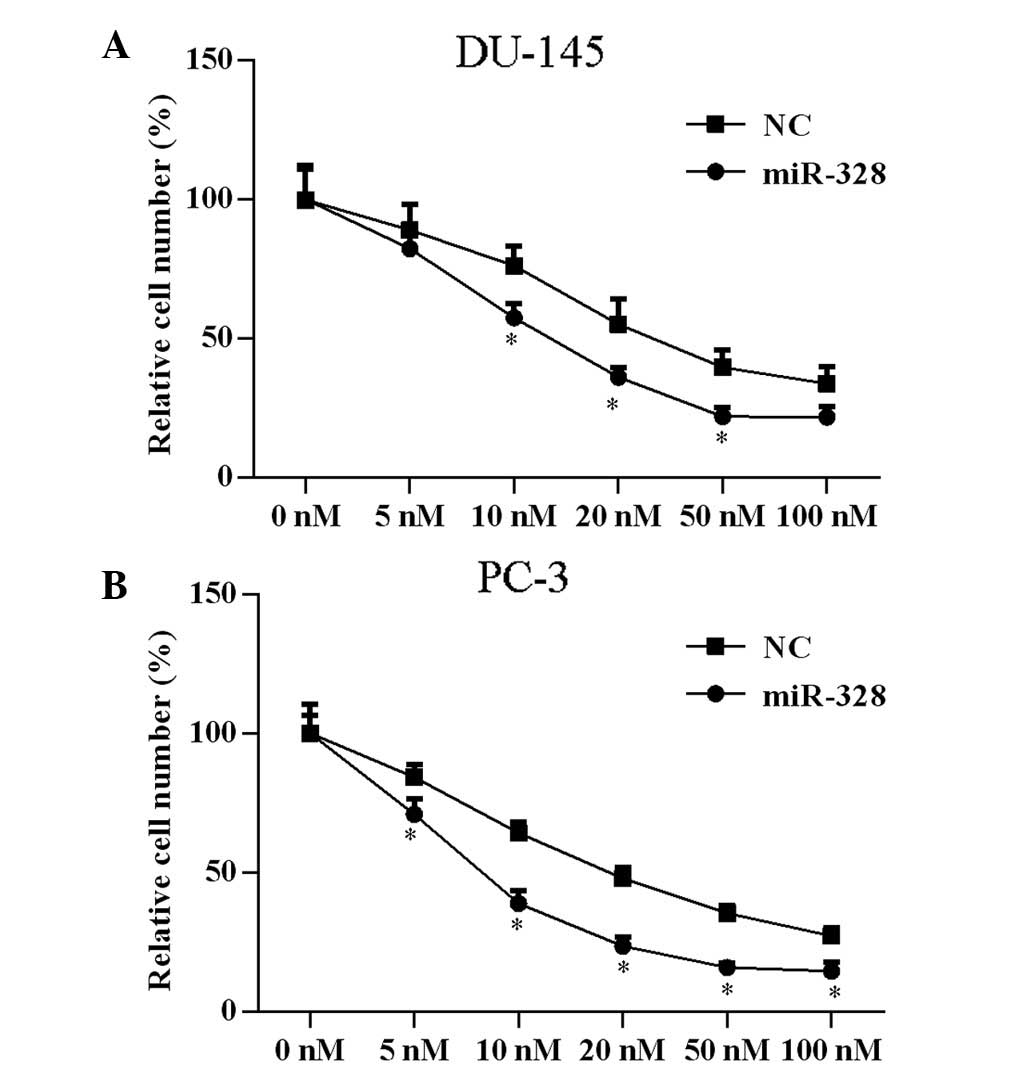
miR-328 enhances docetaxel
sensitivity
A previous study demonstrated that PAK6 knockdown in
prostate cell lines increases docetaxel sensitivity (8). To determine if miR-328 exerts a
similar effect, assessment of docetaxel drug sensitivity following
transfection with miR-328 was conducted. Cells were transfected
with miR-328 or NC, and treated with docetaxel at concentrations of
5, 10, 20, 50 or 100 nM for 24 h. Subsequent to miR-328
transfection, the docetaxel drug concentration that inhibited 50%
of cell proliferation significantly decreased from 25.45 to 8.30 nM
(P<0.05) in PC-3 cells and from 36.63 to 16.78 nM (P<0.05) in
DU-145 cells (Fig. 4).
Discussion
Androgen-deprivation therapy is commonly adopted to
treat metastatic Pca; however, the majority of ADPC cases
inevitably progress to CRPC (14).
Hormone refractory cases and metastasis remain major challenges of
Pca, however the mechanisms of these issues remain unclear.
Therefore, additional studies are necessary to understand the
relevant molecular mechanisms and develop more effective treatment
methods. Recent evidence indicates that PAK6 performs critical
functions in Pca development (6,7). In
the present study, PAK6 overexpression was observed in CRPC
tissues, which suggests that PAK6 contributes significantly to the
progression of ADPC to CRPC. Furthermore, PAK6 overexpression was
observed to be mediated by weak miR-328 expression.
Previous studies have demonstrated that various miRs
are involved in Pca development and progression (15,16).
miR expression is involved in critical biological processes,
including growth, proliferation and apoptosis (17). Pca cell proliferation and apoptosis
is closely associated with tumor malignancy and drug resistance,
and multiple miRs have been demonstrated to regulate Pca cell
proliferation and apoptosis (18).
For example, miR-143 arrests cell proliferation by inhibiting
extracellular signal-regulated kinase-5 (19), and miR-15 and -205 inhibit
proliferation and promote apoptosis in Pca cells by targeting the
bcl-2 gene (20,21).
miR-328 is known to be weakly expressed in certain
types of cancer, including Pca (22–24).
In human breast cancer and glioblastoma cancer stem cells, miR-328
targets breast cancer resistance protein (BCRP/ABCG2) and affects
drug disposition (25,26). In bone marrow cells, mir-328
interacts with heterogeneous ribonucleoprotein E2 and affects
granulocytic maturation (24).
However, miR-328 is also overexpressed in lung adenocarcinoma
(27). In non-small cell lung
cancer, miR-328 overexpression results in increased cell migration
and is associated with brain metastasis (28). In glioma cells, miR-328 targets
secreted frizzled-related protein 1 and promotes cell invasion
(29). These conflicting roles of
miR-328 in different types of cancer indicate the tissue
specificity of miR-328 function. In the present study, forced
miR-328 overexpression markedly enhanced docetaxel sensitivity,
reduced cell proliferation and increased apoptosis in Pca cells
without affecting the cell cycle. miR-328 overexpression increases
levels of caspase-3 and -9 expression, and decreased bcl-2
expression.
In conclusion, miR-328 is weakly expressed in CRPC,
and regulates cell proliferation and apoptosis by targeting PAK6.
In addition, upregulated miR-328 expression enhances docetaxel
sensitivity, inhibits cell proliferation and promotes cell
apoptosis without affecting the cell cycle.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370849, 81300472
and 81202034), the Natural Science Foundation of Jiangsu Province
(grant nos. BL2013032 and BK2012336) and Nanjing City (grant no.
201201053) and Southeast University (grant no. 3290002402), the
Science Foundation of Ministry of Education of China (grant no.
20120092120071), the Fundamental Research Funds for the Central
Universities and Scientific Research Innovation Project of
University in Jiangsu Province (grant no. KYLX_0203).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cookson MS, Roth BJ, Dahm P, Engstrom C,
Freedland SJ, Hussain M, Lin DW, Lowrance WT, Murad MH, Oh WK, et
al: Castration-resistant prostate cancer: AUA Guideline. J Urol.
190:429–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Willard SS and Koochekpour S: Regulators
of gene expression as biomarkers for prostate cancer. Am J Cancer
Res. 2:650–657. 2012.
|
|
4
|
Yang F, Li X, Sharma M, Zarnegar M, Lim B
and Sun Z: Androgen receptor specifically interacts with a novel
p21-activated kinase, PAK6. J Biol Chem. 276:15345–15353. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SR, Ramos SM, Ko A, Masiello D,
Swanson KD, Lu ML and Balk SP: AR and ER interaction with a
p21-activated kinase (PAK6). Mol Endocrinol. 16:85–99. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur R, Yuan X, Lu ML and Balk SP:
Increased PAK6 expression in prostate cancer and identification of
PAK6 associated proteins. Prostate. 68:1510–1516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schrantz N, da Silva Correia J, Fowler B,
Ge Q, Sun Z and Bokoch GM: Mechanism of p21-activated kinase
6-mediated inhibition of androgen receptor signaling. J Biol Chem.
279:1922–1931. 2004. View Article : Google Scholar
|
|
8
|
Wen X, Li X, Liao B, Liu Y, Wu J, Yuan X,
Ouyang B, Sun Q and Gao X: Knockdown of p21-activated kinase 6
inhibits prostate cancer growth and enhances chemosensitivity to
docetaxel. Urology. 73:1407–1411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Siedow M, Saia G and Chakravarti
A: Inhibition of p21-activated kinase 6 (PAK6) increases
radiosensitivity of prostate cancer cells. Prostate. 70:807–816.
2010.PubMed/NCBI
|
|
10
|
Ayub SG, Kaul D and Ayub T:
Microdissecting the role of microRNAs in the pathogenesis of
prostate cancer. Cancer Genet. 208:289–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chua JH, Armugam A and Jeyaseelan K:
MicroRNAs: Biogenesis, function and applications. Curr Opin Mol
Ther. 11:189–199. 2009.PubMed/NCBI
|
|
12
|
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z,
Li P, Zhang W, Wu H, Feng N, et al: miR-143 decreases prostate
cancer cells proliferation and migration and enhances their
sensitivity to docetaxel through suppression of KRAS. Mol Cell
Biochem. 350:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epstein JI, Allsbrook WE Jr, Amin MB and
Egevad LL; ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of Prostatic Carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar
|
|
15
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar
|
|
16
|
McKee TC and Tricoli JV: Epigenetics of
prostate cancer. Methods Mol Biol. 1238:217–234. 2015. View Article : Google Scholar
|
|
17
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar :
|
|
18
|
Deng JH, Deng Q, Kuo CH, Delaney SW and
Ying SY: MiRNA targets of prostate cancer. Mol Biol Methods.
936:357–369. 2013. View Article : Google Scholar
|
|
19
|
Clapé C, Fritz V, Henriquet C, Apparailly
F, Fernandez PL, Iborra F, Avancès C, Villalba M, Culine S and
Fajas L: miR-143 interferes with ERK5 signaling, and abrogates
prostate cancer progression in mice. PLoS One. 4:e75422009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verdoodt B, Neid M, Vogt M, Kuhn V,
Liffers ST, Palisaar RJ, Noldus J, Tannapfel A and
Mirmohammadsadegh A: MicroRNA-205, a novel regulator of the
anti-apoptotic protein Bcl2, is downregulated in prostate cancer.
Int J Oncol. 43:307–314. 2013.PubMed/NCBI
|
|
21
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Peng B, Wang D, Ma X, Jiang D,
Zhao J and Yu L: Human tumor microRNA signatures derived from
large-scale oligo-nucleotide microarray datasets. Int J Cancer.
129:1624–1634. 2011. View Article : Google Scholar
|
|
23
|
Malzkorn B, Wolter M, Liesenberg F,
Grzendowski M, Stühler K, Meyer HE and Reifenberger G:
Identification and functional characterization of microRNAs
involved in the malignant progression of gliomas. Brain pathol.
20:539–550. 2010. View Article : Google Scholar
|
|
24
|
Eiring AM, Harb JG, Neviani P, Garton C,
Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, et al:
miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation
of mRNA translation in leukemic blasts. Cell. 140:652–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan YZ, Morris ME and Yu AM: MicroRNA-328
negatively regulates the expression of breast cancer resistance
protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol.
75:1374–1379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li WQ, Li YM, Tao BB, Lu YC, Hu GH, Liu
HM, He J, Xu Y and Yu HY: Downregulation of ABCG2 expression in
glioblastoma cancer stem cells with miRNA-328 may decrease their
chemoresistance. Med Sci Monit. 16:HY27–HY30. 2010.PubMed/NCBI
|
|
27
|
Dacic S, Kelly L, Shuai Y and Nikiforova
MN: miRNA expression profiling of lung adenocarcinomas: Correlation
with mutational status. Mod Pathol. 23:1577–1582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arora S, Ranade AR, Tran NL, Nasser S,
Sridhar S, Korn RL, Ross JT, Dhruv H, Foss KM, Sibenaller Z, et al:
MicroRNA-328 is associated with (non-small) cell lung cancer
(NSCLC) brain metastasis and mediates NSCLC migration. Int J
Cancer. 129:2621–2631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Delic S, Lottmann N, Stelzl A, Liesenberg
F, Wolter M, Götze S, Zapatka M, Shiio Y, Sabel MC, Felsberg J, et
al: MiR-328 promotes glioma cell invasion via SFRP1-dependent
Wnt-signaling activation. Neuro-oncol. 16:179–190. 2014. View Article : Google Scholar :
|