Introduction
Almost all individuals suffer from a form of
psychological and physical stress, and increased stress can lead to
other health complications. In particular, depression is a chronic
mental disorder that causes, not only psychological and physical
damage, but is also associated with suicide rates (1,2).
Antidepressants in current use include monoamine oxidase
inhibitors, norepinephrine (NE) reuptake inhibitors, selective
serotonin (5′hydroxytryptamine; 5HT) reuptake inhibitors, tricyclic
antidepressants, 5HT and noradrenaline (NA) reuptake inhibitors,
and NA and dopamine reuptake inhibitors (3). Although these antidepressant drugs
show adequate potency, they frequently produce unwanted adverse
effects. Therefore, there has been a focus on identifying and
developing more promising antidepressants through the use of
alternative medicine (4).
Monoaminergic systems have a central function in
neuronal system (5).
Phytoestrogens, which selectively bind estrogen receptor (ER)-β
have been shown to improve mood and 5HT neurotransmission (3). 5HT induces the significant increase
in the expression of hippocampal brain-derived neurotrophic factor
(BDNF) (6). BDNF is a member of
the neurotrophin family, and improves the development and function
of several neuronal systems (7).
In addition, BDNF promotes neuronal cell growth and protects
against hippocampal damage in depression (7). These BDNFs are expressed via the
activation of extracellular signal-regulated kinases (ERKs)
(8).
The inflammatory hypothesis of depression suggests
that elevated circulating levels of pro-inflammatory cytokines
promote the evolution and maintenance of depressive symptoms
(9). Ikwitang (IW) is has been
used for the treatment of inflammatory diseases (10). IW is composed of Liriopis
Tuber, Rehmanniae Radix, Adenophorae Radix, Rhizoma
Polygonati odorati, and Saccharum nigrum.
Liriopis Tuber reduces hyper-responsiveness and airway
inflammation (11).
Rehmanniae Radix has been used in the treatment of
depression in several Asian countries (12). Adenophorae Radix has
regulatory effects on airway inflammation and hyper-responsiveness
(13). However, the effects of IW
on depression remain to be elucidated. The present study aimed to
investigate the antidepressant-like effect of IW, and to determine
the regulatory mechanisms of IW in the central monoaminergic
system. The present study investigated if IW exhibited
antidepressant-like effects though the modulation of monoaminergic
systems.
Materials and methods
Reagents
Avidin peroxidase, bicinchoninic acid (BCA), and
other reagents were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Anti-mouse interleukin (IL)-1β, IL-6 and tumor necrosis
factor (TNF)-α purified antibody (Ab), anti-mouse IL-6, TNF-α and
IL-1β biotin-conjugated Ab, and recombinant mouse (rm) IL-1β, IL-6
and TNF-α Ab were purchased from BD Pharmingen (San Diego, CA,
USA). Abs for BDNF, GAPDH, ERK and phosphorylated ERK (pERK) were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Preparation of IW
A sample of IW was obtained from an oriental drug
store, Noa Pharmacy (Seoul, South Korea), and then authenticated by
Professor HM Kim, College of Korean Medicine, Kyung Hee University
(Seoul, South Korea). A voucher specimen was deposited at the
College of Korean Medicine, Kyung Hee University (IW voucher no.
304047). IW was extracted by decocting the dried herbs (total
58.125 g) with boiling distilled water (D.W; 1 liter) for ~2.5 h.
The decoction was then filtered using 3MM chromatography paper,
lyophilized in a freeze-dryer (Operon Co., Ltd., Kimpo, South
Korea) and stored at 4°C. An IW yield of 9.7% was obtained by
freeze-drying. IW was dissolved in D.W. IW was filtered through a
0.22 µm syringe filter and then diluted with D.W. (1 and 10
mg/ml). The dose range of 0.01, 0.1 and 1 g/kg was selected to
determine whether dose dependency was apparent, in accordance with
a previous report (9).
Forced swimming test (FST)
Male ICR mice (3 weeks old, 10–12 g) were purchased
from the Dae-Han Experimental Animal Center (Daejon, South Korea),
and subsequently housed at the College of Korean Medicine, Kyung
Hee University. The animals were maintained at a temperature of
22±1°C at a relative humidity of 55±10% under a 12:12 light/dark
cycle, lights on at 7:00 throughout the study. Food and water were
available ad libitum. All manipulations were carried out
between 9:00 and 16:00, and no animal was used more than once. All
protocols were approved by the institutional animal care and use
committee of Kyung Hee University (Seoul, South Korea). Following
the first measurement of immobility times, the mice were randomly
separated into control, fluoxetine and IW (0.01, 0.1 and 1 g/kg)
groups, based on the documented immobility times. IW (0.01, 0.1,
and 1 g/kg) was orally administered to mice once per day for 2
weeks using an atraumatic feeding needle. Fluoxetine (10 mg/kg), an
antidepressant of the selective serotonin reuptake inhibitor class,
was used as a positive control. The FST was performed at the end of
the 2-week administration period. During the 6 min FST, the times
of immobility was measured. The instrument comprised two Plexiglas
cylinders (height, 25 cm; diameter, 10 cm; Deoksan Lab, Seoul,
South Korea), placed alongside a Makrolon cage (Dae-Han
Experimental Animal Center) filled with water (10 cm height) at a
temperature of 23–25°C. For the FST, two mice (one from each
experimental group) were assessed concurrently for a 6 min period
of time inside the vertical Plexiglas cylinders. An opaque screen
was placed between the two cylinders to prevent the mice from
seeing each other. The total duration of immobility, following a
stabilizing duration of 2 min, was measured for a period of 4 min.
Each mouse was considered to be immobile when it ceased struggling
and remained floating motionless in the water, making only those
movements necessary to maintain its head above the water surface.
Each group contained five mice. Mice were anesthetized using an
intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4
mg/kg). Following anesthetization, blood was withdrawn from the
hearts of forced swimming-tested mice. Then, the serum was prepared
by centrifugation at 1,900 x g at 4°C for 10 min. The hippocampus
was dissected out of the brain and homogenized with a
homogenization buffer (20 mM HEPES pH 7.5, 1.5 mM MgCl2,
0.2 mM EDTA, 0.1 M NaCl, 0.2 mM DTT). The protein extracts were
prepared by centrifugation at 12,000 x g for 10 min at 4°C.
5HT assay
The 5HT levels were measured, according to the
manufacturer protocol using a Mouse 5HT/ST ELISA kit (MyBiosource,
San Diego, CA, USA).
NA assay
The NA levels were measured according to
manufacturer protocol using an NA Urine ELISA kit (Labor
Diagnostika Nord GmbH & Co. KG, Nordhorn, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissue according to the
manufacturer's specification using easy-BLUE RNA extraction kit
(iNtRON Biotechnology, Inc., Kyungki-Do, South Korea). The
concentration of total RNA in the final elutes was determined by
NanoDrop (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total
RNA (2.5 µg) was heated at 65°C for 10 min and then chilled
on ice. Each sample was reverse-transcribed to cDNA for 90 min at
37°C using First-Strand cDNA Synthesis kit (GE Healthcare Life
Sciences, Chalfont, UK). RT-qPCR was performed using SYBR Green
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.),
and the detection of mRNA was analyzed using an ABI Step One real
time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primer sequences for the reference gene, GAPDH, and the
genes of interest were as follows: GAPDH, forward
5′-TCGACAGTCAGCCGCATCTTCTTT-3′ and reverse
5′-ACCAAATCCGTTGACTCCGACCTT-3′; estrogen receptor (ER)-β, forward
5′-GACTGTAGAACGGTGTGGTC-3′ and reverse 5′-CCTGTGAGGTAGGAATGCGA 3′);
IL-6, forward 5′-AAATTCGGTACATCCTCGACGGCA-3′ and reverse
5′-AGTGCCTCTTTGCTGCTTTCACAC-3′); TNF-α, forward
5′-AGGACGAACATCCAACCTTCCCAA-3′ and reverse
5′-TTTGAGCCAGAAGAGGTTGAGGGT-3′); IL-1β, forward
5′-AAACAGATGAAGTGCTCCTT-3′ and reverse 5′-TGGAGAACACCACTTGTTGC-3′.
These were obtained from the Bioneer Corporation, Daejeon, South
Korea. The thermocycling profile used was as follows: Initial step
of 95°C for 10 min, followed by 95°C for 15 sec and 60°C for 30
sec, for 40 cycles, prior to melting curve analysis. The levels of
target mRNA were normalized to the level of GAPDH and compared with
the control. Data were analyzed using the 2−ΔΔCq method
(14).
Western blot analysis
Hippocampus tissue extracts were used for Western
blot analysis. Western blotting was performed, as previously
described. Samples were heated at 95°C for 5 min and briefly cooled
on ice. Following the centrifugation, protein was estimated using a
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). The
50 µg aliquots were resolved by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 120 V for 2 h. The
resolved proteins were electro-transferred overnight to
nitrocellulose membranes in 25 mM Tris, pH 8.5, 200 mM glycerin,
and 20% methanol at 20 V. Blots were blocked for at least 2 h with
6% bovine serum albumin then incubated with rabbit polyclonal
anti-mouse BDNF (1:500, sc-546), mouse monoclonal anti-mouse GAPDH
(1:500, sc-32233), rabbit polyclonal anti-mouse ERK (1:500, sc-94),
and mouse monoclonal anti-mouse pERK (1:500, sc-7383) Abs for 1 h
at room temperature. The membranes were washed three times with
phosphate-buffered saline containing 0.05% Tween-20. Blots were
developed by peroxidase-conjugated rabbit/mouse secondary Abs
(1:5,000, Santa Cruz, CA, USA) for 30 min, and proteins were
visualized by enhanced chemi-luminescence procedures (GE Healthcare
Life Sciences), according to the manufacturer's protocol.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of cytokines in the serum and in the
hippocampal tissues were analyzed using an ELISA. The ELISA was
performed, as described previously (10). The 96-well plates used were coated
with 100 µl aliquots of anti-mouse IL-1β/IL-6/TNF-α
monoclonal antibodies at a concentration of 1.0 µg/ml in
PBS, respectively and incubated overnight at 4°C. Following 3
washes with PBS containing 0.05% Tween (PBST), 100 µl of
samples or IL-1β/IL-6/TNF-α standards were added and incubated at
37°C for 2 h. The wells were washed 3 times with PBST and
biotinylated anti-mouse IL-1β/IL-6/TNF-α antibodies (1
µg/ml) were added and incubated at 37°C for an additional 2
h. Next, the wells were washed 3 times with PBST and the
avidinperoxidase was added and incubated for 30 min at 37°C. The
wells were then washed with PBST, and a substrate solution was
added. The plates were read at 405 nm. Cytokine levels in the
hippocampus were divided according to the total protein levels,
which were estimated using a Pierce BCA protein assay kit (Thermo
Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The analyses were performed using SPSS v. 11.5 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. Comparison between the
effects of different treatments were analyzed using an independent
t-test and one-way analysis of variance, followed by Tukey's
multiple range tests
Results
Effect of IW on immobility duration in
the FST
The IW (0.01, 0.1 and 1 g/kg) was orally
administered for 2 weeks, following which, the durations of
immobility were determined in a FST. The immobility durations
determined in the IW groups (0.01, 0.1 and 1 g/kg) were
significantly decreased, compared with the durations in the D.W.
groups (Fig. 1A; P<0.05). The
fluoxetine group also showed significantly decreased immobility
durations, compared with the D.W. groups (Fig. 1A; P<0.05).
Effect of IW on the levels of 5HT and NA
in the brain
Monoamine systems have wide-ranging effects on
animal behavior, and noradrenergic and serotonergic systems have
long been implicated in depression (2). The reduced levels of 5HT or NA are
considered to be involved in the underlying pathophysiology of
clinical depression (15).
Antidepressants elevate extracellular levels of monoamines by
inhibiting their degradation or reuptake (3). Thus, the present study analyzed the
levels of 5HT and NA in the brain following FST. As shown in
Fig. 1B and C, the levels of 5HT
and NA in the IW-administered group (1 g/kg) were significantly
increased, compared with those in the D.W.-administered group
(Fig. 1B and C; P<0.05).
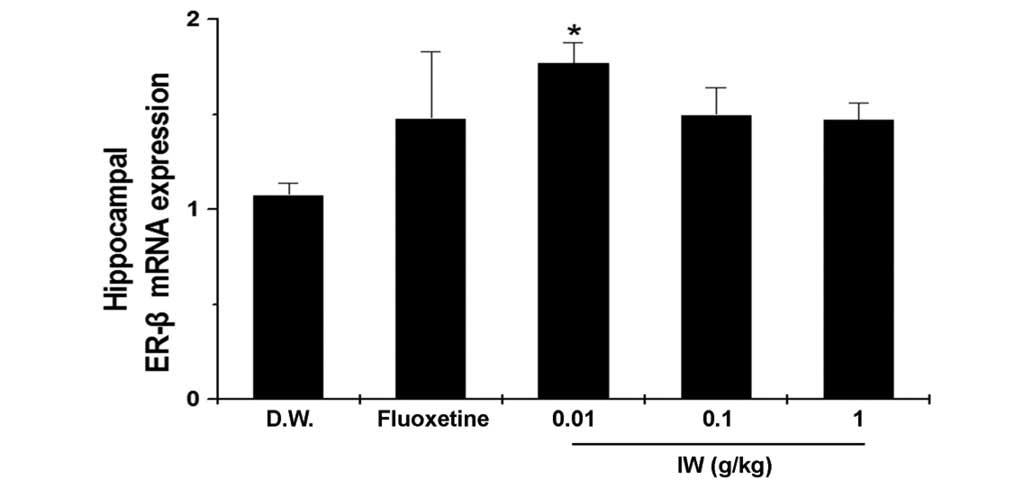
Effect of IW on hippocampal mRNA levels
of ER-β
ER-β has a beneficial effect on depression (16), therefore, the present study
investigated whether the antidepressant effect of IW was associated
with the expression of ER-β. The administration of IW (0.01 g/kg)
significantly increased the mRNA expression of ER-β, compared with
the D.W. group. (Fig. 2,
P<0.05).
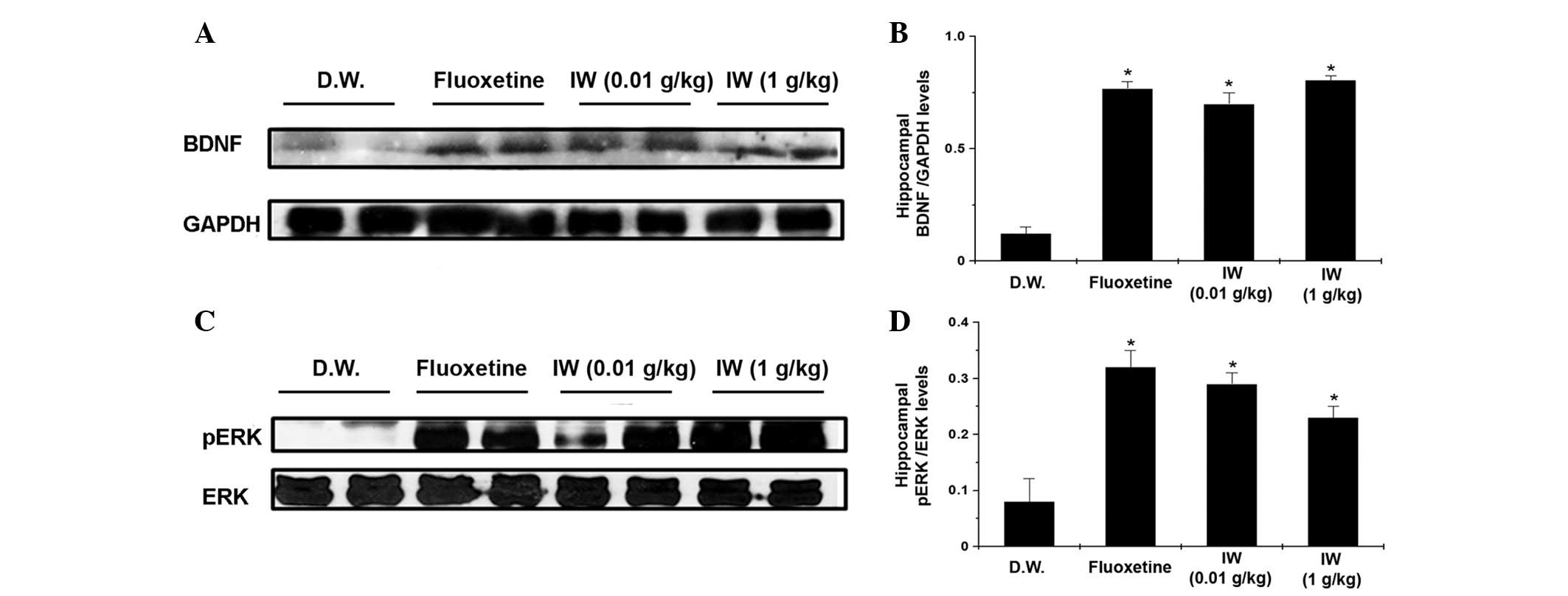
Effect of IW on the protein levels of
hippocampal BDNF and pERK
Reduced the levels of BDNF and pERK in response to
stress can lead to the impaired neurogenesis and depressive
symptoms (17–20). The protein levels of BDNF in the IW
groups were higher than those of the control groups (Fig. 3A and B; P<0.05). The protein
levels of pERK in the IW groups were also higher than the levels in
the control groups (Fig. 3C and D,
P<0.05).
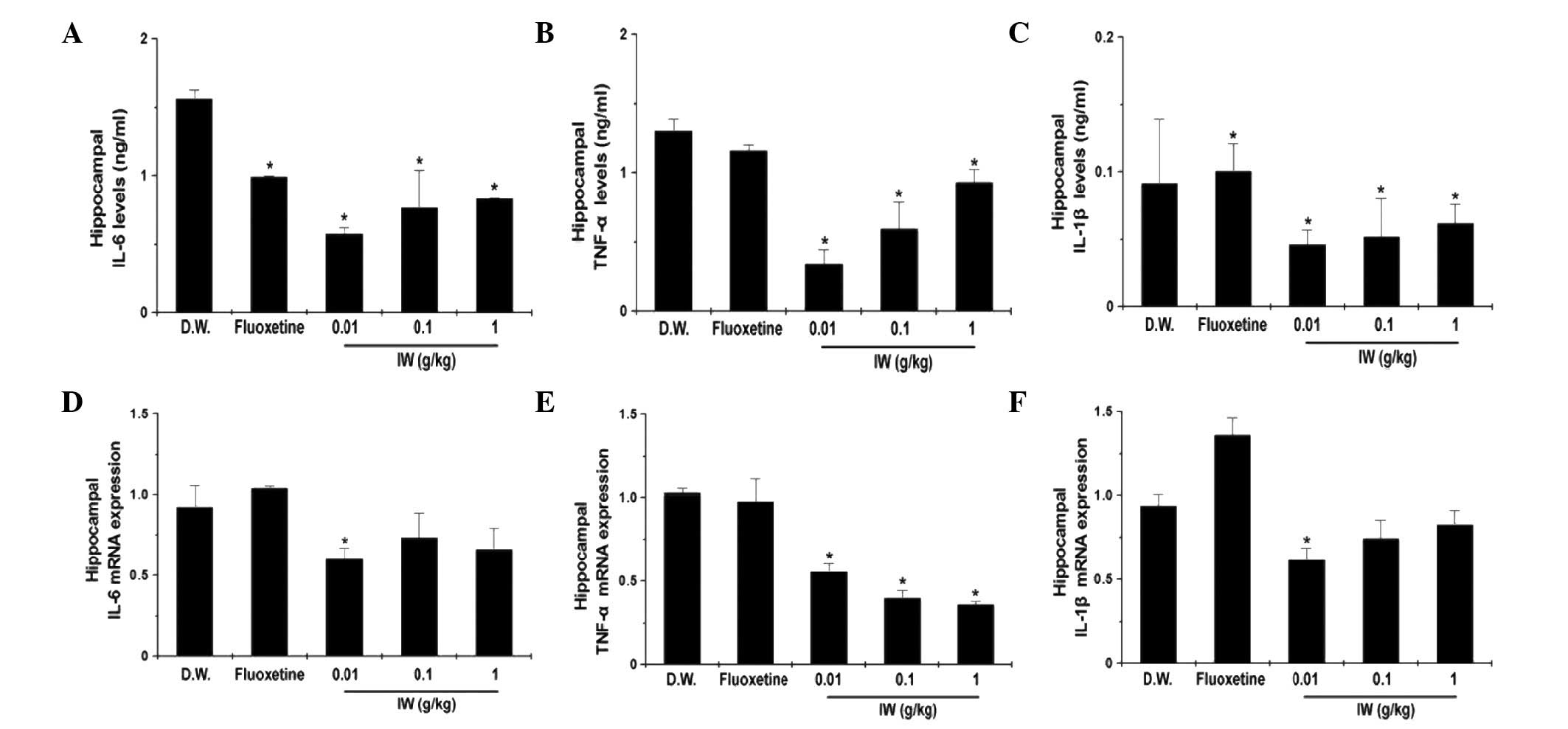
Effect of IW on the protein and mRNA
levels of hippocampal inflammatory cytokines
The overexpression of pro-inflammatory cytokines,
including IL-6, TNF-α, and IL-1β, results in autoimmune or
inflammatory reactions in depression (9). The protein levels of IL-6, TNF-α and
IL-1β were significantly decreased by IW administration in the
hippocampus (Fig. 4A–C;
P<0.05). The present study also measured the mRNA levels of
IL-6, TNF-α, and IL-1β in the hippocampus. As the shown in Fig. 4D–F, IW administration significantly
decreased the mRNA levels of IL-6, TNF-α and IL-1β (P<0.05).
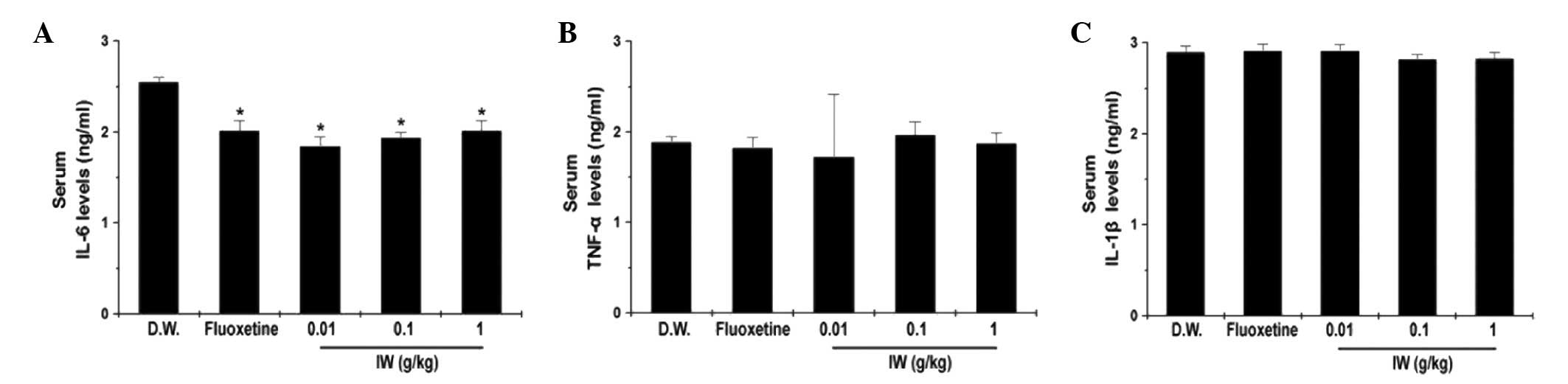
Effect of IW on the levels of serum
inflammatory cytokines
Pro-inflammatory cytokines are important in the
pathophysiology of depression (9).
Therefore, the present study analyzed the protein levels of IL-6,
TNF-α, and IL-1β in the serum. The levels of IL-6 in the serum were
significantly decreased by IW administration, however, no
significant differences were observed in the levels of TNF-α and
IL-1β (Fig. 5A–C; P<0.05).
Discussion
The present study demonstrated that IW exhibited
antidepressant-like effects in the FST animal model. IW
significantly increased the levels of 5HT, NA, ER-β, BDNF and pERK,
and decreased the duration of immobility. In addition, IW
significantly decreased the levels of inflammatory cytokines levels
in the hippocampus and serum.
The FST is an important tool for investigating the
neuro-biological mechanisms involved in antidepressant responses,
and can be used for the screening of potential antidepressant
agents (21). According to a
previous study, monoamine has an important function in depression,
and the major neurochemical process in depression is a reduction of
monoaminergic functions and in the levels of 5HT and NA (22). Antidepressant drugs promote the
availability of these monoamines through increasing neurogenesis
and modulating the monoaminergic functions at the synapse (23). Effective antidepressants increased
the levels of 5HT and NA in the brain, and decrease the duration of
immobility in FSTs (24). In the
present study, it was confirmed that the administration of IW for 2
weeks significantly increased the levels of 5HT and NA in the
brain, and decreased the durations of immobility. Therefore, the
data of the present study indicated IW as a useful
antidepressant-like drug.
17-β estradiol (E2) exerts its actions at
the two types of classical nuclear receptors, ER-α and ER-β. ER-β
is predominant in stress-responsive regions of the hippocampus, the
bed nucleus of the stria terminalis and the paraventricular nucleus
of the hypothalamus (25). Thus,
E2 may act to alter affective behaviors of rodents, in
part, through activation of ER-β. In anxiety and depression-like
behaviors, E2 induces protective effects through the
activation of ER-β (26). The
E2 induces slowing of serotonin clearance via activation
of the ER-β/mitogen-activated protein kinase/ERK signaling cascade
pathways (27). Previous
neurochemical results have suggested that ER-β-knockout mice have
depleted levels of 5HT in the brain (27). In the present study, it was
confirmed that IW-administration upregulated the activation of
ER-β. These results suggested that the antidepressant-like effect
of IW was associated with ER-β activation.
BDNF is distributed extensively in the brain. In
particular, its expression is at high concentrations in the
cerebral cortex and hippocampus (28). However, exposure to stress is
associated with decreased expression of BDNF (29). The levels of BDNF reduced by
depression or stress are increased by administration of
antidepressant in the hippocampus (29). The deletion of BDNF in adult mice
produces chronic pain and symptoms of depression (30). Thus, there is an association
between reduced levels of BDNF and chronic pain associated with
depressive behavior. The data obtained in the present study showed
that the protein levels of BDNF of IW groups were higher, compared
with the protein levels of BDNF in the control groups. ERK is
extensively distributed throughout the central nervous system, and
the ERK pathway is involved in depression (31). The protein levels of pERK in the IW
groups were higher, compared with those in the control groups. This
effect of IW is similar to the mechanism of action of the
anti-depressant drug, fluoxetine. Therefore, these results
suggested that IW has an antidepressant-like effect via activation
of the BDNF/ERK signaling pathway.
Pro-inflammatory cytokines, including IL-6, TNF-α
and IL-1β, are involved in inflammatory processes and increase the
symptoms of depression (32).
IL-1β and IL-6 are responsible for hyperactivation of the
hypothalamic-pituitary-adrenal axis and activation of the
indoleamine 2,3-dioxygenase enzyme in the FST and tail suspension
test (32). Patients with
depression present with significantly higher levels of IL-1b, IL-6
and TNF-α in the brain and blood (9). Alterations in the metabolism of 5HT
and NA have been associated with the potent effects of
pro-inflammatory cytokines on pathways involved in the
pathophysiology of depression (33). The present study showed that the
levels of IL-6, TNF-α and IL-1β were significantly decreased by IW
administration in the hippocampus and serum, suggesting that IW had
an antidepressant-like effect due to the suppression of
inflammation by IW.
According to previous reports (10), IW has anti-inflammatory effects. IW
is composed of the five medicinal herbs, Liriopis Tuber,
Rehmanniae Radix, Adenophorae Radix, Rhizoma Polygonati
odorati and Saccharum nigrum. Previous studies have
reported that each medicinal herb has a different effect, for
example, Rehmanniae Radix has an antidepressant-like effect
(12). Catalpol is a major
bioactive compound of Rehmanniae Radix, and certain studies
have reported that catalpol ameliorates cognitive deficits and has
a neuroprotective effect (34). In
addition, catalpol offers potential as a treatment for
inflammation-associated neurodegenerative diseases (34). Liriopis Tuber and
Adenophorae Radix have been observed to significantly reduce
inflammation and hyper-responsiveness in an asthma animal model
(11,13). In conclusion, the present study
demonstrated that IW had an antidepressant-like effect, via the
BDNF signaling pathway, and suppressed inflammation. In addition,
the results indicated that IW induced antidepressant-like effects
through activation of the serotonin and noradrenaline systems.
Therefore, it was hypothesized that IW may be used as a promising
antidepressant-like drug. However, the active components of IW
require isolation in further experiments, to clarify whether the
components them-selves may also be effective in the treatment of
depression.
Acknowledgments
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea, funded
by the Ministry of Education, Science and Technology (grant no.
2012R1A1A2A10044645).
References
|
1
|
Alfonso J, Frasch AC and Flugge G: Chronic
stress, depression and antidepressants: Effects on gene
transcription in the hippocampus. Rev Neurosci. 16:43–56. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reeves RR and Ladner ME:
Antidepressant-induced suicidality: An update. CNS Neurosci Ther.
16:227–234. 2010.PubMed/NCBI
|
|
3
|
Shively CA, Mirkes SJ, Lu NZ, Henderson JA
and Bethea CL: Soy and social stress affect serotonin
neurotransmission in primates. Pharmacogenomics J. 3:114–121. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang
YH and Li XJ: Antidepressant effects of curcumin in the forced swim
test and olfactory bulbectomy models of depression in rats.
Pharmacol Biochem Behav. 82:200–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee S, Jeong J, Kwak Y and Park SK:
Depression research: Where are we now? Mol Brain. 3:82010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji XW, Wu CL, Wang XC, Liu J, Bi JZ and
Wang DY: Monoamine neurotransmitters and fibroblast growth factor-2
in the brains of rats with post-stroke depression. Exp Ther Med.
8:159–164. 2014.PubMed/NCBI
|
|
7
|
Watanabe K, Hashimoto E, Ukai W, Ishii T,
Yoshinaga T, Ono T, Tateno M, Watanabe I, Shirasaka T, Saito S and
Saito T: Effect of antidepressants on brain-derived neurotrophic
factor (BDNF) release from platelets in the rats. Prog
Neuropsychopharmacol Biol Psychiatry. 34:1450–1454. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patterson M and Yasuda R: Signalling
pathways underlying structural plasticity of dendritic spines. Braz
J Pharmacol. 163:1626–1638. 2011. View Article : Google Scholar
|
|
9
|
Maes M: Evidence for an immune response in
major depression: A review and hypothesis. Prog
Neuropsychopharmacol Biol Psychiatry. 19:11–38. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Go JH, Jung JH, Chang YJ, Kim HM and Chung
YJ: Ikwi-tang for the treatment of allergic rhinitis as a
traditional medicine. TANG. 3:37–42. 2013.
|
|
11
|
Lee YC, Lee JC, Seo YB and Kook YB:
Liriopis tuber inhibit OVA-induced airway inflammation and
bronchial hyperresponsiveness in murine model of asthma. J
Ethnopharmacol. 101:144–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang M, Shin D, Oh JW, Cho C, Lee HJ, Yoon
DW, Lee SM, Yun JH, Choi H, Park S, et al: The anti-depressant
effect of Nelumbinis semen on rats under chronic mild stress
induced depression-like symptoms. Am J Chin Med. 33:205–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Roh SS, Kim SH, Lee YC and Seo YB: Effects
of radix adenophorae and cyclosporine A on an OVA-induced murine
model of asthma by suppressing to T cells activity, eosinophilia
and bronchial hyperresponsiveness. Mediators Inflamm.
2008:7814252008. View Article : Google Scholar
|
|
15
|
Masi G and Brovedani P: The hippocampus,
neurotrophic factors and depression: Possible implications for the
pharmacotherapy of depression. CNS Drugs. 25:913–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walf AA, Koonce CJ and Frye CA: Adult
female wildtype, but not oestrogen receptor beta knockout, mice
have decreased depression-like behaviour during pro-oestrus and
following administration of oestradiol or diarylpropionitrile. J
Psychopharmacol. 23:442–450. 2009. View Article : Google Scholar
|
|
17
|
Schmidt HD and Duman RS: The role of
neurotrophic factors in adult hippocampal neurogenesis,
antidepressant treatments and animal models of depressive-like
behavior. Behav Pharmacol. 18:391–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerrits M, Westenbroek C, Koch T,
Grootkarzijn A and ter Horst GJ: Increased lim-bic phosphorylated
extra-cellular-regulated kinase 1 and 2 expression after chronic
stress is reduced by cyclic 17beta-estradiol administration.
Neuroscience. 142:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong HJ, Kim JH, Kim NR, Yoou MS, Nam SY,
Kim KY, Choi Y, Jang JB, Kang IC, Baek NI and Kim HM:
Antidepressant effect of Stillen. Arch Pharm Res. 38:1223–1231.
2015. View Article : Google Scholar
|
|
20
|
Hughes ZA, Liu F, Platt BJ, Dwyer JM,
Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S and Day
M: WAY-200070, a selective agonist of estrogen receptor beta as a
potential novel anxiolytic/antidepressant agent. Neuropharmacology.
54:1136–1142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petit-Demouliere B, Chenu F and Bourin M:
Forced swimming test in mice: A review of antidepressant activity.
Psychopharmacology (Berl). 177:245–255. 2005. View Article : Google Scholar
|
|
22
|
Zheng M, Fan Y, Shi D and Liu C:
Antidepressant-like effect of flavonoids extracted from Apocynum
venetum leaves on brain monoamine levels and dopaminergic system. J
Ethnopharmacol. 147:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delgado PL: Depression: The case for a
monoamine deficiency. J C Psychiatry. 61(Suppl 6): S7–S11.
2000.
|
|
24
|
Kumar N, Dhayabaran D, Nampoothiri M,
Nandakumar K, Puratchikody A, Lalani N, Dawood K and Ghosh A:
Atypical antidepressant activity of 3,4-Bis(3,4-Dimethoxyphenyl)
Furan-2,5-Dione Isolated from heart wood of cedrus deodara, in
rodents. Korean J Physiol Pharmacol. 18:365–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shughrue PJ, Lane MV and Merchenthaler I:
Comparative distribution of estrogen receptor-alpha and -beta mRNA
in the rat central nervous system. J Comp Neurol. 388:507–525.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rocha BA, Fleischer R, Schaeffer JM,
Rohrer SP and Hickey GJ: 17 Beta-estradiol-induced
antidepressant-like effect in the forced swim test is absent in
estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology
(Berl). 179:637–643. 2005. View Article : Google Scholar
|
|
27
|
Imwalle DB, Gustafsson JA and Rissman EF:
Lack of functional estrogen receptor beta influences anxiety
behavior and serotonin content in female mice. Physiol Behav.
84:157–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Binder DK and Scharfman HE: Brain-derived
neurotrophic factor. Growth Factors. 22:123–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee BH and Kim YK: The roles of BDNF in
the pathophysiology of major depression and in antidepressant
treatment. Psychiatry Investing. 7:231–235. 2010. View Article : Google Scholar
|
|
30
|
Heldt SA, Stanek L, Chhatwal JP and
Ressler KJ: Hippocampus-specific deletion of BDNF in adult mice
impairs spatial memory and extinction of aversive memories. Mol
Psychiatry. 12:656–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Jin C, Li Y, Guan S, Han F and
Zhang S: Catalpol improves cholinergic function and reduces
inflammatory cytokines in the senescent mice induced by
D-galactose. Food Chem Toxicol. 58:50–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Capuron L and Miller AH: Immune system to
brain signaling: Neuropsychopharmacological implications. Pharmacol
Ther. 130:226–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen W, Li X, Jia LQ, Wang J, Zhang L, Hou
D, Wang J and Ren L: Neuroprotective activities of catalpol against
CaMKII-dependent apoptosis induced by LPS in PC12 cells. Br J
Pharmacol. 169:1140–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|