Introduction
Obesity can be defined not only as excess body
weight, but also as an increased accumulation of adipose tissue.
Obesity is a risk factor for chronic diseases, including
hypertension, coronary heart disease, stroke, obstructive sleep
apnea, asthma, hyperlipidemia and type 2 diabetes (1,2).
Current strategies for obesity management include diet, exercise,
drug therapy and bariatric surgery, either alone or in combination.
Anti-obesity drugs are designed to suppress appetite and reduce fat
absorption (1,3). However, these drugs show adverse
effects, including tachycardia, hypertension, arrhythmias,
steatorrhea, fecal urgency and incontinence (4). Due to these undesirable effects of
anti-obesity drugs, particularly the life-threatening safety
concerns of the centrally acting agents, there is an urgent
requirement to identify effective, safe and well-tolerated
anti-obesity drugs. One strategy for weight loss is to induce brown
adipocyte tissue in adult humans. As brown adipocyte tissue
generates heat from fatty acids and increases the breakdown of fat
in the body, brown adipocyte tissue is considered a promising
target to combat obesity (5).
Diabetes mellitus is often associated with obesity,
and its incidence is increasing (6). Diabetes results from a defect of
insulin secretion and/or insulin action, and thaiazolidinediones
(TZDs), including rosiglitazone and pioglitazone, are a class of
anti-diabetic drugs, which work as insulin sensitizers (7,8).
They usually promote adipocyte differentiation and encourage
insulin sensitivity by stimulating the transcriptional activity of
peroxisome proliferator-activated receptor (PPAR)-γ (9,10).
However, its side effects include weight gain, edema with worsening
of cardiac failure, liver toxicity and anemia (11–13),
which has resulted in the withdrawal of certain TZDs from the
market. Therefore, there is a requirement to develop novel
anti-diabetic drugs.
The adipocyte is a central site for the regulation
of energy storage and metabolism (14). Adipocyte differentiation is
triggered by signaling molecules, which induce the conversion of
adipose-derived stem cells (ASCs) to pre-adipocytes, which finally
differentiate into adipocytes. Following a library screen in a
preliminary study, a novel regulator of ASC differentiation and
glucose uptake was identified from the fern Cyclosorus
terminans. Although its traditional use has not been described,
this plant is usually consumed as a vegetable in northern Thailand
(15). Bioactive interruptins A
and B from C. terminans, which exhibit antibacterial,
anticancer and reactive oxygen species (ROS)-scavenging activities,
were identified in our previous study (16). The present study aimed to
investigate whether interruptin B isolated from C. terminans
induces brown adipocyte differentiation of ASCs, whether
interruptin B induces glucose uptake by ASCs, and investigate the
potential mechanism underlying the action of interruptin B in
regulating ASC development. The results from the present study aim
to provide a novel scientific basis to support the use of the
edible vegetable, C. terminans, as a medicinal plant, and to
justify applications of interruptin B for the treatment of obesity
and diabetes.
Materials and methods
Materials
Interruptin B (0.003% w/w) from the fern
Cyclosorus terminans (J. Sm. ex Hook.) Panigrahi, of the
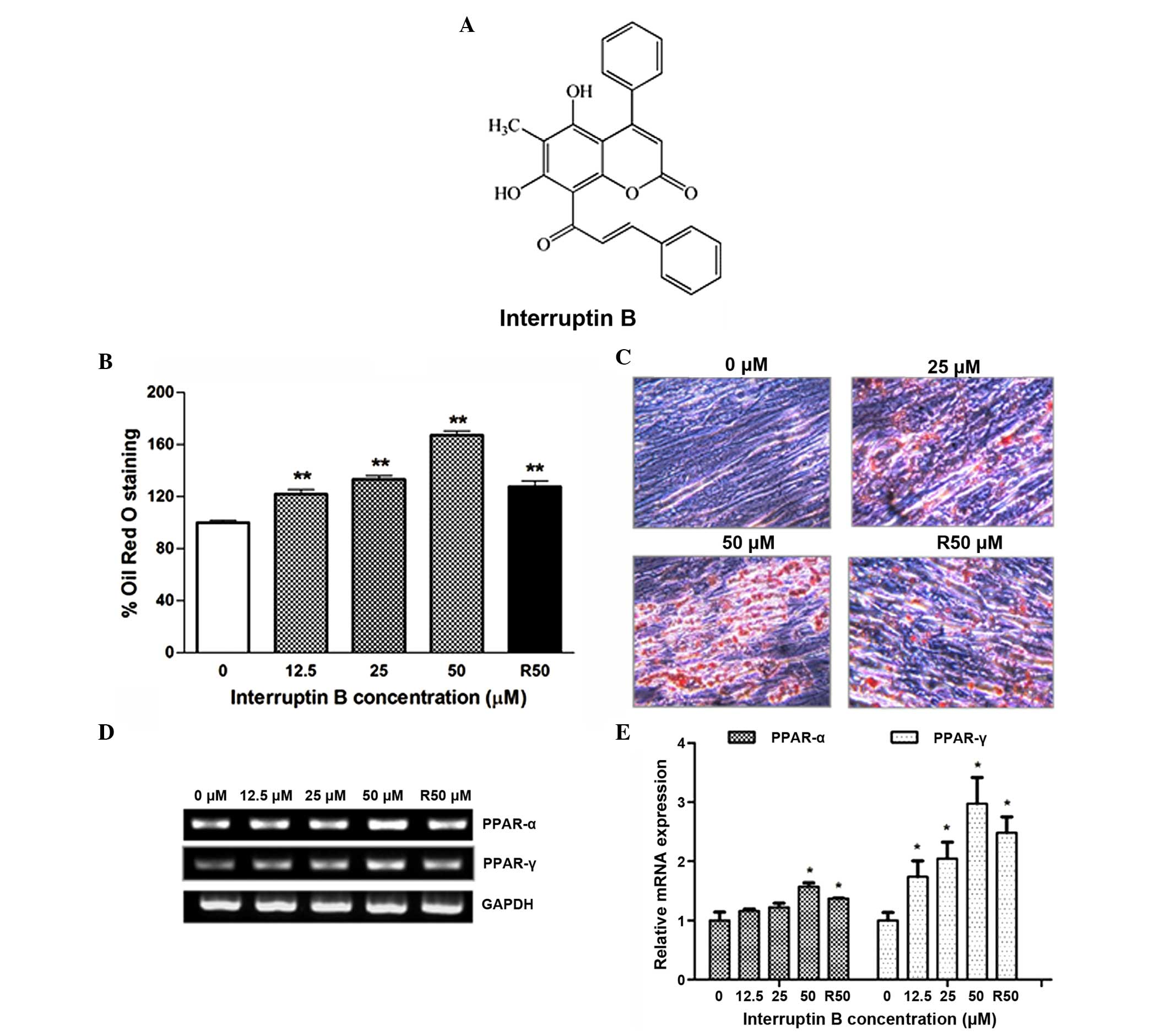
family Thelypteridaceae (Fig. 1A)
was purified by open column chromatography over silica gel, using
the same step gradient of n-hexane with increasing
concentrations of dichloromethane, followed by dichloromethane with
increasing concentrations of ethyl acetate, and then ethyl acetate
with increasing concentrations of methanol, as described in our
previous report (16). The plant
was collected from a forested area at the Prince of Songkla
University (PSU; Songkhla, Thailand) in April 2010 by Miss.
Arpaporn Kaewchoothong and was identified by Professor Thaweesakdi
Boonkerd (Chulalongkorn University, Bankok, Thailand). The voucher
specimen (identification no. SKP 208 03 20 001) is now stored in
the herbarium of the Faculty of Pharmaceutical Sciences, PSU. The
purity of the isolated interruptin B was determined from nuclear
magnetic resonance spectra and high-performance liquid
chromatography data, which revealed no significant impurities.
Rosiglitazone and bisphenol A diglycidyl ether (BADGE) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). GW6471 was
purchased from Tocris Bioscience (Ellisville, MO, USA). All other
chemicals were of reagent grade and obtained from Sigma-Aldrich,
iNtRON Biotechnology (Seoul, South Korea) or Molecular Probes
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
In addition, α-minimum essential medium (α-MEM) was
purchased from Thermo Fisher Scientific, Inc., and fetal bovine
serum (FBS) and penicillin-streptomycin were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Differentiation media and
supplements were purchased from Lonza (Walkerville, MD, USA).
ASC culture
Human ASCs were isolated via the liposuction of
subcutaneous fat, as described previously (17), following the provision of written
informed consent, at Bundang CHA Hospital (Seoul, Korea;
BD2011-152D) and approval by the ethics committee of CHA University
(Pocheon, South Korea). The human ASCs were cultured in α-MEM
supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C
under a humidified 5% CO2 atmosphere.
Adipocyte differentiation
For adipocyte differentiation, the ASCs were plated
in 6-well plates (4×104) and grown in α-MEM containing
10% FBS for 3 days, following which the medium was replaced with
pre-differentiation medium (pre-adipocyte growth medium-2; PGM-2)
comprising pre-adipocyte basal medium-2 supplemented with FBS,
L-glutamine and GA-1000. The cells were further cultured for 6–7
days until reaching confluence. In the subsequent adipogenesis
experiments, the ASCs were treated for 4 days with various
concentrations of interruptin B (0, 12.5, 25 or 50 µM) in
pre-adipocyte differentiation medium-2 (PDM-2) comprising PGM-2
supplemented with h-insulin, dexamethasone,
3-isobutyl-1-methylxanthine and indomethacin. Dimethyl sulfoxide
(DMSO; 0.1%) and rosiglitazone (50 µM) served as negative
and positive controls, respectively.
Oil Red O staining
Following the induction of differentiation of the
ASCs for 4 days, the cells were fixed with 10% formaldehyde (Junsei
Chemical Co., Ltd., Toyko, Japan) in phosphate-buffered saline
(PBS, iNtRON Biotechnology, Seoul, Korea) for 1 h at room
temperature, washed three times with PBS and stained with filtered
Oil Red O (Sigma-Aldrich) solution (0.5% Oil Red O-isopropyl
alcohol: H2O; 3:2, v/v) for 2 h. Following three washes
with distilled water, images of the cells were captured under a
microscope. Lipid was extracted with isopropanol (Sigma-Aldrich)
for 30 min, and the concentration was determined using a microplate
reader (Tecan GmbH, Grodig, Austria) at a wavelength of 492 nm.
Glucose consumption assay
The confluent ASCs were treated with interruptin B
(0, 25 or 50 µM) and rosiglitazone (50 µM) in PDM-2
medium for 4 days. The conditioned medium from the differentiated
ASCs was then removed and assayed for glucose content using a
Glucose Colorimetric Assay kit II, (cat. no. 686–100, BioVision,
Inc., Milpitas, CA, USA).
RNA analysis
The total RNA from the differentiated ASCs treated
with varying concentrations of interruptin B (0, 12.5, 25 or 50
µM) was isolated using an Easy-spin™ (DNA-free) Total RNA
Extraction kit (iNtRON Biotechnology). Subsequently, 500 ng total
RNA from each sample was reverse-transcribed to cDNA using 100 ng
Oligo(dt) 15 Primer (Promega Corporation, Madison, WI, USA) and a
reverse transcription system (Promega Corporation), in accordance
with the manufacturer's protocol. Semi-quantitative polymerase
chain reaction (PCR) analysis was performed to visualize and
compare the expression levels of the genes associated with
adipogenesis. PCR was performed using Solg™ 2X Taq PCR-Pre-Mix
(SolGent Co., Ltd., Daejeon, South Korea) in a total volume of 30
µl for PCR amplification of cDNA, which was
reverse-transcribed from 500 ng total RNA. PCR amplification was
performed for 35 cycles with denaturation at 94°C for 20 sec,
annealing at 54–58°C for 40 sec and polymerization at 72°C for 20
sec, followed by a final extension at 72°C for another 10 min, and
cooled to 4°C. The primer sequences (Bioneer Corporation, Daejeon,
South Korea) used for PCR amplification are listed in Table I. The PCR products were
electrophoresed on 1.5% agarose gels (Qiagen GmbH, Hilden,
Germany), stained with 0.5 µg/ml ethidium bromide
(Sigma-Aldrich), and images were captured using Gel Doc (Bio-Rad
Laboratories, Inc., Milan, Italy). The densitometric analysis of
the bands was determined using Image Lab software (version 2.2.4.0;
MCM Design, Hillerød, Denmark) in order to compare the mRNA
expression levels among samples, using GADPH as the standard gene.
All quantification was performed in triplicate, and compared with
DMSO (0.1%) and rosiglitazone (50 µM).
 | Table IPrimers used for polymerase chain
reaction amplification. |
Table I
Primers used for polymerase chain
reaction amplification.
| Primer | Sequence |
|---|
| PPAR-α_F |
5′-CTGAGCCATGCAGAATTTAC-3′ |
| PPAR-α_R |
5′-TAACAGTTCCCTGAAGAGCA-3′ |
| PPAR-γ_F |
5′-TGGAATTAGATGACAGCGACTTGG-3′ |
| PPAR-γ_R |
5′-CTGGAGCAGCTTGGCAAACA-3′ |
| C/EBP-β_F |
5′-GTTCATGCAACGCCTGGTG-3′ |
| C/EBP-β_R |
5′-AAGCAGTCCGCCTCGTAGTAGAAG-3′ |
| UCP-1_F |
5′-GTGTGCCCAACTGTGCAATG-3′ |
| UCP-1_R |
5′-CCAGGATCCAAGTCGCAAGA-3′ |
| CPT1B_F |
5′-AAACAGTGCCAGGCGGTC-3′ |
| CPT1B_R |
5′-CGTCTGCCAACGCCTTG-3′ |
| COX-2_F |
5′-CCCGCAGTACAGAAAGTATC-3′ |
| COX-2_R |
5′-CCATAGAGTGCTTCCAACTC-3′ |
| GLUT-1_F |
5′-ATCCCTGTTACCCAGAGAAT-3′ |
| GLUT-1_R |
5′-TTCAGGCACATAACCTCTTT-3′ |
| GLUT-4_F |
5′-GAATACCTTCTTCGCTGCTA-3′ |
| GLUT-4_R |
5′-TGGATTTCTTGTCTCCTGTC-3′ |
| GAPDH_F |
5′-CGAGATCCCTCCAAAATCAA-3′ |
| GAPDH_R |
5′-TGTGGTCATGAGTCCTTCCA-3′ |
MitoTracker Red staining
The fluorescence signal of MitoTracker Red was used
as an indicator of mitochondrial number. The differentiated cells
grown on coverslips were incubated with media containing 5% FBS and
200 nM MitoTracker Red CMXRos (Molecular Probes; Thermo Fisher
Scientific, Inc.) for 20 min in a 5% CO2 incubator at
37°C, followed by two washes in PBS. The cells were subsequently
fixed with 4% paraformaldehyde (Bio Basic Canada, Inc., Markham,
ON, Canada) for 15 min, following which the cells were
permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) in PBS for 5
min, and nuclei were stained with 4′,6-diamidino-2-phenyl-indole
(Roche Diagnostics, Indianapolis, IN, USA) at room temperature for
an additional 10 min. Immediately following a final wash with 0.1%
Triton X-100 in PBS, images of the cells were captured under a
Nikon microscope (ECLIPSE E600; Nikon Instruments, Melville, NY,
USA).
Analysis of mitochondria membrane
potential
The mitochondrial membrane potential was measured by
means of 3,3′dihexyloxacarbocyanine iodide (DiOC6 3)
staining (Molecular Probes; Thermo Fisher Scientific, Inc.). The
differentiated cells were harvested, washed three times in
serum-free media and incubated in 200 nM DiOC6 (3)-containing media at 37°C for 20 min.
For each sample, at least 10,000 cells were analyzed for
corresponding fluorescence on a flow cytometer (BD FACSCalibur™; BD
Biosciences, Franklin Lakes, NJ, USA) using the FL1-H channel.
Computational analysis
Interruptin B was independently docked into the
ligand-binding domain (LBD) of PPAR-α (PDB ID: 1I7G) and PPAR-γ
(PDB ID: 4EMA). Ciglitazone, pioglitazone and rosiglitazone were
used as positive controls for the PPAR-γ ligands, according to
standard procedure (18). Docking
was performed using the AutoDock4.2 program suite (19,20)
from Scripps Research Institute (La Jolla, CA, USA). This program
uses a Larmarckian genetic algorithm for the docking of flexible
ligands into protein binding sites, in order to determine the full
range of potential ligand conformers within a rigid protein target.
The AutoDock run parameters used for all of the docking were a
population size, of 150 and an increase in the maximum number of
energy evaluations to 10,000,000 per run. All other run parameters
remained at their default settings. A total of 100 genetic
algorithm runs were performed for each docking, and the final
docked conformations were clustered using a tolerance of 2 Å root
mean square deviation (RMSD).
Transfection and luciferase assay
HepG2 cells [5×105; American Type Culture
Collection (ATCC), Manassas, VA, USA] were seeded onto 12-well
plates and transfected with lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and pGL3 Basic Vector (Promega
Corporation) containing a PPAR response element (PPRE). In all
cases, a β-galactosidase (β-gal) expression plasmid was transfected
to control transfection efficiency. At 24 h post-transfection, the
cells were treated with rosiglitazone (10 µM) or interruptin
B (10 µM) for another 24 h at 37°C. The cells were then
washed twice with PBS and harvested using lysis buffer (Promega
Corporation). Luciferase activity was determined using a Luciferase
Assay system (Promega Corporation) and quantified using the Glomax
9101–002 system (Promega Corporation), according to the
manufacturer's protocol. Luciferase activity was expressed as the
fold induction, which was normalized by β-gal activity. Each
condition was performed in triplicate.
PPAR ligand-binding activity assay
pcDNA3 vectors (Invitrogen; Thermo Fisher
Scientific, Inc.) encoding HA-PPARα and HA-PPARγ were transfected
into HEK 293 cells (2×106/100 mm; ATCC) for 24 h at
37°C, and the cells were lysed with lysis buffer containing 50 mM
HEPES, 150 mM NaCl, 1 mM EDTA, 10% glycerol, Triton X-100, 12 mM
β-glycerophosphate, 10 mM NaF, 1 mM NaOV3, 5 mg/ml
aprotinin and 1 mM PMSF (pH 7.6). Protein extracts were
subsequently incubated with anti-HA agarose beads (Sigma-Aldrich)
and washed with lysis buffer at 4°C. Proteins were eluted using 0.1
M glycine (pH 2.5; Sigma-Aldrich) and immediately neutralized with
1 M Tris-HCl (Bio Basic Canada, Inc.). The proteins were then
dialyzed against PBS containing 20% glycerol and stored at
−70°C.
To analyze the interactions of interruptin B with
binding affinities to myc-PPARα and myc-PPARγ, the surface plasmon
resonance (SPR) technology of the SR7500DC system (Reichart
Technologies, Buffalo, NY, USA) was used. The SPR experiment was
performed according to the manufacturer's protocol. Protein
quantitation was performed using a Bradford assay (Bio Rad
Laboratories, Inc.), according to the manufacturer's instructions,
with 0.6 µg protein diluted in PBS. The proteins diluted in
PBS were immobilized on a CMDH chip (Reichart Technologies), and
interruptin B was loaded in a dose-dependent manner (31.25, 62.5,
125, 250 and 500 µM) for the SPR assay. The equilibrium
dissociation constant (KD) value was determined using
the Scrubber2 program (Informer Technologies, Inc., Dallas, TX,
USA).
Inhibition experiments
The roles of PPAR-α and PPAR-γ were determined by
measuring interruptin B-induced adipogenesis and glucose uptake in
the presence or absence of PPAR-α and PPAR-γ antagonists. The
confluent ASCs were pretreated with 50 and 100 µM GW6471
(PPAR-α inhibitor; Tocris Bioscience) or BADGE (PPAR-γ inhibitor;
Sigma-Aldrich) for 2 h. Subsequently, the media were replaced with
PDM-2, comprised of specific inhibitors and 50 µM
interruptin B. Lipid accumulation and glucose uptake were examined
following culture for 4 days at 37°C.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Results were analyzed and presented using GraphPad
Prism® version 6.00 (GraphPad Software, San Diego, CA,
USA). Comparisons between two groups were performed using Student's
t-test and P<0.05 was considered to indicate a statistically
significant difference.
Results
Interruptin B enhances adipocyte
differentiation
To investigate the effect of interruptin B on
adipocyte differentiation, confluent ASCs were treated with various
concentrations (12.5–50 µM) of interruptin B for 4 days,
following which the ASCs were fixed and stained with Oil Red O in
order to detect accumulated neutral lipid. Microscopic examination
of the stained dishes demonstrated that interruptin B increased the
lipid accumulation in a dose-dependent manner, with an increase of
121.9–167.2%, whereas the increase in the cells treated with
rosiglitazone at 50 µM bwas only 127.7% (Fig. 1B and C).
To further characterize the molecular changes
induced by interruptin B during adipocyte differentiation, the
present study examined the mRNA expression levels of PPAR-γ and
PPAR-α during ASC differentiation (Fig. 1D and E). The mRNA expression levels
of PPAR-α and PPAR-γ were significantly enhanced during interruptin
B-induced ASC differentiation, in a concentration-dependent manner.
Rosiglitazone at 50 µM also produced a positive effect on
the adipogenesis markers. The interruptin B-induced mRNA expression
of PPAR-γ was two-fold higher, compared with that of PPAR-α.
Interruptin B facilitates brown adipocyte
differentiation
There is increasing evidence to suggest that
mitochondria are important during brown adipocyte differentiation,
and that the brown color is a result of the high concentrations of
mitochondria, which are not found in white adipocytes (21,22).
Therefore, the present study examined whether interruptin B had any
effect on mitochondrial number or membrane potential during
differentiation. Fluorescence microscopy using a
mitochondrial-specific dye, MitoTracker Red, showed that
interruptin B increased mitochondrial number in the ASCs, compared
with the control. In addition, interruptin B treatment caused an
enlargement in cell size during ASC differentiation (Fig. 2A). The mitochondrial membrane
potential was examined using DiOC6 (3) staining. The fluorescence intensities
of DiOC6 (3) were
significantly increased by 115.1 and 132.3% in the 25 and 50
µM interruptin B-treated cells, respectively, compared with
the control (Fig. 2B). However,
the mitochondrial membrane potential was not induced by 50
µM rosiglitazone treatment (Fig. 2C).
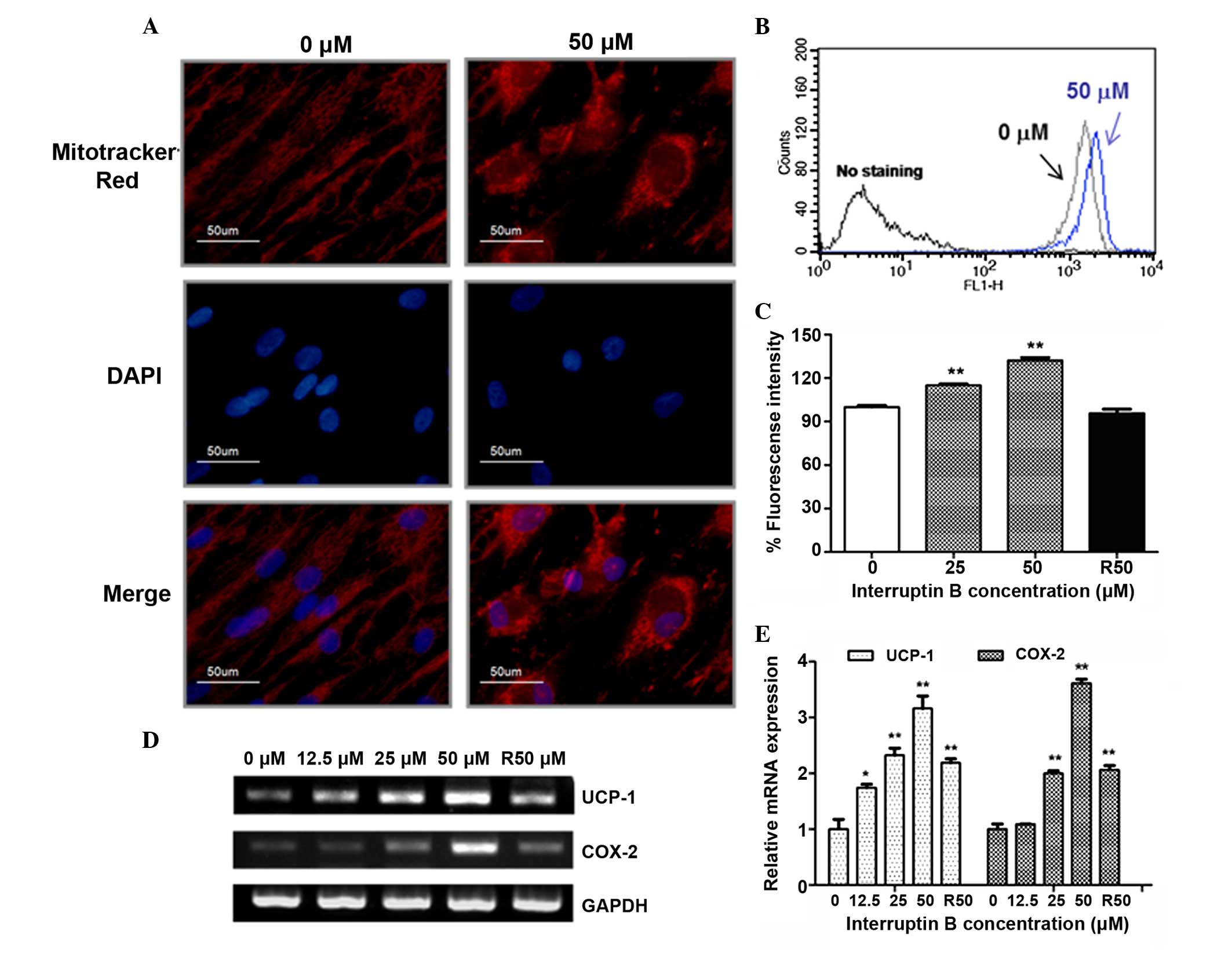 | Figure 2Effect of interruptin B on the brown
adipocyte differentiation of ASCs. ASCs were grown and
differentiated in the absence and presence of interruptin B (0,
12.5, 25 or 50 µM). (A) Signal density of MitoTracker Red
following 4 days treatment with interruptin B (50 µM). (B
and C) Mitochondria membrane potential was measured by
DiOC6 (3) staining
following 4 days of treatment with interruptin B. (D) mRNA
expression levels of UCP-1 and COX-2 were measured following 4 days
treatment with interruptin B and (E) quantified. All values are
presented as the mean ± standard error of the mean (n=3).
*P<0.05 and **P<0.01, compared with the
untreated group. R, rosiglitazone; UCP-1, uncoupling protein-1;
COX, cyclooxygenase; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; DAPI, 4′,6-diamidino-2-phenylindole; ASCs,
adipose-derived stem cells. |
Exposure to interruptin B (12.5–50 µM)
induced the mRNA expression levels of the brown adipocyte markers,
uncoupling protein-1 (UCP-1) and COX-2, compared with the control
(1.7- and 3.6-fold, respectively), and 50 µM rosiglitazone
increased the expression levels 2.2- and 2.0-fold, respectively
(Fig. 2D and E).
Interruptin B increases glucose
consumption
As PPAR-γ is the molecular target for antidiabetic
drugs, which improve insulin sensitivity and glucose tolerance, the
present study examined whether interruptin B treatment affects
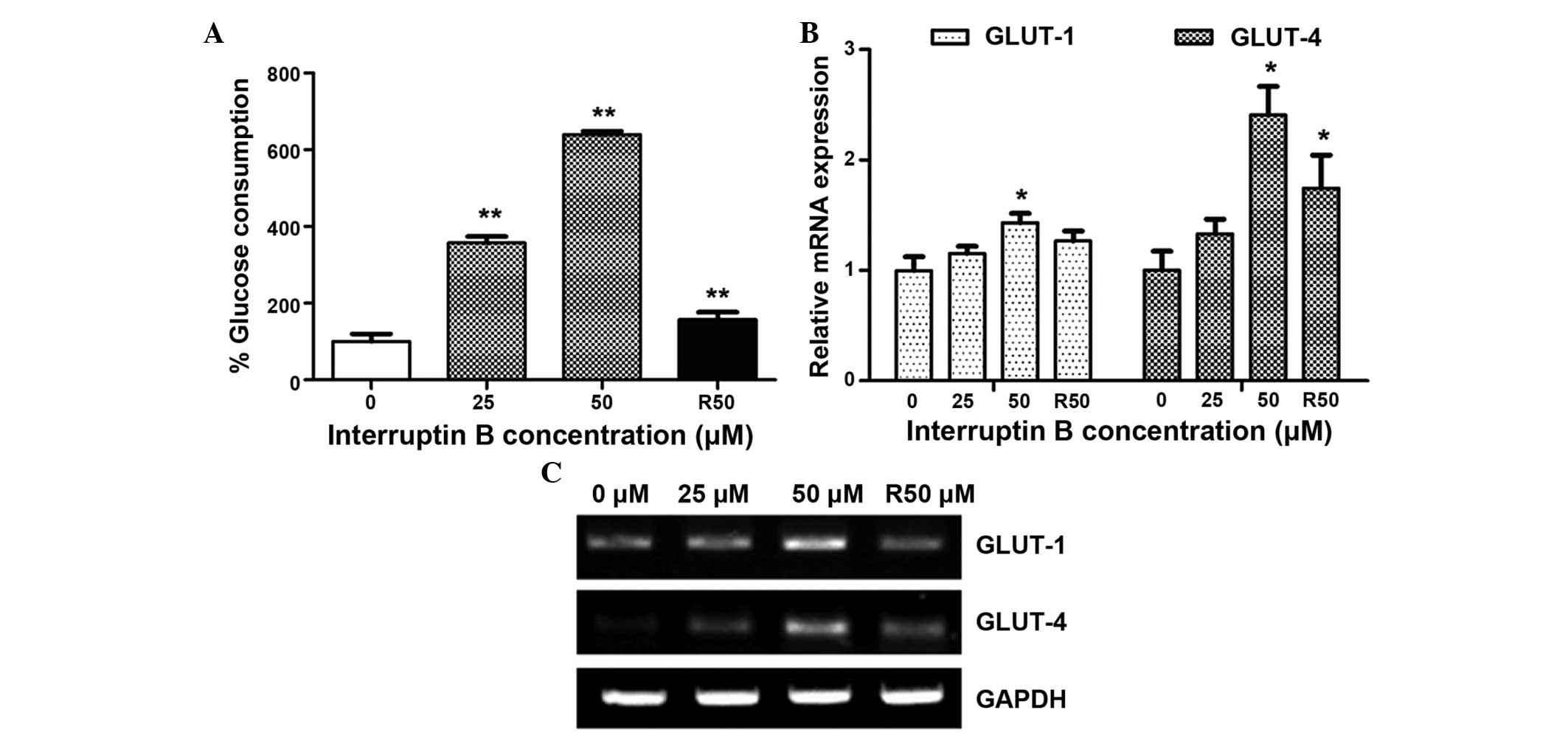
glucose metabolism, compared with rosiglitazone. As shown in
Fig. 3A, glucose consumption was
significantly increased (357–640%) in the differentiated ASCs
treated with 25 and 50 µM interruptin B, which was 2.3–4.1
times higher than the glucose consumption measured following
treatment with 50 µM rosiglitazone (157%).
To understand the mechanism underlying the effects
of interruptin B on glucose consumption, the mRNA expression levels
of a facilitated glucose transporter system were determined. As
shown in Fig. 3B and C, the mRNA
expression levels of GLUT-1 and GLUT-4 were significantly
upregulated in the ASCs exposed to 50 µM of interruptin B,
compared with the untreated control cells.
Interruptin B is a dual PPAR-α and PPAR-γ
ligand
Molecular docking of interruptin B was performed
with AutoDock v4.2 using the X-ray crystal structures PDB ID: 1I7G
and 4EMA as templates for PPAR-α and PPAR-γ, respectively. Each
compound was docked 100 times, and those conformations exhibiting
similar orientation (RMSD <2Å) were clustered. Table II lists the estimated
protein-ligand binding affinities of interruptin B, compared with
ciglitazone, pioglitazone and rosiglitazone (standard PPAR-γ
ligands). A more negative value of binding energy and a higher
percentage of members in a cluster correspond to a higher predicted
binding affinity (23).
Interruptin B was predicted to be a robust PPAR ligand, having
achieved a relatively favorable docking score and relying on the
same receptor pockets as observed for ciglitazone, pioglitazone and
rosiglitazone. The hydrogen-bond contacts of interruptin B
interacted with Thr279 in PPAR-α, and Leu340 and Ser342 in PPAR-γ
(Fig. 4A). In addition, there was
increased binding energy to PPAR-α and PPAR-γ than for
rosiglitazone, which was used as the positive control.
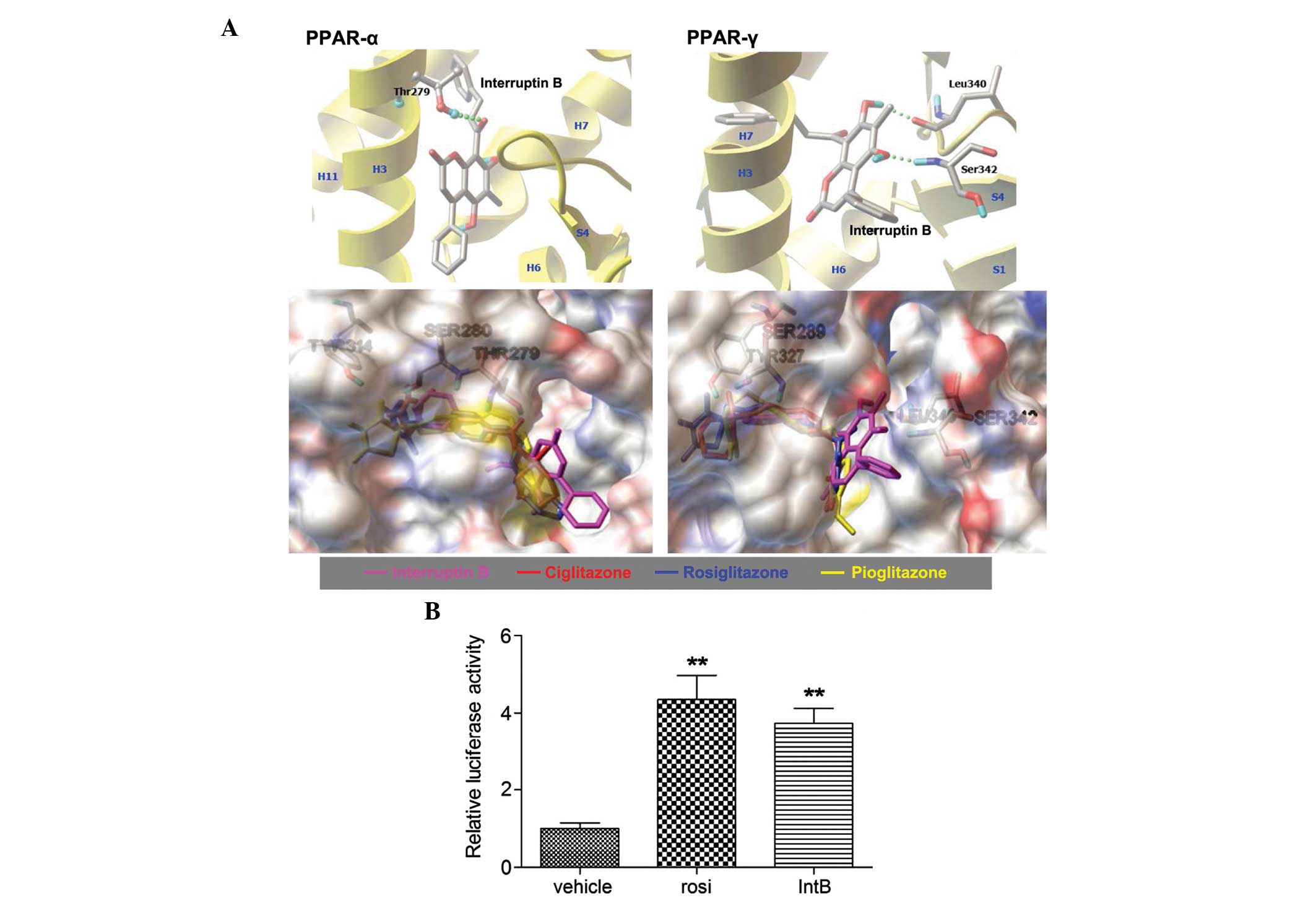 | Figure 4Predicted binding of interruptin B in
the ligand-binding domain of PPAR-α (1I7G) and PPAR-γ (4EMA) by
molecular docking in silico, and the effect of interruptin B
on PPAR activation in HepG2 cells. (A) Structural superposition of
interruptin B, compared with the PPAR-γ ligand. Hydrogen bond
interactions are shown with a green dotted line. Secondary
structure elements are shown in yellow. The structures of
interruptin B, ciglitazone, rosiglitazone, and pioglitazone are
shown in pink, red, blue and yellow, respectively. HepG2 cells were
transfected with PPAR response element-containing reporter
plasmids. (B) Luciferase and β-gal activities were assessed
following 24 h treatment with interruptin B or rosiglitazone (10
µM), All values are presented as the mean ± standard error
of the mean (n=3). **P<0.01, compared with the
untreated group. PPAR, peroxisome proliferator-activated receptor;
IntB, interruptin B; rosi, rosiglitazone. |
 | Table IIIn silico docking results of
interruptin B, compared with the PPAR-γ ligands to PPAR-α and
PPAR-γ, using the AutoDock4.2 program. |
Table II
In silico docking results of
interruptin B, compared with the PPAR-γ ligands to PPAR-α and
PPAR-γ, using the AutoDock4.2 program.
| Compound | PPAR-α (PDB: 1I7G)
| PPAR-γ (PDB: 4EMA)
|
|---|
|
Ebinda (kcal/mol) | Memberb (%) | Interacting
residuec |
Ebinda (kcal/mol) | Memberb (%) | Interacting
residuec |
|---|
| Interruptin B | −9.29 | 59 | Thr279 | −9.36 | 43 | Leu340, Ser342 |
| Ciglitazone | −8.5 | 24 | Thr279, Ser280 | −8.8 | 41 | – |
| Pioglitazone | −9.44 | 28 | Thr279, Ser280,
Tyr314 | −8.5 | 25 | Tyr327 |
| Rosiglitazone | −7.83 | 21 | Thr279, Ser280 | −8.76 | 82 | Ser289 |
To confirm that interruptin B activated PPARs, the
present study assessed its ability to directly activate the PPAR
ligand-binding domain using a chimeric PPRE fusion protein on a
pGL3-dependent luciferase reporter. As shown in Fig. 4B, 10 µM interruptin B
considerably increased PPAR-α and PPAR-γ transcriptional activity
by 3.7-fold, with rosiglitazone causing a 4.4-fold increase. In
addition, the binding activities of interruptin B to purified
PPAR-α and PPAR-γ were determined using an SPR assay (Table III). The results confirmed that
interruptin B bound to PPAR-α and PPAR-γ, with KD values
of 5.32 and 0.10 µM, respectively. These data indicated that
interruptin B acted as a dual PPAR ligand, with superior PPAR-γ to
PPAR-α binding.
 | Table IIIKD values of interruptin B
on PPAR-α and PPAR-γ, determined using a surface plasmon resonance
assay. |
Table III
KD values of interruptin B
on PPAR-α and PPAR-γ, determined using a surface plasmon resonance
assay.
| Protein | KD
(µM) |
|---|
| PPAR-α | 5.32±0.77 |
| PPAR-γ | 0.10±0.00 |
Functional inhibition by PPAR
antagonists
To support the activity of interruptin B as a dual
PPAR-α and PPAR-γ ligand, the present study performed inhibition
experiments in ASC development against specific PPAR-α and -γ
inhibitors. Compared with the control, ASCs treated with 50
µMinterruptin B exhibited increased lipid accumulation, as
determined by Oil Red O staining, which was significantly
attenuated by treatment with 50 and 100 µM GW6471 and BADGE
(PPAR-α and PPAR-γ antagonists, respectively; Fig. 5A and B). Similarly, the increased
glucose consumption, which was induced by 50 µM interruptin
B was reduced considerably by co-treatment with 50 or 100 µM
GW6471, and 100 µM BADGE (Fig.
5C and D). The results suggested that the interruptin B-induced
adipocyte differentiation and glucose consumption were eradicatd in
a dose-dependent manner by GW6471 and BADGE. Taken together, these
observations supported that the induction of adipocyte
differentiation and glucose uptake by interruptin B was dependent
on PPAR-α and PPAR-γ activation.
Discussion
In a preliminary investigation in the present study,
interruptin B not only induced ASC proliferation, but also markedly
enhanced cell size (data not shown). Therefore, whether interruptin
B was involved in the adipogenesis of ASCs to produce brown
adipocyte tissue was subsequently investigated. The results of the
present study demonstrated for the first time, to the best of our
knowledge, that interruptin B induced brown adipocyte
differentiation and increased glucose uptake in ASCs. In addition,
interruptin B showed increased adipogenic differentiation potential
and glucose uptake, compared with rosiglitazone. Although the
effects of natural compounds, including daidzein, equol, magnolol
and arteplilin C, for the induction of adipocyte differentiation
and glucose uptake have been demonstrated, they all act as ligands
of PPAR-γ and originate from cultivated plants (24–26).
To the best of our knowledge, the present study is the first report
to show the stimulation of adipocyte differentiation and glucose
uptake by interruptin B from the wild plant, C. terminans,
mediated by the dual effects of PPAR-α and PPAR-γ binding.
To predict the specific target ASC receptors that
respond to interruptin B in the present study, computational
analysis was performed using the AutoDock4.2 program. In comparison
with standard PPAR-γ sensitizers, ciglitazone, pioglitazone, and
rosiglitazone, molecular docking predicted interruptin B as a dual
PPAR-α and -γ ligand. In addition, a luciferase reporter assay and
SPR technology demonstrated that interruptin B acted as a dual
PPAR-α and PPAR-γ agonist. In the inhibition experiments,
interruptin B-induced adipocyte differentiation and glucose uptake
were significantly inhibited, in a dose-dependent manner, by
co-treatment with the PPAR-α antagonist GW6471 or the PPAR-g
antagonist BADGE. These data confirmed that adipocyte
differentiation and glucose consumption were enhanced by
interruptin B treatment through the PPAR-α and PPAR-γ dependent
pathway.
TZDs have been investigated as potential
antidiabetic drugs. Rosiglitazone is a selective PPAR-γ agonist,
while pioglitazone exerts PPAR-α and PPAR-γ agonistic activity,
which may cause different metabolic effects. However, rosiglitazone
has been associated with an enhanced risk of myocardial infarction,
which led to the withdrawal of this drug from the market in 2010
(27). By contrast, the beneficial
vascular effects of pioglitazone, owing to its dual PPAR-α and
PPAR-γ binding, have been established. Therefore, investigations
are focusing on other dual PPAR-α and PPAR-γ agonists to improve
not only glycemic control, but also lipid levels, potentially
reducing vascular risk (28). The
combined PPAR-α and PPAR-γ stimulation effects of interruptin B
may, therefore, offer an attractive option for developing
antidiabetic or anti-obesity drugs with reduced cardiovascular
risk.
In conclusion, the present study demonstrated that
interruptin B, an ingredient of C. terminans, induced brown
adipocyte differentiation. In addition, the downstream responses to
PPAR-α and PPAR-γ activation included increases in the mRNA
expression levels of GLUT-1 and GLUT-4, resulting in facilitated
glucose uptake. Although its corresponding effects in vivo
remain to be elucidated, these data indicate that interruption B is
a potential natural insulin sensitizer from ferns. These novel
findings support the benefits of C. terminans consumption,
and may assist in the progression between epidemiological
observations and clinical studies on the antidiabetic or
anti-obesity benefits of fern plants.
Acknowledgments
The authors would like to thank Dr Brian Hodgson of
PSU for assistance with English.
References
|
1
|
Hauptman J, Lucas C, Boldrin MN, Collins H
and Segal KR: Orlistat in the long-term treatment of obesity in
primary care settings. Arch Fam Med. 9:160–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halford JC: Pharmacotherapy for obesity.
Appetite. 46:6–10. 2006. View Article : Google Scholar
|
|
4
|
Filippatos TD, Derdemezis CS, Gazi IF,
Nakou ES, Mikhailidis DP and Elisaf MS: Orlistat-associated adverse
effects and drug interactions: A critical review. Drug Saf.
31:53–65. 2008. View Article : Google Scholar
|
|
5
|
Yao X, Shan S, Zhang Y and Ying H: Recent
progress in the study of brown adipose tissue. Cell Biosci.
1(35)2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amos AF, McCarty DJ and Zimmet P: The
rising global burden of diabetes and its complications: Estimates
and projections to the year 2010. Diabet Med. 14(Suppl 5): S1–S85.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takada I and Makishima M: PPARγ ligands
and their therapeutic applications: a patent review (2008 – 2014).
Expert Opin Ther Pat. 25:175–191. 2015. View Article : Google Scholar
|
|
8
|
Nathan DM: Diabetes: Advances in diagnosis
and treatment. JAMA. 314:1052–1062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujimura T, Kimura C, Oe T, Takata Y,
Sakuma H, Aramori I and Mutoh S: A selective peroxisome
proliferator-activated receptor gamma modulator with distinct fat
cell regulation properties. J Pharmacol Exp Ther. 318:863–871.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teboul L, Gaillard D, Staccini L, Inadera
H, Amri EZ and Grimaldi PA: Thiazolidinediones and fatty acids
convert myogenic cells into adipose-like cells. J Biol Chem.
270:28183–28187. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patel C, Wyne KL and McGuire DK:
Thiazolidinediones, peripheral oedema and congestive cardiac
failure: What is the evidence? Diab Vasc Dis Res. 2:61–66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semple RK, Chatterjee VK and O'Rahilly S:
PPAR gamma and human metabolic disease. J Clin Invest. 116:581–586.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diamond GA, Bax L and Kaul S: Uncertain
effects of rosiglitazone on the risk for myocardial infarction and
cardiovascular dealth. Ann Intern Med. 147:578–581. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medina-Gómez G: Mitochondria and endocrine
function of adipose tissue. Best Pract Res Clin Endocrinol Metab.
26:791–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumboonruang N: Fern diversity at Silaphet
waterfall, Pua district, Nan province. (unpublished Master's
Project, M.Ed.). Graduate School, Srinakharinwirot University;
Bangkok, Thailand: 2009
|
|
16
|
Kaewsuwan S, Yuenyongsawad S, Plubr ukarn
A, Kaewchoothong A, Raksawong A, Puttarak P and Apirug C:
Biological activities of interruptins A and B from Cyclosorus
terminans. Songklanakarin J Sci Technol. In press.
|
|
17
|
Kim WS, Park BS, Park SH, Kim HK and Sung
JH: Antiwrinkle effect of adipose-derived stem cell: Activation of
dermal fibroblast by secretory factors. J Dermatol Sci. 53:96–102.
2009. View Article : Google Scholar
|
|
18
|
Sherman W, Day T, Jacobson MP, Friesner RA
and Farid R: Novel procedure for modeling ligand/receptor induced
fit effects. J Med Chem. 49:534–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumari V and Li C: Comparative docking
assessment of glucokinase interactions with its allosteric
activators. Curr Chem Genomics. 2:76–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cannon B and Nedergaard J: Brown adipose
tissue: Function and physiological significance. Physiol Rev.
84:277–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman J: Empowering self-renewal and
differentiation: The role of mitochondria in stem cells. J Mol Med
(Berl). 88:981–986. 2010. View Article : Google Scholar
|
|
23
|
Gallicchio E, Lapelosa M and Levy RM: The
binding energy distribution analysis method (BEDAM) for the
estimation of protein-ligand binding affinities. J Chem Theory
Comput. 6:2961–2977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho KW, Lee OH, Banz WJ, Moustaid-Moussa
N, Shay NF and Kim YC: Daidzein and the daidzein metabolite, equol,
enhance adipocyte differentiation and PPARgamma transcriptional
activity. J Nutr Biochem. 21:841–847. 2010. View Article : Google Scholar
|
|
25
|
Choi SS, Cha BY, Lee YS, Yonezawa T,
Teruya T, Nagai K and Woo JT: Magnolol enhances adipocyte
differentiation and glucose uptake in 3T3-L1 cells. Life Sci.
84:908–914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi SS, Cha BY, Iida K, Lee YS, Yonezawa
T, Teruya T, Nagai K and Woo JT: Artepillin C, as a PPARγ ligand,
enhances adipocyte differentiation and glucose uptake in 3T3-L1
cells. Biochem Pharmacol. 81:925–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nissen SE and Wolski K: Rosiglitazone
revisted: An updated meta-analysis of risk for myocardial
infarction and cardiovascular mortality. Arch Intern Med.
170:1191–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henry RR, Lincoff AM, Mudaliar S, Rabbia
M, Chognot C and Herz M: Effect of the dual peroxisome
proliferator-activated receptor-alpha/gamma agonist aleglitazar on
risk of cardiovascular disease in patients with type 2 diabetes
(SYNCHRONY): A phase II, randomised, dose-ranging study. Lancet.
374:126–135. 2009. View Article : Google Scholar : PubMed/NCBI
|