Introduction
Osteosarcoma (OS), characterized by formation of
cancerous bone tissue, is the most frequent primary malignant tumor
of bone occurring in children and adolescents (1,2). OS
is prone to aggressive biological behavior and development of
distant metastasis (3–5). The currently used treatment for OS is
neo-adjuvant chemotherapy followed by surgical resection, which has
improved the five-year survival rate from 20 to 60% (6,7).
However, OS cells frequently display multi-drug resistance to
chemotherapeutic drugs, which poses a considerable limitation to
the clinical curative effect and long-term survival (8). Induction of apoptosis in target cells
is a key therapeutic strategy for the extermination of cancer
cells; therefore, recent studies have focused on the identification
of novel drugs with the ability to induce and enhance apoptosis
(9–11).
Bufalin, one of the major components of the Chinese
medicine Chan'Su, was shown to exhibit marked anti-tumor activity
against leukemia and various types of solid tumor, including
prostate cancer, lung cancer and hepatoma (12–19).
This anti-tumor activity is achieved by inhibition of proliferation
and induction of apoptosis (18,19).
Bufalin was shown to be potent against human OS tissues and cell
lines via inducing apoptosis (20,21);
however, the specific underlying mechanism has remained elusive.
Mitochondria-mediated signaling pathways have a pivotal role in the
process of apoptosis (22–25), and have been identified to be the
underlying mechanism of bufalin-induced apoptosis in various types
of tumor cell (21). As the
specific mechanisms of action of bufalin vary between different
tumor types or even between different phenotypes of the same tumor
type, the mechanisms of the effects of bufalin on OS cell lines
require to be elucidated. The present study observed the effects of
bufalin on the proliferation and apop-tosis of the U-2OS human OS
cell line and investigated the expression of proteins associated
with the mitochondrial apop-totic pathway in the process of
U-2OS-cell apoptosis induced by bufalin. The apoptosis of U-2OS
cells was explored from the perspective of mitochondrial structure
and function, including the open of permeability transition pore
(PTP) and dissipation of mitochondrial membrane potential
(MMP/ΔΨm).
Materials and methods
Materials and reagents
Bufalin and cyclosporin A (CsA) were purchased from
Sigma-Aldrich (St Louis, MO, USA). The purity of bufalin was
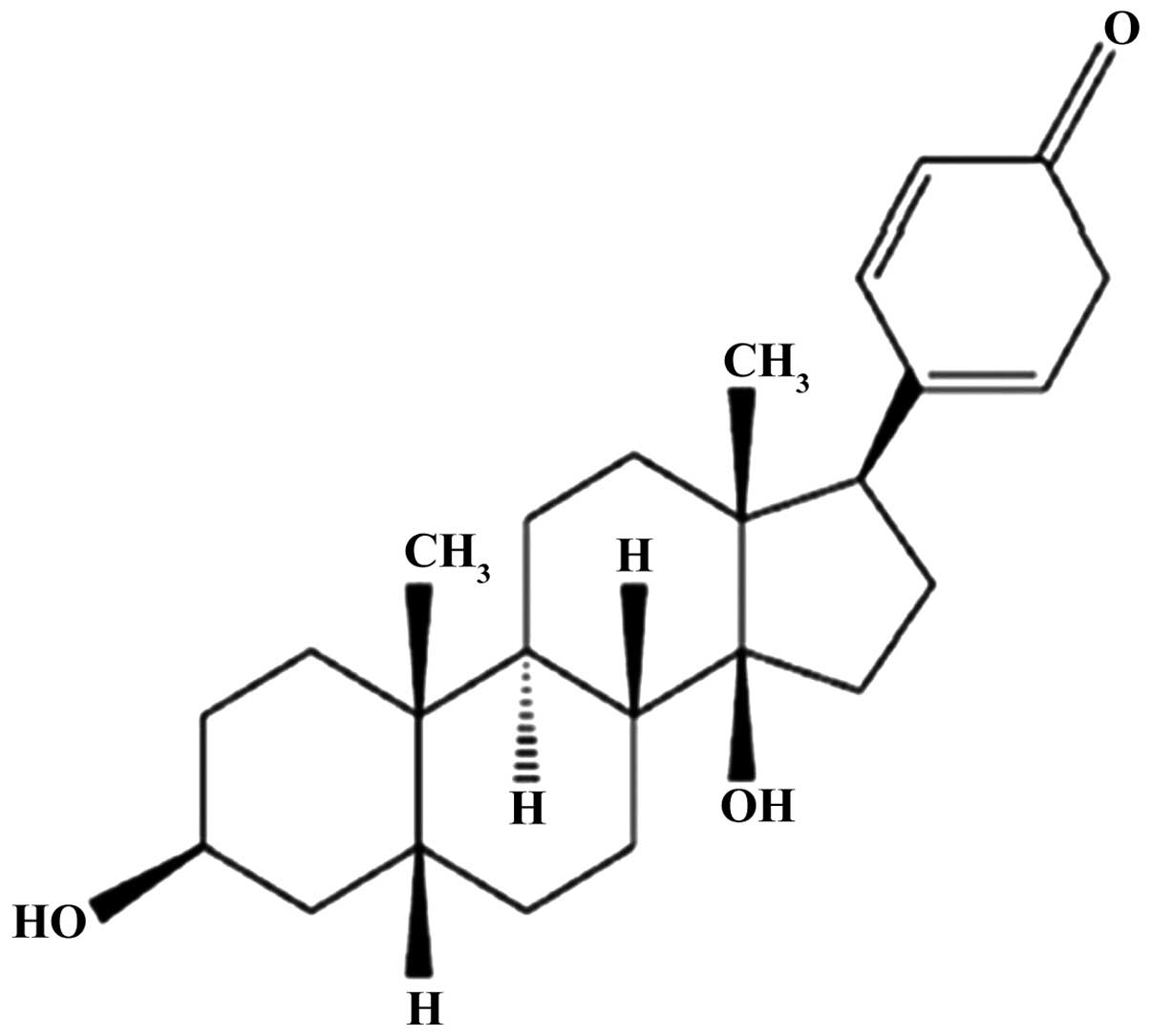
>99% as confirmed by high-performance liquid chromatograph
(Fig. 1). The Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit and the
Hoechst-propidium iodide (PI) staining assay kit were obtained from
BD Biosciences (Franklin Lakes, NJ, USA). The mitochondrial
membrane potential detection kit (JC-1; cat. no. 30001) was from
Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). Antibodies
specific for polyclonal rabbit anti-human B-cell lymphoma 2 (Bcl-2;
cat. no. 2876; 1:1,000), Bcl-2-associated X protein (Bax; cat. no.
2772; 1:1,000), monoclonal mouse anti-rat caspase-3 (1:500),
monoclonal mouse anti-rat caspase-9 (1:500) and anti-human β-actin
(cat. no. 4967; 1:1,000) were purchased from Santa Cruz
Biotechnology (Dallas, TX, USA). Antibodies specific for poly
adenosine diphosphate ribose polymerase (PARP), cleaved PARP and
cytochrome c were supplied by Abcam (Cambridge, UK).
U-2OS cell culture
The U-2OS human OS cell line was provided by the
Shanghai Institutes for Biological Sciences, the Chinese Academy of
Sciences (Shanghai, China). Cells were cultured in 1% Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS), 100 units/ml
penicillin and 100 μg/ml streptomycin (100 Biotech Co.,
Ltd., Hangzhou, China), and grown at 37°C, in a humidified
atmosphere containing 5% CO2. Cells in the logarithmic
growth phase were used in all experiments.
Cell proliferation assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide) MTT
method was used to assess the effects of bufalin on the cell
proliferation. U-2OS cells (2×103 cells/well) in the
logarithmic growth phase were seeded in 96-well plates. Following
24 h incubation at 37°C, the cells were treated with various
concentrations of bufalin (10, 20 or 50 μg/l) for 6, 12 and
24 h, 10 μl MTT (5 mg/ml) was added. Following 4 h of
incubation, the culture media was discarded and 100 μl
dimethylsulfoxide (DMSO) was added to each well to dissolve the
formed formazan crystals. The absorbance was measured at 570 nM
using an ELISA plate reader (Bio-Rad 550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Data were collected from three separate
experiments and the percentage of bufalin-induced cell growth
inhibition was determined by comparison with DMSO-treated control
cells.
Apoptosis assay
The apoptotic rate was determined using Hoechst
33258 staining following fluorescence-microscopic evaluation as
well as by Annexin V-FITC/PI double labeling and flow cytometric
analysis. U-2OS cells (1×105 cells/well) were cultured
in 12-well flat-bottomed microtiter plates for 24 h and
subsequently incubated with 1% DMSO (control cells) or 50
μg/l bufalin for 24 h. After fixing in 4% paraformaldehyde
(Bio-Rad Laboratories, Inc.) for 15 min at room temperature and
staining with 10 ng/ml Hoechst 33258, apoptotic cells were
identified by their characteristic nuclear morphology observed
under an inverted fluorescence microscope (CK40 F200; Olympus
Corporation, Tokyo, Japan). After treatment with various
concentrations (10, 20 or 50 μg/l) of bufalin for various
durations (6, 12 or 24 h), U-2OS cells were harvested, suspended in
phosphate-buffered saline (PBS), and stained with Annexin V-FITC
and PI according to the manufacturer's instructions of the
Apoptosis Detection kit. Flow cytometry (FACSort; BD Biosciences)
was used to quantify apoptotic U-2OS cells.
Flow cytometric detection of ΔΨm
ΔΨm was assessed using
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbo-cyanine
iodide (JC-1). The cells in the logarithmic growth phase were
seeded into 12-well flat-bottomed microtiter plates at a density of
2×105 cells. Following incubation for 24 h to allow for
cell adherence, the cells were treated with 10–50 μg/l
bufalin for 24 h and washed with pre-cooled PBS. The cells were
then collected by centrifugation (4°C, 1,000 × g, 5 min), washed
with PBS and re-suspended in 1 ml PBS. The cells were incubated in
0.5 ml medium containing JC-1 (1.0 μg/ml) for 30 min at 37°C
and staining was quantified using flow cytometry. In mitochondria
with an intact ΔΨm, JC-1 is concentrated to form aggregates with
red fluorescence, while accumulation of JC-1 in mitochondria with
ΔΨm loss is reduced, resulting in single JC-1 molecules with green
fluorescence.
Western blot analysis
For western blot analysis, U-2OS cells were
subjected to the abovementioned drug treatments and total protein
was then extracted using 1% Nonidet P-40 (Pierce Biotechnology,
Inc., Rockford, IL, USA). Each sample was centrifuged at 15,000 x g
for 30 min at 4°C and the supernatant was collected. Protein
concentration was determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Equal amounts
of protein (30 μg) were subjected to 12% sodium dodecyl
sulfate-polyacrylamide gel (Invitrogen; Thermo Fisher Scientific,
Inc.) electrophoresis and transferred onto a poly-vinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Levels of
cytoplasmic cytochrome c, PARP, cleaved PARP, caspase-3, caspase-9,
Bax and Bcl-2 were assessed using western blot analysis. Following
blocking in 5% non-fat dry milk for 1 h, the blots were incubated
with the following primary antibodies: Polyclonal rabbit anti-human
Bcl-2 (1:1,000; cat. no. 2876); polyclonal rabbit anti-human Bax
(1:1,000; cat. no. 2772) antibody; monoclonal mouse anti-rat
caspase-3 (1:500; cat. no. 1992), monoclonal mouse anti-rat caspase
9 (1:500, cat. no. 0620) antibody; poly-clonal mouse anti-human
PARP (1:1,000; cat. no. ab30889); polyclonal mouse anti-human
cleaved PARP (1:1,000; cat. no. ab72805); polyclonal rabbit
anti-human cytochrome c (1:1,000; cat. no. 1116) antibody,
for 12 h at 4°C followed by three 5 min washes in Tris-buffered
saline with Tween-20. Mouse or rabbit secondary antibody (1:2,000;
cat. no. 2357) conjugated with horseradish peroxidase was added to
the membrane and incubated for 1 h at room temperature. Protein
bands were visualized on X-ray films (Sigma-Aldrich) using an
enhanced chemiluminescence detection system (Applygen Technologies,
Inc., Beijing, China). β-actin was used as an internal control for
relative quantification. Grey value analysis of immunoreactive
bands was performed using Quantity One 16.0 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation of three experiments. Statistical comparisons were
performed using one-way analysis of variance. Statistical analysis
was conducted using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference between bufalin-treated groups and the control
group.
Results
Bufalin inhibits the viability of U-2OS
cells
U-2OS cells were exposed to various concentrations
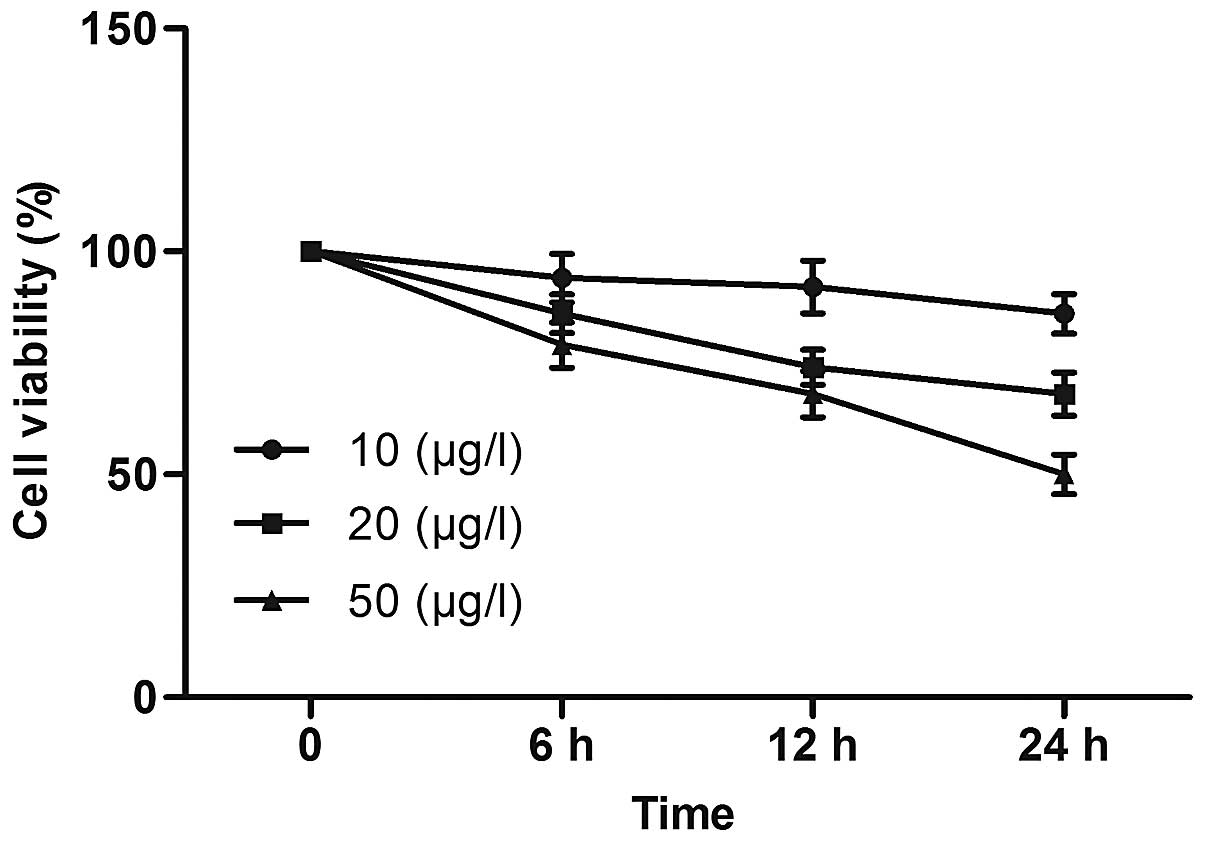
of bufalin for 6, 12 and 24 h and subjected to MTT assays (Fig. 2). Bufalin at 10 μg/l
produced minor decreases of cell viability with increasing time,
while 50 μg/l bufalin induced a marked time-dependent
inhibition of U-2OS-cell viability. Following exposure to 10, 20
and 50 μg/l bufalin for 24 h, the cell viability was reduced
to 86±4, 68±5 and 50±4%, respectively. These results indicated that
bufalin induced a marked concentration- and time-dependent
inhibition of U-2OS cells.
Bufalin induces apoptosis in U-2OS
cells
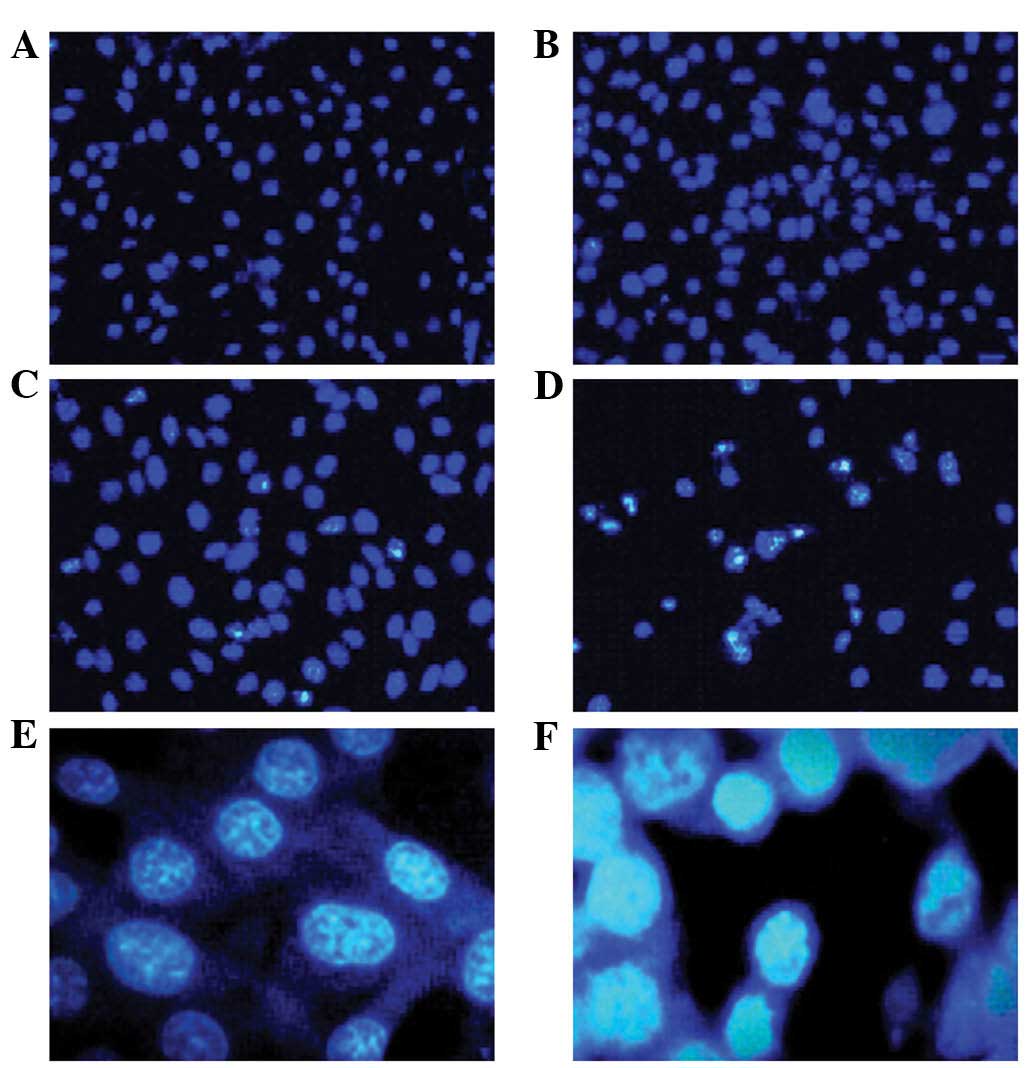
After 24 h of incubation with 10, 20 and 50
μg/l bufalin, U-2OS cells were stained with Hoechst 33258
and observed by fluorescence microscopy. With the increasing
concentration of bufalin, the number of apoptotic cells increased
gradually (Fig. 3A–D).
Morphological observation (magnification, ×400) revealed that U-2OS
control cells exhibited integrated and lightly stained nuclei with
uniform chromatin density, while morphological characteristics of
apoptosis, including cytoplasmic shrinkage, nuclear condensation,
nuclear fragmentation and the formation of apoptotic bodies, were
observed in U-2OS cells treated with bufalin (Fig. 3E and F).
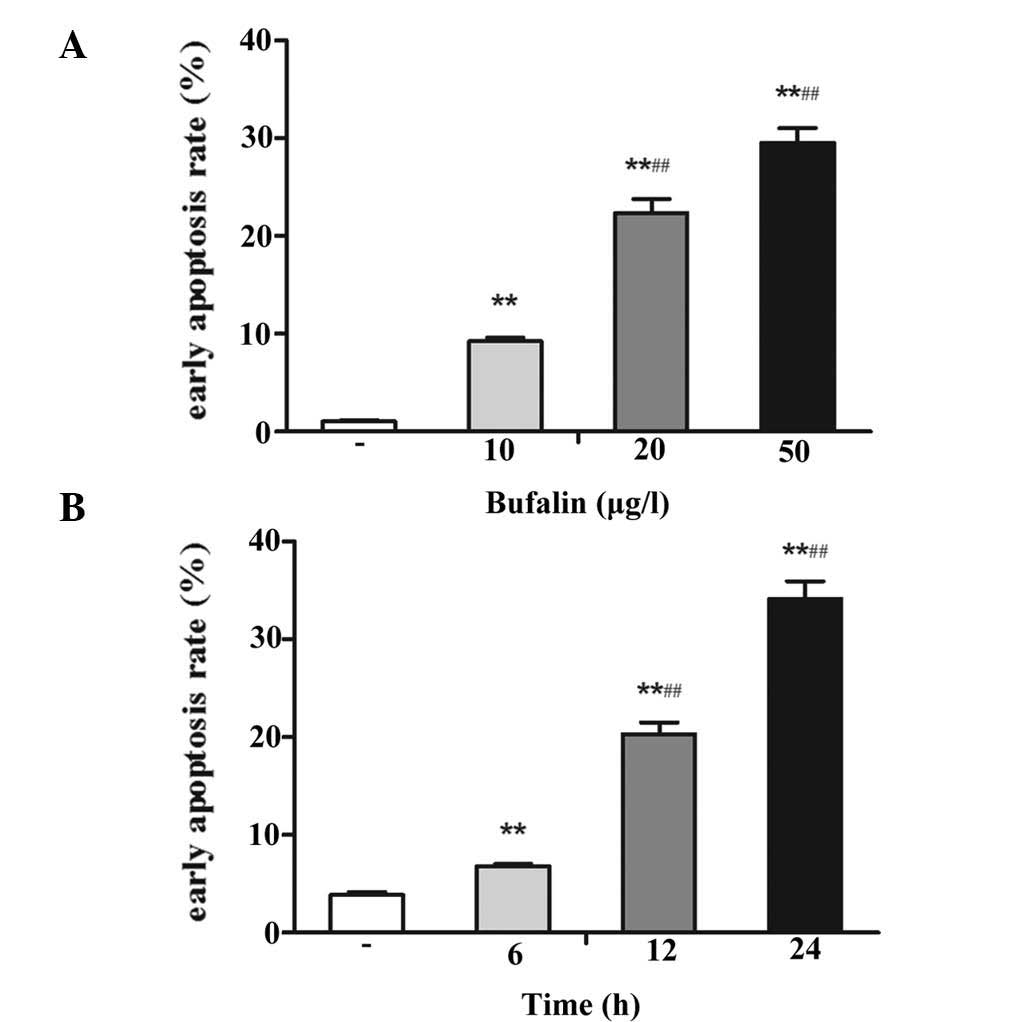
Flow-cytometric determination of the apoptotic rate
revealed that bufalin induced early apoptosis of U-2OS cells in a
dose- and time-dependent manner. Treatment with 10, 20 and 50
μg/l bufalin for 24 h significantly increased the apoptotic
rates to 9.28±0.31, 22.40±1.38 and 29.54±1.55%, respectively
(P<0.05 vs. control group; 1.06±0.11%) (Fig. 4A). In addition, incubation with 50
μg/l bufalin for 6, 12 and 24 h, increased the apoptotic
rate to 6.83±0.17, 20.33±1.19 and 34.17±1.82% (P<0.05 vs.
control group; 3.86±0.33%) (Fig.
4B).
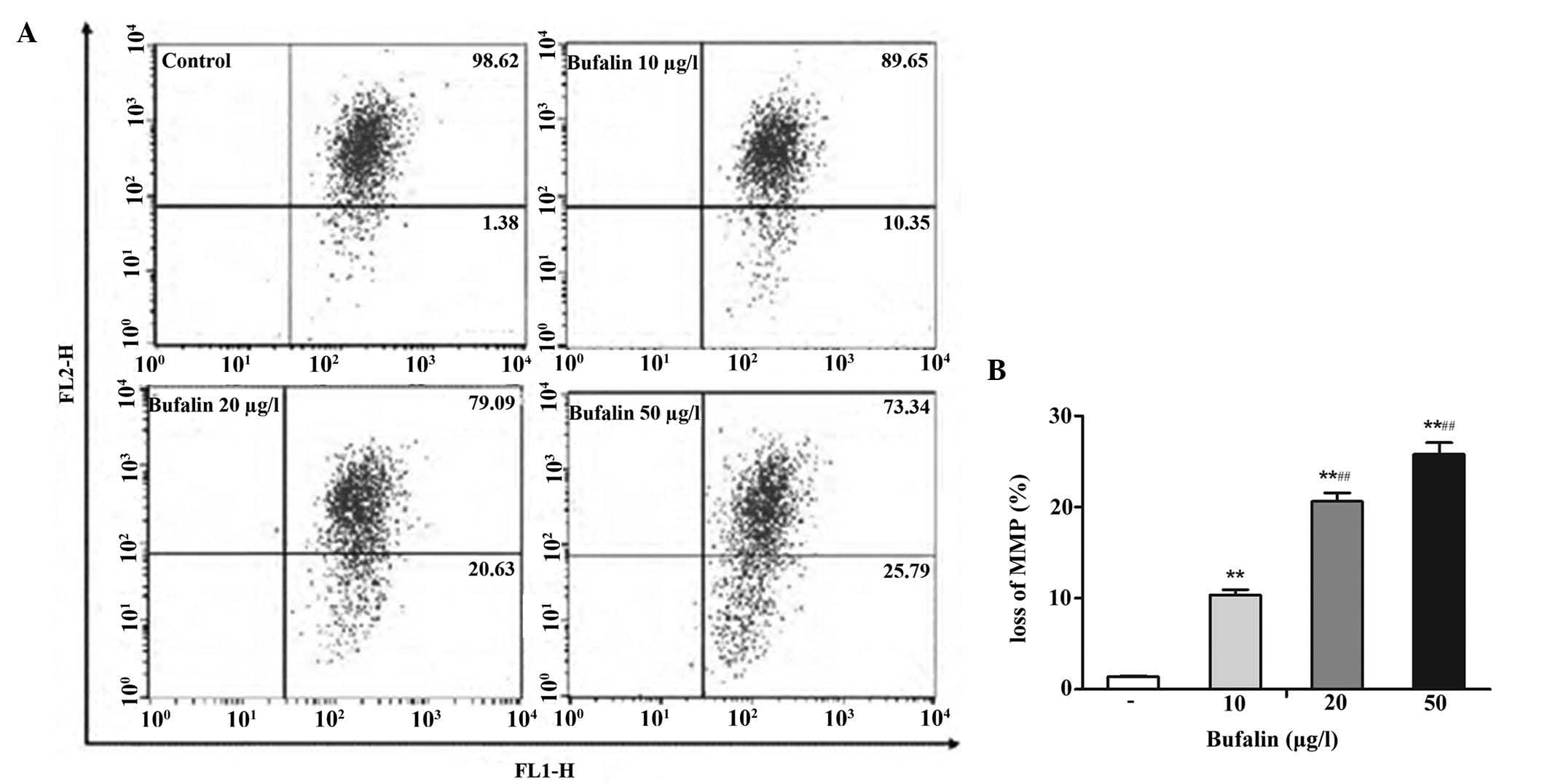
Bufalin decreases ΔΨm
Since mitochondria have an essential role in the
apoptotic process, the present study evaluated whether bufalin
triggered mitochondrial events of apoptosis. The loss of ΔΨm is an
irreversible key event during early apoptosis. In order to explore
the effects of bufalin on the ΔΨm, JC-1 staining followed by
flow-cytometric analysis was performed. As shown in Fig. 5, the red fluorescence (upper right
quadrant) was gradually reduced, while the green fluorescence
(lower right quadrant) was enhanced by increasing doses of bufalin.
This result demonstrated that bufalin promoted the release of JC-1
from the mitochondrial matrix into the cytoplasm, leading to its
disaggregation into green-fluorescent JC-1 monomers, which are a
marker of mitochondrial membrane depolarization and the loss of
ΔΨm. These results indicated that bufalin decreased the ΔΨm by
enhancing the permeability of the mitochondrial membrane to
initiate apoptosis.
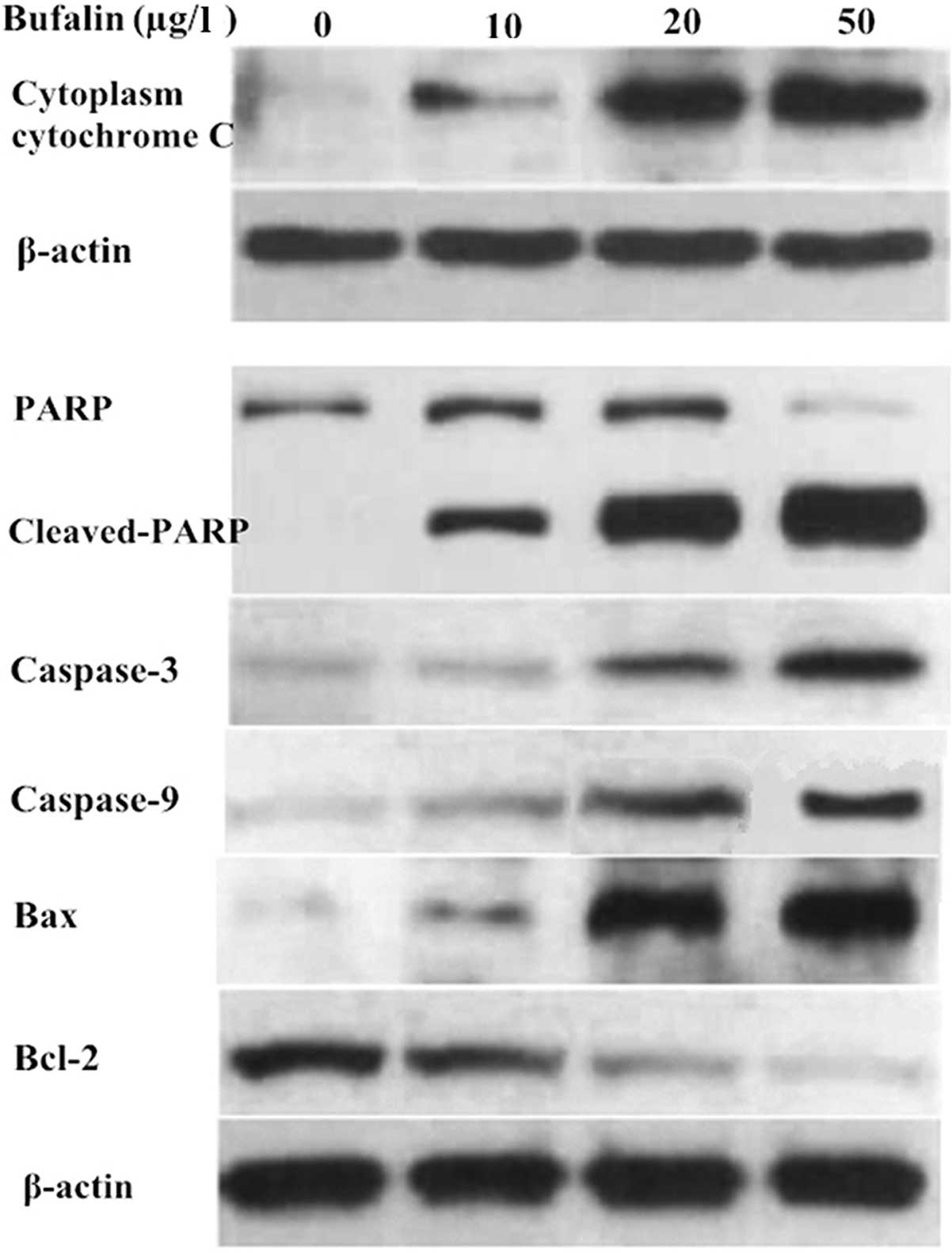
Bufalin affects the expression of
proteins associated with mitochondrial apoptosis
To determine the underlying mechanisms of
bufalin-induced U-2OS-cell apoptosis, the expression of
apoptosis-associated proteins was evaluated by western blot
analysis. With the increasing concentration of bufalin (10, 20 and
50 μg/l), the expression of cytoplasmic cytochrome c,
cleaved PARP, caspase-3, caspase-9 and Bax in the cytosolic
fraction of U-2OS cells was upregulated compared with that in the
control group. By contrast, Bcl-2 and PARP were downregulated
(Fig. 6). These results indicated
that OS-cell apoptosis induced by bufalin is mediated via the
mitochondrial apoptotic signaling pathway.
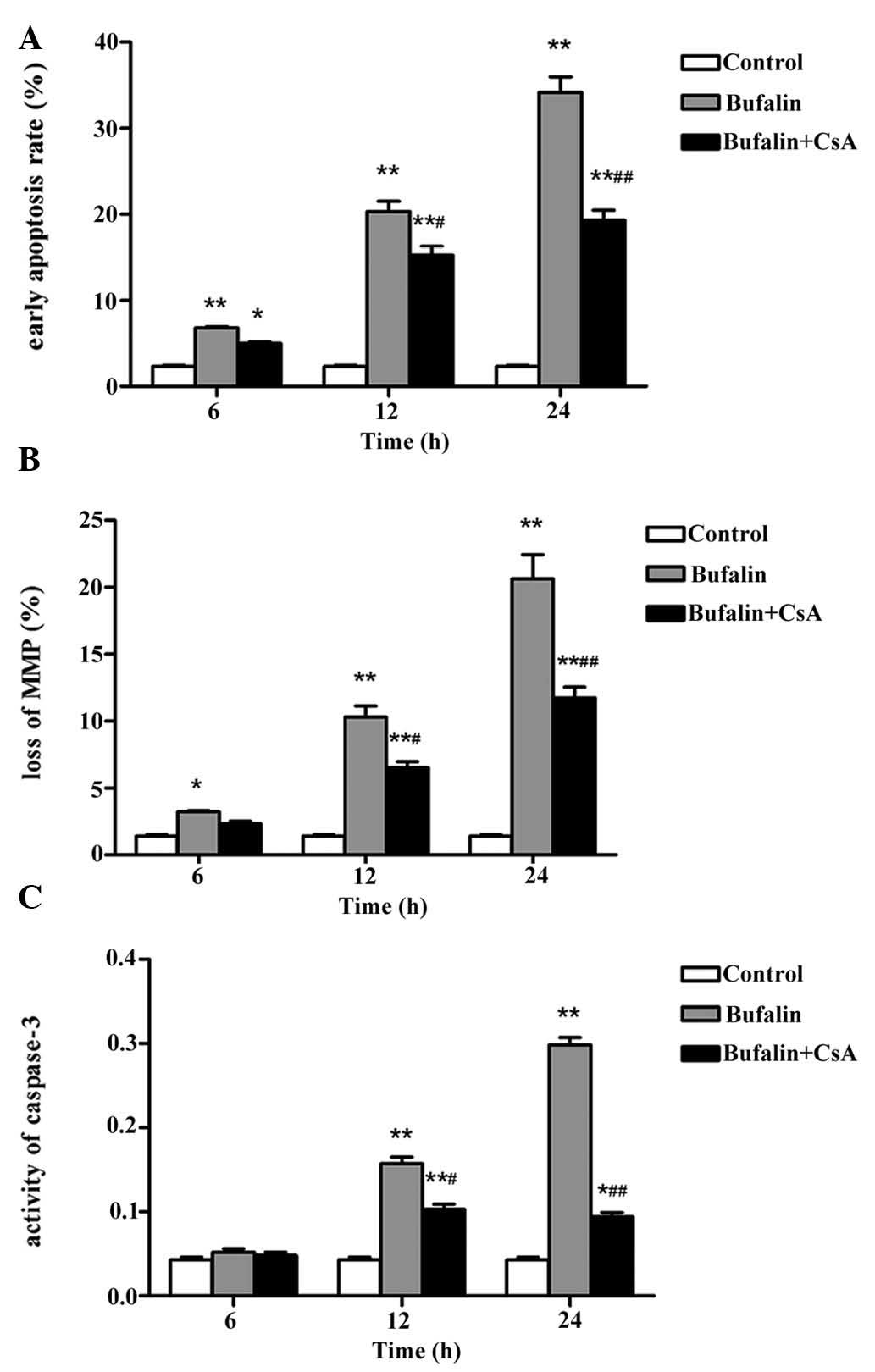
CsA reduces bufalin-induced apoptosis of
U-2OS cells
To further assess the effects of mitochondrial
transmembrane transport in bufalin-induced apoptosis, U-2OS cells
were pre-incubated with CsA, a specific blocker of the PTP. As
shown in Fig. 7A, pre-treatment
with 2 μmol/l CsA for 30 min significantly decreased the
bufalin-induced early apoptotic rate in a time-dependent manner at
12 and 24 h (P<0.05 and P<0.01, respectively). In addition,
CsA pre-treatment significantly reduced the bufalin-induced loss of
ΔΨm in a time-dependent manner at 12 and 24 h (P<0.05 and
P<0.01, respectively) (Fig.
7B). As caspase-3 is a key mediator and executioner of
apoptosis, caspase-3 activity is regarded to be an index of
apoptosis. While the levels of cleaved caspase were significantly
increased following incubation with 50 μg/l bufalin for 12
and 24 h (P<0.01), pre-incubation with 2 μmol/l CsA for
30 min significantly attenuated caspase-3 activation (P<0.05 and
P<0.01, respectively) (Fig.
7C). All of these results indicated that CsA partly inhibited
bufalin-induced apoptosis of U-2OS cells.
Discussion
Bufalin is a biologically active component of the
Traditional Chinese Medicine Chan'Su, which has the potential to
induce differentiation and apoptosis in a wide spectrum of tumor
cells (26,27). Numerous studies have investigated
the potential molecular mechanism of the anti-tumor activity of
bufalin, indicating that the mitochondria-mediated signaling
pathway has a pivotal role in the process of apoptosis induced by
bufalin. In the ASTC-a-1 human lung adenocarcinoma cell line,
bufalin was shown to induce the translocation of Bax from the
cytosol to the mitochondria to activate caspase-3 (28). Masuda et al (29) demonstrated the apoptosis of HL-60
human leukemia cells by altering the expression of
apoptosis-associated genes. In androgen-dependent and -independent
human prostate cancer, bufalin and cinobufagin were shown to
suppress cell proliferation and cause apoptosis via sequence of
apoptotic effectors, including Bax, cytochrome c and
caspases, all of which are implicated in mitochondria-mediated
signaling pathways, which was therefore the predominant apoptotic
mechanism induced by bufalin (30). However, the specific mechanism was
shown to vary between different tumor types and even within
different phenotypes of the same tumor type; therefore, it was
required to determine the molecular mechanism of action of bufalin
in human OS cells.
In the process of apoptosis triggered by the
mitochondria-mediated signaling pathway, the opening of the PTP and
subsequent perforation of the mitochondrial membrane decreases the
ΔΨm and leads to the release of cytochrome c into the
cytoplasm, were it activates caspases, which amplify the apoptotic
signal and disassemble the cytoskeleton (31). The present study revealed that
bufalin reduced the viability and induced apoptosis in the U-2OS
human OS cell line in a time- and dose-dependent manner. Following
24 h of incubation, bufalin reduced the ΔΨm of U-2OS cells in a
concentration-dependent manner. In addition, western blot analysis
revealed that cytochrome c was released from the
mitochondria into the cytoplasm. Furthermore, bufalin
dose-dependently increased the levels of active caspase-3 and -9
with resulting decreases in their substrate PARP and increases in
the product, cleaved PARP.
The Bcl-2 family of proteins comprise important
regulatory factors of cell apoptosis, which include anti-apoptotic
proteins, including Bcl-2 and Bcl extra large protein, as well as
pro-apoptotic proteins, including Bax and Bcl-2-associated death
promoter, with their main site of action being the mitochondrial
outer membrane (32). Bax
translocates from the cytosol to the mitochondria where it
oligomerizes and permea-bilizes the mitochondrial outer membrane to
promote apoptosis, and constantly retro-translocates to the cytosol
depended on pro-survival Bcl-2 family proteins (33). Upon stimulation by apoptotic
signaling in the cell, the Bax/Bcl-2 ratio is enhanced and the
Bax-mediated increases in the permeability of the mitochondrial
outer membrane results in the release of the pro-apoptotic proteins
from the mitochondria, which activates caspase family members and
triggers apoptosis. In the present study, bufalin treatment
enhanced the expression of Bax and downregulated the expression of
Bcl-2 U-2OS cells, which indicated that the apoptosis of human OS
cells induced by bufalin is mediated via the mitochondrial
apoptotic signaling pathway.
The opening of the PTP and the loss of ΔΨm are
considered to be early events in the apoptotic cascade. CsA, a
commonly used specific PTP blocker, effectively suppresses
apoptosis. Its mechanism of action comprises the inhibition of the
combination of mitochondrial matrix protein cyclophilin D and the
multiprotein complex of the PTP, which effects the closing of the
PTP. Interruption of the PTP by CsA is a method for studying the
involvement of the PTP in metabolic processes (34). Based on this principle, the present
study used CsA to explore the role of the PTP in bufalin-induced
apoptosis in U2-OS cells. Pre-treatment of the cells with CsA,
partly inhibited the apop-totic effects of bufalin, as indicated by
attenuation of the early apoptotic rate, loss of ΔΨm and caspase-3
activity. As CsA, which targets cyclophilin D, reduced
bufalin-induced apop-tosis, bufalin may indirectly exert its
effects via cyclophilin D.
In conclusion, bufalin inhibited the viability and
induced apoptosis in U-2OS cells in a time- and dose-dependent
manner. Bufalin-induced apoptosis was accompanied by a significant
reduction of ΔΨm, the release of mitochondrial cytochrome c
into the cytosol, activation of caspase-3 and caspase-9, activating
cleavage of PARP and a decrease of the Bcl-2/Bax ratio. CsA, an
inhibitor of the PTP, attenuated the apoptosis induced by bufalin.
Therefore, bufalin induces apoptosis in the U-2OS human OS cell
line via triggering of the mitochondrial pathway.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Fernandez-Pineda I, Bahrami A, Green JF,
McGregor LM, Davidoff AM and Sandoval JA: Isolated subcutaneous
metastasis of osteosarcoma 5 years after initial diagnosis. J
Pediatr Surg. 46:2029–2031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sampo MM, Tarkkanen M, Kivioja AH,
Taskinen MH, Sankila R and Böhling TO: Osteosarcoma in Finland from
1971 through 1990: A nationwide study of epidemiology and outcome.
Acta Orthop. 79:861–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osborne TS and Khanna C: A review of the
association between osteosarcoma metastasis and protein
translation. J Comp Pathol. 146:132–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaffe N, Carrasco H, Raymond K, Ayala A
and Eftekhari F: Can cure in patients with osteosarcoma be achieved
exclusively with chemotherapy and abrogation of surgery? Cancer.
95:2202–2210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serra M, Scotlandi K, Manara MC, Maurici
D, Lollini PL, De Giovanni C, Toffoli G and Baldini N:
Establishment and char-acterization of multidrug-resistant human
osteosarcoma cell lines. Anticancer Res. 13:323–329.
1993.PubMed/NCBI
|
|
9
|
Li J, Zhang F and Wang S: A polysaccharide
from pomegranate peels induces the apoptosis of human osteosarcoma
cells via the mitochondrial apoptotic pathway. Tumour Biol.
35:7475–7482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie X, Yin J, Jia Q, Wang J, Zou C, Brewer
KJ, Colombo C, Wang Y, Huang G and Shen J: Quercetin induces
apoptosis in the methotrexate-resistant osteosarcoma cell line
U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of
Akt. Oncol Rep. 26:687–693. 2011.PubMed/NCBI
|
|
11
|
Zhao XH, Xu ZR, Zhang Q and Yang YM:
Simvastatin protects human osteosarcoma cells from oxidative
stress-induced apoptosis through mitochondrial-mediated signaling.
Mol Med Rep. 5:483–488. 2012.
|
|
12
|
Cao Hong, Shibayama-Imazu T, Masuda Y,
Shinki T, Nakajo S and Nakaya K: Involvement of Tiam1 in apoptosis
induced by bufalin in HeLa cells. Anticancer Res. 27:245–249.
2007.
|
|
13
|
Dong Y, Yin S, Li J, Jiang C, Ye M and Hu
H: Bufadienolide compounds sensitize human breast cancer cells to
TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway.
Apoptosis. 16:394–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu W, Liu L, Fang FF, Huang F, Cheng BB
and Li B: Reversal effect of bufalin on multidrug resistance in
human hepatocellular carcinoma BEL-7402/5-FU cells. Oncol Rep.
31:216–222. 2014.
|
|
15
|
Hu F, Han J, Zhai B, Ming X, Zhuang L, Liu
Y, Pan S and Liu T: Blocking autophagy enhances the apoptosis
effect of bufalin on human hepatocellular carcinoma cells through
endoplasmic reticulum stress and JNK activation. Apoptosis.
19:210–223. 2014. View Article : Google Scholar
|
|
16
|
Kurosawa M, Tani Y, Nishimura S, Numazawa
S and Yoshida T: Distinct PKC isozymes regulate bufalin-induced
differentiation and apoptosis in human monocytic cells. Am J
Physiol Cell Physiol. 280:C459–464. 2001.PubMed/NCBI
|
|
17
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
18
|
Zhu Z, Li E and Liu Y, Gao Y, Sun H, Wang
Y, Wang Z, Liu X, Wang Q and Liu Y: Bufalin induces the apoptosis
of acute promyelocytic leukemia cells via the downregulation of
survivin expression. Acta Haematol. 128:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of pi3k/akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang Y, Zhao Y, Zhan H, Wei X, Liu T and
Zheng B: Bufalin inhibits the differentiation and proliferation of
human osteosarcoma cell line hMG63-derived cancer stem cells.
Tumour Biol. 35:1075–1082. 2014. View Article : Google Scholar
|
|
21
|
Wang D and Bi Z: Bufalin inhibited the
growth of human osteo-sarcoma MG-63 cells via down-regulation of
Bcl-2/Bax and triggering of the mitochondrial pathway. Tumour Biol.
35:4885–4890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arora S and Tandon S: Achyranthes aspera
root extracts induce human colon cancer cell (colo-205) death by
triggering the mitochondrial apoptosis pathway and s phase cell
cycle arrest. Scientific World Journal. 2014:1296972014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, Miskimen KL, Wang Z, Xie XY,
Brenzovich J, Ryan JJ, Tse W, Moriggl R and Bunting KD: STAT5
requires the N-domain for suppression of miR15/16, induction of
bcl-2 and survival signaling in myeloproliferative disease. Blood.
115:1416–1424. 2010. View Article : Google Scholar :
|
|
25
|
Shimizu S, Takehara T, Hikita H, Kodama T,
Miyagi T, Hosui A, Tatsumi T, Ishida H, Noda T and Nagano H: The
let-7 family of microRNAs inhibits Bcl-xL expression and
potentiates sorafenib-induced apoptosis in human hepatocellular
carcinoma. J Hepatol. 52:698–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang LS, Nakaya K, Yoshida T and Kuroiwa
Y: Bufalin as a potent inducer of differentiation of human myeloid
leukemia cells. Biochemical and biophysical research
communications. 178:686–693. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krenn L and Kopp B: Bufadienolides from
animal and plant sources. Phytochemistry. 48:1–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun L, Chen T, Wang X, Chen Y and Wei X:
Bufalin induces reactive oxygen species dependent bax translocation
and apoptosis in astc-a-1 cells. Evid Based Complement Alternat
Med. 2011:2490902011. View Article : Google Scholar :
|
|
29
|
Masuda Y, Kawazoe N, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: Bufalin induces apoptosis and influences
the expression of apoptosis-related genes in human leukemia cells.
Leuk Res. 19:549–556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naranmandura H, Chen X, Tanaka M, Wang WW,
Rehman K, Xu S, Chen Z, Chen SQ and Suzuki N: Release of apoptotic
cytochrome C from mitochondria by dimethylarsinous acid occurs
through interaction with voltage-dependent anion channel in vitro.
Toxicol Sci. 128:137–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edlich F, Banerjee S, Suzuki M, Cleland
MM, Arnoult D, Wang C, Neutzner A, Tjandra N and Youle RJ: Bcl-x(L)
retrotranslocates Bax from the mitochondria into the cytosol. Cell.
145:104–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andre N, Roquelaure B and Conrath J:
Molecular effects of cyclosporine and oncogenesis: a new model.
Medical hypotheses. 63:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|