Introduction
Mangiferin is isolated from the leaves, stem bark,
fruit peel and root of Mangifera indica L. and other herbal
species (1). Previous studies have
reported that mangiferin exerts numerous beneficial biological
effects, including antioxidant (2), anti-tumor (3), antibacterial, antiviral and
immunomodulatory activities (4).
In addition, previous studies have indicated that mangiferin exerts
markedly antineoplastic effects toward prostate cancer (5), colon cancer (6,7),
leukemia (5,8–11),
and lung cancer (5,6,11).
It has previously been suggested that mangiferin is able to
markedly inhibit the proliferation of K562 leukemia cells, and
induce cell apoptosis via the downregulation of nuclear factor
(NF)-κB activity (9). Furthermore,
mangiferin has been demonstrated to inhibit telomerase activity of
K562 cells in a time- and- dose-dependent manner, induce apoptosis,
and upregulate the mRNA and protein expression of Fas (10).
Cancer is a complex genetic disease that results
from mutations in oncogenes or tumor suppressor genes, thus leading
to altered signaling pathways (11). It is widely acknowledged that
normal cells are able to check and repair DNA damage in response to
external stimuli, otherwise affected cells would undergo cell
death, by mechanisms including apoptosis and autophagy, if the DNA
lesion was irreparable (12).
Dysfunctional methods of repair or insufficient elimination of
damaged cells will eventually lead to malignant transformation;
therefore, programmed cell death modulation may function as a
potential target of cancer treatment by which damaged and
potentially deleterious cells could be cleared. Apoptotic cells
have long been observed to display a series of morphological
characteristics, including nuclear and cytoplasmic shrinkage,
membrane blebbing, and shattering (13–15),
thus suggesting the existence of common pathways involved in
apoptotic cell death. The caspase family has been identified as a
common pathway that is essential for the progression of apoptosis.
Usually, but not exclusively, apoptosis is associated with the
activation of caspase, and both the extrinsic and intrinsic
apoptotic pathways finally converge to a common process, which
initiates a caspase cascade (16).
The present study demonstrated that mangiferin was
able to trigger G2/M phase cell cycle arrest via
downregulating the cyclin-dependent kinase 1 (cdc2)-cyclin B1
signaling pathway, and induce apoptosis by inhibiting the protein
kinase C (PKC)-NF-κB pathway in A549 human lung carcinoma cells. In
addition, mangiferin exerted anticancer effects in vivo,
where it was able to significantly decrease the volume and weight
of subcutaneous tumor mass, and expand the lifespan of A549
xenograft mice.
Materials and methods
Reagents
Mangiferin was purchased from Shanghai Pureone
Biological Technology Co., Ltd. (Shanghai, China). The purity of
mangiferin was >95%, and the chemical structure is presented in
Fig. 1A. The A375 melanoma cells,
HepG2 and 7721 hepatocellular carcinoma cells, MCF-7 breast
carcinoma cells, non-small cell lung cancer A549 cells,
glioblastoma U87 cells and human embryonic lung fibroblast (HELF)
cells were purchased from American Type Culture Collection
(Manassas, VA, USA), and the normal human embryonic lung fibroblast
(HELF) cell line was purchased from The Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). Fetal bovine serum (FBS) was
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). 3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), z-VAD-fmk (pan-caspase inhibitor), z-DEVD-fmk (caspase-3
inhibitor), z-LEHD-fmk (caspase-9 inhibitor), propidium iodide (PI)
and rhodamine-123 were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
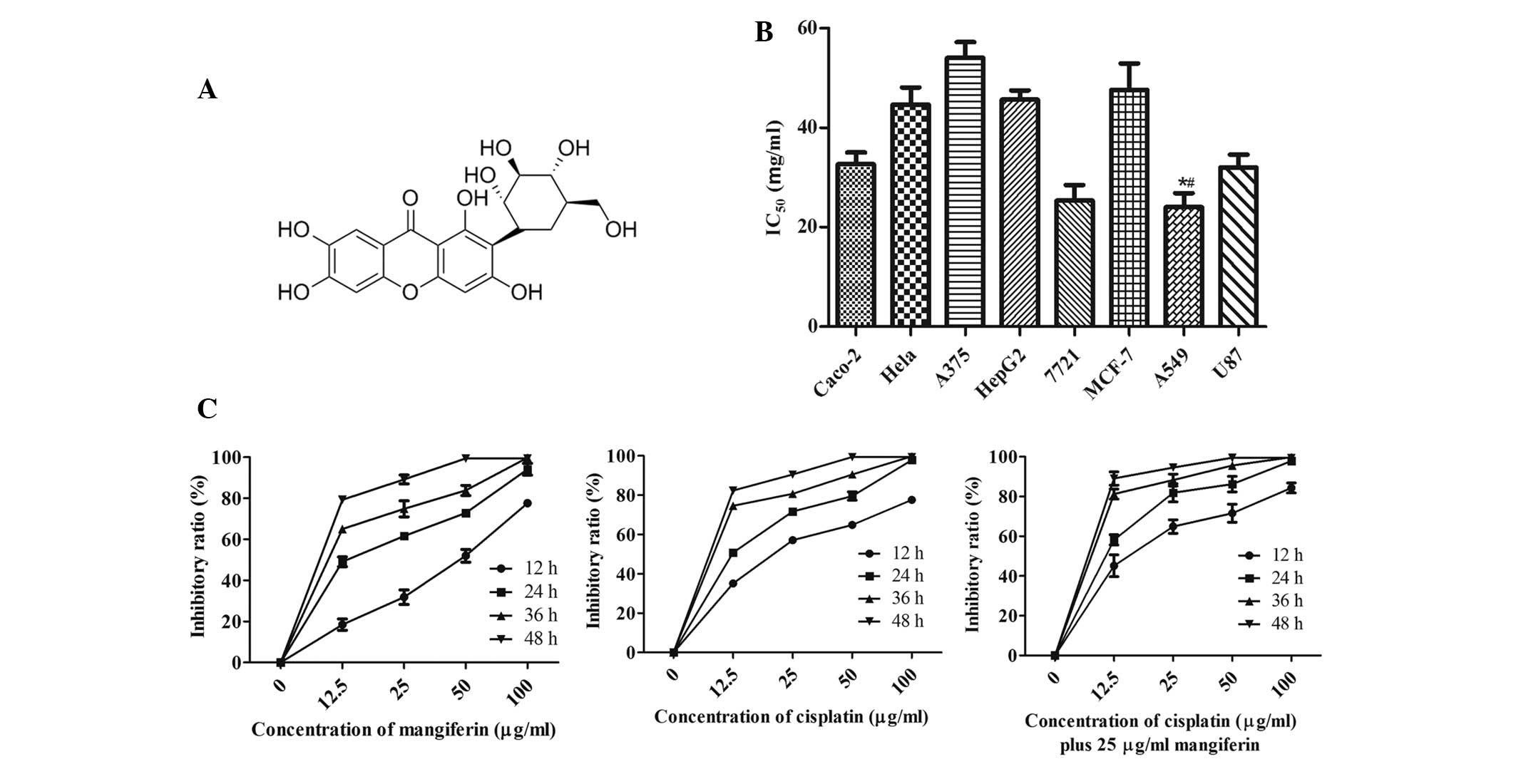 | Figure 1Mangiferin inhibits the proliferation
of A549 cells. (A) Chemical structure of mangiferin. (B) Caco-2
colon carcinoma cells, HeLa cervical carcinoma cells, A549
non-small cell lung cancer cells, HepG2 and 7721 hepatocellular
carcinoma cells, MCF-7 breast carcinoma cells, A375 melanoma cells
and U87 glioblastoma cells were treated with various concentrations
of mangiferin for 24 h, and the half maximal inhibitory
concentration (IC50) values were quantified by MTT
assay. *P<0.05, mangiferin-treated A549 cell group vs
the mangiferin-treated Caco-2 cell group and mangiferin-treated
7721 cell group; #P<0.01, mangiferin-treated A549
cell group vs. all other groups. Data are presented as the mean ±
standard error of the mean (n=3). (C) Inhibitory ratio of A549
cells treated with various concentration of mangiferin (left
panel), cisplatin (middle panel) and cisplatin + 25 µg/ml
mangiferin (right panel) was determined by MTT assay. Data are
presented as the mean ± standard error of the mean (n=3) and
analyzed by two-way ANOVA, P<0.001. |
Cell culture
A549 human lung adenocarcinoma cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS, 100 mg/ml streptomycin
(Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Thermo
Fisher Scientific, Inc.) and 0.03% L-glutamine (Sigma-Aldrich) at
37°C in a humidified atmosphere containing 5% CO2. In
order to generate an in vivo cancer model, a suspension of
A549 cultured human lung adenocarcinoma cells (1.0×107
cells) was inoculated into the neck of 3-month-old male nude mice
(Shanghai Laboratory Animal Research Center, Shanghai, China). The
HELF cells, which were used in the corresponding control group,
were also cultured under the same conditions. For the inhibition of
NF-κB, 100 µM PDTC (Sigma-Aldrich) was incubated with the
cells for 1 h. For inhibition of PKC, 2 µM staurosporine
(Sigma-Aldrich) was incubated with the cells for 1 h.
MTT colorimetric assay
A549 cells at logarithmic growth phase were seeded
in a 96-well plate (3×104 cells per well) and incubated
at 37°C for 24 h. Various concentrations of mangiferin (12.5, 25,
50 and 100 µg/ml) were added to the cells, which were
incubated for a further 12, 24, 36 and 48 h. The control group was
treated with phosphate-buffered saline (PBS; Sigma-Aldrich).
Subsequently, 0.05 mg MTT (10 µl of 5 mg/ml) was added to
each well and incubated at 37°C for 4 h, after which the medium was
removed and the plate was thoroughly agitated for 1 h. Finally,
termination buffer (SDS-HCl) was added to each well and incubated
for 4 h at room temperature. The absorbance was measured at a
wavelength of 570 nm using a spectrophotometer (Model 3550
Microplate Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability was determined using the following equation: Cell
viability (%) = [OD 570 nm (drug)/OD 570 nm (control)] × 100%. OD
indicates optical density.
Observations of cell morphological
changes
A549 and HELF cells were seeded into 6-well culture
plates at a density of 4×105 cells/well in DMEM
containing 10% FBS, and were cultured for 24 h. The control group
was treated with 0.05% dimethyl sulfoxide (DMSO; Sigma-Aldrich),
whereas the mangiferin group was treated with 25 µg/ml
mangiferin. Hoechst 33342 (Sigma-Aldrich) staining was used to
further detect the presence of viable cells. Briefly, cells were
fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min at room
temperature following a 24 h incubation with either mangiferin or
0.05% DMSO. The cells were then washed twice with PBS, and Hoechst
33342 (5 µg/ml) was added to the cells for 15 min, after
which the cells were washed and analyzed immediately using a
fluorescence microscope (Olympus ix73; Olympus Corporation, Tokyo,
Japan).
Measurement of cell cycle progression and
sub-G1 cells
A549 cells treated with 25 µg/ml mangiferin
or 0.05% DMSO at 37°C for 12, 24, 36 and 48 h were harvested and
washed with 0.01 M cold PBS. The cells were then fixed with 70%
ethanol (Sigma-Aldrich) and were incubated at 4°C for 24 h. Cell
pellets were stained with 1 ml PI solution, which consisted of 65
µg/ml PI and 50 µg/ml RNase (Sigma-Aldrich) in 0.01 M
PBS for 30 min at 37°C. The percentage of cells at various phases
of the cell cycle and undergoing apoptosis were evaluated using
FACSCalibur flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA) and analyzed by CellQuest software (version 5.1; Becton
Dickinson, San Jose, CA, USA). In order to further classify the
proportion of early and late apoptotic mangiferin-treated A549
cells, Annexin V-fluorescein isothiocyanate (FITC)/PI double
staining assay was performed using the Annexin V-FITC Apoptosis
Detection kit (BD Pharmingen, San Diego, CA, USA), according to the
manufacturer's protocol.
Caspase assay
A549 cells were seeded into a 6-well culture plate
at a density of 4×105 cells/well for 16 h. Subsequently,
the cells were treated with or without 100 µM z-VAD-fmk
(pan-caspase inhibitor), 50 µM z-DEVD-fmk (caspase-3
inhibitor) and 50 µM z-LEHD-fmk (caspase-9 inhibitor) for 2
h at 37°C. The cells were then treated with 25 µg/ml
mangiferin for 24 h, and the MTT assay was performed as previously
described. In addition, caspase-3 activity was further measured
using a colorimetric assay kit (cat. no. k106-100; BioVision Inc.,
Milpitas, CA, USA), according to the manufacturer's protocol. To
inhibit autophagy, 5 mM 3-methyladenine (3-MA; Sigma-Aldrich) was
incubated with the cells for 6 h. Furthermore, A549 cells underwent
immunofluorescent staining of caspase-3. Briefly, A549 cells were
cultured overnight in a 6-well plate until they reached 90%
confluence. The cells were then treated with 25 µg/ml
mangiferin for 0, 12 and 24 h, and were fixed and stained with
polyclonal rabbit anti-cleaved-caspase-3 antibody (cat. no. 9661;
Cell Signaling Technology, Inc., Beverly, MA, USA). FITC-conjugated
s goat anti-rabbit IgG secondary antibody (cat. no. 11-095-003;
Jackson ImmunoResearch, West Grove, PA, USA) was further applied to
the cells, which were observed under a fluorescent microscope
(Olympus ix73; Olympus Corporation).
Detection of mitochondrial membrane
potential
Mitochondrial membrane potential was measured using
the fluorescent dye rhodamine-123. After treatment with 25
µg/ml mangiferin for 12, 24, 36 and 48 h, the cells were
collected and suspended in 1 ml PBS containing 1 µg/ml
rhodamine-123 for 15 min at 37°C. The fluorescence intensity of the
cells was analyzed by BD FACS Aria II using BD FACS Diva software
(BD Biosciences, San Jose, CA, USA).
Western blot analysis
A549 cells were treated with 25 µg/ml
mangiferin for 12, 24, 36 and 48 h, and both adherent and floating
cells were collected. The cell pellets were resuspended in lysis
buffer and lysed at 4°C for 15 min. The lysis buffer consisted of
50 mmol/l Hepes (pH 7.4), 1% Triton X-100, 2 mmol/l sodium
orthovanadate, 100 mmol/l sodium fluoride, 1 mmol/l edetic acid, 1
mmol/l phenylmethylsulfonyl fluoride, 10 mg/l aprotinin and 10 mg/l
leupeptin (all Sigma-Aldrich). Following centrifugation at 12,000 ×
g for 15 min at 4°C, the protein content of the supernatant was
determined using the Bio-Rad Bradford Protein Assay (Bio-Rad
Laboratories, Inc.). A mitochondria isolation kit (cat. no. 89874;
Thermo Fisher Scientific, Inc.) was applied for the isolation of
mitochondrial protein. Equal quantities of total protein (20
µg) were separated by 4–12% NuPAGE® Bis-Tris gels
(Thermo Fisher Scientific, Inc.) and were transferred to
polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA,
USA). The membranes were soaked in blocking buffer (5% bovine serum
albumin; Sigma-Aldrich), and the proteins of interest were detected
using the following primary antibodies: Rabbit polyclonal
pro-caspase-3 (cat. no. sc-7148; 1:1,000 dilution), rabbit
polyclonal cleaved caspase-3 (cat. no. sc-22171-R; 1:500 dilution),
mouse monoclonal pro-caspase-9 (cat. no. sc-56073; 1:1,000
dilution), goat polyclonal cleaved caspase-9 (cat. no. sc-22182;
1:1,000 dilution), rabbit polyclonal Bax (cat. no. sc-493; 1:500
dilution), rabbit polyclonal Bcl-2 (cat. no. sc-492; 1:1,000
dilution), mouse monoclonal Bcl-XL (cat. no. sc-8392; 1:1,000
dilution), rabbit polyclonal PARP (cat. no. sc-7150; 1:1,000
dilution), rabbit polyclonal cytochrome c (cat. no. sc-7159;
1:1,000 dilution), rabbit polyclonal Prohibitin (cat. no. sc-28259;
1:2,000 dilution), rabbit polyclonal PKC (cat. no. sc-208; 1:1,000
dilution), mouse monoclonal cdc2 (cat. no. sc-54; 1:1,000
dilution), rabbit polyclonal NF-κB (cat. no. sc-109; 1:500
dilution) and mouse monoclonal β-actin (cat. no. sc-47778; 1:5,000
dilution) purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated goat
anti-mouse IgG secondary antibody (cat. no. 11-035-003) and
HRP-conjugated mouse anti-rabbit IgG (cat. no. 211-035-109) were
purchased from Jackson ImmunoResearch Laboratories. The membranes
were incubated with primary antibody overnight at 4°C and then
washed with TBS buffer containing 0.1% Tween-20 (Sigma-Aldrich).
Secondary antibody was incubated at room temperature for 1 h and
washed with TBS buffer containing 0.1% Tween-20. The blots were
visualized using enhanced chemiluminescence (GE Healthcare,
Arlington Heights, IL, USA).
Acute toxicity testing
Acute toxicity testing was performed to determine
the median lethal dose (LD50) of mangiferin. After 16 h
fasting, 80 male nude C57BL mice were randomly divided into eight
groups (n=10 mice/group). Graded doses of mangiferin, dissolved in
PBS (20, 50, 100, 200, 400, 600, 1,000 and 2,000 mg/kg), were
administered intraperitoneally to the mice; the average volume
injected was 0.3 ml. All mice were allowed ad libitum access
to food and water, and the mortality in each group was assessed 24,
48 and 72 h after administration of mangiferin. Percentage
mortality in each group was calculated and plotted against
log10 mangiferin dose. A regression line was fitted by
the method of least squares, and confidence limits for
LD50 values were calculated. Animal handling was in
accordance with the Ethics Committee of Sichuan Academy of Medical
Science & Sichuan Provincial People's Hospital (Medical School,
University of Electronic Science and Technology of China, Chengdu,
China), and all mice were kept under a 12 h light/dark cycle with
ad libitum access to food and water, which is in compliance
with individually ventilated cages requirements at the Sichuan
Academy of Medical Science & Sichuan Provincial People's
Hospital.
Animal group and in vivo xenograft
study
A total of 50 3-month-old male nude C57BL/6 mice
were randomly divided into five groups: Blank control group, mice
administered PBS following injection with A549 cells; high-dose
mangiferin group, mice administered 100 mg/kg mangiferin following
injection with A549 cells; medium-dose mangiferin group, mice
administered 50 mg/kg mangiferin following injection with A549
cells; low-dose mangiferin group, mice administered 10 mg/kg
mangiferin following injection with A549 cells; positive control
(cisplatin) group, mice administered cisplatin (10 mg/kg;
Sigma-Aldrich) following injection with A549 cells. Mangiferin (10,
50 and 100 mg/kg) was intraperitoneally injected into the mice, and
the therapy lasted for 2 weeks. The measurement and calculation of
relative tumor volume, survival rate, inhibitory rate and body
weight was performed as previously described (17). Briefly, tumor volume was determined
using caliper measurements, according to the following formula:
Tvol = length × width × depth × 0.5. Relative
tumor volume (RTV) was calculated as relative increase or decrease
in mean tumor volume from initiation of treatment (V0)
up to value at a given time (Vt) and RTV =
Vt/V0. Inhibitory rate of tumor volume was
determined as follows: Tumor volume inhibition rate =
(Vcontrol × Vt)/Vcontrol × 100%. After 14 days
of treatment, the mice were sacrificed by cervical dislocation, and
subcutaneous tumour mass was determined. Inhibitory rate of tumour
weight was determined as follows: Tumor weight inhibition rate =
(Wcontrol - Wt)/Wcontrol ×
100%.
Statistical analysis
All data are presented as the mean ± standard error
of the mean from at least three independent experiments. Data
analysis was performed using GraphPad Prism 5.0 software (Graphpad
Software, Inc., La Jolla, CA, USA). Statistical significance was
determined by Student's t-test for Figs. 3A, C, E and 4A, 5A
and 6A. Statistical significance
was also determined by two-way analysis of variance for Figs. 1C, 2C, 6B
and 6D. The survival data
(Fig. 6C) were analyzed using the
Kaplan-Meier method.
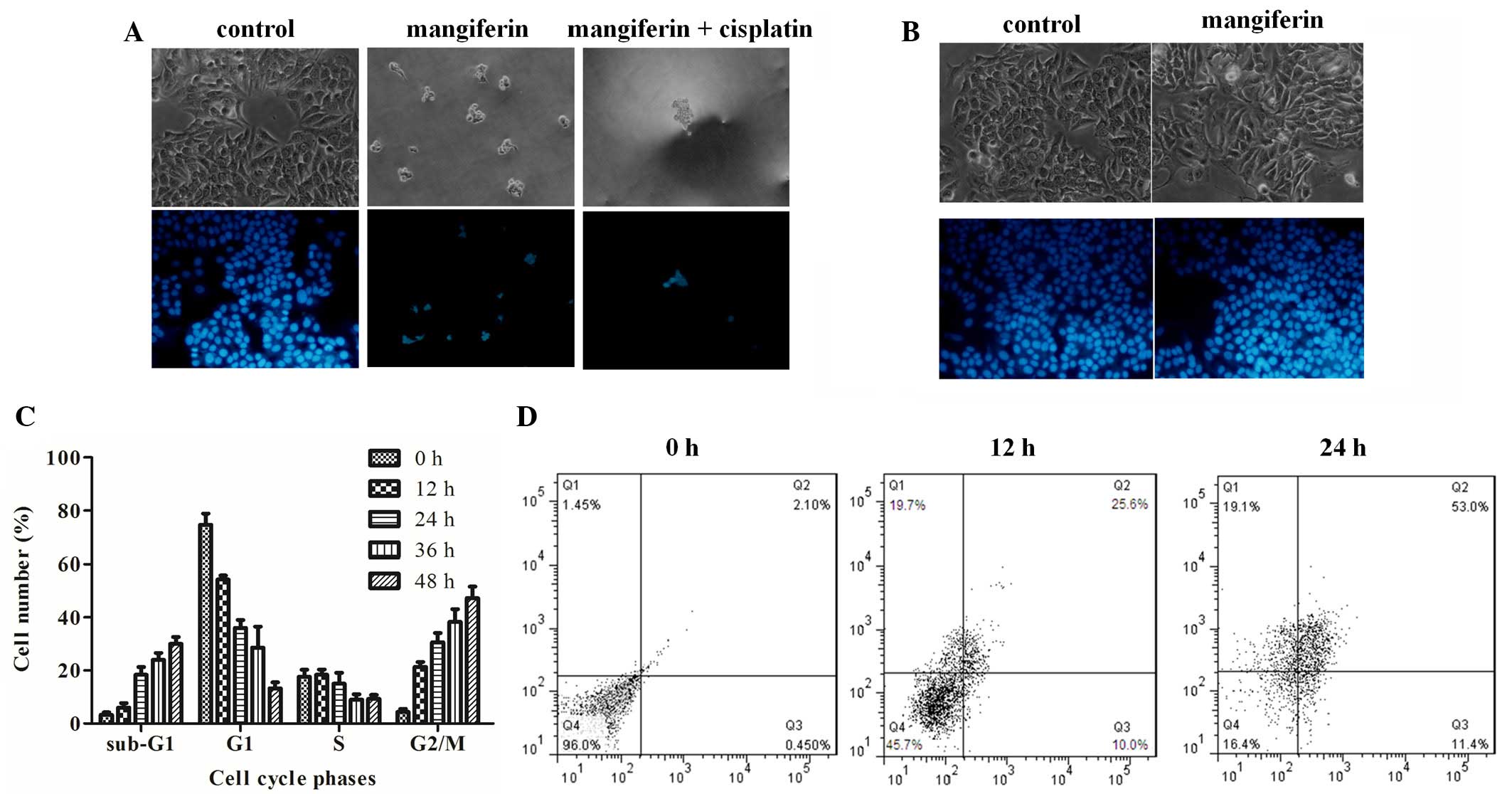 | Figure 2Mangiferin induces apoptosis and
G2/M cell cycle arrest of A549 cells. (A) Apoptotic
morphological observations of A549 cells treated with dimethyl
sulfoxide, 25 µg/ml mangiferin, or mangiferin plus 16
µg/ml cisplatin under phase-contrast microscopy
(magnification, 200×) (upper channel). A549 cells treated with PBS,
50 µg/ml mangiferin, or mangiferin plus 16 µg/ml
cisplatin, were stained with Hoechst 33342 and observed under
fluorescence microscopy (magnification, 200×) (lower channel). (B)
Apoptotic morphological observations of human embryonic lung
fibroblast cells treated with PBS or 25 µg/ml mangiferin
(magnification, ×200; upper channel). Fluorescent staining of HELF
cells treated with either PBS or 25 µg/ml mangiferin,
(magnification, ×200; lower channel). (C) Percentages of A549 cells
within each phase of the cell cycle. The cells were treated with 25
µg/ml mangiferin and were measured by flow cytometry. Data
are presented as the mean ± standard error of the mean (n=3) and
analyzed by two-way ANOVA, P<0.001. (D) A549 cells were treated
with 25 µg/ml mangiferin for 0, 12 or 24 h, and the ratios
of early apoptotic and late apoptotic cells were measured by flow
cytometry using the Annexin V-fluorescein isothiocyanate/propidium
iodide double staining assay. Q1, cell debri; Q, late apoptotic
cells; Q3, early apoptotic cells; Q4, live cells. |
Results
Cytotoxic effects of mangiferin on A549
cells
The growth inhibitory effects of mangiferin were
assessed using the MTT assay on the following human cancer cell
lines: Caco-2 colon carcinoma cells, HeLa cervical carcinoma cells,
A375 melanoma cells, HepG2 and 7721 hepatocellular carcinoma cells,
MCF-7 breast carcinoma cells, non-small cell lung cancer A549 cells
and glioblastoma U87 cells. The half maximal inhibitory
concentration values were 33, 45, 54, 46, 28, 48, 25 and 32
µg/ml, respectively (Fig.
1B). These results indicate that mangiferin was more sensitive
to A549 non-small cell lung cancer cells; therefore, A549 cells
were chosen for further exploration.
To further assess the antitumor properties of
mangiferin in A549 cells, an MTT assay was performed. Mangiferin
was revealed to induce A549 cell death in a dose-dependent manner.
Various doses of mangiferin (0–100 µg/ml) were added to the
culture medium of A549 cells, and even a very low dose of
mangiferin (12.5 µg/ml) exhibited inhibitory effects on cell
proliferation (Fig. 1C). After
incubation with 25 µg/ml mangiferin, the inhibitory rate
reached ~50%, and following treatment with a higher dose of
mangiferin (100 µg/ml), the inhibitory rate reached >60%
at 12 h. These results suggest that the inhibitory efficiency of
mangiferin is comparatively high. Treatment with cisplatin (12.5,
25, 50 or 100 µg/ml) exerted a significant inhibitory effect
on the proliferation of A549 cells, and 12.5 µg/ml cisplatin
resulted in ~50% proliferation inhibition at 24 h (Fig. 1C). Subsequently, a series of doses
of cisplatin in combination with 25 µg/ml mangiferin
(cisplatin + 25 µg/ml mangiferin) were used to treat A549
cells, and cell viability was assessed by MTT assay, at various
time points. Cisplatin + 25 µg/ml mangiferin synergistically
inhibited the proliferation of A549 cells (Fig. 1C); 12.5 µg/ml cisplatin in
combination with 25 µg/ml mangiferin resulted in 50%
inhibitory ratio of A549 cells at 12 h, thus indicating that
mangiferin treatment may amplify the antineoplastic effects of
cisplatin on A549 cells.
Observation of cell morphology and cell
cycle distribution
Subsequently, A549 cells were treated with 0.05%
DMSO, mangiferin alone, or mangiferin plus cisplatin, and cell
morphology was observed under phase contrast microscopy (Fig. 2A, upper channel). Markedly less
viable cells were observed following mangiferin treatment, and even
fewer cells survived mangiferin plus cisplatin treatment. This
result was determined by Hoechst 33342 staining, which marks viable
cells. Conversely, treatment with 25 µg/ml mangiferin did
not result in apparent apoptotic morphology in non-cancerous HELF
cells following a 48 h treatment (Fig.
2B). These results suggest that mangiferin may selectively
induce A549 cells apoptosis, but may not trigger apoptosis of
normal HELF cells. In addition, cell cycle analysis was performed
to determine cell cycle distribution of A549 cells following
treatment with mangiferin. Statistical analysis of the number of
cells at sub-G1, G0/G1, S and
G2/M phase indicated that more cells were arrested in
sub-G1 phase after mangiferin treatment, thus suggesting
that mangiferin may induce apoptosis of A549 cells in a
time-dependent manner. Furthermore, G2/M phase arrest
was observed in the mangiferin-treated cells (Fig. 2C). Annexin V-FITC and PI double
staining was performed to classify the number of viable cells
(Annexin V−/PI−), early apoptotic cells (Annexin V+/PI−), late
apoptotic cells (Annexin V+/PI+) and necrotic cells (Annexin
V−/PI+). As shown in Fig. 2D,
following treatment with 25 µg/ml mangiferin, the percentage
of early apoptotic cells and late apoptotic cells were gradually
enhanced with increasing time. These results indicate that
mangiferin induces apoptotic cell death in A549 cells.
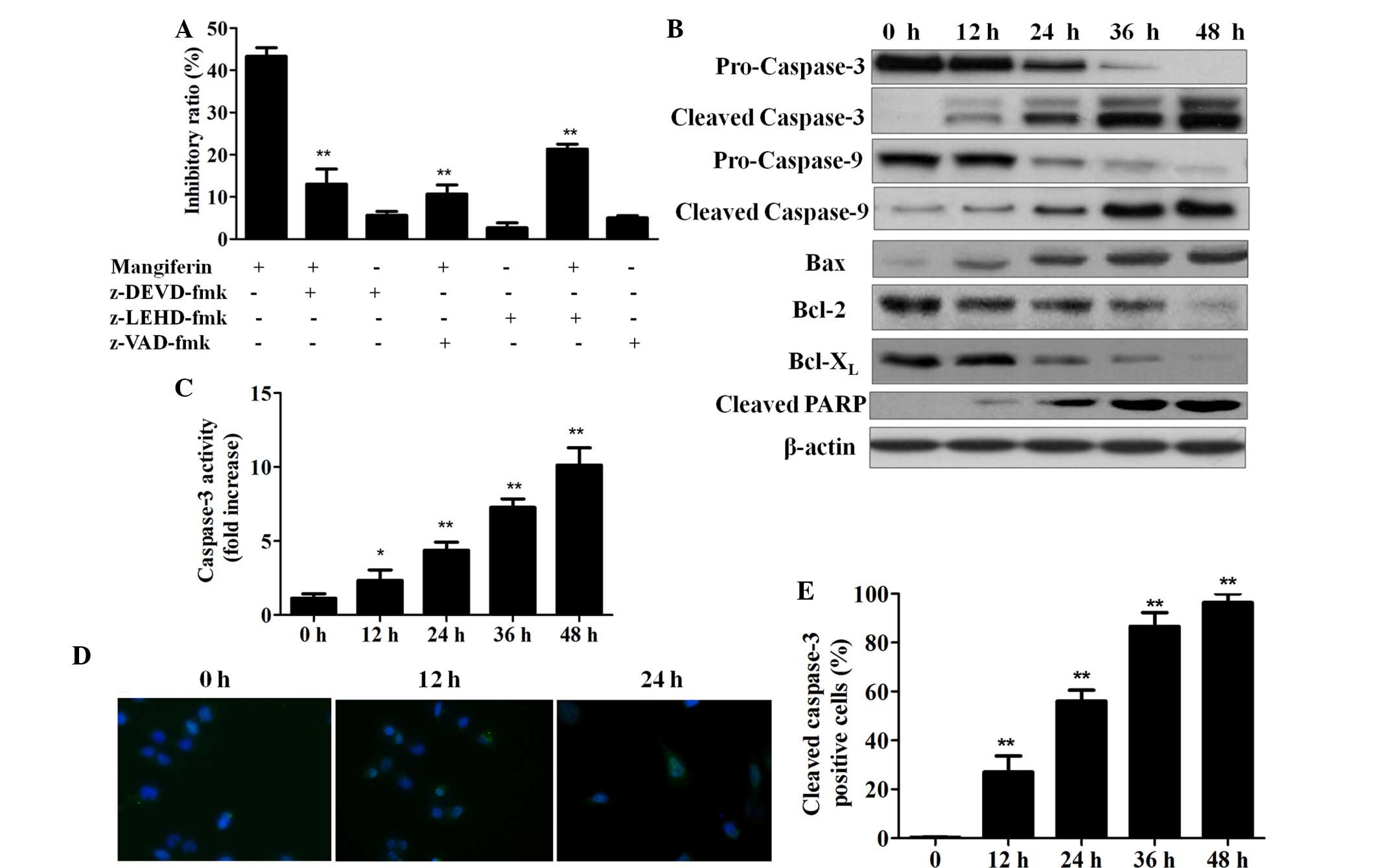
Mangiferin induces apoptosis in a
caspase-dependent manner
To evaluate whether mangiferin-induced cell death
was dependent on the caspase pathway, various caspase inhibitors
were applied as shown in Fig. 3A.
After a 24 h incubation with mangiferin plus caspase inhibitors or
PBS control, cell inhibition of each group was quantified. As
demonstrated by Fig. 3A,
mangiferin-induced cell growth inhibition was completely suppressed
by the addition of pan-caspase, caspase-3 and caspase-9 inhibitors,
thus indicating that mangiferin induced cell apoptosis in a
caspase-dependent manner. Furthermore, western blot analyses
demonstrated that following treatment with 25 µg/ml
mangiferin, caspase-3 and caspase-9 were cleaved, since
procaspase-3 and procaspase-9 expression was decreased whereas
cleaved caspase-3 and cleaved caspase-9 expression was increased
with the duration of mangiferin treatment. In addition,
upregulation of proapoptotic B-cell lymphoma (Bcl) 2-associated X
(Bax) protein, and downregu-lation of anti-apoptotic Bcl-2 and
Bcl-extra large (xL) proteins was also detected following
mangiferin treatment. Treatment with 25 µg/ml mangiferin
also led to enhanced cleavage of poly ADP-ribose polymerase (PARP)
in a time-dependent manner (Fig.
3B).
To further verify that mangiferin-induced apoptosis
is dependent on the caspase pathway, caspase-3 activity was
assessed. As shown in Fig. 3C,
following treatment with the autophagic inhibitor 3-methyladenine
(5 mM), 25 µg/ml mangiferin significantly increased
caspase-3 activity in a time-dependent manner, thus indicating that
mangiferin-induced apoptosis occurs via the activation of common
apoptotic executors such as caspase-3. Furthermore,
immunofluorescent staining of cleaved caspase-3 was conducted
(Fig. 3D) and the number of cells
positively stained for cleaved caspase-3 was manually counted.
Statistical analysis of the percentage of cleaved
caspase-3-positive cells indicated that the longer the duration of
mangiferin treatment, the higher proportion of cleaved
caspase-3-positive cells (Fig.
3E). These results confirm that mangiferin increased the
activities of caspase-3 and caspase-9 in a time-dependent
manner.
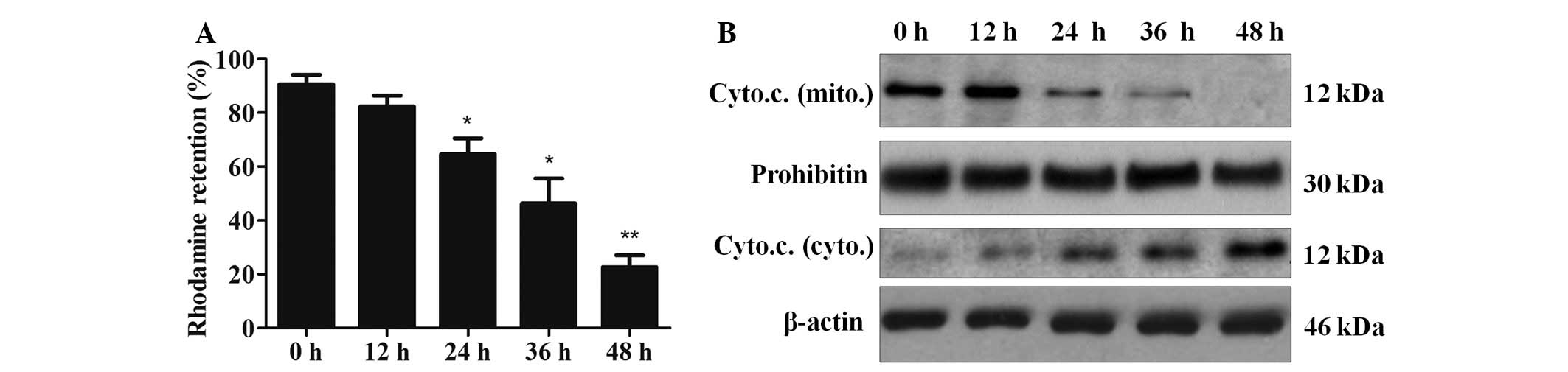
Mangiferin induces apoptosis via the
mitochondrial pathway
To further investigate whether mangiferin induced
apoptosis via the mitochondrial pathway, rhodamine 123 staining
assay was performed to measure the integrity of mitochondrial
membranes. As indicated in Fig.
4A, mangiferin decreased the fluorescence intensity of
rhodamine 123 in a time-dependent manner (control group, 91%; 12 h
group, 82%; 24 h group, 65%; 36 h group, 46%; 48 h group, 23%;
P<0.05, n=3). In addition, detection of cytochrome c in
the cytosol and mitochondria suggested that the amount of
cytochrome c in the mitochondria of the mangiferin-treated
cells was decreased. Furthermore, the amount of cytochrome c
in the cytosol of the cells was increased, thus indicating that
cytochrome c was released from the mitochondria (Fig. 4B). These results clearly indicate
that mangiferin-induced apoptosis in A549 cells may be mediated via
the mitochondrial pathway.
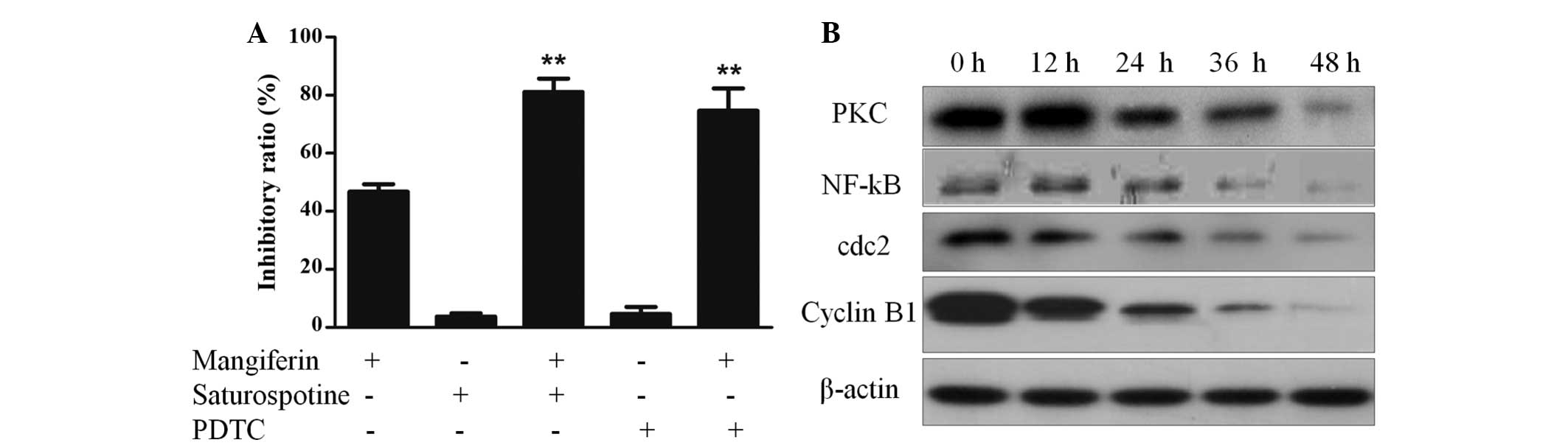
Mangiferin induces apoptosis in A549
cells via inhibition of the PKC-NF-κB pathway
To further explore the mechanism underlying
mangiferin-induced apoptosis in A549 cells, A549 cells were
pretreated with the PKC inhibitor staurosporine or the NF-κB
inhibitor pyrrolidine dithiocarbamate (PDTC), and
mangiferin-induced cell cytotoxicity was measured. As shown in
Fig. 5A, these inhibitors
significantly increased the mangiferin-induced inhibitory ratio and
induced A549 cell survival. These results indicate that the
PKC-NF-κB pathway may have a protective role in mangiferin-induced
A549 cell apoptosis. To further verify that mangiferin-induced cell
apoptosis was induced via inhibition of the PKC-NF-κB pathway, the
protein expression levels of PKC and NF-κB were quantified by
western blotting. As shown in Fig.
5B, A549 cells treated with mangiferin exhibited downregulated
PKC and NF-κB protein expression.
Mangiferin induces G2/M phase
cell-cycle arrest by downregulating the cdc2-cyclin B1 signaling
pathway
To determine whether the cdc2-cyclin B1 signaling
pathway was involved in mangiferin-induced cell cycle arrest, and
whether the cdc2-cyclin B1 signaling pathway was downregulated or
upregulated, A549 cells were treated with 25 µg/ml
mangiferin for various time periods. The mangiferin-treated cells
and control cells were collected, fixed and quantified by flow
cytometry. Analysis of the cell cycle distribution demonstrated
that, as compared with that of the control group, treatment of A549
cells with mangiferin resulted in an increase in the percentage of
G2/M phase cells. This result indicates that mangiferin
may induce G2/M phase arrest (Fig. 2C). Furthermore, to explore the
mechanism underlying mangiferin-induced cell cycle arrest, western
blotting of cell cycle regulatory proteins, such as cyclin B1 and
cdc2 was performed. As shown in Fig.
5B, treatment of A549 cells with mangiferin resulted in
decreased protein expression levels of cyclin B1 and cdc2. These
results indicate that mangiferin may induce G2/M phase
cell cycle arrest by downregulating the expression levels of cdc2
and cyclin B1.
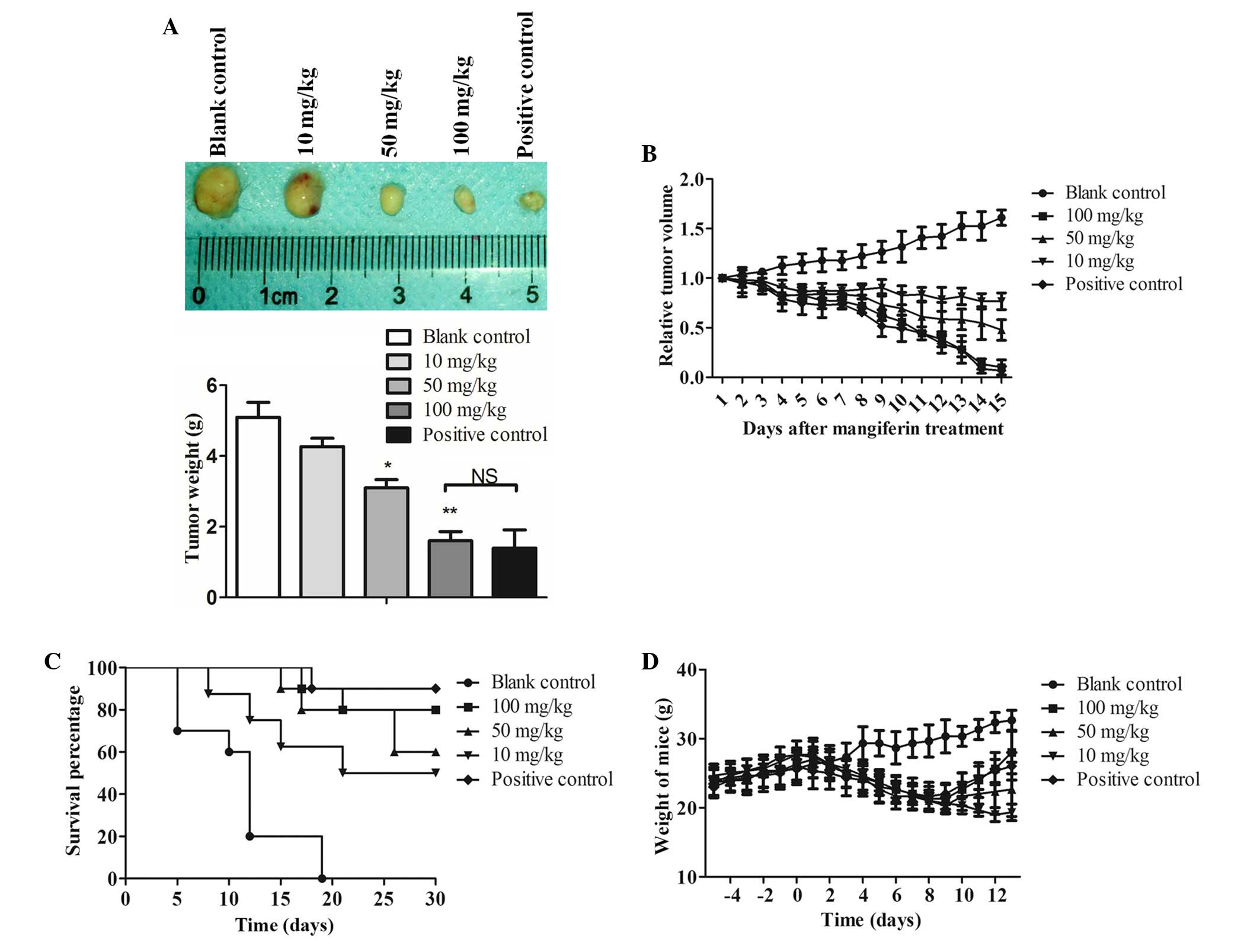
Xenograft tumor growth is inhibited
following mangiferin administration in vivo
To determine whether mangiferin administration
exhibited an antitumor effect in vivo, an A549 xenograft
mouse model was generated. A549 cells were inoculated into the neck
of nude mice, and mangiferin was administered for 2 weeks. Prior to
and after mangiferin treatment, mouse body weight was measured
daily. Subsequently, 5 mice in each group were sacrificed, and 5
mice in each group were raised for survival assay. Following
sacrifice, the subcutaneous tumors were harvested and weighed.
Three dimensional tumor volume was determined using vernier
calipers, and relative tumor volume was calculated as the tumor
volume of the mangiferin-treated group divided by the tumor volume
of the blank control group. As shown in Fig. 6A, after 14 days of treatment,
xenograft tumor volume was significantly decreased in the
mangiferin-treated group in a dose-dependent manner, as compared
with in the blank control group. Furthermore, calculation of the
relative volume demonstrated that 100 mg/kg mangiferin exhibited
similar effects on tumor inhibition to cisplatin, which reached
~90% (Fig. 6B). These results
indicate that mangiferin may be considered a potential effective
antitumor agent.
Further in vivo studies were performed;
including survival assay and body weight assessment, and the
inhibitory ratio was calculated (Table
I). As shown in Fig. 6C,
compared with the blank control group, mangiferin markedly
prolonged the lifespan of A549 cell xenograft mice. Untreated mice
(blank control) succumbed within 40 days of A549 inoculation;
however, mangiferin or cisplatin (positive control) treatment
significantly increased the lifespan of xenograft mice. Even low
dose treatment (10 mg/kg) could partially rescue A549-inoculated
mice, and >60% of mice survived until the end of the in
vivo assay. Furthermore, high dose mangiferin treatment (100
mg/kg) could significantly increase the lifespan of xenograft mice,
as compared with the cisplatin-treated mice. In addition, there was
a significant difference between high dose mangiferin-treated mice
and cisplatin-treated mice (P<0.05). Assessment of body weight
demonstrated that following treatment with various doses of
mangiferin for 14 days, the weight of the mice was markedly
decreased (Fig. 6D; P<0.05). In
addition, the body weight of 100 mg/kg mangiferin-treated mice
almost reached that of the cisplatin-treated mice. Based on the
survival ratio and body weight data, these results indicate that
mangiferin may exhibit antitumor activity in vivo.
 | Table IInhibitory effects of mangiferin on
A549-bearing mice after 14 days (n=10, P<0.05). |
Table I
Inhibitory effects of mangiferin on
A549-bearing mice after 14 days (n=10, P<0.05).
| Group |
Volume/cm3 | Weight/g | Volume inhibition
rate (%) | Weight inhibition
rate (%) |
|---|
| Blank control | 0.57±0.29 | 0.93±0.28 | | | |
| 10 mg/kg | 0.43±0.14 | 0.74±0.35 | 23.9 | | 18.3 |
| 50 mg/kg | 0.28±0.19a | 0.61±0.19b | 29.5 | | 42.7 |
| 100 mg/kg | 0.12±0.06b | 0.38±0.12b | 71.3 | P | 62.3 |
| Positive
control | 0.09±0.09c | 0.27±0.09c | 80.6 | 75.8 |
Discussion
The present study investigated mangiferin-induced
apoptosis of A549 human lung adenocarcinoma cells with the hope of
identifying a therapeutic strategy for the treatment of cancer.
Mangiferin has recently gained more attention due to its
comparatively various biological functions. In the present study,
mangiferin exerted growth-inhibitory and apoptosis-inducing effects
on A549 lung adenocarcinoma cells in vitro and in
vivo. Further experiments regarding the mechanism underlying
mangiferin-induced A549 cell apoptosis demonstrated that, firstly,
mangiferin was able to induce A549 lung adenocarcinoma cell arrest
at G2/M phase by downregulating the cdc2-cyclin B1
signaling pathway. Secondly, mangiferin was able to induce the
apoptosis of A549 cells by inhibiting the PKC-NF-κB pathway.
Finally, mangiferin exerted anticancer and apoptosis-inducing
effects in vivo, as detected by decreases in the volume and
weight of the subcutaneous tumor mass, and the enhanced lifespan of
the mice.
Previous studies have reported the anticancer
effects of mangiferin. Mangiferin has been shown to inhibit tumor
necrosis factor-induced activation of NF-κB in A549 cells (18). In addition, crude extract of
mangiferin suppressed MDA-MB-231 cell proliferation by inhibiting
the NF-κB pathway in A549 cells (5). Administration of mangiferin was also
able to enhance the detoxification of enzymes, including
glutathione transferase, quinone reductase and uridin
5′-diphosphate-glucuronosyl transferase, and reduce DNA damage in
lung cancer-bearing animals (19).
In addition, mangiferin has been reported to inhibit telomerase
activity and induce apoptosis in K562 cells (8,9), and
Mangifera indica extract initiated
G0/G1 phase cell cycle arrest (10).
In the present study, mangiferin was shown to induce
intrinsic mitochondrial-mediated apoptosis in a caspase-3 and
caspase-9 dependent manner. As determined using caspase-3 activity
assays and the analysis of cleaved caspase-3 immunostaining in A549
cells, mangiferin induced apoptosis via the caspase pathway. The
release of cytochrome c from the mitochondria to the
cytoplasm appears to be a central event in apoptosis, since it is
critical for the initiation of the caspase family (16). Cytochrome c expression was
detected in the cytosol and mitochondria using western blotting,
and the results indicated that alongside reduced mitochondrial
membrane potential, cytochrome c release from the
mitochondria into the cytosol was increased following treatment
with mangiferin. These results suggested that treatment with
mangiferin resulted in activation of caspase-3 and -9, release of
cytochrome c into the cytoplasm, reduced mitochondrial
membrane potential, upregulated proapoptotic Bax, downregulated
anti-apoptotic Bcl-2 and Bcl-XL, and cleavage of PARP,
which are characteristics of caspase- and mitochondrial-dependent
apoptosis.
Subsequent studies regarding the cell cycle
progression of A549 cells demonstrated that the percentage of cells
within G2/M and sub-G1 phase were markedly
increased in response to mangiferin treatment in a time-dependent
manner. These results indicated that mangiferin was able to induce
G2/M phase cell cycle arrest and apoptotic cell death in
A549 cells. Previous studies on cell cycle proteins have reported
that in various tumors, the cdc2-cyclin B1 complex is
over-expressed (20–22). Therefore, suppressing expression of
the cdc2-cyclin B1 complex may result in cancer cell growth
inhibition (23). In eukaryotes,
initiation of the cdc2-cyclin B1 complex (M-phase promoting factor,
MPF) has an important role in regulating the entry into mitosis,
and cdc2 is known to be an active sub-unit of MPF (24). Therefore, in the present study, the
protein expression levels of cdc2 and cyclin B1 were investigated,
and the results demonstrated that following treatment with 25
µg/ml mangiferin for 12, 24, 36 and 48 h, the expression
levels of cdc2 and cyclin B1 were decreased. These results
suggested that mangiferin was able to inhibit A549 human lung
carcinoma cell proliferation by initiating G2/M cell
cycle arrest via downregulation of the cdc2-cyclin B1 signaling
pathway.
Further exploration of the molecular mechanisms
underlying mangiferin-induced apoptosis indicated that mangiferin
initiated apoptotic cell death via suppression of the PKC-NF-κB
pathway. PKCε is an oncogenic kinase, which is essential for cell
survival regulation, mitogenesis and invasion, and PKCε is
upregulated in the majority of epithelial cancers, including
prostate and lung cancer (25). In
addition, a strong correlation has been detected between PKC
overexpression and NF-κB activation, and the NF-κB family of
transcription factors has a crucial role in inflammation, as well
as in regulation of the expression of genes involved in cell
survival, proliferation, angiogenesis and invasion (26). Therefore, in the present study,
after pretreatment of A549 cells with the PKC inhibitor
staurosporine and the NF-κB inhibitor PDTC, mangiferin-induced
cytotoxicity was investigated and was shown to be markedly
increased. These findings indicated that PKC and NF-κB have a
protective role in mangiferin-induced A549 cell apoptosis. This
result was further demonstrated by western blotting, the protein
expression levels of PKC and NF-κB were markedly decreased in a
time-dependent manner, indicating that mangiferin induced apoptosis
via suppression of the PKC-NF-κB signaling pathway in A549 human
lung carcinoma cells. Thereby, inhibition of the PKC-NF-κB pathway
may function as an effective strategy in cancer treatment.
As well as investigating the in vitro
anti-cancer activity of mangiferin, mangiferin was verified to
possess antitumor effects in vivo. Treatment with mangiferin
was able to decrease the volume and weight of subcutaneous tumor
mass; after 14 days of treatment tumor volume and weight decreased
by ~90 and 85%, respectively. The highest dose of mangiferin (100
mg/kg) exhibited similar antitumor effects to cisplatin, which
serves as an accepted effective antitumor agent. These results
indicated that mangiferin may possess the potential to inhibit A549
human lung carcinoma cell growth and induce apoptotic cell death
in vitro and in vivo.
In conclusion, the results of the present study
provided evidence regarding the antitumor effects and underlying
mechanisms of mangiferin in A549 cells, and sheds new light on the
application of mangiferin in human lung adenocarcinoma therapy. The
present study is the first, to the best of our knowledge, to report
that mangiferin induced G2/M phase cell cycle arrest via
the cdc2-cyclin B1 pathway, and induced cell apoptosis via the
PKC-NF-κB pathway. Due to the gradual elucidation of the molecular
mechanisms underlying the antitumor activities of mangiferin, novel
therapeutic strategies may be developed that target cell apoptotic
pathways for cancer therapy.
Acknowledgments
The present study was supported in part by the
National Natural Science Foundation of China (grant nos. 81271049
and 30871350), Guangxi Education funding (grant no. 2015-12) and
Key Laboratory Funding (grant no. 12-071-08).
References
|
1
|
Sánchez GM, Re L, Giuliani A, Núñez-Sellés
AJ, Davison GP and León-Fernández OS: Protective effects of
Mangifera indica L. extract, mangiferin and selected antioxidants
against TPA-induced biomolecules oxidation and peritoneal
macrophage activation in mice. Pharmacol Res. 42:565–573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dar A, Faizi S, Naqvi S, Roome T,
Zikr-ur-Rehman S, Ali M, Firdous S and Moin ST: Analgesic and
antioxidant activity of mangiferin and its derivatives: The
structure activity relationship. Biol Pharm Bull. 28:596–600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guha S, Ghosal S and Chattopadhyay U:
Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a
naturally occurring glucosylxanthone. Chemotherapy. 42:443–451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duang XY, Wang Q, Zhou XD and Huang DM:
Mangiferin: A possible strategy for periodontal disease to therapy.
Med Hypotheses. 76:486–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of Mangifera indica L. bark in MDA-MB231 breast cancer
cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noratto GD, Bertoldi MC, Krenek K, Talcott
ST, Stringheta PC and Mertens-Talcott SU: Anticarcinogenic effects
of polyphenolics from mango (Mangifera indica) varieties. J Agric
Food Chem. 58:4104–4112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chieli E, Romiti N, Rodeiro I and Garrido
G: In vitro effects of Mangifera indica and polyphenols derived on
ABCB1/P-glycoprotein activity. Food Chem Toxicol. 47:2703–2710.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng P, Peng ZG, Yang J and Song SJ: The
effect of mangiferin on telomerase activity and apoptosis in
leukemic K562 cells. Zhong Yao Cai. 30:306–309. 2007.In Chinese.
PubMed/NCBI
|
|
9
|
Peng ZG, Luo J, Xia LH, Chen Y and Song
SJ: CML cell line K562 cell apoptosis induced by mangiferin.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 12:590–594. 2004.In Chinese.
PubMed/NCBI
|
|
10
|
Percival SS, Talcott ST, Chin ST, Mallak
AC, Lounds-Singleton A and Pettit-Moore J: Neoplastic
transformation of BALB/3T3 cells and cell cycle of HL-60 cells are
inhibited by mango (Mangifera indica L.) juice and mango juice
extracts. J Nutr. 136:1300–1304. 2006.PubMed/NCBI
|
|
11
|
Chari NS, Pinaire NL, Thorpe L, Medeiros
LJ, Routbort MJ and McDonnell TJ: The p53 tumor suppressor network
in cancer and the therapeutic modulation of cell death. Apoptosis.
14:336–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartwell LH and Kastan MB: Cell cycle
control and cancer. Science. 266:1821–1828. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein JC, Waterhouse NJ, Juin P, Evan
GI and Green DR: The coordinate release of cytochrome c during
apoptosis is rapid, complete and kinetically invariant. Nat Cell
Biol. 2:156–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li CY, Wang Y, Wang HL, Shi Z, An N, Liu
YX, Liu Y, Zhang J, Bao JK and Deng SP: Molecular mechanisms of
Lycoris aurea agglutinin-induced apoptosis and G2/M cell cycle
arrest in human lung adenocarcinoma A549 cells, both in vitro and
in vivo. Cell Prolif. 46:272–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar A, Sreenivasan Y, Ramesh GT and
Manna SK: beta-D-Glucoside suppresses tumor necrosis factor-induced
activation of nuclear transcription factor kappaB but potentiates
apoptosis. J Biol Chem. 279:33768–33781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Protective role of mangiferin against Benzo(a)pyrene induced
lung carcinogenesis in experimental animals. Biol Pharm Bull.
31:1053–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Touny LH and Banerjee PP: Identification
of both Myt-1 and Wee-1 as necessary mediators of the
p21-independent inactivation of the cdc-2/cyclin B1 complex and
growth inhibition of TRAMP cancer cells by genistein. Prostate.
66:1542–1555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoffmann TK, Trellakis S, Okulicz K,
Schuler P, Greve J, Arnolds J, Bergmann C, Bas M, Lang S, Lehnerdt
G, et al: Cyclin B1 expression and p53 status in squamous cell
carcinomas of the head and neck. Anticancer Res. 31:3151–3157.
2011.PubMed/NCBI
|
|
22
|
Chen H, Huang Q, Dong J, Zhai DZ, Wang AD
and Lan Q: Overexpression of CDC2/CyclinB1 in gliomas, and CDC2
depletion inhibits proliferation of human glioma cells in vitro and
in vivo. BMC Cancer. 8:292008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vairapandi M, Balliet AG, Hoffman B and
Liebermann DA: GADD45b and GADD45g are cdc2/cyclinB1 kinase
inhibitors with a role in S and G2/M cell cycle checkpoints induced
by genotoxic stress. J Cell Physiol. 192:327–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Huang Q, Dong J, Zhai DZ, Wang AD
and Lan Q: Overexpression of CDC2/CyclinB1 in gliomas, and CDC2
depletion inhibits proliferation of human glioma cells in vitro and
in vivo. BMC Cancer. 8:292008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reyland ME: Protein kinase Cdelta and
apoptosis. Biochem Soc Trans. 35:1001–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mezzano S, Aros C, Droguett A, Burgos ME,
Ardiles L, Flores C, Schneider H, Ruiz-Ortega M and Egido J:
NF-kappaB activation and overexpression of regulated genes in human
diabetic nephropathy. Nephrol Dial Transplant. 19:2505–2512. 2004.
View Article : Google Scholar : PubMed/NCBI
|