Introduction
The peroxisome proliferator-activated receptor
(PPAR)-γ coactivator-1 (PGC-1) family of coactivators is an
extensively regulated group of proteins that are highly responsive
to a variety of environmental cues, including temperature,
nutritional status and physical activity. This family of
coactivators serves a crucial role in integrating signaling
pathways and adapts them to best suit the varying cellular and
systemic environment. The first identified member of the PGC-1
family was PGC-1α, which was initially identified as a
PPARγ-interacting protein from brown fat (1). PGC-1α is a transcription cofactor
that contains binding sites for a number of nuclear hormone
receptors. It regulates oxidative reactions and mitochondrial
energy metabolism in various cells and tissues (1). PGC-1α is expressed at high levels in
numerous human and rodent tissues, including brown fat, skeletal
muscle, heart, kidney, liver and brain, and additionally in
vascular endothelial cells (1–4).
Under ischemic and hypoxic conditions, the
expression and transcriptional regulation activity of PGC-1α is
enhanced in rat cardiac myocytes (5,6) and
brain cells (7,8), rabbit renal tubular cells (9), and human skeletal muscle cells
(10,11). A previous study demonstrated that
PGC-1α is able to regulate an angiogenic program including vascular
endothelial growth factor (VEGF) and additional angiogenic factors,
in cultured muscle cells and skeletal muscle in vivo
(12). Transgenic expression of
PGC-1α in skeletal muscle markedly increased microvascular density
and was protective in a model of skeletal muscle ischemia (12). PGC-1α may represent a novel
therapeutic target to aid in improving the treatment of ischemic
and hypoxic diseases (13).
Neoangiogenesis is a common pathophysiological
feature of numerous diseases. Ischemia and hypoxia induce the
formation of new blood vessels by altering the balance between
angiogenesis-promoting factors and angiogenesis-inhibiting factors.
Nascent blood vessels grow from existing ones by sprouting. In
certain diseases, new blood vessels frequently have abnormal vessel
walls, which may lead to complications. Retinal neovascularization
(NV) occurs in various ocular disorders including proliferative
diabetic retinopathy, retinopathy of prematurity and secondary
neovascular glaucoma, which are a major cause of blindness
worldwide (14).
Considering that PGC-1α is able to mediate
angiogenesis in skeletal muscle, it has been suggested that it may
do so in additional tissues, such as the retina. The retina is a
highly metabolic neural tissue with the highest oxygen consumption
per unit weight of any human tissue (15). PGC-1α may serve a role in retinal
neovascularization as a regulator of angiogenesis. In order to
investigate this hypothesis, the current study used human retinal
vascular endothelial cells (hRVECs) and a mouse model of
oxygen-induced ischemic retinopathy (OIR) to explore the potential
role of PGC-1α in mediating retinal neovascularization in
vitro and in vivo. In the current study, hRVECs and OIR
mice were treated with recombinant PGC-1α and the effect on VEGF
expression, cell proliferation, endothelial cell tube formation and
retinal neovascularization was investigated.
Materials and methods
Cell culture and treatment
Human retinal vascular endothelial cells
(HUM-CELL-0112; Wuhan PriCells Biomedical Technology Co., Ltd.,
Wuhan, China) were cultured in 6-well plates with Dulbecco's
modified Eagle's medium containing 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in a 5%
CO2, 20% O2 environment. Subsequently, 24 h
following plating and once cells had reached the logarithmic growth
phase, media was replaced and the cells were divided into the
following groups: Normoxia PGC-1α, normoxia control, hypoxia PGC-1α
and hypoxia control. A total of 5 µl (0.25
µg/µl) recombinant PGC-1α (Abnova Corporation,
Taipei, Taiwan, R.O.C.) was added to each well of cells in the
normoxia PGC-1α and hypoxia PGC-1α groups. A total of 5 µl
phosphate-buffered saline (PBS) was added to the cells in the
normoxia control and hypoxia control groups. At 24 h following
treatment, the cells were left in the normoxic conditions or placed
into a hypoxic environment (1% O2, 5% CO2 and
94% N2) and cultured for a further 16 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) to measure VEGF mRNA expression
in hRVECs
RNA was extracted from cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
total of 1 µg template was reverse-transcribed using the
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.). The primers used in the qPCR were as follows:
Human VEGF (124 bp), forward 5′-CTGTCTAATGCCCTGGAGCC-3′ and reverse
5′-ACGCGAGTCTGTGTTTTTGC-3′; and human β-actin (169 bp), forward
5′-TCTGGCACCACACCTTCTAC-3′ and reverse 5′-GATAGCACAGCCTGGATAGC-3′.
The PCR reaction conditions were as follows: Denaturation at 94°C
for 3 min followed by 40 cycles of 94°C for 30 sec, 59°C for 30
sec, and 72°C for 45 sec. The 2−ΔΔCq method was used to
analyze the relative changes in gene expression from the RT-PCR
data.
Measurement of VEGF protein expression in
hRVECs using ELISA
The amount of VEGF secreted into the cell culture
supernatant was measured using an enzyme-linked immunosorbent assay
(ELISA) kit (USCN, Houston, TX, USA). The standard wells were
filled with 100 µl VEGF standard at various concentrations,
blank wells were filled with 100 µl buffer and sample wells
were filled with 100 µl of sample. The microplates were then
covered and incubated at 37°C for 2 h. Each well was filled with
100 µl working solution A and the microplates were covered
again and incubated at 37°C for 1 h. Subsequently, 100 µl
working solution B was added to each well and the microplates were
covered and incubated at 37°C for 30 min. A total of 90 µl
substrate solution was then added to each well and the microplates
were covered and incubated at 37°C for 20 min in the dark.
Following this, each well was filled with 50 µl stop
solution and the optical density at 450 nm was measured using a
plate reader (Infinite 200 Pro; Tecan, Männedorf, Switzerland).
Cell proliferation assay
hRVECs were seeded in 96-well tissue culture plates
and incubated for 24 h. Following this, cells were starved in M199
medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 2%
FBS in the absence of endothelial cell growth supplements for a
further 16 h. Following starvation, cells were treated with
recombinant PGC-1α or PBS for 24 h, followed by culturing under
normoxic or hypoxic conditions for a further 16 h. Cell
proliferation was determined using a Cell Proliferation ELISA,
bromodeoxyuridine (BrdU) kit (Roche Diagnostics, Indianapolis, IN,
USA.) based on the colorimetric detection of the incorporation of
BrdU, following the manufacturer's instructions.
Tube formation assay
At 24 h following treatment with recombinant PGC-1α
or PBS, the media was replaced with endothelial cell basal medium
and the cells were added to plates coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). At 30–60 min prior to
plating the cells, 100 µl Matrigel was placed at the bottom
of each well in a 96-well chamber, avoiding bubbles. The plate was
incubated at 37°C for 30–60 min to allow the Matrigel to solidify.
Subsequently, cells were dissociated and counted, and 100–200
µl cells/well were added on top of the Matrigel. Cells were
placed under normoxic (20% O2) or hypoxic (1%
O2) conditions and cultured for a further 24 h. The
tubes formed were observed and images were captured using an
optical microscope (DM5000B; Leica Microsystems, Wetzlar, Germany).
The number of tubes in each well were quantified by a blinded
observer.
Animal model
C57BL/6J mice were used in the current study and
were treated in accordance with the Association for Research in
Vision and Ophthalmology (ARVO) Statement for the Use of Animals in
Ophthalmic and Vision Research. OIR was generated in C57BL/6J mice
(16), as previously described by
Smith et al (17). The
present study was approved by the ethics committee of Xiangya
Hospital, Central South University (Changsha, China). Mice,
obtained from the Experimental Animal Center, Chinese Academy of
Sciences, were selected at postnatal day 7 (P7) to optimize the
balance in retinal development between hyaloid regression and
incomplete retinal vascularization. P7 mice and their mothers were
placed in an oxygen chamber and exposed to an oxygen concentration
of 75±2% as monitored by an oxygen analyzer (CY-7B; Mei Cheng
Electrical Analysis Instrument Factory, Jiande, China) for 5 days.
The mice were exposed to 12 h cyclical broad spectrum light. The
room temperature was maintained at 23±2°C. On P12, the mice were
removed to room air from P12 to P17 or P21, when the retinas were
assessed for the maximum neovascular response. Age-matched C57BL/6J
mice maintained in room air were used as the controls. The mice
were randomly divided into normal, OIR control and OIR PGC-1α
groups. A total of 114 C57BL/6J mice were used for in vivo
studies.
Intravitreal injection of recombinant
PGC-1α
OIR mice were anesthetized intraperitoneally with 1%
pentobarbital sodium (30 mg/kg body weight) and given an
intravitreal injection of recombinant PGC-1α or PBS. The tip of a
10 mm 34-gauge steel needle, mounted on a 5 µl Hamilton
syringe, was pushed through the sclera, 1 mm posterior to the
corneoscleral limbus, into the vitreous body (18). OIR mice received an intravitreal
injection of 1 µl (0.25 µg/µl) recombinant
PGC-1α (OIR PGC-1α group) or 1 µl PBS (OIR control group) at
P11, and were returned to room air at P12. Mice in the normal group
were not subjected to an intravitreal injection.
Angiography using
fluorescein-dextran
At P17, 10 mice from each group were sacrificed
through overdose of 1% pentobarbital sodium at 30 mg/kg body weight
and were then perfused through the left ventricle with 1 ml PBS,
containing 50 mg of 2×106 molecular weight
fluorescein-dextran (Sigma-Aldrich, St. Louis, MO, USA). Eyes were
enucleated and fixed in 4% formaldehyde for 10 min. The retina was
dissected free of the lens and cornea, and placed in 4%
formaldehyde for 5 min. The peripheral retina was then cut in four
places and flat-mounted with glycerol/PBS (50/50).
Cross-sectional analysis of NV
At P17, 10 mice from each group were sacrificed and
the eyes were enucleated and fixed in 4% formaldehyde for 24 h,
prior to embedding in paraffin. Serial sections (5 µm) of
whole eyes were cut sagittally through the cornea and parallel to
the optic nerve, and stained with hematoxylin and eosin (Boster
Systems, Inc., Wuhan, China). A total of 10 nonserial sections were
analyzed per eye. Sections including the optic nerve were excluded,
and the nuclei of new vessels extending from the retina into the
vitreous were counted.
RT-qPCR analysis of the expression of
VEGF in the retina
Total RNA was prepared from each group of mouse
retinas at P17, to measure the expression of VEGF mRNA. Each RNA
sample was obtained from two retinas. In brief, retinas were lysed
in TRIzol reagent and RNA was extracted and purified, according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 1 µg template was
reverse-transcribed using the RevertAid First Strand cDNA Synthesis
kit. The primers used in qPCR were as follows: Mouse VEGF (240 bp),
forward 5′-CATCTTCAAGCCGTCCTGT-3′ and reverse
5′-GAGGAAAGGGAAAGGGTCA-3′; and mouse β-actin (203 bp), forward
5′-TTCCTTCTTGGGTATGGAAT-3′ and reverse 5′-GAGCAATGATCTTGATCTTC-3′.
The PCR reaction conditions were as follows: Denaturation at 95°C
for 5 min followed by 40 cycles of 94°C for 20 sec, 60°C for 20 sec
and 72°C for 20 sec. The 2−ΔΔCq method was used to
analyze the relative changes in gene expression from the RT-PCR
data.
Western blot analysis of VEGF protein
expression in the retina
Proteins were prepared from each group of mouse
retinas at P17 to measure the expression of VEGF. Each protein
sample was obtained from four retinas from the same group. The
retinas were homogenized in ice-cold lysis buffer, containing 20 mM
HEPES (pH 7.5), 1% Triton X-100, 1 mM EDTA and 0.1 mol/L NaCl), and
the lysates were centrifuged at 15,000 × g for 15 min at 4°C. The
supernatant was collected and the protein concentration was
determined with Bradford assay. For each sample, 100 µg
protein was fractionated using 10% SDS-PAGE (Pierce Biotechnology,
Inc., Rockford, IL, USA), and transferred onto polyvinylidene
membranes (Pierce Biotechnology, Inc). The membranes were gently
agitated in the blocking solution (5% skim milk) for 1 h at room
temperature and then incubated overnight with primary antibodies at
4°C with agitation. The primary antibodies used were as follows:
1:200 polyclonal goat anti-mouse VEGF164 (cat. no. AF-493-NA; Novus
Biologicals LLC, Littleton, CO, USA) and 1:1,000 polyclonal rabbit
anti-mouse β-actin (cat. no. sc-130656; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The membranes were washed with PBS,
incubated with the secondary antibodies for 1 h at room temperature
and washed again. Antibodies bound to the membranes were detected
using enhanced chemiluminescence (Pierce Biotechnology, Inc.) and
exposed to X-ray films (Kodak, Rochester, NY, USA) in the dark. The
intensity of the protein bands was analyzed using BandScan
software, version 5.0 (Glyko, Inc., Novato, CA, USA). The relative
levels of VEGF were calculated as VEGF band intensity/β-actin band
intensity.
Statistical analysis
One-way analysis of variance was used for
comparisons across all groups, and pair-wise comparisons between
groups were conducted using the Fisher's least significant
difference test. SPSS software version 19.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Data from two groups
were compared by paired Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
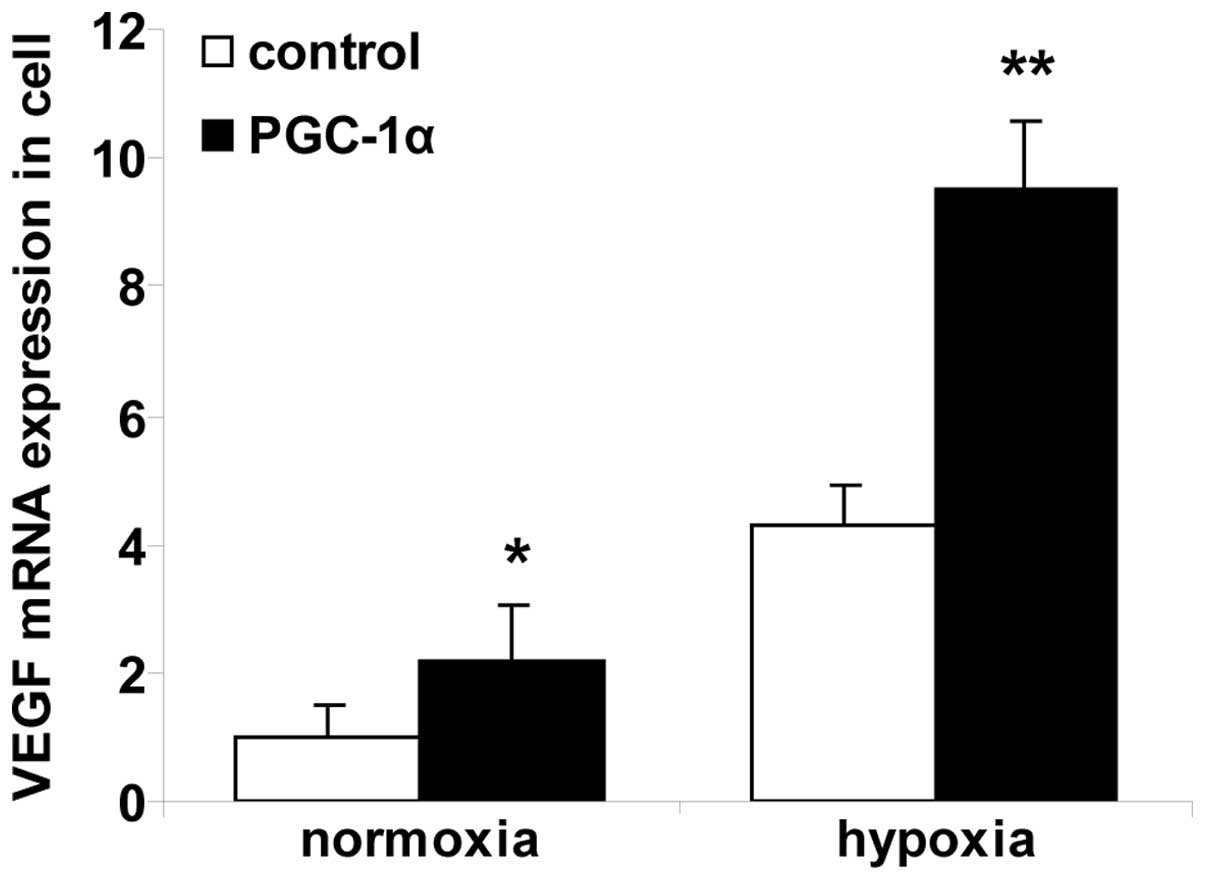
Alterations in VEGF mRNA expression in
hRVECs
RT-qPCR was used to measure the levels of VEGF mRNA
(Fig. 1). The expression of VEGF
mRNA was significantly upregulated in the cells in the normoxia
PGC-1α group compared with the cells in the normoxia control group
(P<0.01). Cells in the hypoxia control group expressed
significantly greater levels of VEGF mRNA compared with the cells
in the normoxia control group. The cells in the hypoxia PGC-1α
group expressed significantly greater levels of VEGF mRNA compared
with the cells in the hypoxia control group (P<0.01).
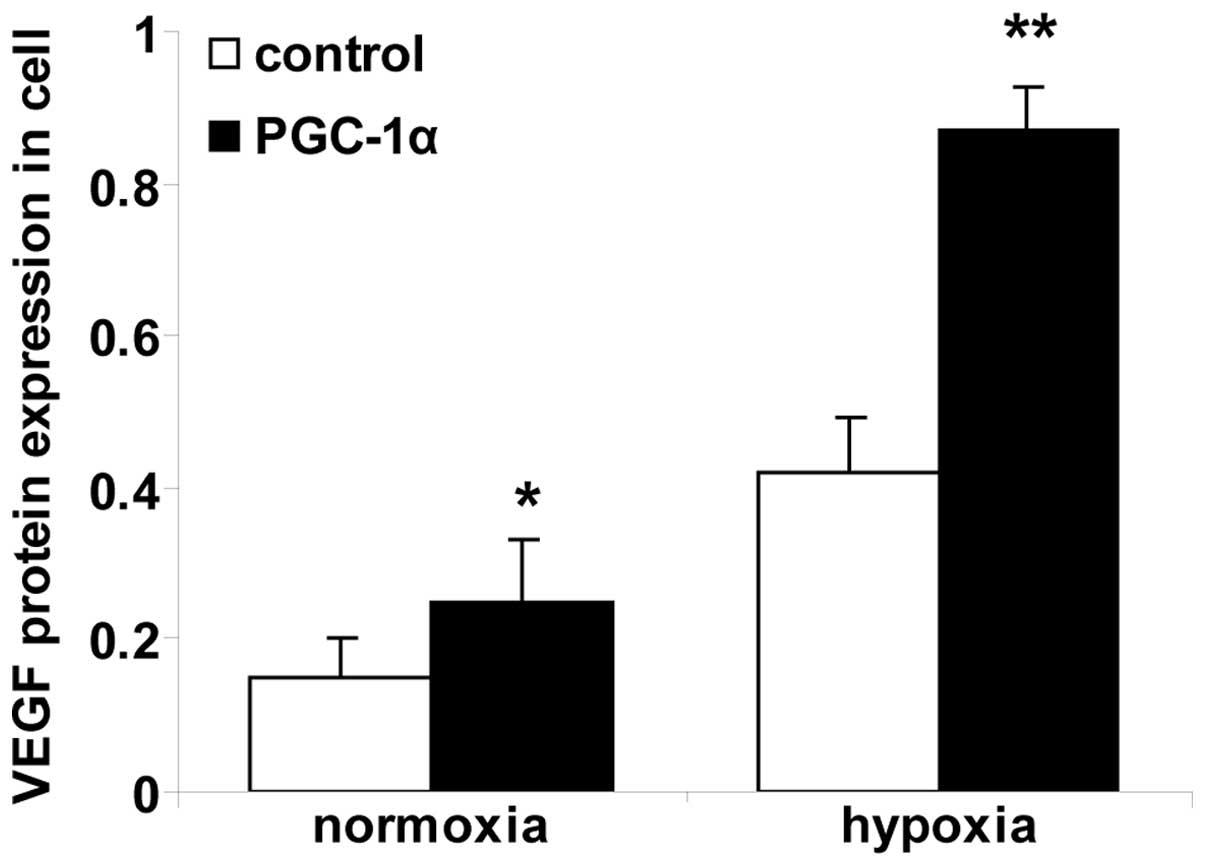
Alterations in VEGF protein levels in
hRVECs
ELISA was used to measure the VEGF protein levels
(Fig. 2). The levels of VEGF
protein were significantly greater in the cells in the normoxia
PGC-1α group compared with the cells in the normoxia control group
(P<0.01). Cells in the hypoxia control group expressed
significantly greater levels of VEGF protein compared with cells in
the normoxia control group. Cells in the hypoxia PGC-1α group
expressed significantly greater levels of VEGF protein compared
with the cells in the hypoxia control group (P<0.01).
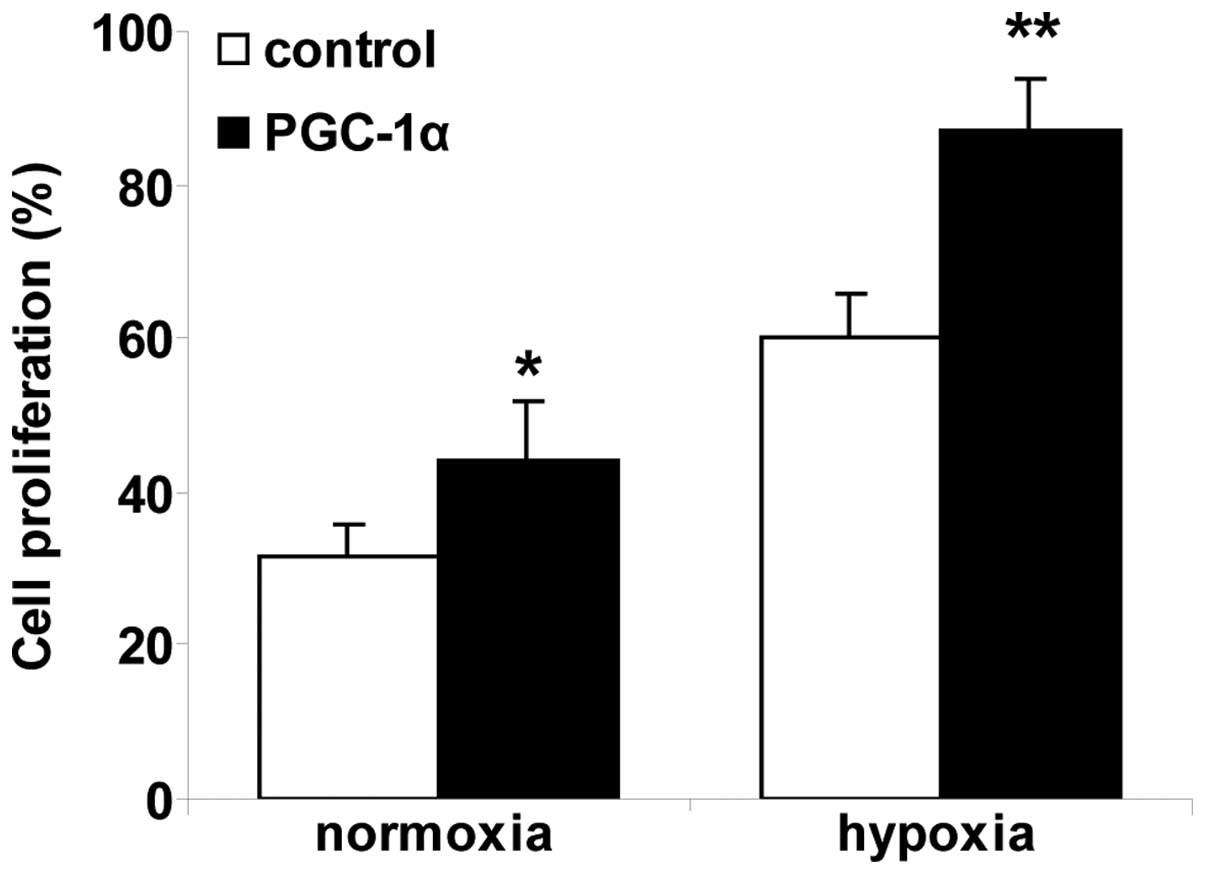
Recombinant PGC-1α promotes cell
proliferation in hRVECs
Endothelial cell proliferation is an essential step
in angiogenesis. To investigate the angiogenic activity of PGC-1α,
the effect of PCG-1α on cell proliferation in hRVECs was analyzed
(Fig. 3). The percentage of
BrdU-labeled cells was significantly increased in the normoxia
PGC-1α group compared with the normoxia control group (P<0.01).
Cell proliferation in the hypoxia control group was enhanced
significantly compared with the cells in the normoxia control
group. The percentage of BrdU-labeled cells was significantly
increased in the hypoxia PGC-1α group compared with the hypoxia
control group (P<0.01). Recombinant PGC-1α significantly
promoted cell proliferation in hRVECs under normoxic and hypoxic
conditions.
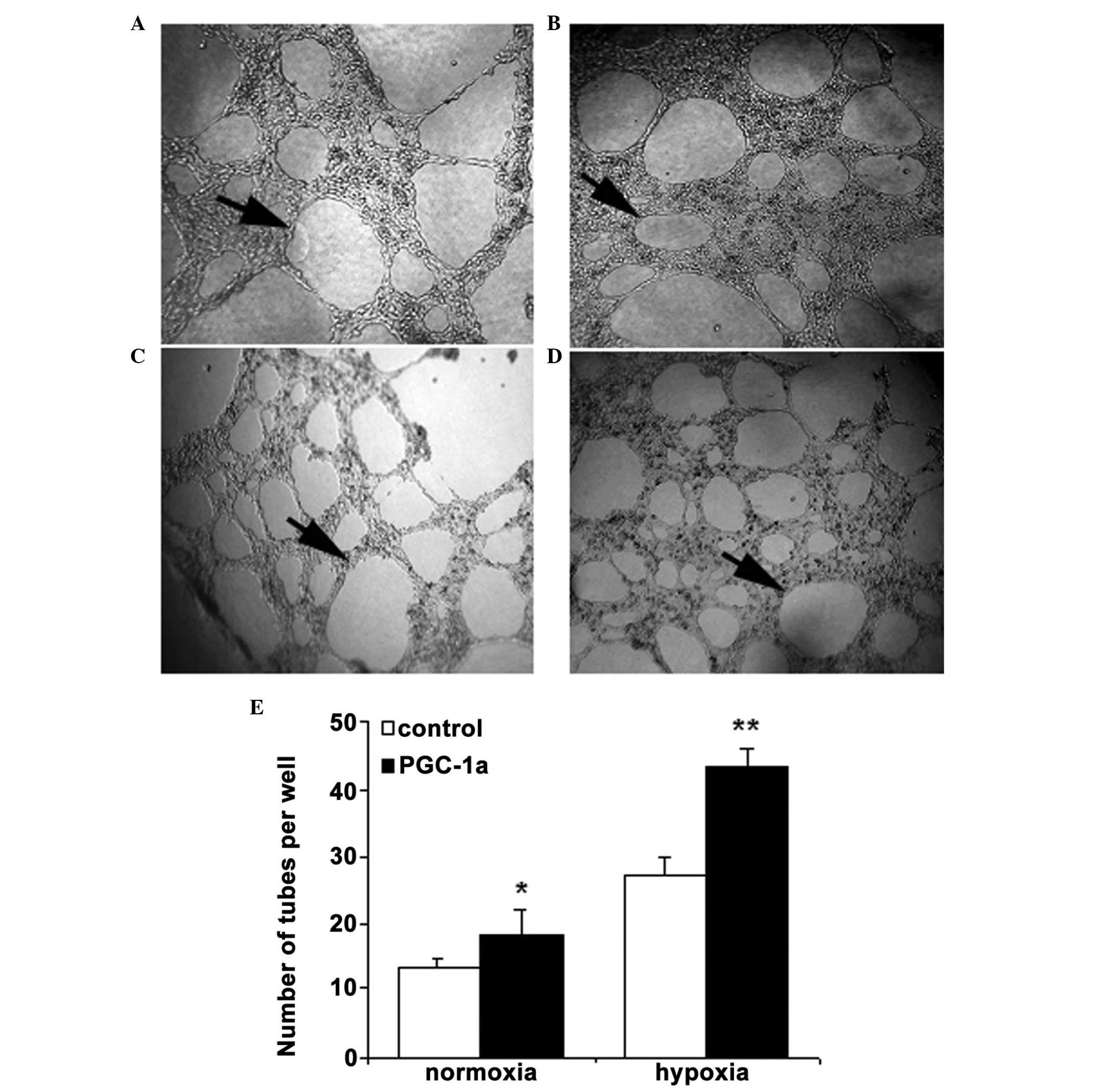
Recombinant PGC-1α promotes cell tube
formation in hRVECs
Following the culture of hRVECs in Matrigel-coated
plates, cells began forming tubes at 6 h, with the tubes appearing
to be stabilized by 24 h. Recombinant PGC-1α induced significantly
greater tube formation compared with PBS (18.2±2.8 and 13.5±1.2
tubes/well, respectively; P<0.01) under normoxic condition in
hRVECs. Similar results were obtained from the hypoxia PGC-1α group
and the hypoxia control group (43.6±2.5 and 27.1±3.7 tubes/well,
respectively; P<0.01). Cells in the hypoxia control group
exhibited markedly enhanced tube formation compared with the cells
in the normoxia control group. Taken together, these results
suggest that PGC-1α is able to directly upregulate angiogenic
activity in hRVECs (Fig. 4).
Effects of recombinant PGC-1α on retinal
NV
Following the exposure of P7 mice to hyperoxia, the
initial response of the retinal vasculature was reversible central
vasoconstriction followed by nonperfusion. Exposure of P7 mice to 5
days of 75% oxygen followed by a return to room air lead to
relative ischemia in the central retina. As a result of hypoxia,
the larger central radial vessels became tortuous and engorged. NV
at the junction between the vascularized and nonvascularized retina
then occurred., determined using retinal flat-mount angiography
using fluorescein dextran.
Following the observation that recombinant PGC-1α
induced VEGF expression and promoted cell proliferation and tube
formation in vitro, it was investigated whether recombinant
PGC-1α is able to induce NV in vivo. No mice used in the
current study developed signs of infection or retinal detachment.
The patterns of vascular development and NV were observed in
retinal flat-mounts following fluorescein-dextran perfusion
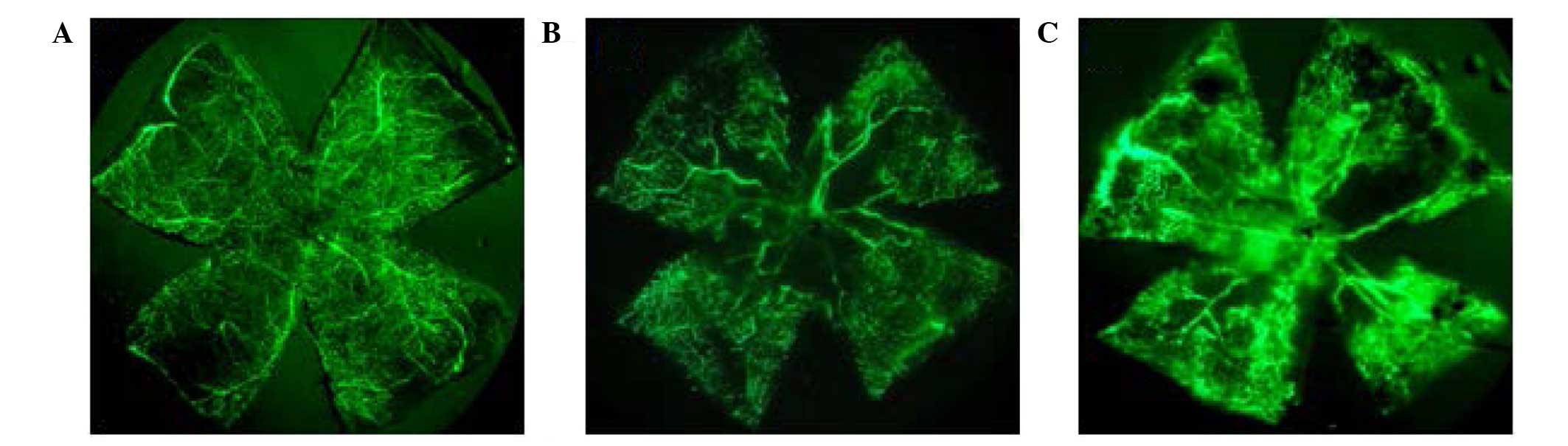
(Fig. 5). The retinas of P17 mice
from the normal group exhibited superficial and deep vascular
layers that extended from the optic nerve to the periphery. The
vessels formed a fine radial branching pattern in the superficial
retinal layer and a polygonal reticular pattern in the deep retinal
layer (Fig. 5A). The retinal
vascular pattern in mice exposed to hyperoxia was characterized by
a central nonperfused region and neovascular tufts (Fig. 5B). At P17, the results indicated
that in the OIR PGC-1α group, the neovascular tufts were increased
compared with the OIR control group, and fluorescein leakage was
additionally aggravated (Fig.
5C).
To further investigate the effect of recombinant
PGC-1α, examination of 5 µm paraffin-processed
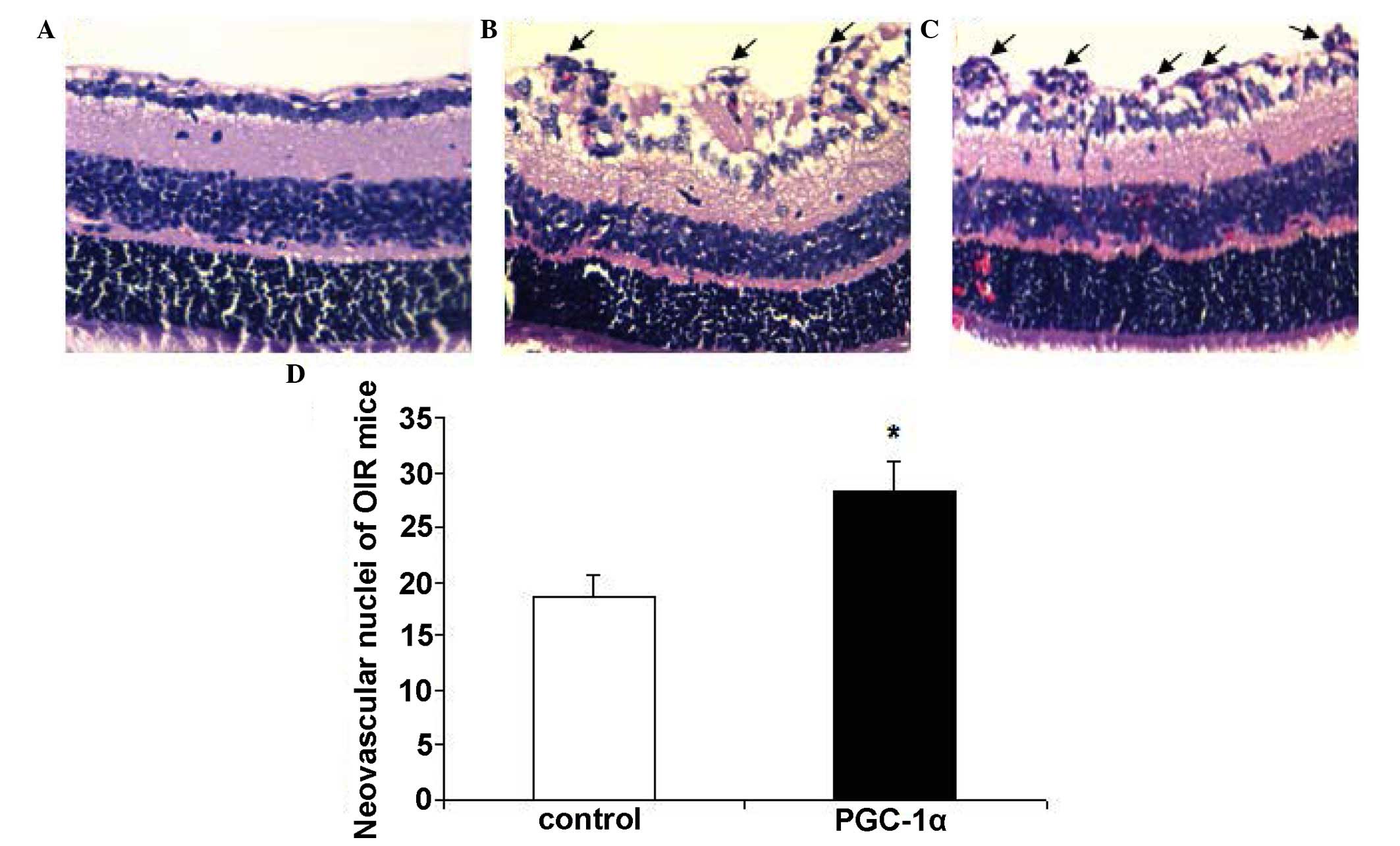
cross-sections of mouse eyes was conducted. Neovascular tufts are
defined as vascular cells extending beyond the internal limiting
membrane into the vitreous. The degree of NV was quantified in
cross-sections by counting the number of vascular cell nuclei on
the vitreal side of the internal limiting membrane. There were no
neovascular nuclei in the normal group (Fig. 6A). The mean number of nuclei per
cross-section in OIR PGC-1α group (28.2±2.9) was significantly
increased compared with the OIR control group (18.6±2.1);
(P<0.01; Fig. 6).
Regulation of the mRNA expression of VEGF
in the retina
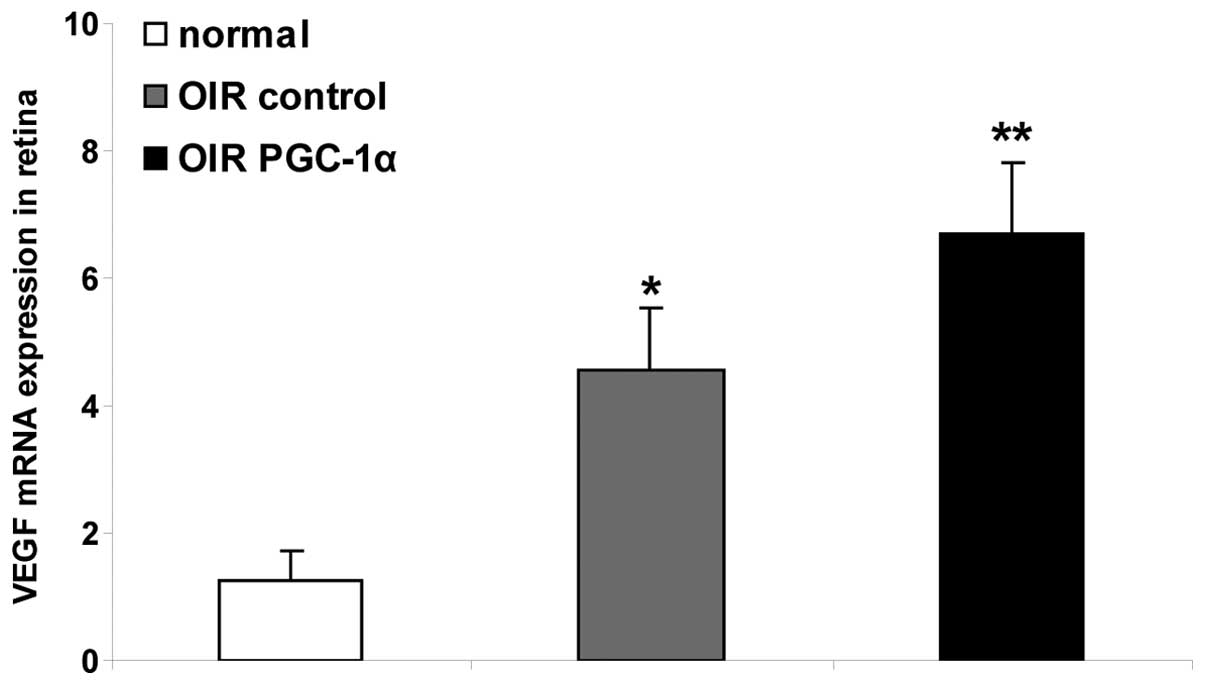
The results of the RT-qPCR analysis demonstrated
that the level of VEGF mRNA in the OIR control group retinas was
significantly upregulated, compared with that in the normal group
(P<0.01). Following the treatment with recombinant PGC-1α, the
retinas in the OIR PGC-1α group were observed to have significantly
higher mRNA expression levels of VEGF, compared with the levels in
the retinas in the OIR control group (P<0.01, Fig. 7).
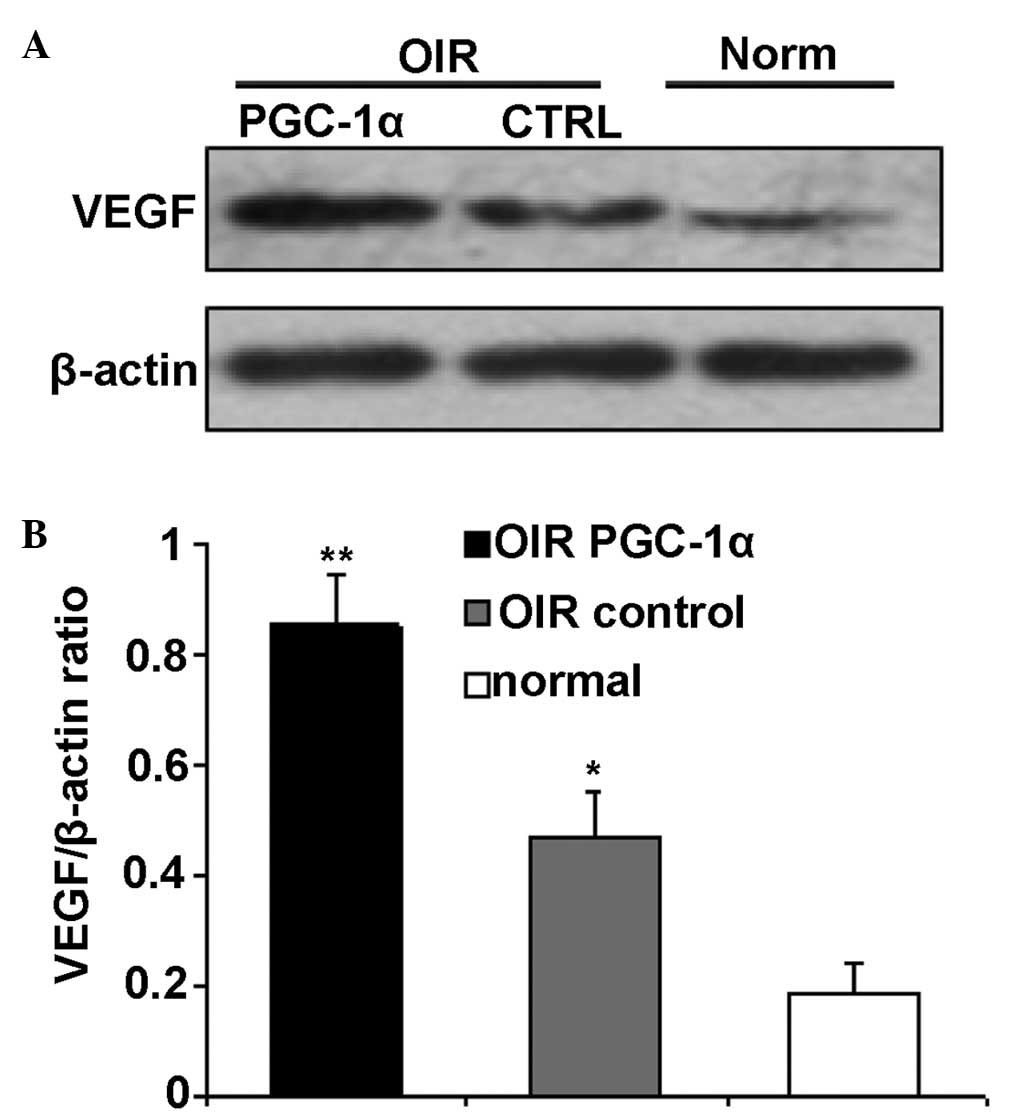
Western blot analysis in the retina
Retinal VEGF levels were measured by western blot
analysis. The VEGF protein levels in the OIR control group retinas
was significantly upregulated compared with the normal group
(P<0.01; Fig. 8). The protein
expression levels of VEGF were significantly increased in the OIR
PGC-1α group compared with the OIR control group (P<0.01;
Fig. 8).
Discussion
Retinal NV is the abnormal proliferation and
migration of new blood vessels from pre-existing vessels in the
retina. It occurs in several disease processes including
proliferative diabetic retinopathy, retinopathy of prematurity and
secondary neovascular glaucoma, and is a major cause of blindness.
Neoangiogenesis is the formation of new blood vessels from
pre-existing vessels, usually small veins, by sprouting, and occurs
in wound healing and additional pathological conditions. In the
current study, a mouse model of OIR was used to recapitulate
certain aspects of neovascularization in ocular diseases in humans.
OIR is characterized by vessel loss followed by vascular regrowth
and hypoxia-induced neovascularization (17).
During angiogenesis, vascular endothelial cells
secrete platelet-derived growth factor to stimulate the
proliferation of mesenchymal cells, promoting the maturation of the
vascular wall (19). Vascular
endothelial cells form a barrier between the vascular wall and the
blood, and injuries to endothelial cells lead to numerous vascular
diseases (19). In wound healing,
inflammation and cancer, endothelial cells proliferate and form new
blood vessels. The abnormal growth of new vessels results in
vessels which are very fragile, that leak and are susceptible to
hemorrhage. The resultant hemorrhage or accumulation of blood in
ocular cavities, such as in the vitreous, leads to the further
blockade of light transportation and a reduction in visual acuity.
For these reasons, retinal vascular endothelial cells represent an
important research tool in the in vitro investigation of
angiogenesis-associated ocular diseases.
PGC-1α is a transcriptional coactivator identified
as an upstream regulator of lipid catabolism, mitochondrial number
and function (20). Consistent
with its emerging role as a central regulator of energy metabolism,
PGC-1α is abundantly expressed in tissues with high metabolic
rates. PGC-1α is a potent modulator of oxidative metabolism in
numerous settings (21). In
particular, PGC-1α regulates oxidative phosphorylation,
mitochondrial biogenesis and respiration (22,23).
Numerous previous studies have suggested that ischemia and hypoxia
significantly induce the expression of PGC-1α (5–11,24,25).
Arany et al (12) reported
that PGC-1α was able to upregulate the expression of angiogenic
factors such as VEGF, and promote the formation of new blood
vessels in skeletal muscle. In the current study, hRVECs were
cultured in a hypoxic environment to simulate hypoxia in
vivo. hRVECs cultured in the hypoxic environment grew well and
exhibited no overt cell death, suggesting that hypoxia did not
affect the growth or survival of endothelial cells. The expression
of VEGF was significantly upregulated at the mRNA and protein
levels under hypoxic conditions in hRVECs and in OIR mice,
confirming that hypoxia is able to upregulate the expression of
VEGF in vitro and in vivo.
In order to investigate the regulatory effects of
PGC-1α on VEGF in vitro and in vivo, hRVECs and OIR
mice were treated with recombinant PGC-1α. Following treatment with
recombinant PGC-1α, VEGF mRNA and protein expression levels were
significantly increased, indicating that PGC-1α regulates VEGF
expression in hRVECs and mice. VEGF is a specific mitogen for
endothelial cells and is a key inducer of angiogenesis. It induces
the migration and proliferation of endothelial cells and increases
the permeability of the endothelium (26). VEGF acts directly on vascular
endothelial cells to increase vascular permeability, leading to the
extravasation of fibrin, which forms a fibrin gel with fibronectin
and serves as a temporary matrix for the migration and invasion of
fibroblasts, endothelial cells and additional cells. These cells
are subsequently incorporated into the vasculature (27). In the current study, the cell
proliferation assay demonstrated that hypoxia increased the
proliferation of hRVECs via the stimulation of VEGF expression.
Following treatment with recombinant PGC-1α, VEGF expression in
hRVECs was upregulated, followed by an enhancement of cell
proliferation. Therefore, recombinant PGC-1α may promote cellular
proliferation in hRVECs.
To further investigate the molecular mechanisms of
recombinant PGC-1α responsible for the reinforcement of angiogenic
activity in hRVECs, the potential effect of PGC-1α on angiogenic
activity in vitro was investigated by tube formation assays.
This assay mimics numerous key steps of the angiogenic process,
including endothelial cell adhesion, migration, differentiation and
growth (28). In the current
study, Matrigel coating provided the necessary extracellular matrix
for human endothelial cells to form a network of tubes. The
endothelial cells began to form tubes at 6 h following addition to
the Matrigel-coated plates, with the tubes becoming stable by 24 h.
The endothelial cells formed tubes in normoxic and hypoxic
environments, however greater numbers of tubes formed in the
hypoxic environment, suggesting that hypoxia is able to promote
tube formation. A previous study indicated that PGC-1α promotes
blood vessel formation by increasing the expression of VEGF
(12), therefore, the current
study investigated whether PGC-1α is able to increase tube
formation. Cells treated with recombinant PGC-1α under normoxic and
hypoxic conditions formed greater numbers of tubes compared with
their corresponding control PBS treated cells, suggesting that
recombinant PGC-1α upregulates VEGF, thereby increasing tube
formation. Therefore, the in vitro experiments suggest that
recombinant PGC-1α is able to promote angiogenesis.
In a previous study, PGC-1α-knockout mice subjected
to oxygen-induced retinopathy exhibited reduced expression of VEGFA
and were protected against pathological revascularization (29). As demonstrated in the present
study, recombinant PGC-1α was able to induce cell proliferation and
tube formation in hRVECs, which indicates its potential for
promoting retinal neovascularization. This was demonstrated by
overexpression of PGC-1α in vivo in the mouse model of OIR
with retinal neovascularization. From the in vivo
experiments, the current study indicated that the intravitreal
injection of recombinant PGC-1α is able to increase the neovascular
tufts in the retina, and specific protein levels were consistently
greater in PGC-1α-treated eyes. Furthermore, treatment with
recombinant PGC-1α exacerbated retinopathy by increasing the
invasion of new vessels beyond the inner-limiting membrane of the
retina according to the analysis of eye sections, and aggravating
fluorescein leakage according to the angiography of retinas. The
data presented here demonstrates that recombinant PGC-1α is able to
increase the expression of VEGF through the PGC-1α-VEGF pathway,
thereby promoting retinal neovascularization.
In conclusion, recombinant PGC-1α is able to
increase the expression of VEGF in hRVECs and retinas, thereby
promoting cellular proliferation, tube formation and retinal
neovascularization, suggesting an important role of PGC-1α in
regulating vascular growth. This indicates that PGC-1α may be
considered as a potential anti-angiogenic target in retinal
neovascularization.
Acknowledgments
The current study was supported by the National
Natural Science Fundation of China (grant no. 81000387) and the
Ph.D. Programs Foundation of the Ministry of Education of China
(grant no. 20100162120050).
References
|
1
|
Puigserver P, Wu Z, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Larrouy D, Vidal H, Andreelli F, Laville M
and Langin D: Cloning and mRNA tissue distribution of human
PPARgamma coactivator-1. Int J Obes Relat Metab Disord.
23:1327–1332. 1999. View Article : Google Scholar
|
|
3
|
Valle I, Alvarez-Barrientos A, Arza E,
Lamas S and Monsalve M: PGC-1α regulates the mitochondrial
antioxidant defense system in vascular endothelial cells.
Cardiovasc Res. 66:562–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borniquel S, Valle I, Cadenas S, Lamas S
and Monsalve M: Nitric oxide regulates mitochondrial oxidative
stress protection via the transcriptional coactivator PGC-1alpha.
FASEB J. 20:1889–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barger PM, Browning AC, Garner AN and
Kelly DP: p38 mitogen-activated protein kinase activates peroxisome
proliferator-activated receptor α: A potential role in the cardiac
metabolic stress response. J Biol Chem. 276:44495–44501. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mascareno E, Manukyan I, Das DK and
Siddiqui MA: Downregulation of cardiac lineage protein (CLP-1)
expression in CLP-1 +/− mice affords cardioprotection against
ischemic stress. J Cell Mol Med. 13:2744–2753. 2009. View Article : Google Scholar
|
|
7
|
Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ,
Liou CW and Chuang YC: Protective effects of peroxisome
proliferator-activated receptors gamma coactivator-1 alpha against
neuronal cell death in the hippocampal CA1 subfield after transient
global ischemia. J Neurosci Res. 88:605–613. 2010. View Article : Google Scholar
|
|
8
|
Gutsaeva DR, Carraway MS, Suliman HB,
Demchenko IT, Shitara H, Yonekawa H and Piantadosi CA: Transient
hypoxia stimulates mitochondrial biogenesis in brain subcortex by a
neuronal nitric oxide synthase-dependent mechanism. J Neurosci.
28:2015–2024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasbach KA and Schnellmann RG: Signaling
of mitochondrial biogenesis following oxidant injury. J Biol Chem.
282:2355–2362. 2007. View Article : Google Scholar
|
|
10
|
Yamaguchi T, Omori M, Tanaka N and Fukui
N: Distinct and additive effects of sodium bicarbonate and
continuous mild heat stress on fiber type shift via
calcineurin/NFAT pathway in human skeletal myoblasts. Am J Physiol
Cell Physiol. 305:C323–C333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi T, Suzuki T, Arai H, Tanabe S
and Atomi Y: Continuous mild heat stress induces differentiation of
mammalian myoblasts, shifting fiber type from fast to slow. Am J
Physiol Cell Physiol. 298:C140–C148. 2010. View Article : Google Scholar
|
|
12
|
Arany Z, Foo SY, Ma Y, Ruas JL,
Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J,
Rangwala SM, et al: HIF-independent regulation of VEGF and
angiogenesis by the transcriptional coactivator PGC-1alpha. Nature.
451:1008–1012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmeliet P and Baes M: Metabolism and
therapeutic angiogenesis. N Engl J Med. 358:2511–2512. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gariano RF and Gardner TW: Retinal
angiogenesis in development and disease. Nature. 438:960–966. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu DY and Cringle SJ: Oxygen distribution
and consumption within the retina in vascularised and avascular
retinas and in animal models of retinal disease. Prog Retin Eye
Res. 20:175–208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bucolo C, Melilli B, Piazza C, Zurria M
and Drago F: Ocular pharmacokinetics profile of different
indomethacin topical formulations. J Ocul Pharmacol Ther.
27:571–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–110.
1994.PubMed/NCBI
|
|
18
|
Jiang J, Xia XB, Xu HZ, Xiong Y, Song WT,
Xiong SQ and Li Y: Inhibition of retinal neovascularization by gene
transfer of small interfering RNA targeting HIF-1alpha and VEGF. J
Cell Physiol. 218:66–74. 2009. View Article : Google Scholar
|
|
19
|
Saint-Geniez M and D'Amore PA: Development
and pathology of the hyaloid, choroidal and retinal vasculature.
Int J Dev Biol. 48:1045–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suwa M, Nakano H and Kumagai S: Effects of
chronic AICAR treatment on fiber composition, enzyme activity,
UCP3, and PGC-1 in rat muscles. J Appl Physiol. 95:960–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Handschin C and Spiegelman BM:
Metabolic control through the PGC-1 family of transcription
coactivators. Cell Metab. 1:361–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and
Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and
respiration through the thermogenic coactivator PGC-1. Cell.
98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
St-Pierre J, Lin J, Krauss S, Tarr PT,
Yang R, Newgard CB and Spiegelman BM: Bioenergetic analysis of
peroxisome proliferator-activated receptor gamma coactivators
1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol
Chem. 278:26597–26603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaillou T, Koulmann N, Meunier A, Chapot
R, Serrurier B, Beaudry M and Bigard X: Effect of hypoxia exposure
on the recovery of skeletal muscle phenotype during regeneration.
Mol Cell Biochem. 390:31–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao J, Li J, Liu Y, Lu P, Sun X, Sugumaran
PK and Zhu D: The key role of PGC-1α in mitochondrial biogenesis
and the proliferation of pulmonary artery vascular smooth muscle
cells at an early stage of hypoxic exposure. Mol Cell Biochem.
367:9–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrara N: VEGF as a therapeutic target in
cancer. Oncology. 69:11–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arnaoutova I, George J, Kleinman HK and
Benton G: The endothelial cell tube formation assay on basement
membrane turns 20: State of the science and the art. Angiogenesis.
12:267–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saint-Geniez M, Jiang A, Abend S, Liu L,
Sweigard H, Connor KM and Arany Z: PGC-1α regulates normal and
pathological angiogenesis in the retina. Am J Pathol. 182:255–265.
2013. View Article : Google Scholar :
|