Introduction
Allergic conjunctivitis is a common ocular allergic
disease, with a high incidence of ~20% of the total population in
China (1). It predominantly occurs
as a result of type I and IV hypersensitivity, of which the main
symptoms include ocular itching, frequently with conjunctival
hyperemia and edema (2). Allergic
conjunctivitis is divided into the following clinical subtypes: i)
Seasonal allergic conjunctivitis; ii) perennial allergic
conjunctivitis; iii) vernal keratoconjunctivitis; iv) atopic
keratoconjunctivitis; and v) giant papillary conjunctivitis
(3). Diagnosis and classification
of allergic conjunctivitis is predominantly based on clinical
features, laboratory or pathological tests (4).
The early reactions in the pathogenesis of allergic
conjunctivitis are mediated by mast cells and T cells. Following
contact with the allergen, the antigen is combined with specific
immunoglobulin E (IgE), resulting in mast cell degranulation and
the release of inflammatory mediators (5). Mast cell degranulation activates
endothelial cells, promoting the expression of chemokines and
adhesion molecules (6). This
attracts inflammatory cells to the conjunctival membrane, and
activates conjunctival fibroblasts and epithelial cells to
participate in the generation of conjunctivitis, with this process
occurring within a few seconds following contact with the antigen,
and the effects lasting from tens of minutes to several hours
(7). In addition, interleukins
(ILs) are released by fibroblasts, and mast cells are activated and
release secondary messengers, promoting the allergic reaction to
enter the late phase (8). The
released cytokines, including IL-4, IL-5, IL-6, IL- 8, IL-13, tumor
necrosis factor-α (TNF-α) and vascular cell adhesion molecule-1,
act on the conjunctiva and recruit inflammatory cells, including
eosinophils, basophils, neutrophils and helper T lymphocytes,
producing the second peak of immune inflammatory reaction (9).
Naphazoline hydrochloride is an adrenergic drug,
stimulating adrenergic α-receptors resulting in vasoconstriction
(10). Clinically, it is
predominantly used for allergic and inflammatory nasal congestion,
acute and chronic rhinitis and eye congestion. Additionally, it is
also used for bacterial and allergic conjunctivitis and reduces
blepharospasm (11). Olopatadine
is a drug with dual effects, as a selective antagonist of histamine
1 receptors and a stabilizer of mast cells, and works faster than
non-steroidal anti-inflammatory agents and mast cell stabilizers
(12). However, the effects and
mechanisms of olopatadine and naphazoline hydrochloride on allergic
conjunctivitis remain to be fully elucidated. The current study
hypothesized that olopatadine and naphazoline hydrochloride are
able to reduce allergic conjunctivitis in mice, with the mechanism
involved associated with effects on inflammation, nerve growth
factor (NGF) and vascular endothelial growth factor (VEGF).
Materials and methods
Animals
A total of 40 female wild-type BALB/c mice (4–5
weeks; 18 g ± 2 g) were housed in the facilities of the Health
Sciences Center of The People's Hospital of Guangxi (Guangxi,
China) and maintained following the Use of Animals in Research and
the internal animal use guidelines (13). All mice were maintained at 23±2°C
and 55% humidity with a 12/12 h light/dark cycle, and received
sterilized food and water ad libitum. The present study was
approved by the Ethics Committee of Guangxi People's Hospital.
Study groups
The mice were divided into five groups: i) Control
group (Con; n=8), mice received physiological saline [0.1 m/100 g,
intraperitoneal injection (i.p.)]; ii) allergic conjunctivitis
model group (AC; n=8), mice were induced by histamine (30
µl, 0.1 mg/ml; Sigma-Aldrich) or ovalbumin (OVA; 30
µl); iii) olopatadine (Sigma-Aldrich, St. Louis, MO, USA)
group (OLO; n=8), allergic conjunctivitis mice received 0.1%
olopatadine solutions (10 µl per eye) (14); iv) naphazoline hydrochloride
(Sigma-Aldrich) group (NH; n=8), allergic conjunctivitis mice
received 0.2 mg/ml naphazoline hydrochloride (10 µl per eye)
(15); v) olopatadine and
naphazoline hydrochloride group (OLO + NH; n=8), allergic
conjunctivitis mice received 1% olopatadine solutions and 0.2 mg/ml
of naphazoline hydrochloride (10 µl per eye) (11).
Histamine-induced conjunctival vascular
hyperpermeability in mice
The mice were narcotized with pentobarbital (50
mg/kg, i.p.), then injected with 30 µl of histamine (0.1
mg/ml) in the upper subconjunctiva following the intravenous
injection of 1.5% w/v Evans blue solution. At 30 min following
treatment, the mice were sacrificed by decollation following
anesthesia with pentobarbital sodium (50 mg/kg; Sigma-Aldrich) and
the treated eye was immediately removed. The tissue sample was
extracted using formamide at 45°C for 5 min and the absorbance was
determined using a spectrophotometer (3550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 625 nm. Values were analyzed as per
weight of each eye.
Antigen-induced conjunctival vascular
hyperpermeability in passively sensitized mice
The mice were narcotized with pentobarbital (50
mg/kg, i.p.), then injected with 30 µl anti-OVA antiserum
(cat. no. C6534; rabbit anti-chicken; Sigma-Aldrich) in the upper
subconjunctiva. Subsequently, 48 h later the animals were subjected
to a challenge by an intravenous injection of OVA (2 mg/ml) with
1.5% w/v Evans blue solution. At 30 min following treatment, the
mice were sacrificed and the treated eye was immediately removed.
Normal saline (10–20 µl) was applied to the tissue sample
for 30 min and the absorbance was determined using a
spectrophotometer (3550; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at 625 nm. Values were analyzed as per weight of each
organization.
Measurement of inflammation
The peripheral blood was collected from the tail
vein and centrifuged at 12,000 × g for 10 min at 4°C and the
supernatants were collected. TNF-α, IL-1β and IL-6 levels were
measured using commercially available enzyme-linked immunosorbent
assay (ELISA) kits according to the manufacturer's instructions
(Lianshuo Biological Technology Co., Ltd., Shanghai, China).
Measurement of cytokine levels
The peripheral blood was collected from the tail
vein and centrifuged at 12,000 × g for 10 min at 4°C and the
supernatants were collected. Interferon (IFN)-γ and IL-4 levels
were measured using commercially available ELISA kits according to
the manufacturer's instructions (Lianshuo Biological Technology
Co., Ltd.).
Measurement of IgE levels
The peripheral blood was collected from the tail
vein and centrifuged at 12,000 × g for 10 min at 4°C and the
supernatants were collected. The IgE levels were measured using a
commercially available ELISA kit according to the manufacturer's
instructions (Lianshuo Biological Technology Co., Ltd.).
Measurement of granulocyte-macrophage
colony-stimulating factor (GMCSF)
Conjunctivas were removed at room temperature. The
removed tissue was immediately homogenized in phosphate-buffered
saline (pH 7.4) containing a protease inhibitor (Shanghai Sangon
Biological Engineering Technology, Shanghai, China). The samples
were centrifuged at 12,000 × g for 10 min at 4°C and the
supernatants were collected. The GMCSF level was measured using a
commercially available ELISA kit according to the manufacturer's
instructions (Lianshuo Biological Technology Co., Ltd.).
Measurement of NGF level
Conjunctivas were removed at room temperature. The
removed tissue was immediately homogenized in phosphate buffered
saline (pH 7.4) containing a protease inhibitor. The samples were
centrifuged at 12,000 × g for 10 min at 4°C and the supernatants
were collected. The NGF level was measured using a commercially
available ELISA kit according to the manufacturer's instructions
(Lianshuo Biological Technology Co., Ltd.).
Western blot analysis
Conjunctivas were removed at room temperature and
immediately homogenized in phosphate buffered saline (pH 7.4)
containing a protease inhibitor. The samples were centrifuged at
12,000 × g for 10 min at 4°C and the supernatants were collected.
The protein content of the samples was quantified using a
bicinchoninic acid assay (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Equal amounts of proteins (50
µg) were separated using 12% sodium dodecyl sulfate
polyacrylamide gel (Sangon Biotech Co., Ltd., Shanghai, China)
electrophoresis and transferred to a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). The membrane was incubated with
antibodies against goat polyclonal anti-VEGF (1:1,000; cat. no.
sc-1876; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and goat
polyclonal β-actin (1:500; cat. no. sc-1616; Santa Cruz
Biotechnology, Inc.) overnight at 4°C with agitation. The proteins
were detected using horseradish peroxidase-conjugated anti-rabbit
secondary antibodies (1:5,000; cat. no. sc-2793; Santa Cruz
Biotechnology, Inc.) at room temperature and visualized with an
enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ,
USA).
Statistical analysis
Values are presented as the mean ± standard error.
SPSS software, version 17 (SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. Statistical analysis was performed
using a one way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
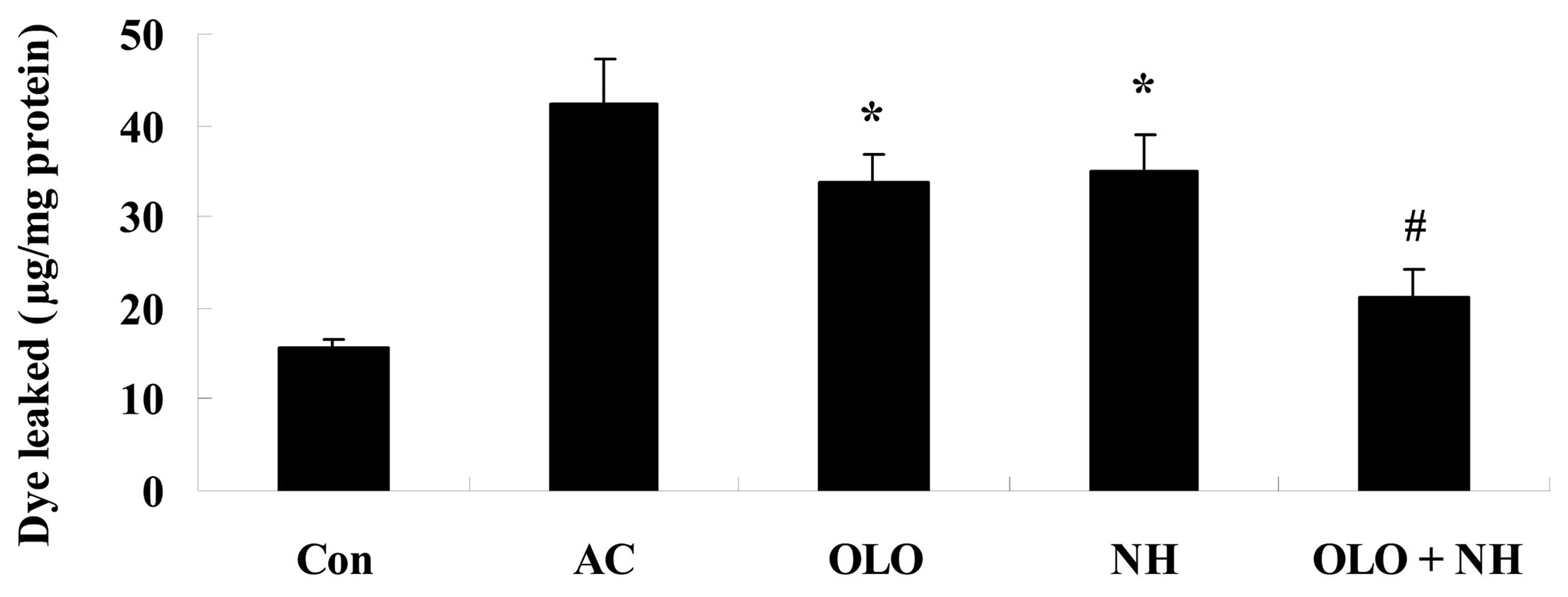
Olopatadine and naphazoline hydrochloride
reduce histamine-induced conjunctival vascular hyperpermeability in
mice
To investigate the effects of olopatadine and
naphazoline hydrochloride on histamine-induced conjunctival
vascular hyperpermeability, mice were induced with histamine. The
amount of conjunctival dye leakage following the injection of
histamine was increased. Treatment with olopatadine or naphazoline
hydrochloride reduced the levels of conjunctival dye leakage
compared with the AC group (Fig.
1). Combined treatment with olopatadine and naphazoline
hydrochloride further reduced conjunctival dye leakage in
histamine-induced mice, compared with the olopatadine alone group
(Fig. 1).
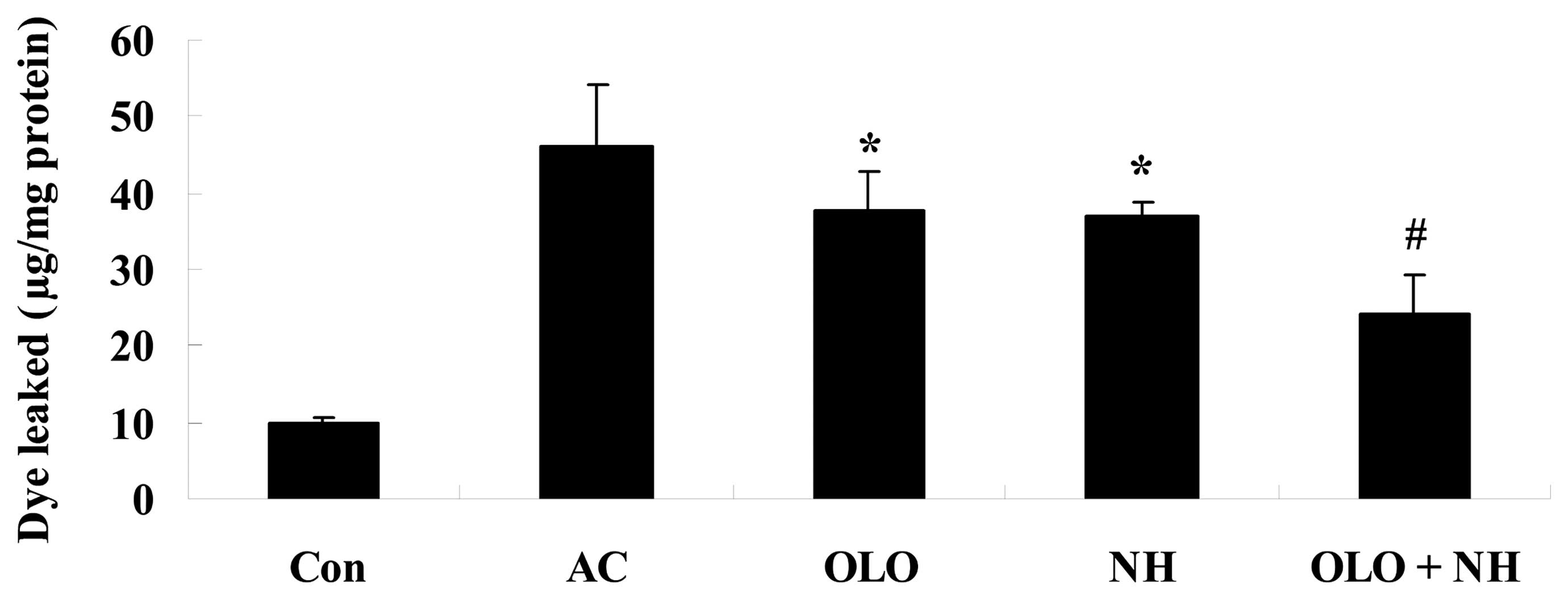
Olopatadine and naphazoline hydrochloride
reduce antigen-induced conjunctival vascular hyperpermeability in
mice
To investigate whether treatment with olopatadine
and naphazoline hydrochloride reduces antigen-induced conjunctival
vascular hyperpermeability, mice were induced using OVA. The amount
of conjunctival dye leaked following the injection of the OVA
antigen was significantly increased. Treatment with olopatadine or
naphazoline hydrochloride reduced the level of conjunctival dye
leakage compared with the AC group (Fig. 2). Combined treatment with
olopatadine and naphazoline hydrochloride further reduced
conjunctival dye leakage in antigen-induced mice, compared with the
olopatadine alone group (Fig.
2).
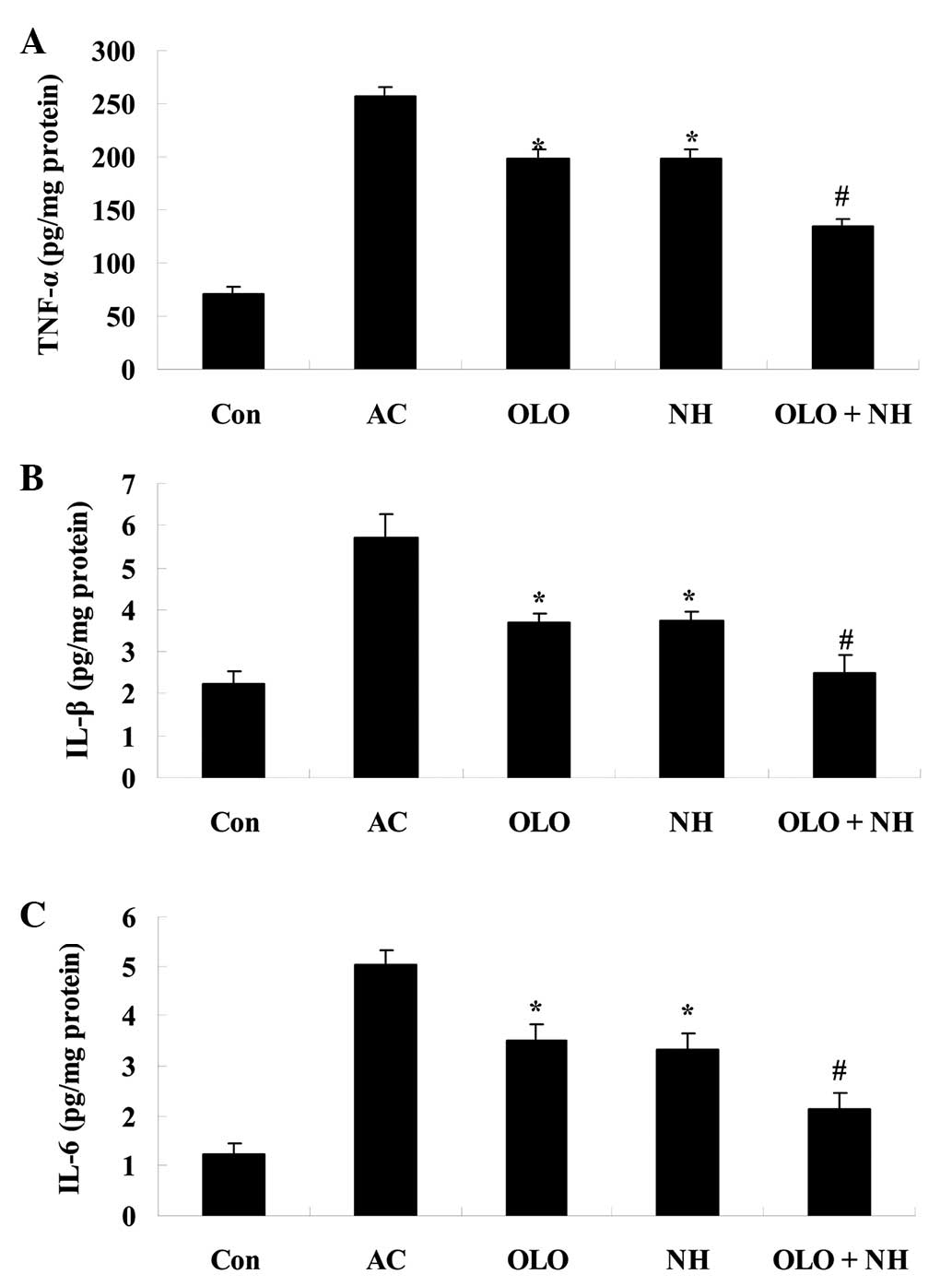
Olopatadine and naphazoline hydrochloride
reduce inflammation in mice with antigen-induced conjunctival
vascular hyperpermeability
The effect of olopatadine and naphazoline
hydrochloride on inflammatory factors was investigated in mice with
antigen-induced conjunctival vascular hyperpermeability. Following
OVA antigen induction, the levels of TNF-α, IL-1β and IL-6 were
observed to increase. Treatment with olopatadine or naphazoline
hydrochloride reduced the levels of the inflammatory factors
compared with the AC group (Fig.
3). Combined treatment with olopatadine and naphazoline
hydrochloride further reduced the levels of the inflammatory
factors in antigen-induced mice, compared with the olopatadine
alone group (Fig. 3).
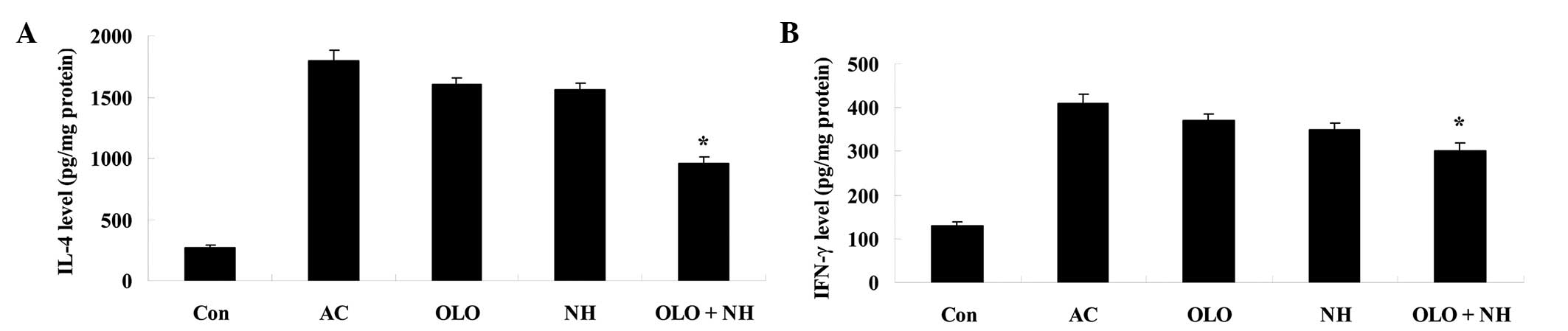
Olopatadine and naphazoline hydrochloride
reduce cytokine levels in mice with antigen-induced conjunctival
vascular hyperpermeability
To further explore the effects of olopatadine and
naphazoline hydrochloride, the cytokine levels were measured in
mice with antigen-induced conjunctival vascular hyperpermeability.
The IFN-γ and IL-4 levels were increased in antigen-induced mice.
Treatment with olopatadine or naphazoline hydrochloride resulted in
a reduction in the cytokine levels, however this was not a
statistically significant difference compared with the AC group
(Fig. 4). Combined treatment with
olopatadine and naphazoline hydrochloride significantly reduced the
levels of IFN-γ and IL-4 in antigen-induced mice, compared with the
olopatadine alone group (Fig.
4).
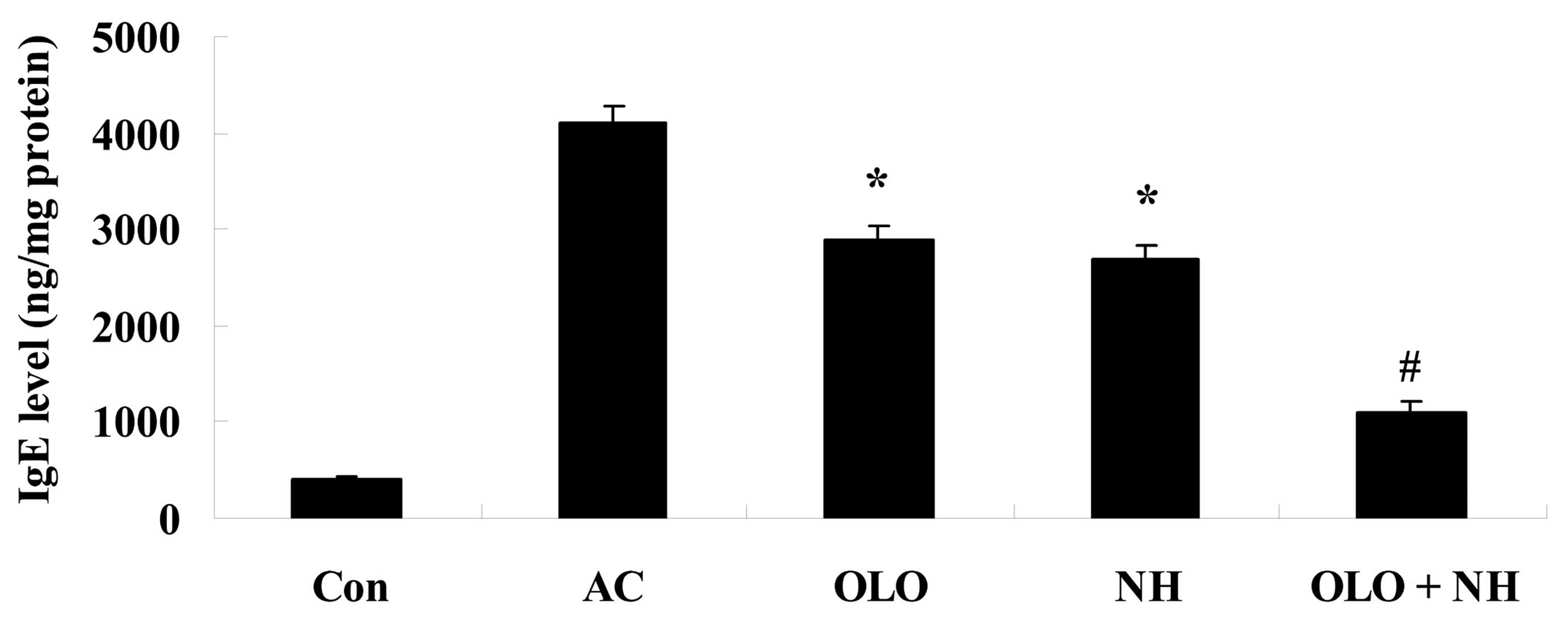
Olopatadine and naphazoline hydrochloride
reduce the levels of IgE in mice with antigen-induced conjunctival
vascular hyperpermeability
Fig. 5 indicates
that the levels of IgE were increased in antigen-induced mice.
Treatment with olopatadine or naphazoline hydrochloride reduced the
levels of IgE compared with the AC group (Fig. 5). Combined treatment with
olopatadine and naphazoline hydrochloride further reduced the
levels of IgE level in antigen-induced mice, compared with the
olopatadine alone group (Fig.
5).
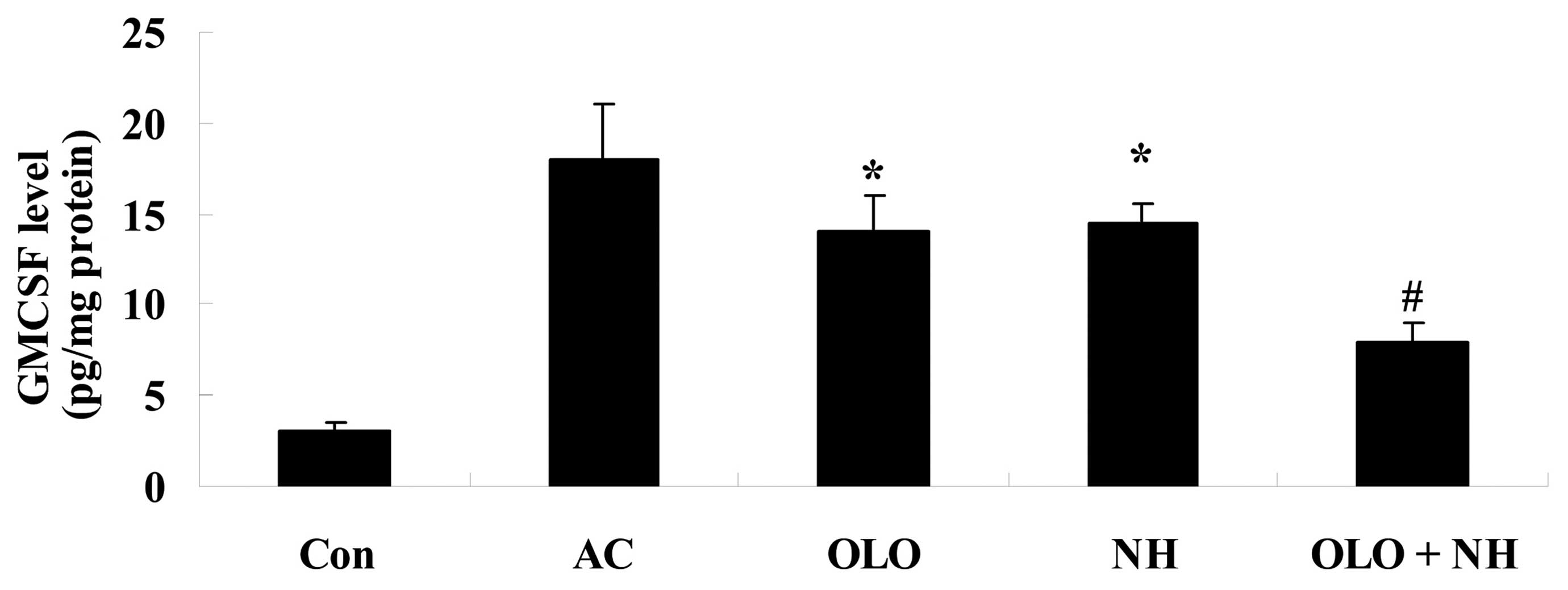
Olopatadine and naphazoline hydrochloride
reduce the levels of GMCSF level in mice with antigen-induced
conjunctival vascular hyperpermeability
Fig. 6 indicates
that the levels of GMCSF were increased in antigen-induced mice.
Treatment with olopatadine or naphazoline hydrochloride reduced the
levels of GMCSF compared with the AC group (Fig. 6). Combined treatment with
olopatadine and naphazoline hydrochloride further reduced the
levels of GMCSF in antigen-induced mice, compared with the
olopatadine alone group (Fig.
6).
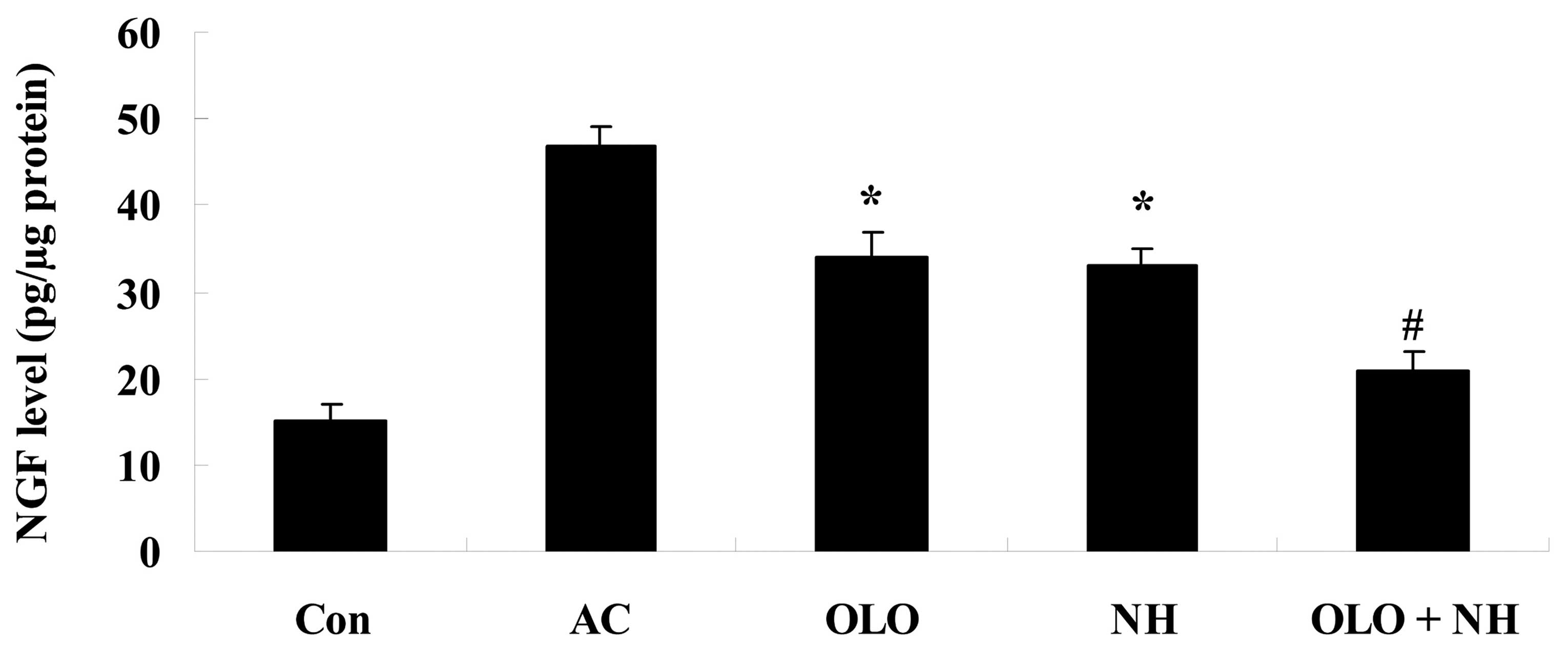
Olopatadine and naphazoline hydrochloride
reduce the levels of NGF in mice with antigen-induced conjunctival
vascular hyperpermeability
Fig. 7 indicates
that the levels of NGF were increased in antigen-induced mice.
Treatment with olopatadine or naphazoline hydrochloride reduced the
levels of NGF compared with the AC group (Fig. 7). Combined treatment with
olopatadine and naphazoline hydrochloride further reduced the
levels of NGF in antigen-induced mice, compared with the
olopatadine alone group (Fig.
7).
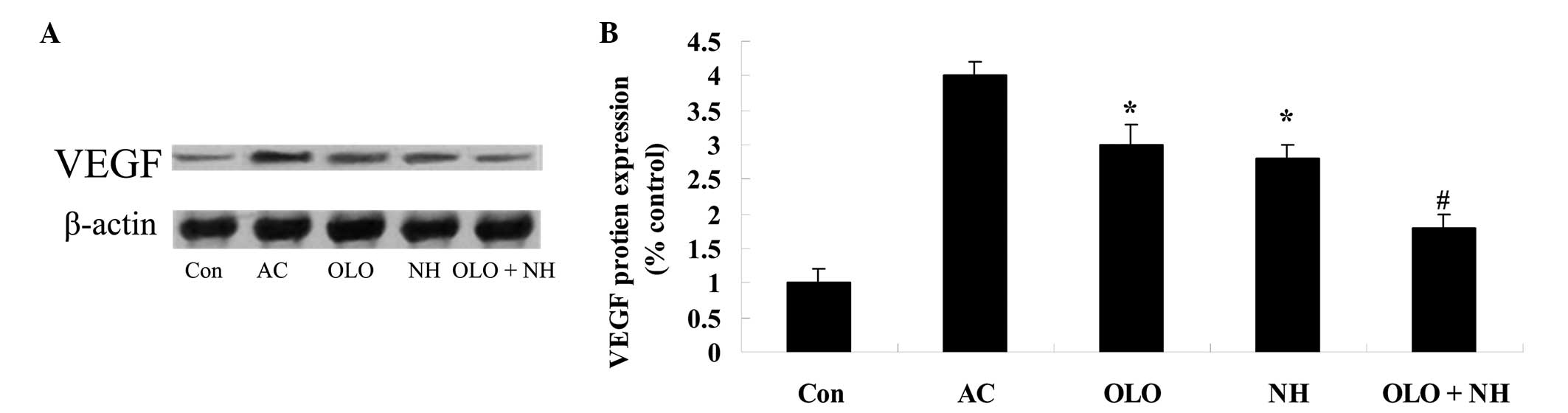
Olopatadine and naphazoline hydrochloride
reduce the expression levels of VEGF in mice with antigen-induced
conjunctival vascular hyperpermeability
To investigate the effects of olopatadine and
naphazoline hydrochloride upon VEGF protein expression in
antigen-induced mice, the VEGF expression levels were measured
using western blot analysis. Fig.
8 indicates that antigen induction increased the expression
levels of VEGF. Treatment with olopatadine or naphazoline
hydrochloride suppressed the elevation of VEGF protein expression
compared with the AC group (Fig.
8). Combined treatment with olopatadine and naphazoline
hydrochloride further reduced the VEGF expression levels in
antigen-induced mice, compared with the olopatadine alone group
(Fig. 8).
Discussion
Conjunctivitis is a common ocular surface disease
with various etiologies, which present with similar symptoms
including eye irritation, eye watering, increased secretions and
congestion (16). The symptoms of
conjunctivitis have a rapid onset and may result in a degree of
damage to the ocular tissue, and in severe cases may result in
blindness, and additional adverse consequences (17). Severe bacterial keratitis is a
sight-threatening disease, which may result in various degrees of
vision loss, with lesions in the central corneal area potentially
resulting in reductions in vision, and in severe cases, corneal
ulcers and corneal perforation, which may eventually require
corneal transplant (18). The
present study demonstrated that olopatadine and naphazoline
hydrochloride significantly reduced the level of conjunctival dye
leakage in mice with histamine or antigen-induced conjunctival
vascular hyperpermeability.
Conjunctival infection may result in systemic
inflammatory responses, and induce increases in the levels of the
inflammatory cytokines, IL-1β, IL-6 and TNF-α (19). Previous studies have indicated that
IL-1β is an important inflammatory cytokine, contributing to
inflammatory injuries and the occurrence of autoimmune diseases,
and participating in an autocrine loop composed of inflammatory
mediators including IL-6, TNF-α and neutrophils (20–22).
The cytokines IL-6 and TNF-α exhibit important biological
activities and are generated by a variety of immune cells in the
body, such as monocyte-macrophage cells (23). In the present study, TNF-α, IL-1β
and IL-6 levels were reduced following treatment with olopatadine
and naphazoline hydrochloride in mice with antigen-induced
conjunctival vascular hyperpermeability. Murota et al
(24) demonstrated that
olopatadine reduced the elevated levels of inflammatory markers in
NC/Nga mice (24). Tamura et
al (25) showed that
olopatadine significantly reduced IL-1β and IL-6 levels induced by
the repeated topical application of oxazolone in rats. However, in
the present study the effect of naphazoline hydrochloride on
inflammatory factors was unremarkable. The effect of olopatadine
solutions and naphazoline hydrochloride on inflammatory factors may
be predominantly concerned with olopatadine.
Previous studies have demonstrated that cluster of
differentiation (CD)4+ T cells may be further divided into Th1-type
and Th2-type cells, according to the surface identity and function
of the T lymphocyte cell subsets (26–28).
Under normal conditions, the Th1/Th2 system of the body maintains a
state of equilibrium; Th1 cell subsets secrete IL-2, IFN-γ and
TNF-α to activate macrophages, with these cells involved in
mediating delayed hypersensitivity responses, which may inhibit the
function of Th2 cells in stimulating B lymphocytes for IgE
synthesis (29). Th2 cells
generate IL-4, 5, 6, 13, 10 and GMCSF, which induces the
differentiation and recruitment of eosinophils, inducing the
transformation of B cell immunoglobulin subtypes and promoting IgE
synthesis and mediating humoral immune responses (30). The present study demonstrated that
the combined treatment with olopatadine and naphazoline
hydrochloride reduced cytokine levels (IFN-γ and IL-4) in mice with
antigen-induced conjunctival vascular hyperpermeability. Tamura
et al (25) previously
demonstrated that olopatadine is able to ameliorate IFN-γ and IL-4
levels in a rat model of experimental cutaneous inflammation
(31).
Allergic conjunctivitis is a type-1
hypersensitivity, mediated by IgE, and eosinophil cationic protein
is the biomarker of eosinophil activity and is able to damage
mucosa epithelium (32). In
addition, eosinophilic basic protein may result in severe allergic
damage (31). A previous study
observed that the levels of total IgE and specific IgE are raised
in the plasma and tears of patients with allergic conjunctivitis.
In addition, B lymphocytes that express CD23, CD21 and CD40 in
conjunctival lymphoid follicles were activated, suggesting that
this type of B lymphocyte may be the precursor cells for IgE
synthesis, and the conjunctiva may serve a catalytic role in IgE
synthesis (33). In the present
study, it was observed that the IgE levels in the olopatadine and
naphazoline hydrochloride treated group was lower compared with the
olopatadine or naphazoline hydrochloride alone groups. Cook et
al (34) reported that
olopatadine inhibits the levels of IgE in human conjunctival mast
cells.
GMCSF is able to stimulate the proliferation and
differentiation of early pluripotent hematopoietic stem cells and
granulocyte-monocyte progenitor cells, and enhance the
collaboration capabilities of mature neutrophils, eosinophils and
monocyte-macrophages (35,36). Additionally, GMSCF stimulates the
growth of erythroid and granulocyte-macrophage progenitor cells in
addition to proliferative hematopoetic progenitor cells, which
prolong the survival time of macrophages and enhance the anti-tumor
capabilities. Furthermore GMCSF is able to stimulate endothelial
cell growth and prevent the apoptosis of various cells (37). GMCSF is an important indicator of
inflammatory stress within the body (38). In the present study, the GMCSF
levels in the olopatadine and naphazoline hydrochloride combination
treatment group were lower compared with the olopatadine or
naphazoline hydrochloride alone groups. Tamura et al
(39) indicated that olopatadine
hydrochloride reduced the inflammatory rebound phenomenon via the
suppression of IL-1β, IL-4, IL-18 and GMCSF levels in mice with
chronic contact hypersensitivity.
Under normal physiological conditions, NGF is a
neurotrophic factor (40). The
elevated levels of NGF in inflammatory reactions have been
indicated to result in the aggravation of pain (41). Previous studies have indicated that
proinflammatory cytokines (IL-1β, TNF-α) are closely associated
with NGF, and promote an increase in the levels of NGF (42–44).
In addition, NGF may act on proinflammatory cytokines in turn
(45). It has been demonstrated
that NGF is able to inhibit inflammation in inflammatory bowel
disease, with increased levels of NGF serving a protective role in
the inflammatory reaction, and suggesting that NGF may in turn act
on proinflammatory cytokines, to inhibit the excessive expression
of proinflammatory cytokines in a potential bio-feedback regulation
mechanism (45–47). In the current study, the NGF levels
were reduced by the treatment with olopatadine and naphazoline
hydrochloride in mice with antigen-induced conjunctival vascular
hyperpermeability. Tamura et al (48) demonstrated that repeated
pretreatment with olopatadine inhibited NGF and VEGF production in
rats (48).
VEGF is a growth factor with heparin-binding
activity, which was purified from the substrate of follicular
stellate cells of bovine pituitary by Ferrara in 1989 for the first
time (49,50). VEGF exerts a strong mitogenic
effect on endothelial cells and is regarded as a vascular
endothelial cell-specific mitogen, inducing the proliferation of
vascular endothelial cells and promoting angiogenesis (51). The biological activity of VEGF
depends on the expression levels, the distribution of the target
cell receptors and its integration with the receptors (52). Previous studies have demonstrated
that hypoxia results in the high expression of VEGF, and that the
alteration in retinal microvascular permeability in diabetic
retinopathy is associated with VEGF (52,53).
In addition, VEGF and the corresponding receptor are integrated,
resulting in retinal endothelial cell proliferation, migration and
contributing to the formation of new blood vessels which destroy
the blood-retina shielding protection in retinal endothelial cells
(54). The current study observed
that olopatadine and naphazoline hydrochloride were able to reduce
the expression levels of VEGF in mice with antigen-induced
conjunctival vascular hyperpermeability. Tamura et al
(55) suggested that the
anti-allergic activity of olopatadine reduced VEGF levels in
sensitized rats.
In conclusion, the current study demonstrated that
treatment with olopatadine and naphazoline hydrochloride reduces
histamine or antigen-induced conjunctival vascular
hyperpermeability in mice. In addition, treatment with olopatadine
and naphazoline hydrochloride reduces inflammatory reactions and
the levels of IL-1β, IL-6, IFN-γ and IL-4. Furthermore, treatment
with olopatadine and naphazoline hydrochloride reduces the levels
of IgE, GMCSF, NGF and VEGF in antigen-induced conjunctival
vascular hyperpermeability mice. These results suggest that
olopatadine and naphazoline hydrochloride are potential treatments
for non-bacterial conjunctivitis.
References
|
1
|
Fujishima H, Ohashi Y and Takamura E:
Efficacy of epinastine hydrochloride ophthalmic solution in
allergic conjunctivitis by conjunctival cedar pollen allergen
challenge. Ann Allergy Asthma Immunol. 113:476–481. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irkec MT and Bozkurt B: Molecular
immunology of allergic conjunctivitis. Curr Opin Allergy Clin
Immunol. 12:534–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams JI, Kennedy KS, Gow JA,
Torkildsen GL, Abelson MB, Gomes PJ and McNamara TR; Bepotastine
Besilate Ophthalmic Solutions Study Group: Prolonged effectiveness
of bepotastine besilate ophthalmic solution for the treatment of
ocular symptoms of allergic conjunctivitis. J Ocul Pharmacol Ther.
27:385–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sgrulletta R and Bonini S, Lambiase A and
Bonini S: Allergy and infections: Long-term improvement of vernal
keratoconjunctivitis following viral conjunctivitis. Eur J
Ophthalmol. 16:470–473. 2006.PubMed/NCBI
|
|
5
|
Chen H, Yang HW, Wei JF and Tao AL: In
silico prediction of the T-cell and IgE-binding epitopes of Per a 6
and Bla g 6 allergens in cockroaches. Mol Med Rep. 10:2130–2136.
2014.PubMed/NCBI
|
|
6
|
Lopes-Ferreira M, Gomes EM, Bruni FM,
Ferreira MJ, Charvet P and Lima C: First report of interruption of
mast cell degranulation and endothelial cells activation by
anti-inflammatory drugs controlling the acute response provoked by
Pseudoplatystoma fasciatum fish venom. Toxicon. 90:237–248. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam DS, Fan DS, Ng JS, Yu CB, Wong CY and
Cheung AY: Ocular hypertensive and anti-inflammatory responses to
different dosages of topical dexamethasone in children: A
randomized trial. Clin Experiment Ophthalmol. 33:252–258. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kase S, Yokoi M, Ishida S and Kase M:
Measurement of interleukins in vitreous infusion fluid. Biomed Rep.
3:818–820. 2015.PubMed/NCBI
|
|
9
|
Waisbourd M, Levinger E, Varssano D,
Moisseiev E, Zayit-Soudri S, Barak A, Loewenstein A and Barequet I:
High-dose topical bevacizumab for corneal neovascularization.
Pharmacology. 92:310–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pemberton JD, MacIntosh PW, Zeglam A and
Fay A: Naphazoline as a confounder in the diagnosis of carotid
artery dissection. Ophthal Plast Reconstr Surg. 31:e33–35. 2015.
View Article : Google Scholar
|
|
11
|
Greiner JV and Udell IJ: A comparison of
the clinical efficacy of pheniramine maleate/naphazoline
hydrochloride ophthalmic solution and olopatadine hydrochloride
ophthalmic solution in the conjunctival allergen challenge model.
Clin Ther. 27:568–577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaurin EB, Marsico NP, Ackerman SL,
Ciolino JB, Williams JM, Villanueva L and Hollander DA: Ocular itch
relief with alcaftadine 0.25% versus olopatadine 0.2% in allergic
conjunctivitis: Pooled analysis of two multicenter randomized
clinical trials. Adv Ther. 31:1059–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamura T: Olopatadine ophthalmic solution
suppresses substance P release in the conjunctivitis models. Asia
Pac Allergy. 2:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Souri E, Amanlou M, Farsam H and Afshari
A: A rapid derivative spectrophotometric method for simultaneous
determination of naphazoline and antazoline in eye drops. Chem
Pharm Bull (Tokyo). 54:119–122. 2006. View Article : Google Scholar
|
|
15
|
Berger WE, Ratner PH, Casale TB, Meltzer
EO and Wall GM: Safety and efficacy of olopatadine hydrochloride
nasal spray 0.6% in pediatric subjects with allergic rhinitis.
Allergy Asthma Proc. 30:612–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curran JP, Nunez JR and Visconti E:
Primary meningococcal conjunctivitis. N Y State J Med. 89:634–635.
1989.PubMed/NCBI
|
|
17
|
Joob B and Wiwanitkit V: Rasanjana Madhu
Ashchyotana for a mucopurulent conjunctivitis. Ayu. 33:1462012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo PC, Lin JY, Chen LC, Fang YT, Cheng
YC, Wu HY, Tsai CY, Chen YS, Lin SY, Wu CL and Ling QD: Molecular
and immunocytochemical identification of coxsackievirus A-24
variant from the acute haemorrhagic conjunctivitis outbreak in
Taiwan in 2007. Eye (Lond). 24:131–136. 2010. View Article : Google Scholar
|
|
19
|
Rank RG, Bowlin AK, Tormanen KI, Wang Y
and Maurelli AT: Effect of inflammatory response on in vivo
competition between two chlamydial variants in the guinea pig model
of inclusion conjunctivitis. Infect Immun. 80:612–619. 2012.
View Article : Google Scholar :
|
|
20
|
Fernandez-Robredo P, Recalde S,
Moreno-Orduña M, García-García L, Zarranz-Ventura J and
García-Layana A: Azithromycin reduces inflammation in a rat model
of acute conjunctivitis. Mol Vis. 19:153–165. 2013.PubMed/NCBI
|
|
21
|
Li Y, Zhang Z, Zhao J, Li H, Ren K and
Xing R: Influences of Bushen Xingnao Decoction on expression of
vascular endothelial growth factor, IL-1β and tumor necrosis
factor-α in vascular dementia rats. Pak J Pharm Sci. 28:2317–2320.
2015.PubMed/NCBI
|
|
22
|
Borkenstein A, Faschinger C, Maier R,
Weger M, Theisl A, Demel U, Graninger W, Irene H and Mossböck G:
Measurement of tumor necrosis factor-alpha, interleukin-6, Fas
ligand, interleukin-1α, and interleukin-1β in the aqueous humor of
patients with open angle glaucoma using multiplex bead analysis.
Mol Vis. 19:2306–2311. 2013.
|
|
23
|
Cavone L, Muzzi M, Mencucci R, Sparatore
B, Pedrazzi M, Moroni F and Chiarugi A: 18β-glycyrrhetic acid
inhibits immune activation triggered by HMGB1, a pro-inflammatory
protein found in the tear fluid during conjunctivitis and
blepharitis. Ocul Immunol Inflamm. 19:180–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murota H, El-latif MA, Tamura T, Amano T
and Katayama I: Olopatadine hydrochloride improves dermatitis score
and inhibits scratch behavior in NC/Nga mice. Int Arch Allergy
Immunol. 153:121–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamura T, Matsubara M, Amano T and Chida
M: Olopatadine ameliorates rat experimental cutaneous inflammation
by improving skin barrier function. Pharmacology. 81:118–126. 2008.
View Article : Google Scholar
|
|
26
|
Liu L, Rich BE, Inobe J, Chen W and Weiner
HL: Induction of Th2 cell differentiation in the primary immune
response: Dendritic cells isolated from adherent cell culture
treated with IL-10 prime naive CD4+ T cells to secrete
IL-4. Int Immunol. 10:1017–1026. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Luo Z, Shen H, Ren C, Li Z, Tang
J, Wang J and Wu T: Research on the roles of transcription factors
T-bet and GATA-3 in aplastic anemia. Clin Lab. 60:291–295.
2014.PubMed/NCBI
|
|
28
|
Krzyzowska M, Polanczyk M, Bas M, Cymerys
J, Schollenberger A, Chiodi F and Niemialtowski M: Mousepox
conjunctivitis: The role of Fas/FasL-mediated apoptosis of
epithelial cells in virus dissemination. J Gen Virol. 86:2007–2018.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mello CB, Ramos L, Gimenes AD, Andrade TR,
Oliani SM and Gil CD: Immunomodulatory effects of galectin-1 on an
IgE-mediated allergic conjunctivitis model. Invest Ophthalmol Vis
Sci. 56:693–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z and Davies JD: CD8 blockade
promotes the expansion of antigen-specific CD4+
FOXP3+ regulatory T cells in vivo. Int Immunopharmacol.
7:249–265. 2007. View Article : Google Scholar :
|
|
31
|
Eperon S, Berguiga M, Ballabeni P,
Guex-Crosier C and Guex-Crosier Y: Total IgE and eotaxin (CCL11)
contents in tears of patients suffering from seasonal allergic
conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 252:1359–1367.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Acar N, Toker E and Kazokoglu H: Tear and
serum eosinophil cationic protein levels in seasonal allergic
conjunctivitis. Eur J Ophthalmol. 13:671–675. 2003.PubMed/NCBI
|
|
33
|
Mimura T, Amano S, Funatsu H, Yamagami S,
Araie M, Kaji Y, Arimoto A, Ishida Y, Usui T and Okamoto S:
Correlations between allergen-specific IgE serum levels in patients
with allergic conjunctivitis in spring. Ocul Immunol Inflamm.
12:45–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cook EB, Stahl JL, Barney NP and Graziano
FM: Olopatadine inhibits TNFalpha release from human conjunctival
mast cells. Ann Allergy Asthma Immunol. 84:504–508. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou W, Chu D, Sha W, Fu L and Li Y:
Effects of granulocyte-macrophage colony-stimulating factor
supplementation in culture medium on embryo quality and pregnancy
outcome of women aged over 35 years. J Assist Reprod Genet. Dec
10–2015.Epub ahead of print.
|
|
36
|
Son BK, Sawaki D, Tomida S, Fujita D,
Aizawa K, Aoki H, Akishita M, Manabe I, Komuro I, Friedman SL, et
al: Granulocyte macrophage colony-stimulating factor is required
for aortic dissection/intramural haematoma. Nat Commun. 6:69942015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang G, Sun T, Zhang L, Wu Q, Zhang K,
Tian Q and Huo R: Combined application of alginate dressing and
human granulocyte-macrophage colony stimulating factor promotes
healing in refractory chronic skin ulcers. Exp Ther Med.
7:1772–1776. 2014.PubMed/NCBI
|
|
38
|
Min L, Isa SA, Fam WN, Sze SK, Beretta O,
Mortellaro A and Ruedl C: Synergism between curdlan and GM-CSF
confers a strong inflammatory signature to dendritic cells. J
Immunol. 188:1789–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tamura T, Matsubara M, Hasegawa K, Ohmori
K and Karasawa A: Olopatadine hydrochloride suppresses the rebound
phenomenon after discontinuation of treatment with a topical
steroid in mice with chronic contact hypersensitivity. Clin Exp
Allergy. 35:97–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Priyanka HP, Sharma U, Gopinath S, Sharma
V, Hima L and ThyagaRajan S: Menstrual cycle and reproductive aging
alters immune reactivity, NGF expression, antioxidant enzyme
activities, and intracellular signaling pathways in the peripheral
blood mononuclear cells of healthy women. Brain Behav Immun.
32:131–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu CP, Wu XR, Li QG, Sun ZW, Wang AP, Feng
JT and Wang J: Proteomic analysis of NGF-induced
transdifferentiation of adrenal medullary cells. Int J Mol Med.
32:347–354. 2013.PubMed/NCBI
|
|
42
|
Mita S, Shimizu Y, Sato A, Notsu T, Imada
K and Kyo S: Dienogest inhibits nerve growth factor expression
induced by tumor necrosis factor-α or interleukin-1β. Fertil
Steril. 101:595–601. 2014. View Article : Google Scholar
|
|
43
|
Taishi P, Churchill L, De A, Obal F Jr and
Krueger JM: Cytokine mRNA induction by interleukin-1beta or tumor
necrosis factor alpha in vitro and in vivo. Brain Res. 1226:89–98.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antonelli A, Lapucci G, Vigneti E, Bonini
S and Aloe L: Human lung fibroblast response to NGF, IL-1beta, and
dexamethsone. Lung. 183:337–351. 2005. View Article : Google Scholar
|
|
45
|
Gruber HE, Hoelscher GL, Bethea S and
Hanley EN Jr: Interleukin 1-beta upregulates brain-derived
neurotrophic factor, neurotrophin 3 and neuropilin 2 gene
expression and NGF production in annulus cells. Biotech Histochem.
87:506–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vieira KP, de Almeida e Silva Lima Zollner
AR, Malaguti C, Vilella CA and de Lima Zollner R: Ganglioside GM1
effects on the expression of nerve growth factor (NGF), Trk-A
receptor, proinflammatory cytokines and on autoimmune diabetes
onset in non-obese diabetic (NOD) mice. Cytokine. 42:92–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ryan VH, German AJ, Wood IS, Hunter L,
Morris P and Trayhurn P: NGF gene expression and secretion by
canine adipocytes in primary culture: upregulation by the
inflammatory mediators LPS and TNFalpha. Horm Metab Res.
40:861–868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tamura T and Kimoto N: Efficacy of
repeated pretreatment with olopatadine hydrochloride on rhinitis
induced by intranasal instillation of toluene-2,4-diisocyanate in
rats. Pharmacology. 84:288–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang WJ, Yang YN, Cao J, Man ZH, Li Y and
Xing YQ: Paxillin regulates vascular endothelial growth factor
A-induced in vitro angiogenesis of human umbilical vein endothelial
cells. Mol Med Rep. 11:1784–1792. 2015.
|
|
50
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L,
Rosen EM and Fan S: Sanguinarine is a novel VEGF inhibitor involved
in the suppression of angiogenesis and cell migration. Mol Clin
Oncol. 1:331–336. 2013.
|
|
52
|
Sahin E, Baycu C, Koparal AT, Burukoglu
Donmez D and Bektur E: Resveratrol reduces IL-6 and VEGF secretion
from co-cultured A549 lung cancer cells and adipose-derived
mesenchymal stem cells. Tumour Biol. Dec 18–2015.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bills VL, Salmon AH, Harper SJ, Overton
TG, Neal CR, Jeffery B, Soothill PW and Bates DO: Impaired vascular
permeability regulation caused by the VEGF(1)(6)(5)b splice variant
in pre-eclampsia. BJOG. 118:1253–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Okabe K, Kobayashi S, Yamada T, Kurihara
T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M
and Kubota Y: Neurons limit angiogenesis by titrating VEGF in
retina. Cell. 159:584–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tamura T: Investigation of the
antiallergic activity of olopatadine on rhinitis induced by
intranasal instillation of antigen in sensitized rats using
thermography. Asia Pac Allergy. 1:138–144. 2011. View Article : Google Scholar : PubMed/NCBI
|