Introduction
Dendranthema morifolium (Ramat.) Tzvel. cv.
Hangju of the genus Chrysanthemum and the Asteraceae family is
widely used in south China, either as a fragrant floral tea or as
an anti-inflammatory herb in Traditional Chinese Medicine.
According to component analysis, D. morifolium contains
various nutrients, vitamins A and B, and flavonoids (1,2). The
main flavonoid components include
luteolin-7-O-β-D-glucoside, apigenin-7-O-β-9
D-glucoside, acacetin-7-O-β-D-glucoside and anthocyanins
(1,3). It has been reported that these
components have a hypotensive effect in rats under normal
conditions (4) and increase the
contractility and the coronary flow in isolated and re-perfused rat
hearts, thereby protecting the heart against ischemia/reperfusion
injury (5,6). However, to the best of our knowledge,
the possible pharmacological effects of D. morifolium
components on vessels and the underlying mechanism of action have
not been studied. The present study hypothesized that total
flavones extracted from D. morifolium (Ramat.) Tzvel. cv.
Hangju (FDM) may have a direct relaxant effect on vasculature. The
vasorelaxant effects of FDM and the potential underlying mechanisms
of action were therefore investigated.
As a cluster of cardiovascular risk factors, the
metabolic syndrome is increasingly being recognized as an important
factor in the pathophysiological mechanisms underlying
atherosclerosis and is a target of therapies (7). The metabolic syndrome is also termed
syndrome of insulin resistance. Insulin can stimulate the
over-proliferation of vascular smooth muscle cells (VSMCs) through
the mitogen-activated protein kinase (MAPK) pathway and the
phosphatidyl inositol-3 kinase (PI3K)-Akt pathway (8). The over-proliferation of VSMCs is one
of the key factors in atherosclerosis and the restenosis following
percutaneous transcoronary angioplasty (9,10).
It has been reported that the flavones and flavonoids extracted
from other plants inhibit the proliferation of VSMCs and leiomyomal
cells (11,12). Therefore, the present study
examined the effects of FDM on the proliferation of VSMCs.
Materials and methods
Drugs and chemicals
Acetylcholine (ACh), phenylephrine (PE) and phorbol
12,13-diacetate were purchased from Sigma-Aldrich (St. Louis, MO,
USA). FDM was prepared by extraction (with 70% ethanol) of the
dried flowers of D. morifolium (Ramat.) Tzvel. cv. Hangju,
which were obtained from Tongxiang City (China), as described in a
previous study (13). The FDM was
identified by Professor Huidi Jiang (Department of Pharmaceutical
Analysis, College of Medicine, Zhejiang University, Hangzhou,
China).
Preparation of rat aortic rings
Male Sprague Dawley (SD) rats (weight, 240–300 g;
age, 6–8 weeks) were purchased from the Animal Center, Zhejiang
Academy of Medical Sciences (Hangzhou, China). All procedures were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals and the experimental protocol of the present
study was approved by the ethics committee of Zhejiang University.
Rats were anesthetized with chloral hydrate (0.4 g/kg
intraperitoneally (i.p.); Shenggong Biotech Co., Shanghai, China)
and sacrificed by decapitation. Thoracic aortas were immediately
harvested and maintained in a Krebs solution with the following
composition: 118 mmol/l NaCl, 25 mmol/l NaHCO3, 1.2
mmol/l KH2PO4, 1.2 mmol/l MgSO4,
4.7 mmol/l KCl, 1.25 mmol/l CaCl2 and 10 mmol/l glucose
(Shenggong Biotech Co.) at 4°C. Aortas were cleared of adipose and
connective tissue and then cut into 3 mm-wide rings. A forceps was
inserted into the lumen of the aortic ring and the endothelium was
gently removed. Rings were suspended from an iron hook connected
with a force transducer in chambers containing 10 ml Krebs solution
at 37°C and with streaming of 95% O2 and 5%
CO2 gas. The preparations were passively stretched to an
optimal tension of 2 g after 15 min of equilibration with no
tension. The equilibration time was 60 min and the Krebs solution
was refreshed every 15 min. Isometric tension was recorded by
transducers connected to a recording system (MacLab, ADInstruments,
Bella Vista, Australia) (14). The
function of the rings was first tested in the presence of KCl (60
mmol/l; Shenggong Biotech Co.) at least three times until a
reproducible and stable contractile response was obtained. The
function of the endothelium was tested using ACh (10−5
mol/l; Shenggong Biotech Co.) to induce at least 70% relaxation
pre-contraction with PE (10−6 mol/l).
Culture of VSMCs and cell proliferation
assay
According to the protocol of a previous study
(15), chloral hydrate (0.4 g/kg,
i.p.) was used to anesthetize the male SD rats (240–300 g) prior to
sacrifice by decapitation. Thoracic aortas were rapidly removed
under aseptic conditions and stored in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich) supplemented with 10% heat-inactivated
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and antibiotics (100 g/ml streptomycin and 100 U/ml
penicillin; Sigma-Aldrich). The aortas were dissected,
longitudinally opened and cut into 1 × 1-mm pieces following
removal of the endothelium and adventitia. Cell cultures were
maintained under 5% CO2 at 37°C in an incubator and the
medium was replaced twice a week. VSMCs were identified by a
typical 'hill and valley' growth pattern and confirmed by
immunofluorescence staining for α-smooth muscle actin
(Sigma-Aldrich), which is identified by green fluorescence.
Confluent cells at passage 3–7 were used in all experiments.
The effects of FDM on VSMC proliferation were
assayed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
method with the MTT Cell Proliferation and Cytotoxicity Assay kit
(Beyotime Institute of Biotechnology, Haimen, China) as previously
described (16). The VSMCs were
transferred onto a 96-well plate. After 20 h of culture, 15
µl MTT (5 mg/ml) was added to each well and the culture
medium was replaced with dimethyl sulfoxide (DMSO; Sigma-Aldrich)
after 4 h. The absorbance of every well was measured at a
wavelength of 570 nm using a spectrophotometer (Multiskan MK3;
Thermo Fisher Scientific, Inc.).
Assessment of the effects of FDM on PE-
and KCl-induced vascular contraction
After a steady contraction of the aortic rings with
or without endothelium induced by PE (10−6 mol/l) or KCl
(60 mmol/l) was achieved, FDM (0.005, 0.015, 0.05, 0.15 or 0.5
g/l), dissolved in DMSO, was cumulatively added to the Krebs
solution, and the results were used to generate a
concentration-response curve.
Assessment of the effects of
extracellular and intracellular calcium on FDM-induced reduction of
vasocontraction
In the first series of experiments, aortic rings
were first incubated in Ca2+-free Krebs solution
containing 50 mol/l ethylene glycol
bis(aminoethylether)-tetraacetic acid (EGTA; Shenggong Biotech Co.)
and 60 mmol/l KCl for 20 min. FDM at 5×10−2 g/l was then
added to the Krebs solution. In order to obtain a
concentration-response curve, Ca2+ (0.25–5 mmol/l) was
then cumulatively added after 10 min.
In the second series of experiments, after
endothelium-denuded rings were incubated in Ca2+-free
Krebs solution containing 50 µmol/l EGTA for 15 min, PE
(10−6 mol/l) was used to induce the first transient
contraction. In order to allow for the restoration of intracellular
Ca2+ levels, Ca2+-free Krebs solution was
then replaced with normal Krebs solution and the aortic rings were
incubated in normal Krebs solution for at least 40 min. After the
solution was rapidly replaced with Ca2+-free solution,
the rings were incubated for another 15 min. In order to test the
effects of FDM on the second contraction induced by 10−6
mol/l PE, 5×10−2 g/l FDM was added to the solution after
15 min.
In the third series of experiments,
2×10−3 mol/l caffeine (Shenggong Biotech Co.) was used
to elicit a transient contractile response in endothelium-scraped
rings immersed in Ca2+-free Krebs solution.
5×10−2 g/l FDM was added to the solution and the second
contraction was induced again.
Assessment of the effects of FDM on
phorbol ester-induced vasocontraction
In order to induce a stable contraction in
endothelium-scraped rings, 10−6 mol/l phorbol ester
(phorbol 12,13-diacetate; Shenggong Biotech Co.), an activator of
protein kinase C, was added to Ca2+-free Krebs solution
containing 50 µmol/l EGTA, which the rings were immersed in.
FDM was cumulatively added and the effects were recorded.
Assessment of the effects of FDM on the
proliferation of cultured VSMCs
VSMCs at passages 3–7 and 70–90% confluence in 10-cm
dishes were transferred to a 96-well plate. The growth of VSMCs was
arrested by incubation with serum-free DMEM for 24 h prior to
use.
In the first set of experiments, synchronized cells
were treated in the presence or absence of FDM (0.0025, 0.01, 0.02,
0.04, 0.08, 0.16, 0.32, 0.64, 1.28 and 2.56 g/l) for 24 h, and the
effects of FDM on the cell proliferation were assessed using the
MTT method as described above.
In the second set of experiments, cells were
pre-incubated with insulin (100 nmol/l; Shenggong Biotech Co.) for
24 h. FDM (0.32 or 0.64 g/l) was added to the culture medium for
another 24 h and the proliferation of the VSMCs was assessed using
the MTT method.
Statistical analysis
Values are expressed as the mean ± standard
deviation. An unpaired Student's t-test was used to compare
differences between two groups. One-way analysis of variance and
the Newman-Keuls test, followed by a post-hoc least-significant
differences test were used to determine significant differences
between multiple groups. SPSS 15.0 (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism
software version 5.0 (GraphPad Software Inc., San Diego, CA, USA)
was used for curve fitting.
Results
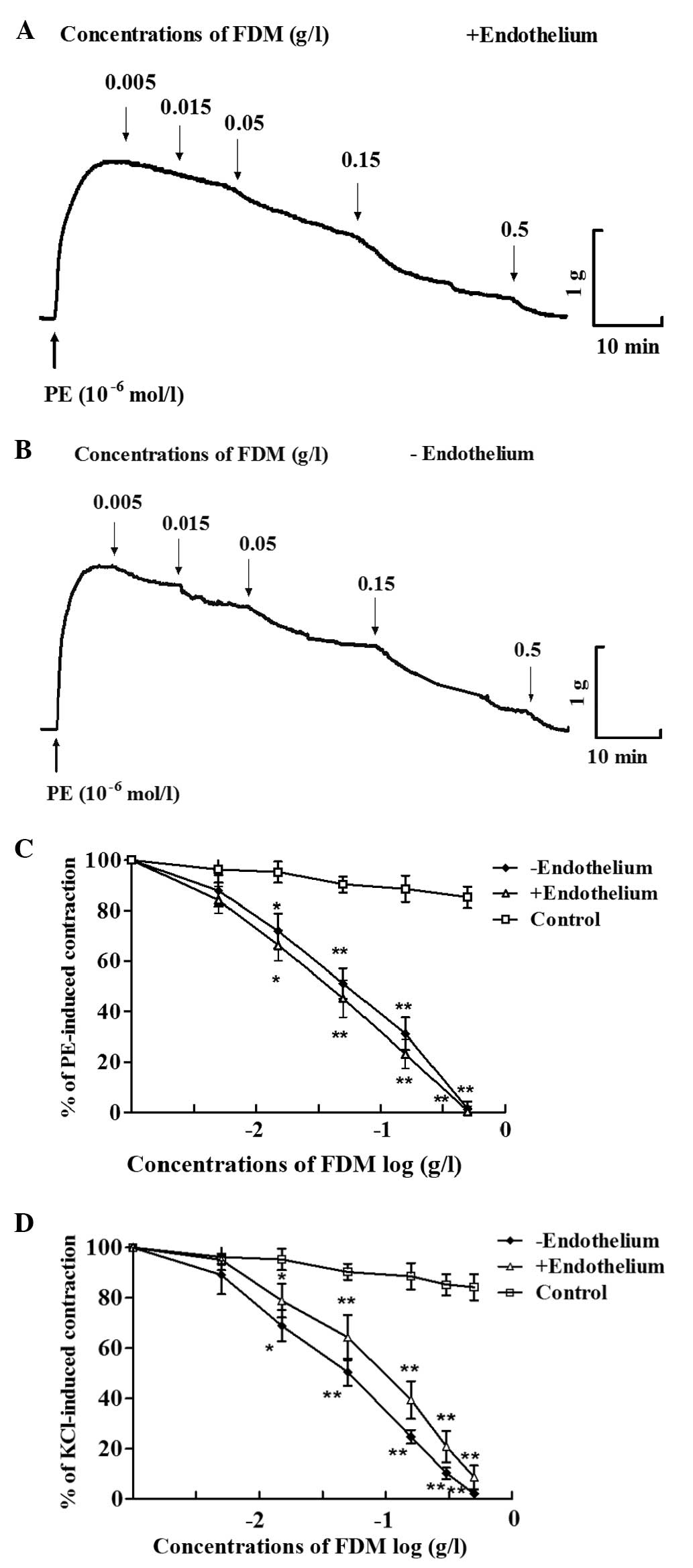
FDM exerts concentration-dependent
vasorelaxant effects
After a steady contraction elicited by
10−6 mol/l PE was achieved, FDM was added to the aortic
rings and was revealed to concentration-dependently induce vascular
relaxation (Fig. 1). FDM at 0.5
g/l induced complete relaxation of aortic rings in
endothelium-intact as well as endothelium-denuded arotic rings
(Fig. 1A–C). Furthermore, FDM
caused a concentration-dependent decrease of vasoconstriction
induced by KCl (60 mmol/l), in endothelium-intact as well as
-denuded aortic rings (Fig.
1D).
FDM reduces vasocontraction induced by
extracellular and intracellular calcium
After a steady contraction of endothelium-scraped
aortic rings was induced by KCl (60 mmol/l) in Ca2+-free
Krebs solution, Ca2+ was cumulatively added to the
solution, which was shown to concentration-dependently enhance
vasoconstriction. The Ca2+-induced vasoconstriction of
the aortic rings was significantly inhibited by incubation with
5×10−2 g/l FDM for 10 min (Fig. 2A).
In another series of experiments, a contraction of
endothelium-scraped aortic rings was transiently induced by
10−6 mol/l PE in Ca2+-free Krebs solution. A
second contraction was further induced by PE with or without
5×10−2 g/l FDM. As shown in Fig. 2, the ratio of the second to the
first contraction was significantly reduced by pre-treatment with
FDM for 10 min. Pre-treatment with FDM also reduced the ratio of
the second to the first contraction elicited by 2×10−3
mol/l caffeine (Fig. 2B).
FDM does not affect phorbol ester-induced
vasocontraction in a Ca2+-free solution
As shown in Fig. 3,
a sustained contraction of endothelium-scraped rings evoked by
phorbol ester in Ca2+-free Krebs solution with 50
µmol/l EGTA was not effected by FDM.
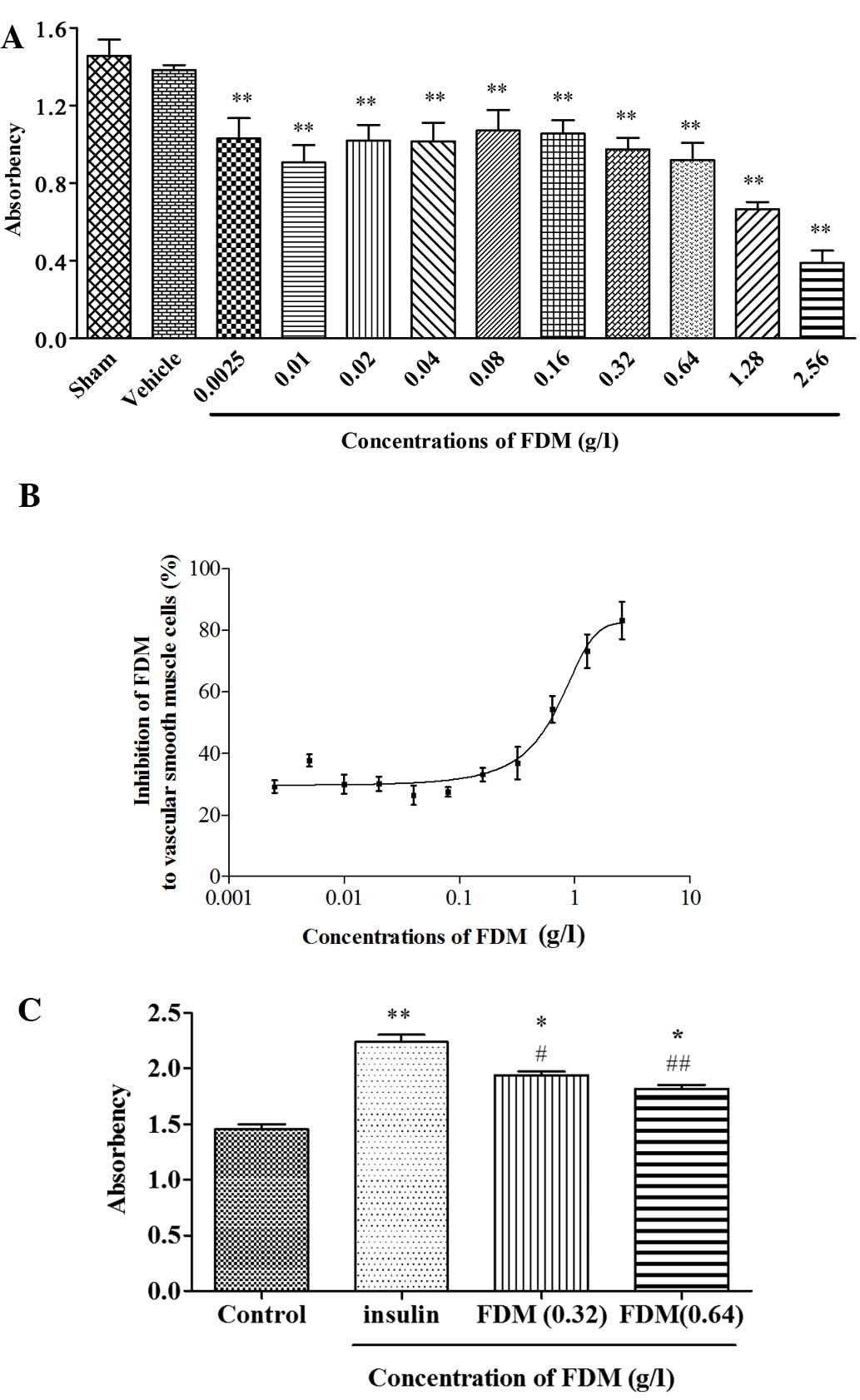
FDM inhibits the proliferation of
cultured VSMCs
FDM dose-dependently inhibited the proliferation of
cultured VSMCs. It also reduced, but did not fully abrogate,
insulin-induced increases in the proliferation of VSMCs (Fig. 4).
Discussion
Although D. morifolium is widely used in
southeast China, its pharmacological action on blood vessels has
not been assessed, to the best of out knowledge. The present study
revealed the dose-dependent vasorelaxant effects of FDM in aortic
rings from rats and investigated the potential underlying
mechanisms. Furthermore, it was demonstrated that insulin-induced
growth stimulation of VSMCs was inhibited by FDM.
The results of the present study revealed that FDM
exerted a vascular relaxation effect and inhibited the contractions
of aortic rings from rats evoked by either PE or KCl. The
vasodilation induced by FDM in aortic rings was shown to be
endothelium-independent. Therefore, the mechanism of action of FDM
is unlikely to be mediated via any endogenous vasodilators,
including prostacyclin and nitric oxide from endothelial cells.
Ajay et al (17)
demonstrated that the release of nitric oxide was partly involved
in the vascular dilation effects of most of the flavonoids. In
addition, Jiang et al (18)
reported that the nitric oxide synthase (NOS) activity of aortic
rings was significantly increased by pre-treatment with an ethyl
acetate extract of D. morifolium. Whether FDM causes any
variation in NO levels and/or NOS activation requires further
study.
The two main types of Ca2+ channel,
voltage-dependent and receptor-operated channels, can be activated
by KCl and PE, respectively. KCl causes vascular contraction mainly
by inducing Ca2+ influx upon de-polarization of the cell
membrane and activating voltage-dependent L-type Ca2+
channels (19).
In the present study, FDM inhibited KCl-induced
vasoconstriction in endothelium-denuded rat aortas as well as
Ca2+-induced contraction of aortic rings de-polarized by
KCl, which demonstrated that FDM exerts its vascular dilation
effect in part by inhibiting the voltage-dependent L-type
Ca2+ channel. In addition, FDM significantly inhibited
the PE-induced vascular contraction, indicating that it may also
reduce influx of Ca2+ through receptor-operated
Ca2+ channels.
PE, as a selective α-adrenoceptor agonist, induces
vasoconstriction by opening receptor-operated Ca2+
channels. More importantly, it evokes Ca2+ release from
the sarcoplasmic reticulum by activating phospholipase C and the
generation of diacylglycerol (DG) that activates myosin light chain
kinase through protein kinase C (PKC) and inositol triphosphate
(IP3) (20). Therefore, the
present study investigated whether FDM exerts the vasorelaxant
effects through inhibiting the intracellular mobilization of
Ca2+ from the sarcoplasmic reticulum and
diglyceride-dependent PKC-activated myosin light chain kinase in
aortic rings. In order to demonstrate that the transient
contraction response to PE was mainly due to IP3-induced
Ca2+ release from the sarcoplasmic reticulum, several
experiments of the present study were performed in a
Ca2+-free Krebs solution. FDM inhibited the PE-induced
vascular contraction response in endothelium-scraped aortic rings
in an extracellular Ca2+-free environment, probably due
to the inhibition of intracellular Ca2+ release.
However, the vascular contraction response to phorbol ester was not
affected by FDM in the concentration range that relaxed aortic
rings induced to contract by pre-treatment with PE or KCl,
suggesting that the relaxation response to FDM was not mediated
through the PKC pathway.
Insulin can stimulate the overproliferation of VSMCs
through the MAPK and the PI3K-Akt pathway (7). This over-proliferation is a key
factor in atherosclerosis and the restenosis following stent
implantation (8,9). The results of the present study
showed that FDM inhibited the proliferation of VSMCs under normal
conditions and also inhibited insulin-induced over-proliferation.
Therefore, FDM has useful implications in the clinical treatment of
diseases associated with the over-proliferation of VSMCs, including
as atherosclerosis and restenosis after stent implantation. Further
study is required to explore the underlying mechanisms of the
inhibitory effects of FDM on the proliferation of VSMCs.
In conclusion, the present study indicated that FDM
exerted a vasorelaxant effect on rat aortic rings in a
concentration-dependent and endothelium-independent manner. The
underlying mechanism is likely to include a reduction of the
Ca2+ influx by FDM through voltage-dependent as well as
receptor-operated channels to inhibit the release of intracellular
Ca2+ in VSMCs. It was also demonstrated that FDM
inhibited the proliferation of VSMCs under normal conditions and
the over-proliferation induced by insulin.
Acknowledgments
The present study was funded by grants from the
Natural Science Foundation of Zhejiang province (Project for Young
Scientists, no. LQ13H020004) and the Health Bureau of Zhejiang
Province (no. 201353645).
References
|
1
|
Qin S and Wen X: Simultaneous
determination of 6 active components in Chrysanthemum morifolium by
HPLC. China Journal of Chinese Materia Medica. 36:1474–1477.
2011.In Chinese.
|
|
2
|
Yu SK, Zhang Y and Wu XQ: Nutrition
component and bioactivity of Dendranthema morifolium (Ramat.)
Tzvel. cv. Hangju. Chinese Food and Nutrition. 2:50–51. 2002.
|
|
3
|
Liang YN, Guo QS, Zhang ZY, Wang SX and
Wang T: Study on dynamic accumulation of secondary metabolites
content and isoenzyme activity during blossoming stages in
Chrysanthemum morifolium originating from Wenxian county. Zhongguo
Zhong Yao Za Zhi. 32:199–202. 2007.In Chinese. PubMed/NCBI
|
|
4
|
Dai M, Liu Q, Li D and Liu L: Research of
material bases on antifebrile and hypetensive effects of flos
chrysanthemi. Zhong Yao Cai. 24:505–506. 2001.In Chinese.
PubMed/NCBI
|
|
5
|
Jiang H, Xia Q, Xu W and Zheng M:
Chrysanthemum mori-folium attenuated the reduction of contraction
of isolated rat heart and cardiomyocytes induced by
ischemia/reperfusion. Pharmazie. 59:565–567. 2004.PubMed/NCBI
|
|
6
|
Huang H, Guo HY and Jin HF: Effects of
water extract of Dendranthema morifolium on myocardial ischemia in
canines. Zhongguo Zhong Yao Za Zhi. 21:307–310. 2011.
|
|
7
|
Guo S: Insulin signaling, resistance and
metabolic syndrome: Insights from mouse models into disease
mechanisms. J Endocrinol. 220:T1–T23. 2014. View Article : Google Scholar
|
|
8
|
Cersosimo E, Xu X and Musi N: Potential
role of insulin signaling on vascular smooth muscle cell migration,
proliferation, and inflammation pathways. Am J Physiol Cell
Physiol. 302:C652–C657. 2012. View Article : Google Scholar
|
|
9
|
Chen J, Dai M and Wang Y: Paeonol inhibits
proliferation of vascular smooth muscle cells stimulated by high
glucose via Ras-Raf-ERK1/2 signaling pathway in coculture model.
Evid Based Complement Alternat Med. 2014:4842692014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang K, Wen L, Peng W, Li H, Zhuang J, Lu
Y, Liu B, Li X, Li W and Xu Y: Vinpocetine attenuates neointimal
hyper-plasia in diabetic rat carotid arteries after balloon injury.
PLoS One. 9:e968942014. View Article : Google Scholar
|
|
11
|
Xing J, Peng K, Cao W, Lian X, Wang Q and
Wang X: Effects of total flavonoids from Dracocephalum moldavica on
the proliferation, migration, and adhesion molecule expression of
rat vascular smooth muscle cells induced by TNF-α. Pharm Biol.
51:74–83. 2013. View Article : Google Scholar
|
|
12
|
Jiang D, Li D and Wu W: Inhibitory effects
and mechanisms of luteolin on proliferation and migration of
vascular smooth muscle cells. Nutrients. 5:1648–1659. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang H, Xia Q, Xu W and Zeng M:
Chrysanthemum mori- folium attenuated the reduction of contraction
of isolated heart and cardiomyocytes induced by
ischemia/reperfusion. Pharmazie. 59:565–567. 2004.PubMed/NCBI
|
|
14
|
Kim SH, Kang KW, Kim KW and Kim ND:
Procyanidins in crataegus extract evoke endothelium-dependent
vasorelaxation in rat aorta. Life Sci. 67:121–131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Yang Y, Kone BC, Allen JC and
Kahn AM: Insulin-stimulated cyclic guanosine monophosphate inhibits
vascular smooth muscle cell migration by inhibiting
Ca/calmodulin-dependent protein kinase II. Circulation.
107:1539–1544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ta ka hashi S, Abe T, Gotoh J and Fukuuchi
Y: Substrate-dependence of reduction of MTT: A tetrazolium dye
differs in cultured astroglia and neurons. Neurochem Int.
40:441–448. 2002. View Article : Google Scholar
|
|
17
|
Ajay M, Gilani AU and Mustafa MR: Effects
of flavonoids on vascular smooth muscle of the isolated rat
thoracic aorta. Life Sci. 74:603–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang HD, Cai J, Xu JH, Zhou XM and Xia Q:
Endothelium-dependent and direct relaxation induced by ethyl
acetate extract from Flos Chrysanthemi in rat thoracic aorta. J
Ethnopharmacol. 101:221–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji J, Benishin CG and Pang PK: Nitric
oxide selectively inhibits intracellular Ca2+ release elicited by
inositol trisphosphate but not caffeine in rat vascular smooth
muscle. J Pharmacol Exp Ther. 285:16–21. 1998.PubMed/NCBI
|
|
20
|
Amberg GC and Navedo MF: Calcium dynamics
in vascular smooth muscle. Microcirculation. 20:281–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|