Introduction
Stem cell transplantation is a promising novel
treatment strategy for ischemic cardiomyopathy, potentially
resulting in cardiac repair and regeneration. Various different
types of stem and progenitor cells are being investigated to assess
their therapeutic effect (1).
Mesenchymal stem cells (MSCs) are easily isolated and expanded, and
exhibit low immunogenicity (2).
However, their low survival rate after transplantation into damaged
myocardium hinders their therapeutic potential. It has been
demonstrated that the majority of donor MSCs, transplanted into the
challenging microenvironment induced by acute myocardial infarction
(AMI), undergo apoptosis in vivo. In vitro, hypoxia
and serum deprivation (hypoxia/SD) are utilized to imitate the
ischemic environment and have been shown to induce apoptosis in
MSCs (3,4). Therefore, improvement of MSC survival
in an ischemic environment is a key challenge in developing
MSC-mediated treatments in hearts following AMI.
Macrophage migration inhibitory factor (MIF) is a
pleiotropic cytokine expressed in various cell types, including
monocytes/macrophages, smooth muscle cells of the vascular system
and cardiomyocytes (5–7). In addition, it acts as a regulatory
factor for inflammation, apoptosis, autophagy, and glucose
catabolism (8–11). Furthermore, MIF provides cardiac
protection by promoting energy uptake under stress (9,12).
MIF has been identified as an anti-apoptotic agent in various cell
types (8,13), and has recently been demonstrated
to protect cardiomyocytes from apoptosis by modulating autophagy
under stress conditions (9).
Autophagy is induced by MIF in conditions such as inflammation and
starvation to counter the stress (14). Previous studies have indicated that
MIF is involved in the maintenance of cardiac contractile function
during starvation and protects cardiomyocytes from apoptosis by
regulating autophagy (9). The
above-mentioned studies demonstrate that MIF may also function in a
protective capacity against hypoxia/SD-induced apoptosis in MSCs
via autophagic regulation (15).
Autophagy is a catabolic process that maintains
cellular homeostasis despite a variety of cellular stresses,
including nutrient starvation, infection, damaged organelles and
protein aggregates (16,17). Autophagy and apoptosis are two
major pathways utilized in a cell in response to stress. They are
commonly co-regulated, but result in opposite cellular outcomes
(18). Autophagy is a
self-catabolic process where double-membrane vacuoles, called
autophagosomes, form around components of the cell and degrade them
utilizing lysosomal machinery (19). Autophagy is also critical in
adaptive survival during periods of metabolic starvation or stress
by maintaining nutrient availability and energy levels. By
contrast, apoptosis is the self-elimination of a damaged or
non-functional cell (20).
Numerous studies have identified that stressors may trigger either
process depending on the cellular context (21,22).
Inhibition of either pathway, by genetic or chemical methods,
results in the activation of the other; blocking apoptosis in cells
that would usually die triggers autophagy and survival, whereas
blocking induction of autophagy in cells that would usually survive
rapidly induces apoptosis (23,24).
Recent studies have confirmed that autophagy has a protective
effect on MSCs under stress conditions (25). Therefore, modulation of autophagy
may provide a novel mechanism to prevent apoptosis in MSCs under
stress conditions.
Notably, previous studies have demonstrated the
AMP-activated protein kinase/mammalian target of rapamycin
(AMPK/mTOR) signaling pathway is activated by MIF to regulate
autophagy and protect cells from apoptosis (9,13,26,27).
In hypoxia/SD, or other energy shortage situations, AMPK may sense
the cellular energy change and become activated by a decreased
ATP/AMP ratio, thus indirectly activating mTOR, one of its major
downstream targets (9). Activation
of the AMPK/mTOR signaling pathway may in turn stimulate autophagy
and exert an anti-apoptotic effect (15). The AMPK/mTOR signaling pathway has
been demonstrated to exert a protective role in MSCs (15,28).
Thus, activation of the AMPK/mTOR signaling pathway in MSCs may be
a positive regulator of autophagy resulting in MSCs that are
resistant to hypoxia/SD-induced apoptosis.
Materials and methods
Animals
Male Sprague Dawley rats, weighing 60–80 g, were
cared for in accordance with the U.S. National Institutes of Health
(NIH) guidelines (29). All of the
study procedures were approved by the Wenzhou Medical University
Institutional Animal Care and Use Committee (Wenzhou, China).
Furthermore, the present study was conducted in compliance with the
Guide for the Care and Use of Laboratory Animals, published by the
National Academy Press (NIH; revised 1996). Ethical approval was
obtained from Wenzhou Medical University Ethical Research
Committee. The mice were raised in the Wenzhou Medical University
Laboratory Animals Center, and raised separately, and fed with
nourishment supplied by Research Diets, Inc. (New Brunswick, NJ,
USA). The animals were housed in a light/dark cycle of 12h
light/12h dark at 20–25°C.
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from GE Healthcare Life Sciences
(Hyclone; Logan, UT, USA). The Annexin V-fluorescein isothiocyanate
(FITC) Apoptosis Detection kit was obtained from BD Pharmingen (San
Diego, CA, USA). Rabbit anti-rat monoclonal antibodies,
LC3BI/LC3BII (1:1,000; cat. no. 3868), Beclin-1 (1:750; cat. no.
3495), autophagy protein 5 (Atg5; 1:1,000; cat. no. 5831), AMPK
(1:1,000; cat. no. 5831), phosphorylated (p)-AMPK (1:500; cat. no.
2325), p-mTOR (1:500; cat. no. 2971) and mTOR (1:1,000; cat. no.
2972) were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Mouse polyclonal antibody anti-β-actin (1:1,000; cat. no.
TA-09) was purchased from Beijing Zhongshan Goldenbridge
Biotechnology, Co., Ltd. (Beijing, China). Anti-mouse/anti-rabbit
secondary antibody horseradish peroxidase conjugate (1:1,000; cat.
no. sc-2954) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) and 3-methyladenine (3-MA) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). AMPK inhibitor, Compound C was
obtained from Merck Millipore (Darmstadt, Germany). Rat recombinant
MIF was obtained from Prospec-Tany TechnoGene, Ltd. (East
Brunswick, NJ, USA).
Cell culture and treatment
Bone marrow-derived (BM)-MSCs were isolated from the
femurs and tibias of Sprague-Dawley rats, as described in a
previous study (30). Bone marrow
cells were flushed from the femurs and tibias with 5 ml DMEM/F12
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
After the red blood cells were lysed with red cell lysis buffer
(Beyotime Institute of Biotechnology, Inc., Jiangsu, China) and
removed, 5×105 cells were seeded in a 25-cm2
flask with 6 ml DMEM/F12 supplemented with 10% FBS and 1% 0.06 mL
penicillin/streptomycin, (Beyotime Institute of Biotechnology,
Inc.) and incubated at 37°C with 5% CO2. Following three
days of culture, non-adherent cells were removed, the medium was
replaced and adherent MSCs were grown in the medium, which was
replaced every three days. Upon reaching 80–90% confluence,
adherent cells were trypsinized (Beyotime Institute of
Biotechnology, Inc.) and expanded at 2:3 or 1:2 dilutions. All of
the cells used in the assay were from passages three to five.
Induction of apoptosis in vitro by
hypoxia/SD, which was designed to mimic the in vivo
conditions of the ischemic myocardium, was initiated, as described
in a previous study (4). Cells
exposed to hypoxia/SD alone served as apoptotic controls, with
hypoxia induced by incubating MSCs in serum-free media in a glove
box (Plas Labs 855-AC; Plas Labs, Inc., Lansing, MI, USA) under a
controlled anaerobic atmosphere at 37°C to scavenge free oxygen.
MIF (100 ng/ml) was added to the medium at the start of the
hypoxia/SD exposure and incubation continued for 24 h under hypoxic
conditions. The concentration of MIF used in the current study was
based on the concentration administered in previous studies
(8,13).
Cells were preincubated with 10 mmol/l AMPK
inhibitor, Compound C or 5 mmol/l autophagy inhibitor, 3-MA in
complete medium under normoxic conditions for 90 min. MIF (100
ng/ml) was then added to the cultures and the cells were placed
under conditions of hypoxia/SD for 24 h.
Flow cytometric analysis of cell
apoptosis
Apoptosis was determined by detecting
phosphatidylserine on cell membranes using the fluorescent dye,
Annexin V-FITC Apoptosis Detection kit (BD Pharmingen), according
to the manufacturer's protocols. This assay differentiates between
intact [Annexin V−/propidium iodide (PI)−],
early apoptotic (Annexin V+/PI−), late
apoptotic (Annexin V+/PI+) and necrotic
(Annexin V−/PI+) cells. Cells were harvested
and washed three times in ice-cold phosphate-buffered saline (PBS;
Beyotime Institute of Biotechnology, Inc.), resuspended in 300
μl binding buffer and incubated with 5 μl Annexin
V-FITC solution for 30 min at 4°C in the dark. This was followed by
further incubation with 5 μl PI for 5 min and immediate
analysis by bivariate flow cytometry using the BD FACSCantoII
equipped with FACSDiva software (642218 Rev.A; BD Biosciences,
Franklin Lakes, NJ, USA). Approximately 1–5×105 cells
were analyzed in each experiment.
Toxicity assay
The potential toxicity of MIF at varying
concentrations was investigated in cultured MSCs. MSCs were
incubated for 24 h at 37°C in culture medium with MIF at increasing
concentrations from 1 to 1,000 ng/ml. Trypan Blue (Beyotime
Institute of Biotechnology, Inc.) was added to the medium, and
cells were incubated at 37°C for 15 min, phase contrast
photomicrographs were taken using an inverted microscope (DMI4000B;
Leica, Wetzlar, Germany), and the Trypan Blue-positive cells were
counted. Five fields were randomly selected from each culture dish
and at least three dishes were counted for each MIF
concentration.
Western blot analysis
Western blot analysis was conducted, as previously
described (31). Cells were washed
twice with ice-cold PBS and lysed with lysis buffer (Beyotime
Institute of Biotechnology, Inc.) containing 20 mM Tris-HCl, 150 mM
NaCl, 1% Triton X-100, and protease and phosphatase inhibitors.
Cell extracts were centrifuged for 5 min at 12,000 × g and
supernatants were collected. Cellular proteins (20 μg) were
resolved with SDS-PAGE (Beyotime Institute of Biotechnology, Inc.)
at 1.5 mA/cm2 for 90 min and transferred onto
polyvinylidene fluoride membranes (Beyotime Institute of
Biotechnology, Inc.). The membranes were blocked for 1 h with 5%
skimmed milk in Tris-buffered saline containing 0.1% Tween-20
(Beyotime Institute of Biotechnology, Inc.) and incubated overnight
at 4°C with the above-mentioned primary antibodies. The membranes
were washed and incubated for 1 h with the appropriate secondary
antibodies conjugated to horseradish peroxidase. The membranes were
developed using chemiluminescence substrates (Beyotime Institute of
Biotechnology, Inc.), photographed with Bio-Rad ChemiDoc XRS
equipment (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
quantified and analyzed with Quantity One software (v4.62; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are expressed as means ± standard deviations.
Differences among groups were assessed by one-way analysis of
variance. Comparisons between two groups were evaluated using
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
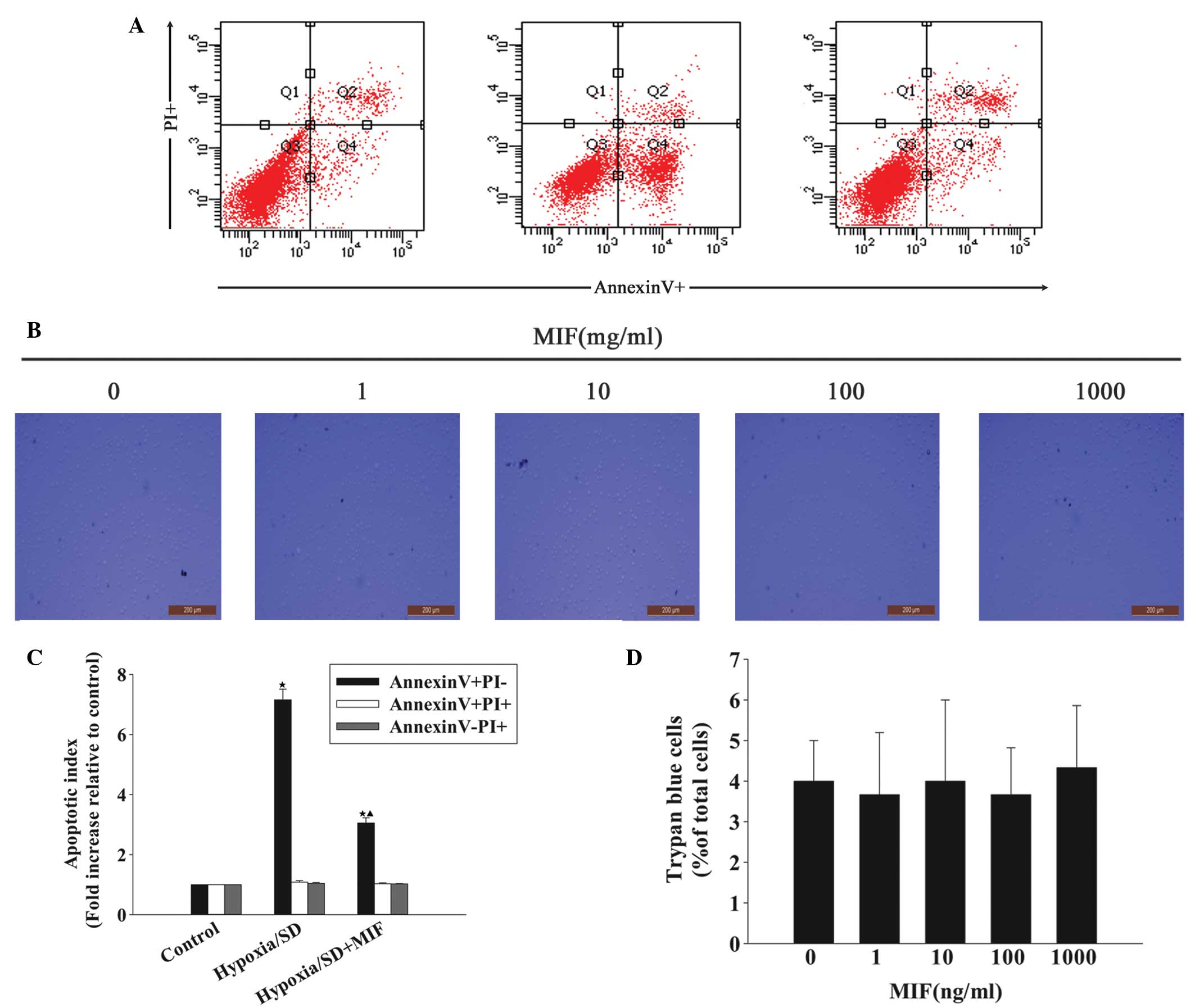
MIF protects MSCs from hypoxia/SD-induced
apoptosis
Previous studies have indicated that the maximal
induction of early apoptosis by hypoxia/SD in MSCs occurs at 24 h
(30). The current study
investigated whether MIF protects MSCs from this process. MSCs were
incubated with 100 ng/ml MIF during exposure to hypoxia/SD for 24
h, and cell apoptosis was then determined by fluorescence-activated
cell sorting (Fig. 1). MIF
efficiently blocked apoptosis and, compared with the hypoxia/SD
cells, the fold increase in the apoptotic index decreased following
exposure to MIF (3.05±0.17 vs. 7.16±0.35; P<0.05; Fig. 1A and C). To the best of our
knowledge, the potential toxicity of MIF to MSCs at these
concentrations has not been investigated, thus, the current study
examined the effect of increasing concentrations of MIF on MSC
viability. Trypan Blue assays indicated that MIF ≤1,000 ng/ml
exerted no adverse effect on the viability of MSCs (Fig. 1B and D).
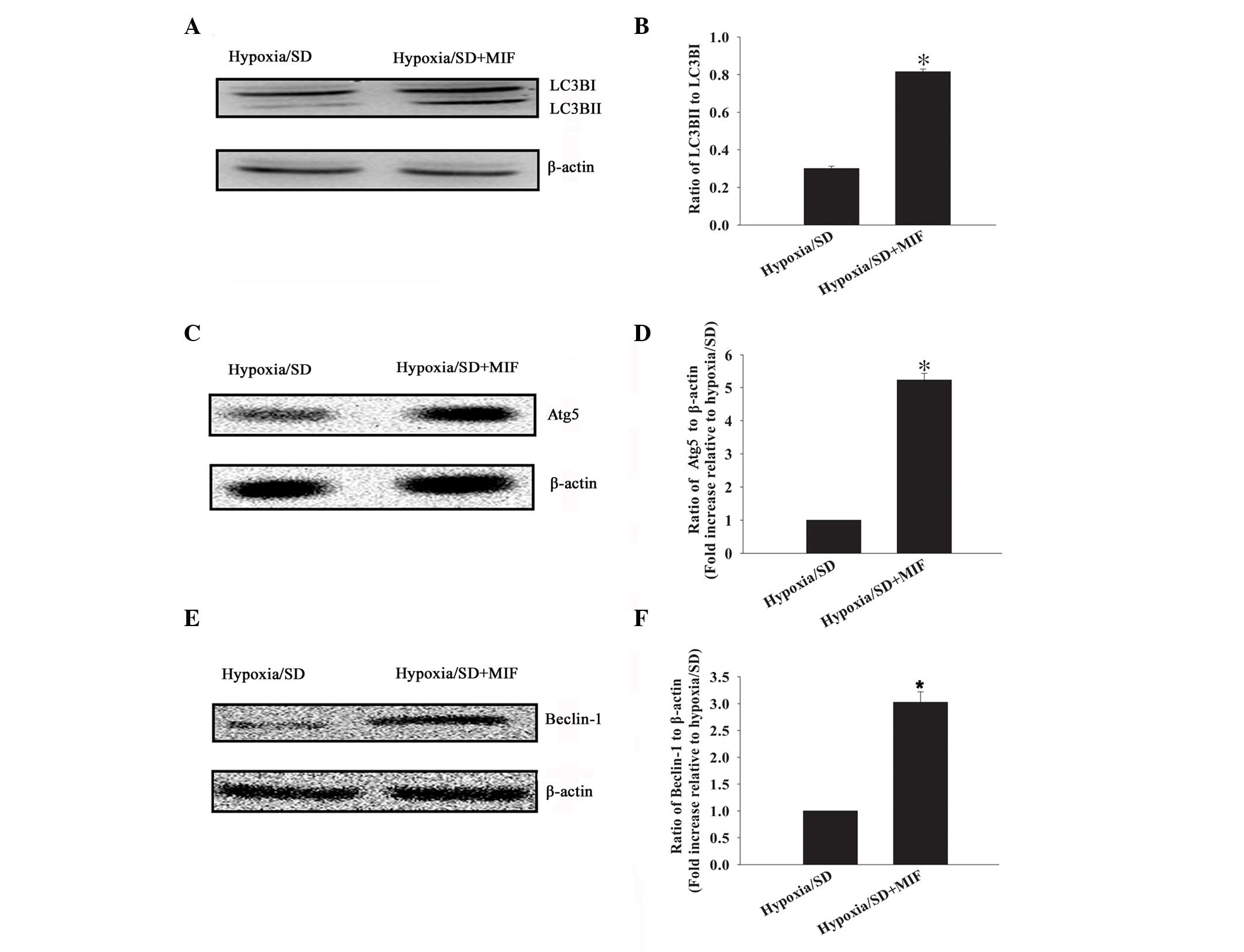
MIF promotes autophagy in MSCs under
hypoxia/SD
Previous studies have demonstrated MIF induces
autophagy in various cell lines under stress conditions (14). The present study investigated
whether MIF, at the concentration that protected MSCs from
apoptosis under hypoxia/SD, also promoted autophagy. MIF was
observed to promote autophagy, as demonstrated by the conversion of
LC3BI to LC3BII (ratio of LC3BII to LC3BI), compared with the
hypoxia/SD+MIF cells: 0.82±0.01 vs. 0.30±0.01 (P<0.05; Fig. 2A and B). MIF-induced activation of
MSC autophagy was further confirmed by the increased expression
level of Atg5 (5.23±0.20 vs. 1.00±0.00; P<0.05; Fig. 2C and D) and Beclin-1 (3.03±0.19 vs.
1.00±0.00; P<0.05; Fig. 2E and
F).
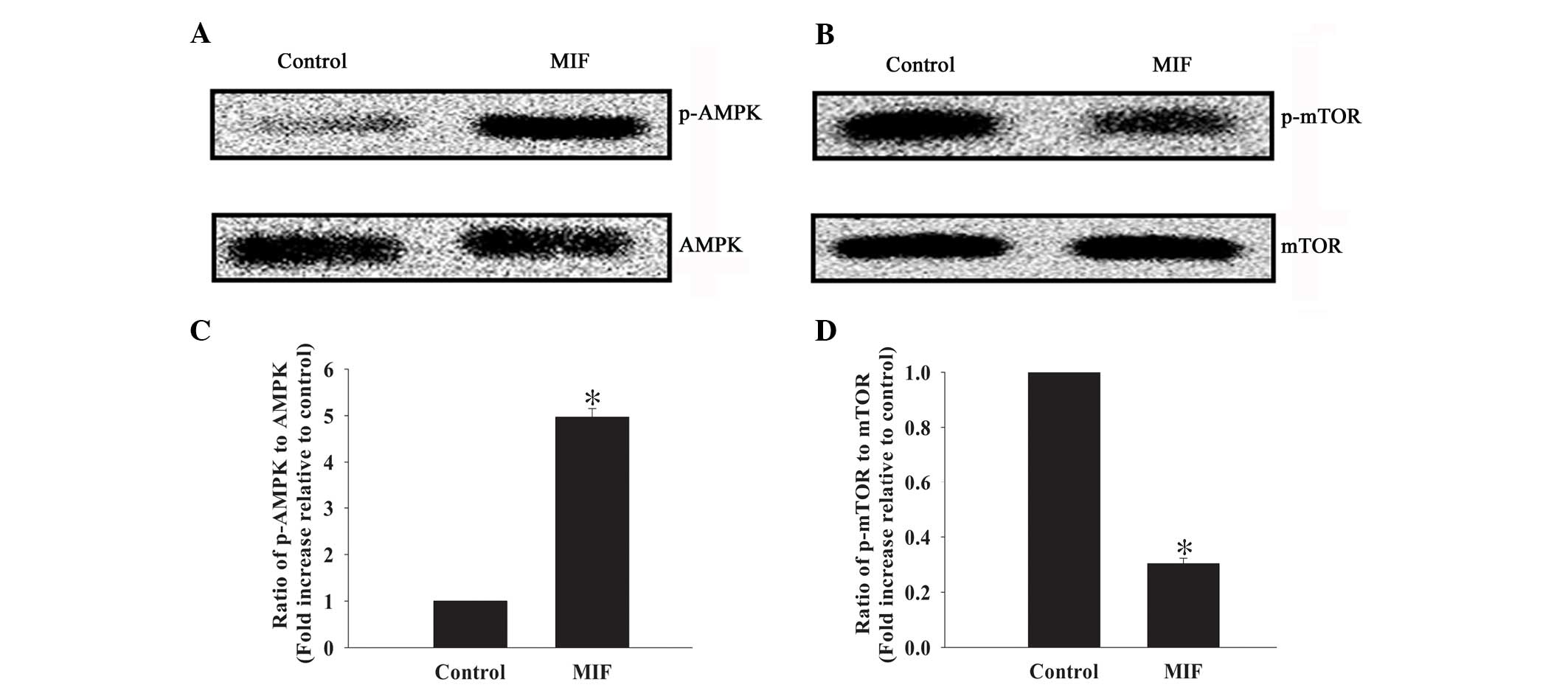
MIF exposure activates the AMPK/mTOR
signaling pathway
The AMPK/mTOR signaling pathway is an important
regulator of autophagy in multiple cell types (15,32).
Therefore, the current study investigated whether this signaling
pathway mediates the anti-apoptotic effect of MIF in MSCs (Fig. 3). Western blot analysis revealed
low but detectable levels of p-AMPK in control cells, but high
levels of p-mTOR. By contrast, MIF exposure induced a marked
increase in the ratio of p-AMPK to AMPK (4.97±0.18 vs. 1.00±0.00;
P<0.05; Fig. 3A and C), but a
decreased ratio of p-mTOR to mTOR (0.30±0.02 vs. 1.00±0.00;
P<0.05; Fig. 3B and D).
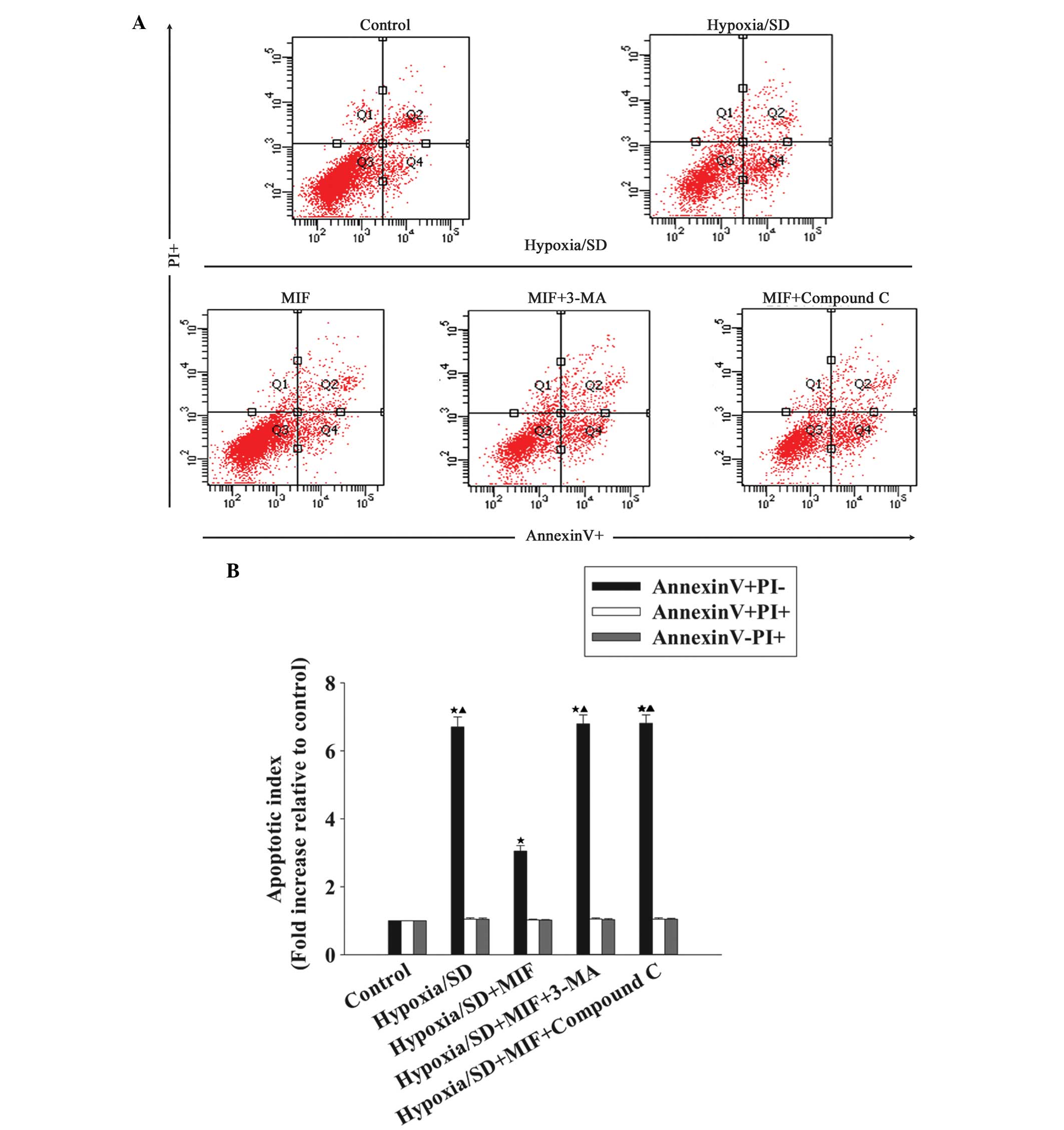
Anti-apoptotic effect of MIF is induced
by autophagy and mediated by the AMPK/mTOR signaling pathway
Previous studies have identified that autophagy
induced by the AMPK/mTOR signaling pathway is important in
resistance to apoptosis (15). To
investigate whether MIF activates the AMPK/mTOR signaling pathway
and induces autophagy to exert an anti-apoptotic effect in MSCs,
the AMPK inhibitor, Compound C or the autophagy inhibitor, 3-MA was
administered to MSCs prior to treatment with MIF. Subsequently, the
cells were exposed to hypoxia/SD. Apoptosis was measured using flow
cytometric assays, which indicated that the fold increase in
apoptotic index relative to the control, following hypoxia/SD
exposure, was decreased by MIF treatment (6.70±0.30 vs. 3.05±0.17;
P<0.05; Fig. 4). This effect
was reversed by the autophagy inhibitor, 3-MA (6.81±0.25 vs.
3.05±0.17; P<0.05) and by the AMPK inhibitor, Compound C
(6.79±0.27 vs. 3.05±0.17; P<0.05), suggesting that autophagy is
induced by the AMPK/mTOR signaling pathway and is involved in the
protection of MSCs against hypoxia/SD-induced apoptosis.
Discussion
Autologous MSCs are effective as therapeutic agents
when administered to regenerate the damaged myocardium, and restore
heart function following transplantation into an ischemic or
infarcted heart. Autologous MSCs are easily prepared from adult
patients and are immunologically safe (2). However, the extent of MSC engraftment
is particularly low despite large numbers of implanted cells, this
is primarily due to low cell survival in the ischemic
microenvironment (33). Upon
transplantation into ischemic tissue, MSCs encounter nutrient and
oxygen deprivation and undergo apoptosis; thus, improving MSC
survival rates under these conditions is a key issue in MSC-based
therapy. In the present study, autophagy induced by MIF is
important in protecting MSCs from hypoxia/SD-induced apoptosis via
AMPK/mTOR-associated signaling pathways, and may enable MSCs to
withstand the growth factor fluctuations and nutrient deprivation
that occurs in the ischemic microenvironment, particularly
following AMI.
MIF is a pluripotent cytokine that is important in
the cellular response to stress (14). MIF contributes to proliferation and
survival by preventing cellular apoptosis. Previous studies
observed that MIF enhances B cell survival, and demonstrated that
MIF promotes cell survival and the proliferation of neural stem
cells, suggesting that MIF may be an effective anti-apoptotic agent
(8,13). Furthermore, MIF regulates key
functions in myocardial ischemia injury and exhibits
cardioprotective activity (12,34,35).
During myocardial ischemia, MIF modulates the activity of various
proteins involved in apoptosis and oxidative stress, including AMPK
and mTOR (36). Myocardial MIF may
function as an endogenous protection mechanism against ischemic
injury; in a previous study of patients with AMI, the serum
concentration of MIF was elevated, suggesting that this pathway is
active during cardiac ischemia (37). Circulating MIF may provide
cardioprotection by elevating energy uptake, but also by protecting
cardiomyocytes from apoptosis (9,12).
The present study suggests that elevating MIF may enhance MSC
survival via activation of pro-survival signaling, and thus may
provide cardioprotection during stem cell-based therapy.
Decreased cell survival under ischemic stress is an
obstacle for stem cell-based therapy. One cellular response, which
is critical for cell survival under metabolic stress and energy
starvation, is autophagy, which is a catabolic process that
delivers cytoplasmic components to lysosomes for degradation
(38). Hypoxia and SD are
energy-limiting stressors and, under these conditions, adaptive
autophagy is essential for cell survival by increasing cellular
energy supply, and eliminating damaged organelles and free radicals
(39,40). Recently MIF has been implicated in
the control of autophagy as a regulator of cellular fate (9). Under stress conditions, MIF induced
autophagy to maintain heart function and protect cardiomyocytes
from apoptosis (9). Similarly, in
the present study, MIF protected MSCs from apoptosis, which was
accompanied by an increase in autophagy. The protective effect of
MIF was eliminated by the autophagy inhibitor, 3-MA, confirming
that MIF exerted its anti-apoptotic effect by modulating autophagy
in MSCs.
AMPK is a stress-signaling kinase, and a key
regulator of energy generation and consumption pathways, which
protect cells against hypoxic injury and cell death (32,41).
The release of endogenous MIF from ischemic myocardium has been
demonstrated to stimulate AMPK activation in a paracrine manner,
leading to enhanced glucose uptake and a beneficial
tissue-protective response (12).
A major mechanism underlying the effect of AMPK is the modulation
of autophagy under stress conditions (9). During hypoxia/SD or other energy
shortage situations, AMPK may act as a sensor of cellular energy
change and become activated by a decreased ATP/AMP ratio (32,41).
In addition, mTOR, a major downstream target of AMPK, stimulates
autophagy by inactivating mTOR complex 1, an inhibitor of the
autophagy pathway (42). Thus, the
AMPK/mTOR signaling pathway is hypothesized to be a positive
regulator of autophagy during hypoxia, starvation, or other energy
stress events (14,42). The current study indicated that MIF
increased AMPK phosphorylation and decreased mTOR phosphorylation,
while inhibition of AMPK blocked the anti-apoptotic effect of MIF.
These results suggest that the AMPK/mTOR signaling pathway serves
as a potential underlying mechanism by which MIF promotes autophagy
in MSCs to resist hypoxia/SD-induced apoptosis.
In conclusion, MIF may be able to promote MSC
survival under conditions that mimic the ischemic myocardium. The
results of the current study suggest that MIF protects MSCs from
hypoxia/SD-induced apoptosis by regulating autophagy via the
AMPK/mTOR signaling pathway. These results highlight potential
novel therapeutic strategies for protecting MSCs from apoptosis,
and provide a basis for the clinical application of MIF and MSCs in
cardiac regeneration therapeutic strategies.
References
|
1
|
Segers VF and Lee RT: Stem-cell therapy
for cardiac disease. Nature. 451:937–942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nesselmann C, Ma N, Bieback K, Wagner W,
Ho A, Konttinen YT, Zhang H, Hinescu ME and Steinhoff G:
Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 12(5B):
1795–1810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li RK, Mickle DA, Weisel RD, Rao V and Jia
ZQ: Optimal time for cardiomyocyte transplantation to maximize
myocardial function after left ventricular injury. Ann Thorac Surg.
72:1957–1963. 2001. View Article : Google Scholar
|
|
4
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar
|
|
5
|
Burger-Kentischer A, Goebel H, Seiler R,
Fraedrich G, Schaefer HE, Dimmeler S, Kleeman R, Bernhagen J and
Ihling C: Expression of macrophage migration inhibitory factor in
different stages of human atherosclerosis. Circulation.
105:1561–1566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calandra T, Bernhagen J, Mitchell RA and
Bucala R: The macrophage is an important and previously
unrecognized source of macrophage migration inhibitory factor. J
Exp Med. 179:1895–1902. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willis MS, Carlson DL, Dimaio JM, White
MD, White DJ, Adams GA IV, Horton JW and Giroir BP: Macrophage
migration inhibitory factor mediates late cardiac dysfunction after
burn injury. Am J Physiol Heart Circ Physiol. 288:H795–H804. 2005.
View Article : Google Scholar
|
|
8
|
Ohta S, Misawa A, Fukaya R, Inoue S,
Kanemura Y, Okano H, Kawakami Y and Toda M: Macrophage migration
inhibitory factor (MIF) promotes cell survival and proliferation of
neural stem/progenitor cells. J Cell Sci. 125:3210–3220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Pacheco BD, Leng L, Bucala R and Ren
J: Macrophage migration inhibitory factor plays a permissive role
in the maintenance of cardiac contractile function under starvation
through regulation of autophagy. Cardiovasc Res. 99:412–421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calandra T, Bernhagen J, Metz CN, Spiegel
LA, Bacher M, Donnelly T, Cerami A and Bucala R: MIF as a
glucocorticoid-induced modulator of cytokine production. Nature.
377:68–71. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benigni F, Atsumi T, Calandra T, Metz C,
Echtenacher B, Peng T and Bucala R: The proinflammatory mediator
macrophage migration inhibitory factor induces glucose catabolism
in muscle. J Clin Invest. 106:1291–1300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller EJ, Li J, Leng L, McDonald C,
Atsumi T, Bucala R and Young LH: Macrophage migration inhibitory
factor stimulates AMP-activated protein kinase in the ischaemic
heart. Nature. 451:578–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gore Y, Starlets D, Maharshak N,
Becker-Herman S, Kaneyuki U, Leng L, Bucala R and Shachar I:
Macrophage migration inhibitory factor induces B cell survival by
activation of a CD74-CD44 receptor complex. J Biol Chem.
283:2784–2792. 2008. View Article : Google Scholar
|
|
14
|
Chuang YC, Su WH, Lei HY, Lin YS, Liu HS,
Chang CP and Yeh TM: Macrophage migration inhibitory factor induces
autophagy via reactive oxygen species generation. PLoS One.
7:e376132012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Yang YJ, Wang H, Dong QT, Wang
TJ, Qian HY and Xu H: Autophagy activation: A novel mechanism of
atorvastatin to protect mesenchymal stem cells from hypoxia and
serum deprivation via AMP-activated protein kinase/mammalian target
of rapamycin pathway. Stem Cells Dev. 21:1321–1332. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar :
|
|
18
|
Ferraro E and Cecconi F: Autophagic and
apoptotic response to stress signals in mammalian cells. Arch
Biochem Biophys. 462:210–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nunnari J and Suomalainen A: Mitochondria:
in sickness and in health. Cell. 148:1145–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74. 2005.
View Article : Google Scholar
|
|
24
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herberg S, Shi X, Johnson MH, Hamrick MW,
Isales CM and Hill WD: Stromal cell-derived factor-1β mediates cell
survival through enhancing autophagy in bone marrow-derived
mesen-chymal stem cells. PLoS One. 8:e582072013. View Article : Google Scholar
|
|
26
|
Nepal S and Park PH: Activation of
autophagy by globular adiponectin attenuates ethanol-induced
apoptosis in HepG2 cells: Involvement of AMPK/FoxO3A axis. Biochim
Biophys Acta. 1833:2111–2125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warr MR, Binnewies M, Flach J, Reynaud D,
Garg T, Malhotra R, Debnath J and Passegué E: FOXO3A directs a
protective autophagy program in haematopoietic stem cells. Nature.
494:323–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Li S, Cui M, Gao X, Sun D, Qin X,
Narsinh K, Li C, Jia H, Li C, et al: Rosuvastatin enhances the
therapeutic efficacy of adipose-derived mesenchymal stem cells for
myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res
Cardiol. 108:3332013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin
L, Zhang MM and Yu B: Novel mechanism of inhibition of dendritic
cells maturation by mesenchymal stem cells via interleukin-10 and
the JAK1/STAT3 sinaling pathway. PLoS One. 8:e554872013. View Article : Google Scholar
|
|
30
|
Hou M, Cui J, Liu J, Liu F, Jiang R, Liu
K, Wang Y, Yin L, Liu W and Yu B: Angiopoietin-like 4 confers
resistance to hypoxia/serum deprivation-induced apoptosis through
PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells.
PloS one. 9:e858082014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Baydoun AR, Xu R, Deng L, Liu X,
Zhu W, Shi L, Cong X, Hu S and Chen X: Lysophosphatidic acid
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Stem Cells. 26:135–145. 2008.
View Article : Google Scholar
|
|
32
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with Akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koga K, Kenessey A, Powell SR, Sison CP,
Miller EJ and Ojamaa K: Macrophage migration inhibitory factor
provides cardioprotection during ischemia/reperfusion by reducing
oxidative stress. Antioxid Redox Signal. 14:1191–1202. 2011.
View Article : Google Scholar
|
|
35
|
Ma H, Wang J, Thomas DP, Tong C, Leng L,
Wang W, Merk M, Zierow S, Bernhagen J, Ren J, et al: Impaired
macrophage migration inhibitory factor-AMP-activated protein kinase
activation and ischemic recovery in the senescent heart.
Circulation. 122:282–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haldar SM and Stamler JS: S-nitrosylation:
Integrator of cardiovascular performance and oxygen delivery. J
Clin Invest. 123:101–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi M, Nishihira J, Shimpo M, Mizue
Y, Ueno S, Mano H, Kobayashi E, Ikeda U and Shimada K: Macrophage
migration inhibitory factor as a redox-sensitive cytokine in
cardiac myocytes. Cardiovasc Res. 52:438–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiang L, Wu C, Ming M, Viollet B and He
YY: Autophagy controls p38 activation to promote cell survival
under genotoxic stress. J Biol Chem. 288:1603–1611. 2013.
View Article : Google Scholar :
|
|
39
|
Zhang H, Bosch-Marce M, Shimoda LA, Tan
YS, Baek JH, Wesley JB, Gonzalez FJ and Semenza GL: Mitochondrial
autophagy is an HIF-1-dependent adaptive metabolic response to
hypoxia. J Biol Chem. 283:10892–10903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hamacher-Brady A, Brady NR, Logue SE,
Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA and Gustafsson AB:
Response to myocardial ischemia/reperfusion injury involves Bnip3
and autophagy. Cell Death Differ. 14:146–157. 2007. View Article : Google Scholar
|
|
41
|
Nagendran J, Waller TJ and Dyck JR: AMPK
signalling and the control of substrate use in the heart. Mol Cell
Endocrinol. 366:180–193. 2013. View Article : Google Scholar
|
|
42
|
Kapahi P, Chen D, Rogers AN, Katewa SD, Li
PW, Thomas EL and Kockel L: With TOR, less is more: A key role for
the conserved nutrient-sensing TOR pathway in aging. Cell Metab.
11:453–465. 2010. View Article : Google Scholar : PubMed/NCBI
|