Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of malignancy in men worldwide (1). Its occurrence has a clear
geographical distribution, with the highest incidence in East Asia
and sub-Saharan Africa (2).
Determining the molecular mechanisms underlying the pathogenesis of
HCC is important for early detection and treatment.
Programmed cell death 5 (PDCD5), also designated
TF-1 cell apoptosis-related gene-19 (TFAR19), was identified in
TF-1 cells undergoing apoptosis (3). Decreased expression of PDCD5 has been
characterized in human tumors, including breast cancer (4), gastric cancer (5), and hepatocellular carcinoma (6). Recombinant human PDCD5 (rhPDCD5) has
been shown to enter a variety of cells by clathrin-independent
endocytosis (CIE) (7,8). Endocytosis is a process used by cells
to communicate between their interior and the surrounding
environment (9). Although CIE has
been characterized in numerous cell types and multiple pathways,
few studies have demonstrated the roles of CIE in HCC cells.
Clathrin is a protein complex of three identical 190 kDa clathrin
heavy chains arranged in a trimer (10). Dutta et al (11) showed that CIE of two different
cargo proteins, clathrin heavy chain and amphiphysin, was inhibited
by Pitstop2.
This study, aimed to use Pitstop2 to block
clathrin-dependent endocytosis (CDE) in order to examine the
effects of blocking CDE on the antitumor roles of rhPDCD5 in HCC
cells.
Materials and methods
Blood samples and measurement of PDCD5 in
the serum
All patients approved the use of blood samples for
clinical research and the study was approved by the Ethical
Committee of the China Medical University (Shenyang, China).
Peripheral blood was obtained from 32 patients undergoing surgical
resection of primary HCC without previous chemotherapeutic
treatment or radiotherapy at the Department of General Surgery,
First Affiliated Hospital of China Medical University between
January 2009 and December 2011. Preoperative and postoperative
samples were clotted for 30 min and then centrifuged for 10 min at
1,000 x g. The concentration of PDCD5 in the serum was assayed
using an enzyme-linked immunosorbent assay (ELISA) kit for PDCD5
(USCN Life Science Inc., Houston, TX, USA).
Cell culture
HepG2 and Hep3B human liver cancer cell lines
(American Type Culture Collection, Manassas, VA, USA) were cultured
in Dulbecco's modified Eagle's medium (Hyclone, Logan, UT, USA)
containing 10% fetal bovine serum (Gibco, Thermo Fisher Scientific
Inc., Waltham, MA, USA) and incubated in a 5% CO2
incubator at 37°C.
3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazoliumbromide (MTT)
assay
Cell viability was assayed using MTT assays
(Sigma-Aldrich, St. Louis, MO, USA). Briefly, cells were plated in
96-well plates (1,500 cells/well). After 24 h, cells were treated
with various concentrations of rhPDCD5 protein (0, 5, 10, 20 and 40
µg/ml). After 24 h, 0.5 mg/ml MTT was added to each well.
After 4 h, cells were lysed with dimethyl sulfoxide (DMSO;
Sigma-Aldrich) and absorbance rates were measured at 550–560 nm
using a microplate reader (Bio-Rad 3550; Bio-Rad, Hercules, CA,
USA).
Pitstop2 inhibition assay
Cells were incubated with 30 mM Pitstop2 (Abcam,
Cambridge, UK) for 15 min at 37°C. Cells were then incubated for an
additional 15 min at 37°C in fresh medium.
Cell apoptosis assay and cell cycle
assay
For the apoptosis assay, 5×105 cells were
collected without EDTA and washed with phosphate-buffered saline
(PBS). Then, 500 µl binding buffer, 5 µl Annexin
V-fluorescein isothiocyanate (FITC) and 5 µl propidium
iodide (PI; KeyGen, Nanjing, China) were added to the suspension
and mixed at room temperature in the dark for 10 min. Examination
was performed by flow cytometry (FACSCanto II; BD Biosciences,
Baltimore, MD, USA). For the cell cycle assay, cells were treated
and then collected by trypsinization (trypsin/EDTA; KeyGen). After
washing with PBS three times, the cell suspension was fixed with
70% ethanol and incubated with RNAse A (Beyotime Institute of
Biotechnology, Haimen, China) at 37°C. Cells were stained with 400
µl PI and the suspension was evaluated by flow
cytometry.
Endocytosis assay
FITC labeling of recombinant PDCD5 protein was
conducted as described previously (7). Cells were detached from the dish with
5 mM EDTA and incubated with 1 µM rhPDCD5-FITC for 30 min at
37°C. The cells were fixed with 4% paraformaldehyde for 30 min.
Then, cells were washed in PBS, mounted in FluoroGuard (Bio-Rad),
and observed using a confocal laser-scanning microscope (Leica
TCS4D; Leica Microsystems, Oberkochen, Germany).
Transmission electron microscopy
Cells were immersed in 2% cacodylate-buffered
glutaraldehyde (Sigma-Aldrich), rinsed in cacodylate buffer
supplemented with 15% sucrose, post-fixed with 1%
phosphate-buffered OsO4, dehydrated with alcohol,
clarified in propylene oxide, and embedded in Epon using flat
molds. Ultrathin sections were made with an ultra-microtome and
stained with uranyl acetate and a saturated solution of bismuth
subnitrate. They were then observed under a JEOL JSM 6400 scanning
electron microscope (JEOL, Tokyo, Japan).
In vivo effects of rhPDCD5 on liver
cancer xenografts
The Ethics Committee of the China Medical University
(Shenyang, China) approved the protocol of the present study. NOD
SCID mice (age, 4–6-weeks; NOD.CB17-Prkdcscid/NcrCrl) were obtained
from the Charles River Laboratories (Wilmington, MA, USA).
Throughout the experiment, mice were housed in groups of three in a
room with controlled temperature (22°C) and humidity (50%) under a
12-h light/dark cycle. Standard rat chow (BetterBiotechnology Co.,
Ltd. Nanjing, China) and tap water were available ad
libitum. HepG2 or Hep3B cells (3×107 cells in 200
µl PBS) were injected subcutaneously into the axilla. After
the tumor diameters reached 3–5 mm, the mice were divided randomly
into three groups [HepG2, rhPDCD5 (40 µg/ml) and rhPDCD5 (40
µg/ml) + Pitstop2 (30 mM)] and received a 100 µl
intratumoral injection of PBS, rhPDCD5 or rhPDCD5 + Pitstop2. The
tumors were resected, and the tumor weight and volume were
determined at 0, 5, 10, 15, 20, 25 and 30 days. Tumors were
measured using calipers (Kraftwelle, Hangzhou, China), and tumor
volumes were calculated using the following formula: Tumor volume =
length x width2 x 0.52. Mice (n=180) were used to
establish xenografts for observing survival time (30 mice in each
treatment group). The survival status of the mice was observed; all
mice had died at day 30, at which the experiment was
terminated.
Immunostaining
For immunohistochemical staining, endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 30
min in the tumor sections. Antigen retrieval was performed in
citrate buffer (10 mM, pH 6.0) for 30 min at 95°C in a pressure
cooker. Primary antibodies (Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) were incubated with sections at 1:100 dilution
overnight at 4°C. The following primary antibodies were used:
cyclin A (rabbit polyclonal IgG; cat no. sc-751); CDK2 (rabbit
polyclonal IgG; cat no. sc-163); Ki67 (goat polyclonal IgG; cat no.
sc-7846) and caspase 3 (rabbit polyclonal IgG; cat no. sc-7148).
Sections were then incubated with a biotinylated secondary antibody
(mouse anti-rabbit IgG, cat. no. sc-2491; mouse anti-goat IgG, cat.
no. sc-53799; Santa Cruz Biotechnology Inc.) for 1 h at room
temperature, followed by incubation with a streptavidin horseradish
peroxidase complex (Beyotime Institute of Biotechnology) for 1 h at
room temperature. Bound antibody was visualized with
3,3′-diaminobenzidine tetrahydrochloride (Beyotime Institute of
Biotechnology). Sections were counterstained with hematoxylin
(Beyotime Institute of Biotechnology).
Statistical analysis
Statistical analyses were performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Values are presented as
the mean ± standard deviation. Statistical significance was
calculated with a Mann-Whitney-Wilcoxon statistical test. The
Kaplan-Meier estimator was used to compare different groups, and
P-values were calculated using the log-rank (Mantel-Cox) test.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Preoperative and postoperative serum
levels of PDCD5 detection in patients with HCC
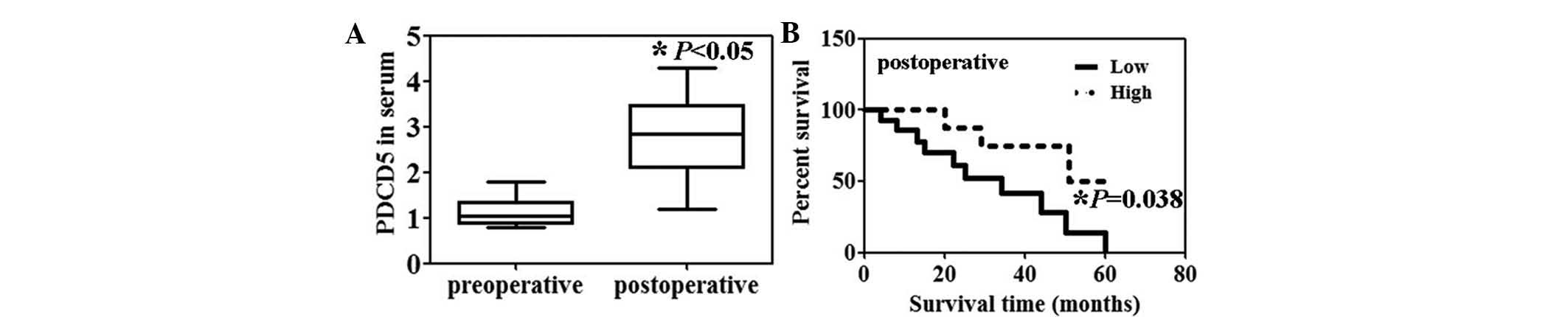
Preoperative serum levels of PDCD5 protein in the
patients with HCC were significantly lower than the postoperative
serum levels (Fig. 1A, P<0.05).
Moreover, the serum PDCD5 levels were significantly correlated with
portal invasion (P=0.0003) and lymph node metastasis (P=0.0103)
(Table I). Patients with HCC with
high serum levels of PDCD5 expression were associated with a
significantly higher survival rate compared with those with low
serum PDCD5 expression (Fig. 1B,
P=0.038).
 | Table ICorrelation between postoperative
serum PDCD5 with demographic and biological parameters in 32
hepatocellular carcinoma samples. |
Table I
Correlation between postoperative
serum PDCD5 with demographic and biological parameters in 32
hepatocellular carcinoma samples.
| Clinicopathological
feature | n | Postoperative serum
PDCD5
| χ2 | P-value |
|---|
| Low | High |
|---|
| Gender | | | | 0.039 | 0.8439 |
| Female | 12 | 8 | 4 | | |
| Male | 20 | 14 | 6 | | |
| Age (years) | | | | 0.264 | 0.6071 |
| <55 | 10 | 8 | 2 | | |
| ≥55 | 22 | 14 | 8 | | |
| Tumor number | | | | 0.009 | 0.9234 |
| Multiple | 18 | 13 | 5 | | |
| Solitary | 14 | 9 | 5 | | |
| Differentiation | | | | 0.191 | 0.6623 |
| Differentiated | 13 | 10 | 3 | | |
|
Undifferentiated | 19 | 12 | 7 | | |
| Portal invasion | | | | 12.959 | 0.0003 |
| − | 10 | 2 | 8 | | |
| + | 22 | 20 | 2 | | |
| Lymph node
metastasis | | | | 6.579 | 0.0103 |
| − | 6 | 1 | 5 | | |
| + | 26 | 21 | 5 | | |
| Tumor size (cm) | | | | 0.004 | 0.9477 |
| <5 | 5 | 4 | 1 | | |
| ≥5 | 27 | 18 | 9 | | |
| HBV infection | | | | 1.901 | 0.1680 |
| − | 12 | 6 | 6 | | |
| + | 20 | 16 | 4 | | |
rhPDCD5 exhibits antitumor activity in
HCC cells by CDE
An MTT assay showed that the proliferation rate of
cells treated with rhPDCD5 was decreased compared with the
untreated cells (Fig. 2A,
P<0.05). In addition, the IC50 value for rhPDCD5 was
40 µg/ml for HepG2 and Hep3B cells. The results of Annexin
V-FITC and PI double staining showed that the apoptotic ratio was
10–20 times higher in rhPDCD5 treated cells compared with untreated
cells (Fig. 2B). PI staining
revealed that HCC cells with rhPDCD5 treatment were arrested in S
phase (Fig. 2C). The antitumor
effects of rhPDCD5 were offset using Pitstop2. It was hypothesized
that rhPDCD5 exhibits antitumor activity in HCC cells due to CDE.
With Pitstop2 treatment, fluid phase pinocytosis in liver cancer
cells was almost completely inhibited, and rhPDCD5 internalization
was reduced (Fig. 2D). Using
electron microscopy, the cytoplasmic face of the plasma membrane
was examined following Pitstop2 treatment. It was confirmed that
rhPDCD5 internalization was inhibited by Pitstop2 (Fig. 2E).
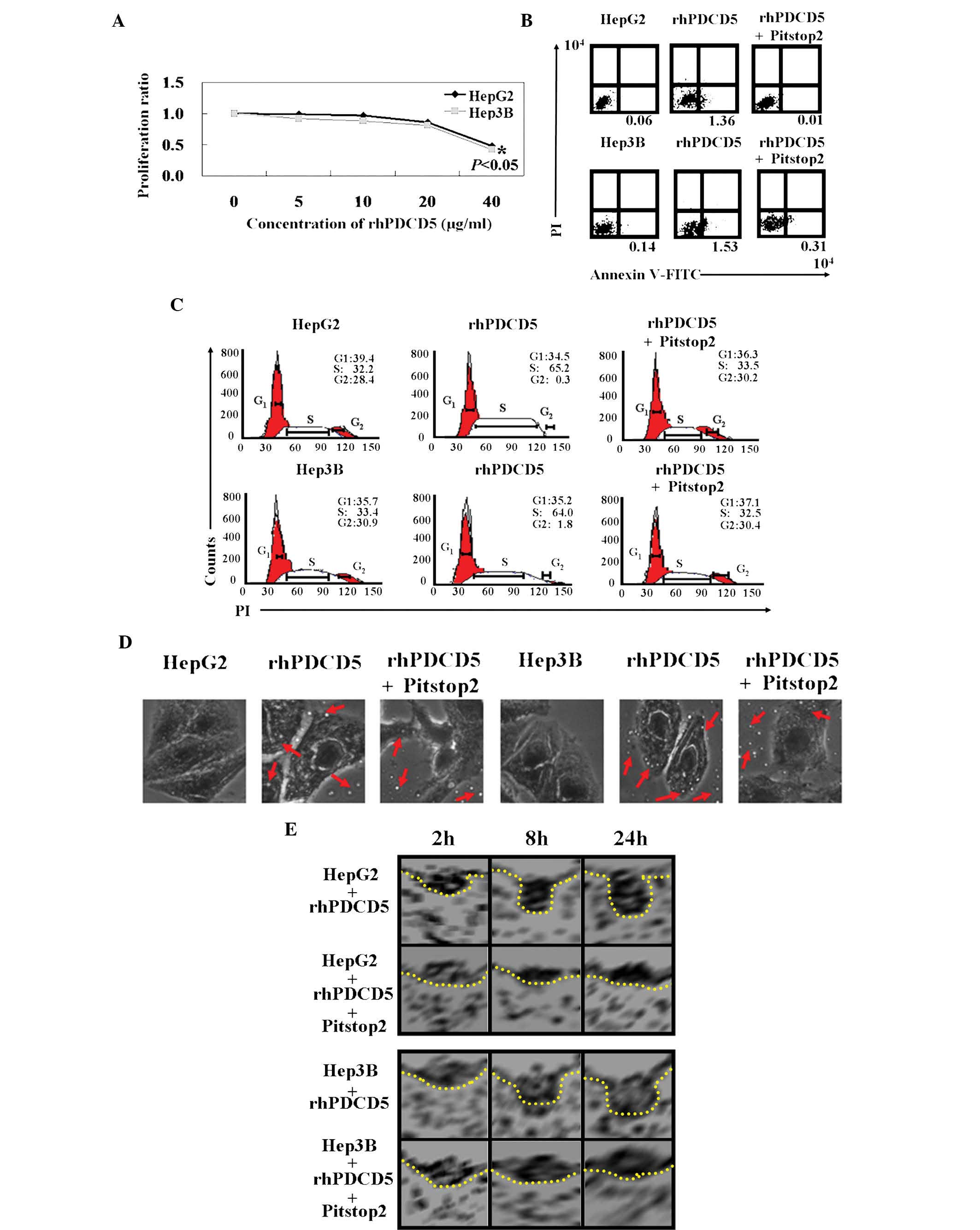 | Figure 2Antitumor roles of rhPDCD5 in HCC
cells. (A) 3-(4,5-Dimethylthiazolyl)-2,5-diphenyltetrazoliumbromide
assays were performed to determine the proliferation ratio of HCC
cells treated with rhPDCD5 compared with untreated cells. (B) The
proportion of apoptotic cells (early apoptosis) was determined by
double-staining with Annexin-V/fluorescein isothiocyanate and PI.
(C) Cells were stained with PI to analyze the cell cycle
distribution of each cell type by flow cytometry. (D) Effect of
rhPDCD5 on endocytosis of HCC cells was assayed using
immunofluorescence (magnification, ×400). The red arrows indicate
fluorescein isothiocyanate-rhPDCD5. (E) Effect of rhPDCD5 on the
morphology of clathrin-coated pits was assayed by using an electron
microscope. HepG2, untreated HepG2 cells; rhPDCD5, HepG2 treated
with rhPDCD5; rhPDCD5+Pitstop2, HepG2 treated with
rhPDCD5+Pitstop2; Hep3B, untreated Hep3B cells; rhPDCD5, Hep3B
treated with rhPDCD5; rhPDCD5+Pitstop2, Hep3B treated with
rhPDCD5+Pitstop2. rhPDCD5, recombinant human programmed cell death
5; HCC, hepatocellular carcinoma; PI, propidium iodide. |
rhPDCD5 has a prominent antitumor effect
in vivo
As shown above, rhPDCD5 exhibits an inhibitory
effect on liver cancer cell activity in vitro. Therefore, in
this study, the antitumor properties of rhPDCD5 were further
evaluated using xenograft tumor models. A significant inhibition of
tumor volume was observed in tumor cells treated with rhPDCD5,
while no effects of rhPDCD5 on HCC cells were observed with
Pitstop2 treatment (Fig. 3A).
Correspondingly, the weights of untreated, rhPDCD5, and
rhPDCD5+Pitstop2-treated tumors followed the same trend at day 5
(448±13, 215±14 and 452±21 mg, respectively) (Fig. 3B, P<0.05). In addition, the
survival rate of mice with tumors treated with rhPDCD5 was
significantly increased (Fig. 3C,
P<0.05).
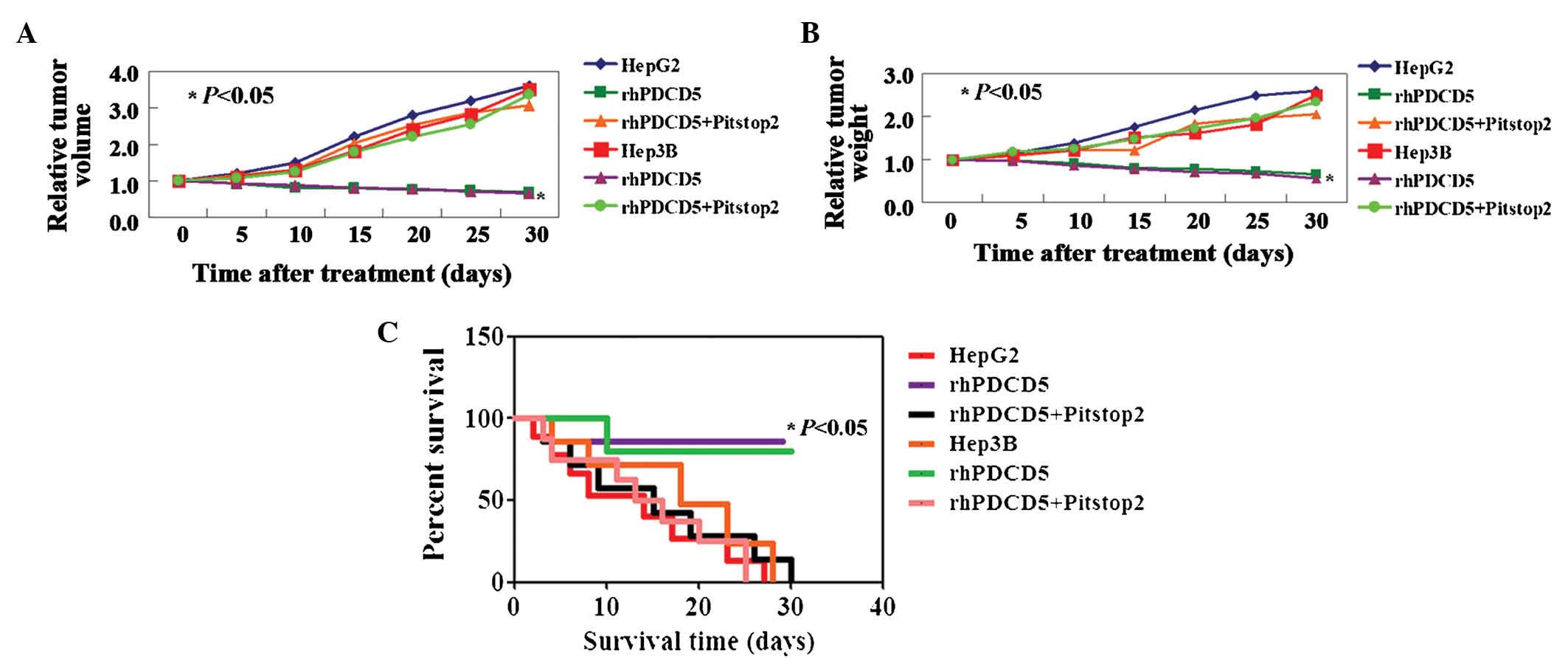 | Figure 3rhPDCD5 suppresses hepatocellular
carcinoma cell growth in vivo. Tumor xenografts were
directly injected with phosphate-buffered saline, rhPDCD5 or
rhPDCD5+Pitstop2. Relative (A) Tumor volume and (B) tumor weight
measured on days 5, 10, 15, 20, 25 and 30. (C) Kaplan-Meier
survival curves for mice in each group as described above. HepG2,
untreated HepG2 cells; rhPDCD5, HepG2 treated with rhPDCD5;
rhPDCD5+Pitstop2, HepG2 treated with rhPDCD5+Pitstop2; Hep3B,
untreated Hep3B cells; rhPDCD5, Hep3B treated with rhPDCD5;
rhPDCD5+Pitstop2, Hep3B treated with rhPDCD5+Pitstop2. rhPDCD5;
recombinant human programmed cell death 5. |
Mechanism(s) of rhPDCD5 in the mouse
xenograft model
In order to identify the mechanism underlying the
effect of rhPDCD5, protein expression of cyclin A, CDK2, Ki67 and
caspase3 was detected by immunostaining. A decrease in cyclin A,
CDK2, and Ki67 protein, and an increase in caspase3 protein levels
was identified in the tumor tissues of the mice treated with
rhPDCD5 (Fig. 4). Compared with
untreated tissue, no changes of these proteins were observed in the
tumor tissues from the mice treated with rhPDCD5+Pitstop2 (Fig. 4).
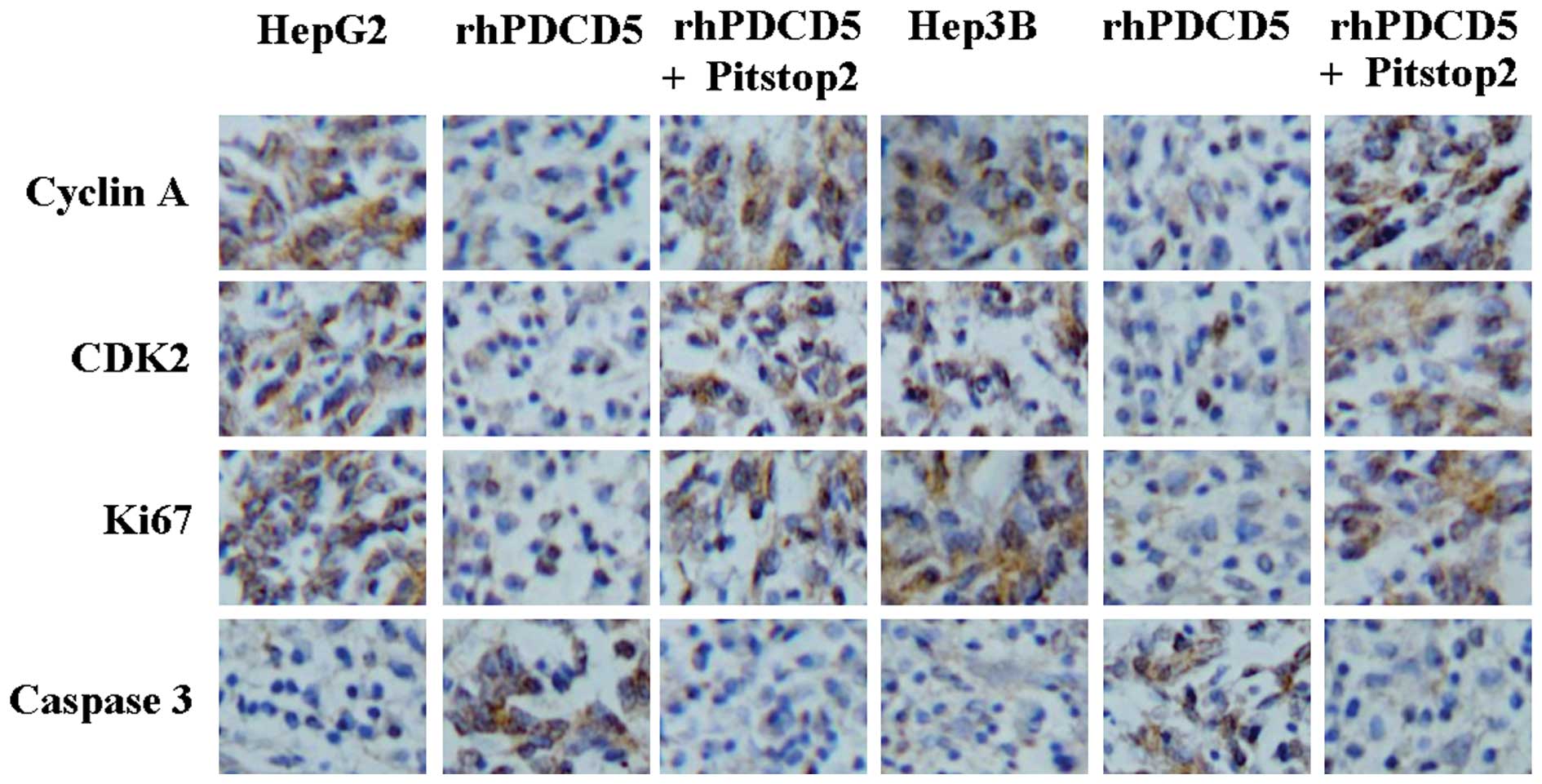 | Figure 4Immunohistochemical staining of cyclin
A, CDK2, Ki67, and caspase3 using specific antibodies
(magnification, ×200). Bound antibody is detected with DAB and
appears brown. HepG2, untreated HepG2 cells; rhPDCD5, HepG2 treated
with rhPDCD5; rhPDCD5+Pitstop2, HepG2 treated with
rhPDCD5+Pitstop2; Hep3B, untreated Hep3B cells; rhPDCD5, Hep3B
treated with rhPDCD5; rhPDCD5+Pitstop2, Hep3B treated with
rhPDCD5+Pitstop2. CDK2, cyclin-dependent kinase 2; DAB,
3,3′-diaminobenzidine tetrahydrochloride; rhPDCD5; recombinant
human programmed cell death 5. |
Discussion
PDCD5 is an apoptosis-related gene cloned from TF-1
cells undergoing cytokine deprivation-induced apoptosis (3). In a study by Wang et al
(12), no statistically
significant difference was observed between the serum PDCD5
concentrations in healthy patients and the patients with breast
cancer, gastrointestinal cancer or lung cancer. In this study, it
was demonstrated that preoperative serum levels of PDCD5 protein in
the patients with HCC were significantly lower than the
postoperative serum levels.
Wang et al (7) found that exogenous addition of
hrPDCD5 to the culture medium of TF-1 cells or HL-60 cells can
enhance programmed cell death triggered by growth factor
deprivation in TF-1 cells or serum deprivation in HL-60 cells.
Notably, it was observed that rhPDCD5 could induce apoptosis and S
phase arrest in HCC cells. Certain previous studies have
demonstrated that PDCD5 is not only an apoptotic accelerator but
also an apoptotic trigger (6,13).
rhPDCD5 has been shown to enter a variety of cells by CIE and exert
biological activities (7,8). However, in this study, it was found
that inhibition of clathrin could inhibit rhPDCD5 internalization.
Clathrin is a protein complex of three identical 190 kDa clathrin
heavy chains arranged in a trimer of three 'legs' connected by
their C-termini at a central vertex (10,14).
This study used the clathrin inhibitor, Pitstop2, to demonstrate
rhPDCD5 internalization via CDE.
In conclusion, the principal findings of this study
are that: i) Preoperative serum levels of PDCD5 protein in patients
with HCC were significantly lower than postoperative serum levels;
ii) the serum PDCD5 levels were correlated statistically with
portal invasion, lymph node metastasis and patient prognosis; iii)
rhPDCD5 could inhibit cell proliferation, induce apoptosis and S
phase arrest in HCC cells and suppress tumor growth in established
xenograft tumor models; and iv) the antitumor roles of rhPDCD5 in
HCC cells occur through CDE. The present study provided a
theoretical basis for the clinical use of rhPDCD5 for the treatment
of HCC patients. Further study of the effects of rhPDCD5 for the
treatment of other types of cancer is also an area of great
interest.
Acknowledgments
The authors would like to thank Dr Miao Yu (Science
Experiment Center, China Medical University, Shenyang, China) for
technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
6:69–90. 2011. View Article : Google Scholar
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen
G, Tang J and Ma D: TFAR19, a novel apoptosis-related gene cloned
from human leukemia cell line TF-1, could enhance apoptosis of some
tumor cells induced by growth factor withdrawal. Biochem Biophys
Res Commun. 254:203–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hedenfalk I, Duggan D, Chen Y, Radmacher
M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M,
Kallioniemi OP, et al: Gene-expression profiles in hereditary
breast cancer. N Engl J Med. 344:539–548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Zhao M, Li WM and Lu YY, Chen YY,
Kang B and Lu YY: Expression of programmed cell death 5 gene
involves in regulation of apoptosis in gastric tumor cells.
Apoptosis. 11:993–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu DZ, Cheng Y, He H, Liu HY and Liu YF:
PDCD5 expression predicts a favorable outcome in patients with
hepatocellular carcinoma. Int J Oncol. 43:821–830. 2013.PubMed/NCBI
|
|
7
|
Wang Y, Li D, Fan H, Tian L, Zhong Y,
Zhang Y, Yuan L, Jin C, Yin C and Ma D: Cellular uptake of
exogenous human PDCD5 protein. J Biol Chem. 281:24803–24817. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Shi L, Song Q, Zhang M, Lou Y,
Zeng Y, Ma D, Wang Y and Ke X: Recombinant human PDCD5 protein
enhances chemosensitivities of hematologic malignancies. Chin Sci
Bull. 54:3981–3990. 2009. View Article : Google Scholar
|
|
9
|
Doherty GJ and McMahon HT: Mechanisms of
endocytosis. Annu Rev Biochem. 78:857–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Royle SJ: The cellular functions of
clathrin. Cell Mol Life Sci. 63:1823–1832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dutta D, Williamson CD, Cole NB and
Donaldson JG: Pitstop 2 is a potent inhibitor of
clathrin-independent endocytosis. PLoS One. 7:e457992012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wang GH and Zhang QY:
Determination of PDCD5 in peripheral blood serum of cancer
patients. Chin J Cancer Res. 23:224–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han XR, Sun Y and Bai XZ: The anti-tumor
role and mechanism of integrated and truncated PDCD5 proteins in
osteosarcoma cells. Cell Signal. 24:1713–1721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fotin A, Cheng Y, Sliz P, Grigorieff N,
Harrison SC, Kirchhausen T and Walz T: Molecular model for a
complete clathrin lattice from electron cryomicroscopy. Nature.
432:573–579. 2004. View Article : Google Scholar : PubMed/NCBI
|