Introduction
Gastric cancer (GC), one of the most common
gastrointestinal malignancies in the world, accounts for ~8% of all
cancers (1) and is the second
leading cause of cancer-associated mortality (2). Highest incidence rates are in Eastern
Asia, Eastern Europe and South America, and lowest incidence rates
are in North America and the majority of Africa (1). The genesis and progression of GC are
multi-step and multi-stage processes. In spite of the treatments
available for GC, including surgery, radiotherapy, chemotherapy and
gene therapy (3–8), the outcome is dismal due to relapses
originating from the residual nidus being common. To improve the
outcome for patients with GC and enhance the efficacy of GC
treatments, novel drugs are urgently required.
Piperlongumine (PL), a primary constituent of long
peppers, is a natural alkaloid found in the fruit as well as the
roots of the plant (9). PL has
broad biological activities, including bactericidal and
insecticidal capabilities (10).
Furthermore, it exhibits anti-atherosclerotic, anti-inflammatory,
anti-platelet, cardioprotective, anti-depressant and analgesic
effects (11–17). Of note, accumulating evidence has
shown that PL has anti-cancer properties. For example, Randhawa
et al (18) reported that
PL could suppress the proliferation of colon cancer cells in a
concentration- and time-dependent manner through the
mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase pathway. Furthermore, Dhillon et al
(19) demonstrated that PL
inhibited the proliferation of pancreatic cancer cells by
upregulating the levels or reactive oxygen species, which caused
DNA damage. In addition, PL was reported to have anti-proliferative
and apoptosis-inducing effects on human ovarian cancer cells
(20).
However, to the best of our knowledge, the effects
of PL on GC cells have not been demonstrated to date. Therefore,
the present study evaluated the effects of PL on the MKN45 and AGS
GC cell lines and explored the underlying mechanisms. The results
demonstrated that PL inhibited the proliferation, cell cycle
progression as well as cell invasion and migration of GC cells
through suppression of the Janus kinase (JAK)1,2/signal transducer
and activator of transcription (STAT)3 signaling pathway.
Materials and methods
Cell lines and reagents
The MKN45 and AGS human GC cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA). All
cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaillé,
France) in a humidified atmosphere containing 5% CO2 at
37°C. PL was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell proliferation assay
Cell proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, MKN45 and AGS cells were seeded into 96-well
plates at a density of 1×104 cells/well and cultured for
24 h. The cells were then treated with various concentrations of PL
(0, 10, 20 or 40 µM) for 24, 48, 72 or 96 h. Subsequently,
20 µl MTT solution (Sigma-Aldrich) was added to each well,
followed by incubation at 37°C for 4 h. The medium was carefully
removed and 100 µl dimethyl sulfoxide (Sigma-Aldrich) was
added into each well. The absorbance at 490 nm was determined using
a spectrophotometer (GENESYS™ 20; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Each experiment was performed at least three
times.
Cell cycle assay
After treatment for 24 h with PL (0, 10, 20 or 40
µM), the cells were trypsinized and fixed overnight in 70%
ethanol (Sigma-Aldrich) at −20°C. Next, the cells were collected
and re-suspended in staining solution containing 50 mg/l propidium
iodide (Sigma-Aldrich) and 100 mg/l RNase A (Sigma-Aldrich),
followed by incubation in the dark for 30 min at room temperature.
The cell cycle distribution was then analyzed with a flow cytometer
(BD FACSAria II; BD Biosciences, Franklin Lakes, NJ, USA).
Cell invasion and migration assays
The invasive and migratory capacity of GC cells was
detected using 24-well Transwell chambers (containing filters with
8-µm pore size; Corning, Inc., Corning, NY, USA) according
to the manufacturer's instructions. For the invasion assay, the
insert membrane was coated with Matrigel (Invitrogen; Thermo Fisher
Scientific, Inc.), while it was kept in its original condition for
the migration assay. MKN45 or AGS cells (1×105) were
seeded in the upper chambers and cultured in serum-free medium
containing PL (0, 10, 20 or 40 µM) for 24 h. Culture medium
containing 10% FBS was added to the lower chamber. After 24 h of
incubation at 37°C, the cells on the upper surface of the membrane
were scraped off with cotton swabs, while the cells on the lower
surface of the membrane were fixed with methanol (Sigma-Aldrich)
and stained with 0.1% crystal violet (Sigma-Aldrich), followed by
counting under a microscope (CX22; Olympus Corporation, Tokyo,
Japan). At least three independent experiments were conducted.
Western blot analysis
MKN45 cells were treated with 0, 10, 20 and 40
µM PL for 24 h and then lysed at 4°C for 20 min in
radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing 1%
Nonidet P40, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium
deoxycholate, 1 µM sodium orthovanadate, 0.03% aprotinin and
10 ng/ml phenylmethylsulfonyl fluoride. The total protein was
separated by 10% SDS-polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Hercules, CA, USA) and then transferred onto a
polyvinylidene difluoride membrane (Pierce Biotechnology, Inc.,
Rockford, IL, USA). The membranes were blocked with 5% non-fat milk
in Tris-buffered saline containing Tween 20 (TBST; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h prior
to incubation with the primary antibodies as follows: Rabbit
polyclonal anti-JAK1 (1:1,500; Cell Signaling Technology, Inc.,
Danvers, MA, USA; cat. no. 3332); rabbit polyclonal
anti-phosphorylated (p)-JAK1 (1:1,500; Cell Signaling Technology,
Inc.; cat. no. 3331); rabbit polyclonal anti-JAK2 (1:1,500; Cell
Signaling Technology, Inc.; cat. no. 3773); rabbit polyclonal
anti-p-JAK2 (1:1,500; Cell Signaling Technology, Inc.; cat. no.
3774); mouse monoclonal anti-STAT3 (1:1,500; Cell Signaling
Technology, Inc.; cat. no. 9139), mouse monoclonal anti-p-STAT3
(1:1,500; Cell Signaling Technology, Inc.; cat. no. 9138); and
mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Following washing with TBST for 10 min, the membranes were
incubated for 1 h at room temperature in goat anti-mouse
horseradish peroxidase-conjugated (1:3,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-2302) and rabbit anti-mouse
horseradish peroxidase-conjugated secondary antibody (1:2,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-358920) and then washed
with TBST three times. The protein bands were visualized using a
Pierce ECL Western Blotting kit (Thermo Fisher Scientific, Inc.).
The absorbance values of target proteins were analyzed with Gel-Pro
Analyzer software (version 4.0; Media Cybernetics, Inc., Rockville,
MD, USA).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was isolated from the PL-treated MKN45
cells with TRIzol reagent (Invitrogen) in accordance with the
manufacturer's instructions and then transcribed into complementary
DNA using a PrimeScript RT reagent kit (Takara Bio Inc., Otsu,
Japan). Primers for gene amplification were from Invitrogen (Ki-67,
cat. no. Mm01278617_m1; Cyclin D1, cat. no. Mm00487804_ m1; MMP-9,
cat. no. Mm00600163_m1; Twist, cat. no. Mm00442036_m1; Hprt1, cat.
no. Mm00446968_m1). Hprt1 was used as the control. PCR
amplification was performed in a 7300 RT-PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the following
conditions: Initial denaturation at 94°C for 3 min; 40 cycles of
denaturation at 94°C for 10 min, annealing at 55°C for 30 sec and
extension at 72°C for 20 sec. Melt curve analysis was conducted
from 65 to 95°C. The mixture contained 5 µl SsoFast EvaGreen
Supermix (Bio-Rad Laboratories, Inc.), 1 µl cDNA (diluted
1:50), and 2 µl each forward and reverse primers (1
µM) to a final volume of 20 µl. The experiment was
performed at least three times. Relative expression values were
calculated using the 2−ΔΔCq method as previously
described (21).
Statistical analysis
All experiments were performed at least three times.
Values are expressed as the mean ± standard deviation. Differences
between groups were compared using analysis of variance using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
PL suppresses the proliferation of GC
cells
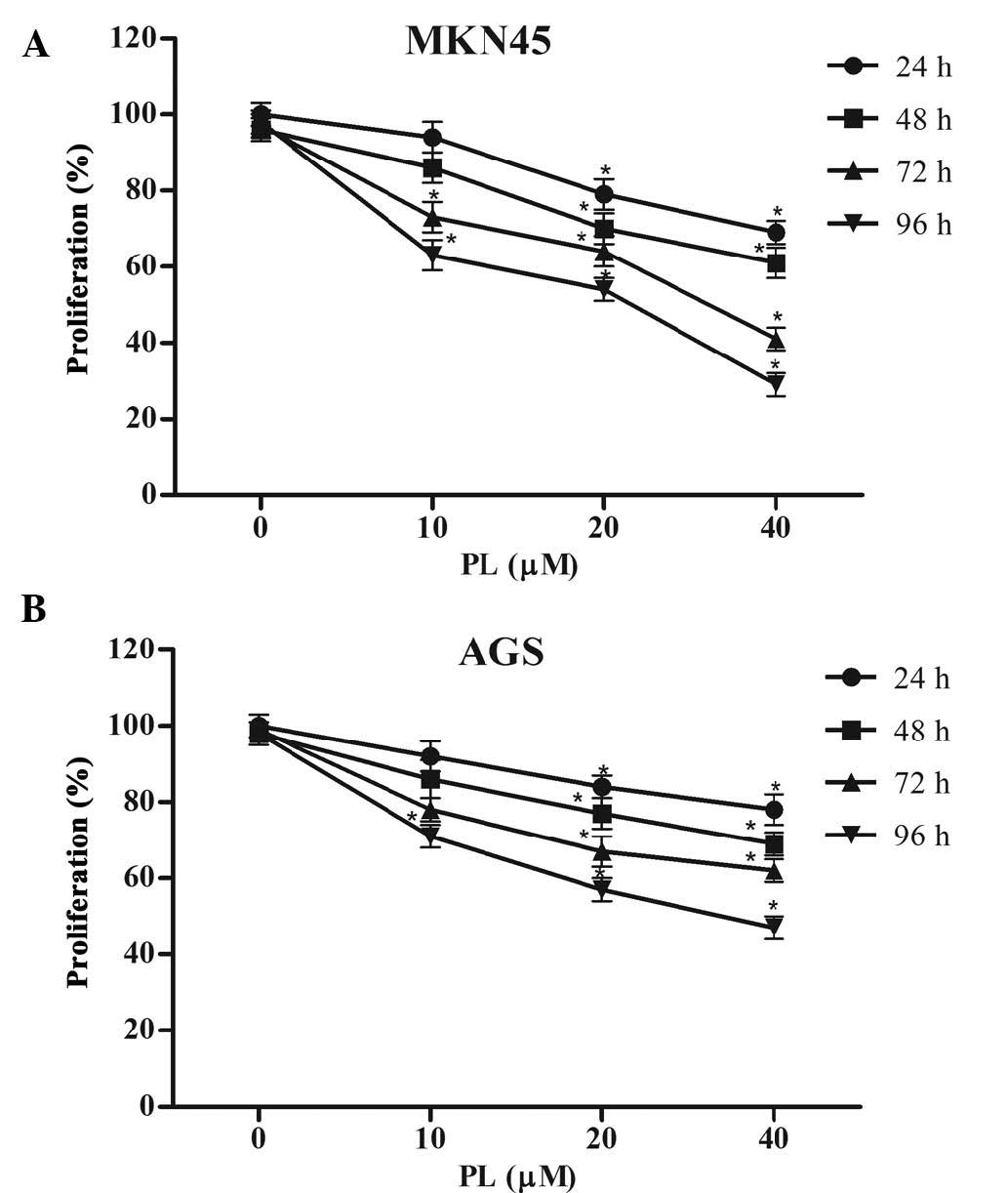
The anti-proliferative effects of PL on GC cells
were determined using an MTT assay. As shown in Fig. 1, PL significantly suppressed
proliferation of MKN45 and AGS cells in a concentration- and
time-dependent manner.
PL induces cell cycle arrest in G2/M
phase in GC cells
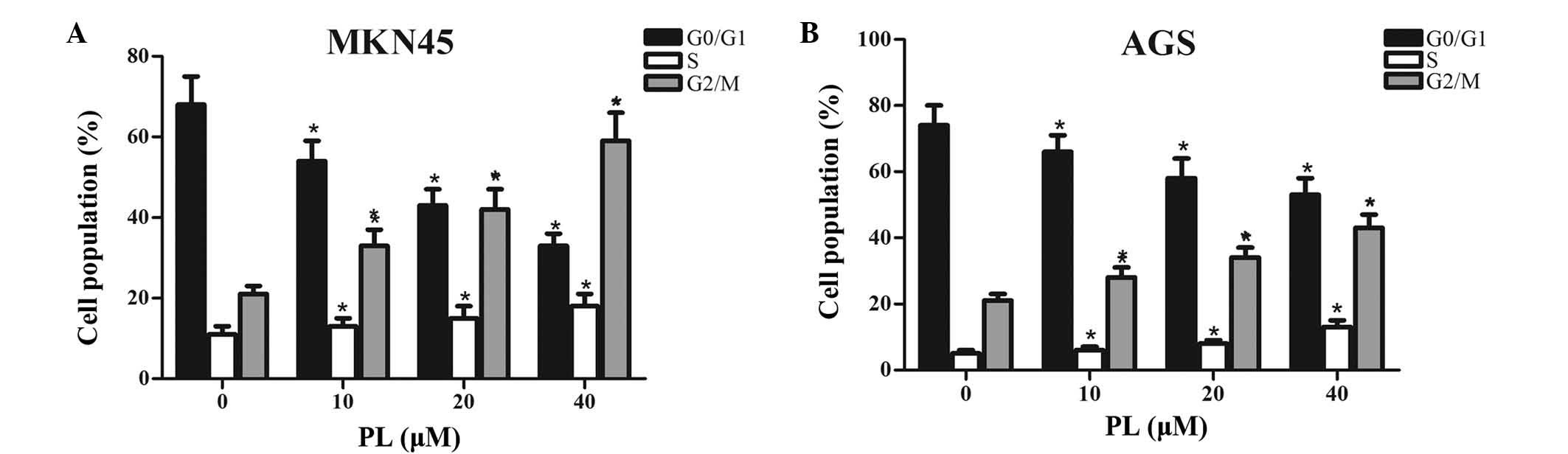
To investigate whether PL inhibited the
proliferation of GC cells via interfering with cell cycle
progression, MKN45 and AGS cells treated with PL for 24 h were
subjected to flow cytometric cell cycle analysis. As shown in
Fig. 2, an increase in the
G2/M-phase population from 21 to 28, 34 and 43, along with a
decrease in G0/G1 phase from 74 to 66, 58 and 53% was observed in
MKN45 cells, after 24 h of treatment with PL at 0, 10, 20 or 40
µM, respectively. Similarly, an increase in the G2/M-phase
population from 21 to 33, 42 and 59%, along with a decrease in
G0/G1 phase from 68 to 54, 43 and 33% was found in AGS cells after
24 h of treatment with PL at 0, 10, 20 or 40 µM,
respectively. Furthermore, slight dose-dependent increase in the
number of MKN45 and AGS cells in S phase after treatment with PL
was observed. All of these results suggested that PL
dose-dependently induced G2/M-phase arrest in GC cells.
PL suppresses the invasion and migration
of GC cells
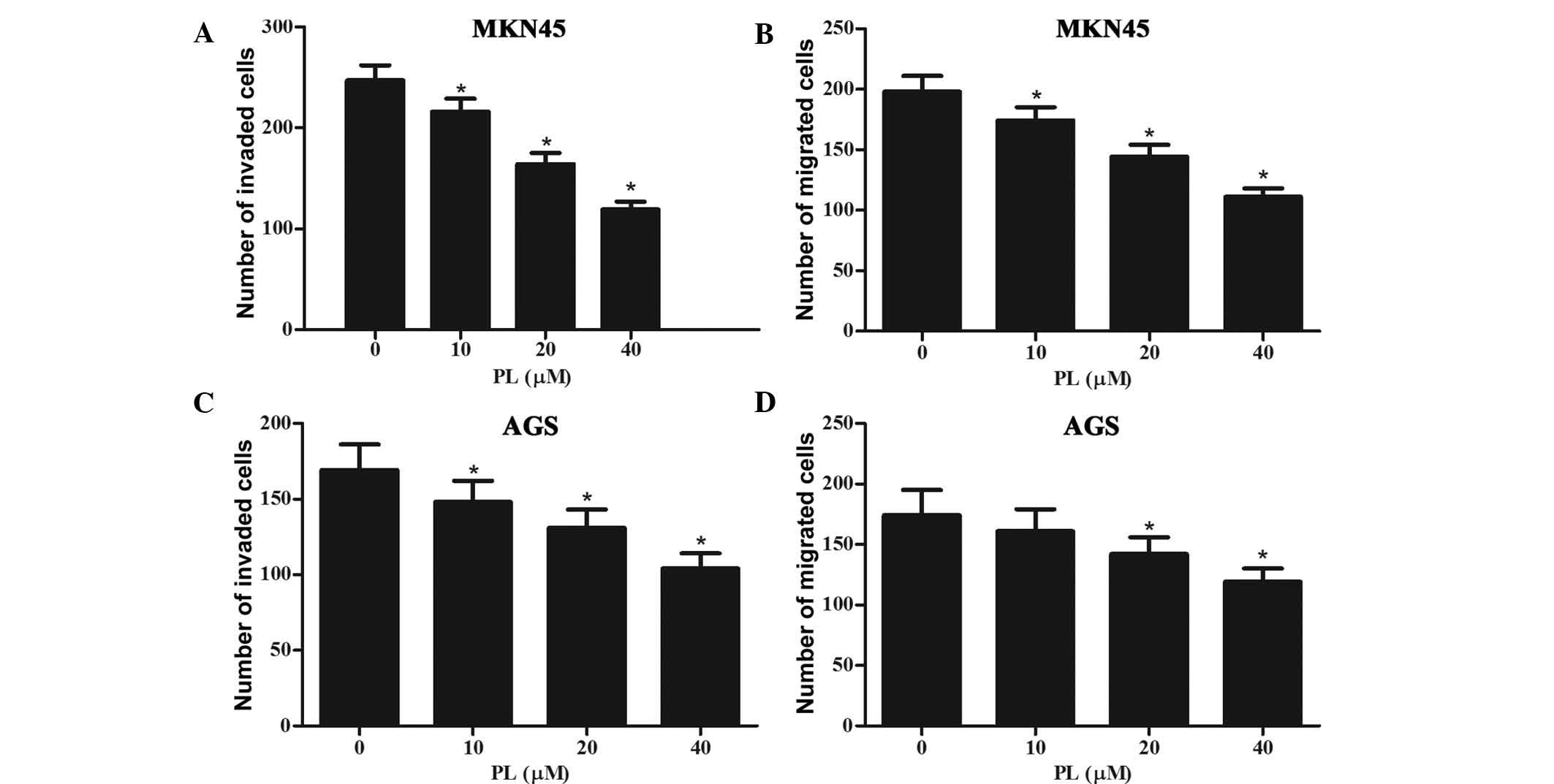
Subsequently, the effects of PL on the invasion and
migration of GC cells were assessed. Invasion of GC cells was
assessed using Transwell chambers with Matrigel-coated membranes,
while the membranes were kept in their original condition for the
migration assay. As shown in Fig. 3A
and B, PL dose-dependently inhibited the invasion and migration
of MKN45 cells. Furthermore, the invasion and migration of AGS
cells was also inhibited by PL in a concentration-dependent manner
(Fig. 3C and D).
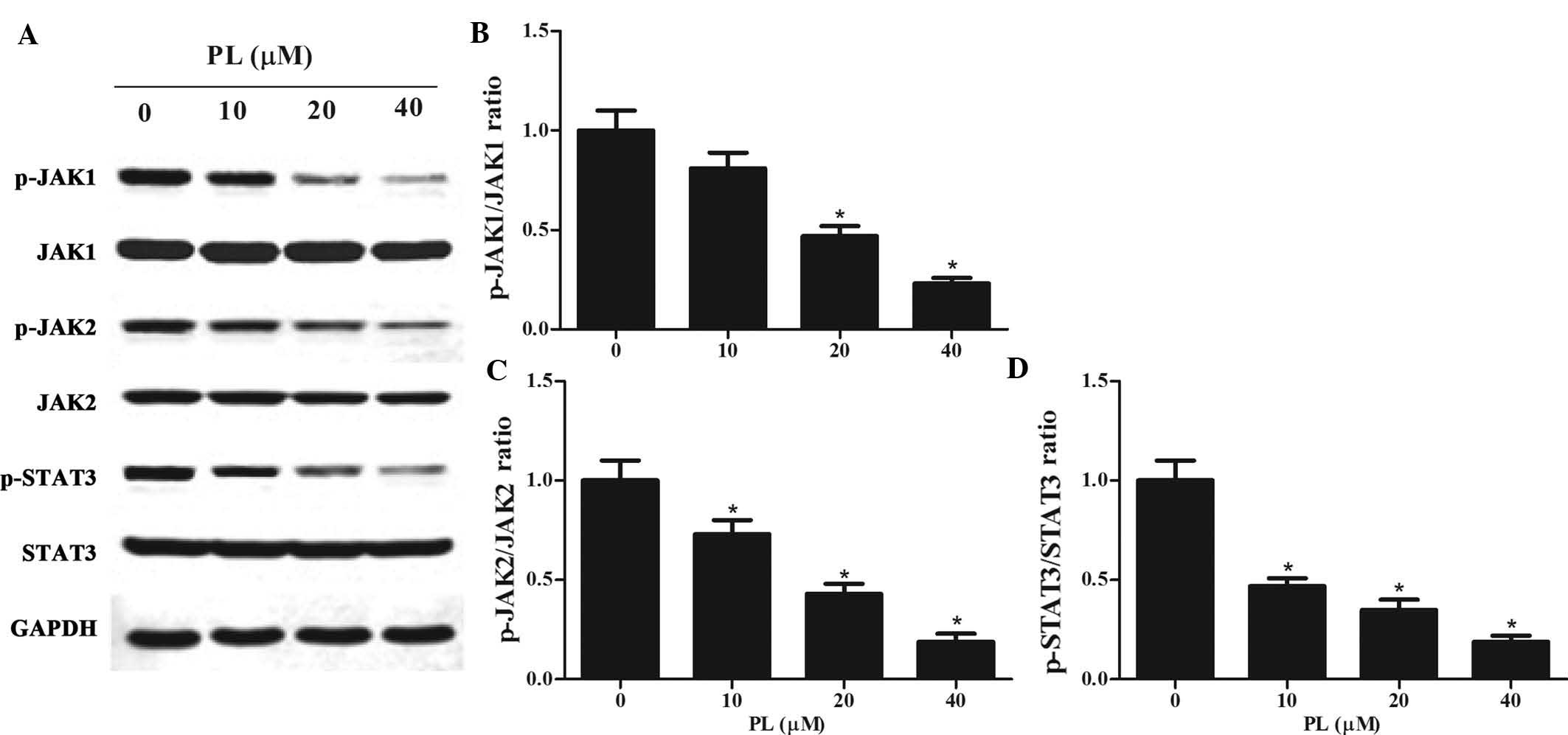
PL de-activates STAT3 activity by
downregulating JAK1/2 activity in GC cells
STAT3 has been reported to be constitutively
activated in GC cells (22,23).
As activation of STAT3 is associated with the activation of
upstream JAKs (24), the present
study examined whether PL affected the JAK/STAT3 pathway. Western
blot analysis was performed on lysates of PL-treated MKN45 cells,
revealing that PL treatment downregulated p-JAK1, p-JAK2 and
p-STAT3 in a concentration-dependent manner (Fig. 4). However, total protein expression
of JAK1, JAK2 and STAT3 was not altered by PL treatment.
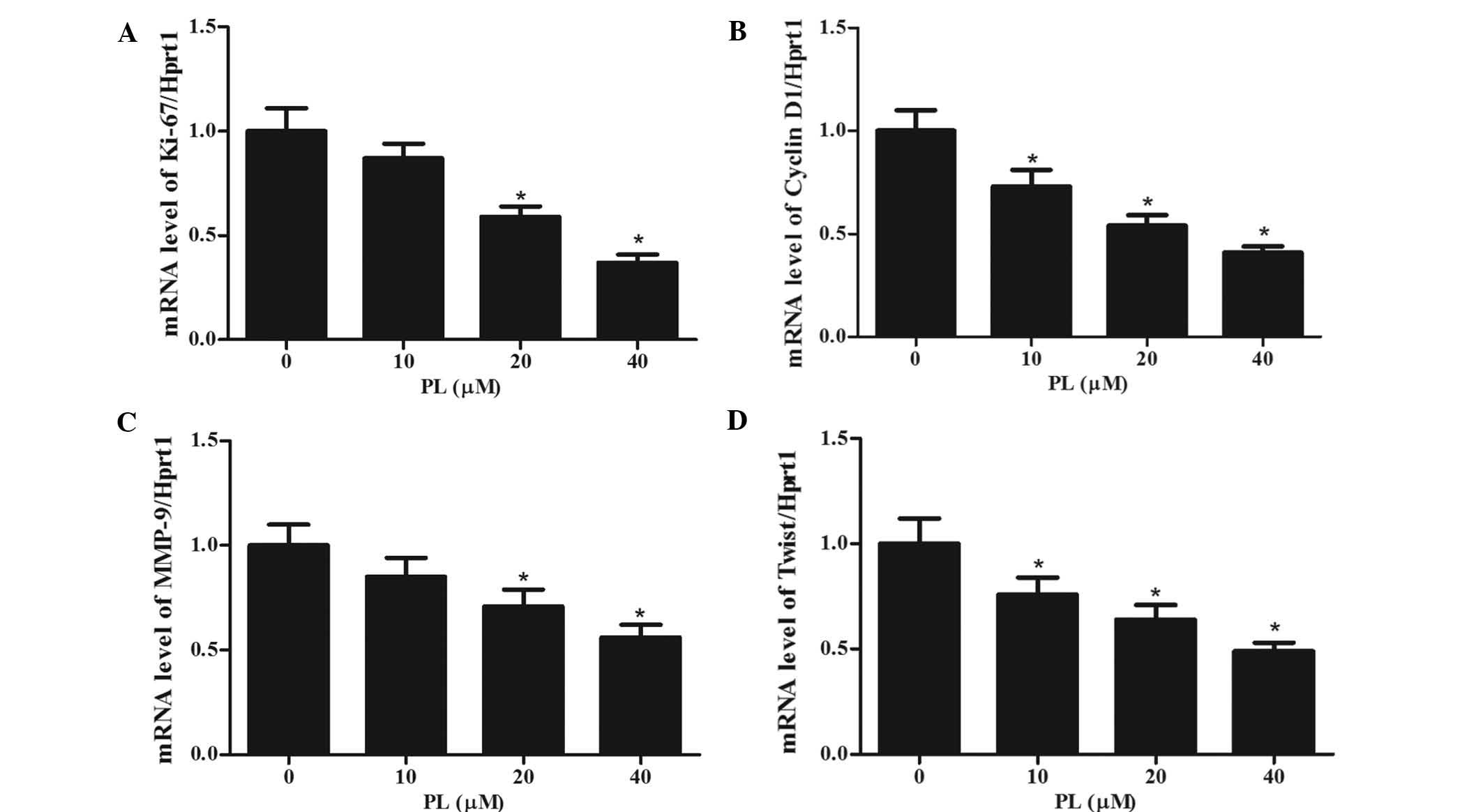
PL decreases the expression of
STAT3-dependent tumor-associated genes
To investigate whether PL showed a specific effect
on STAT3-regulated genes, MKN45 cells were treated with different
concentrations of PL for 24 h, followed by RT-qPCR analysis of the
mRNA expression of the STAT3 target genes Ki-67 (a proliferation
marker), Cyclin D1 (a cell cycle regulator), MMP-9 and Twist
(associated with invasion) (25–27).
As shown in Fig. 5, the mRNA
expression levels of all of these genes were decreased by PL in a
concentration-dependent manner. These results indicated that by
inhibiting STAT3, PL interferes with the expression of these
STAT3-dependent genes, which may be associated with its mechanism
of action.
Discussion
At present, treatments for GC have limitations due
to the occurrence of relapses. Therefore, it is urgently required
to develop novel treatment modalities to improve the outcome of GC
therapies. The present study demonstrated the inhibitory effects of
PL on GC cells and assessed the underlying mechanisms. The results
showed that PL suppressed the proliferation, cell cycle progression
as well as invasion and migration of GC cells through inhibition of
the JAK1,2/STAT3 signaling pathway.
JAKs, comprised of JAK1, JAK2, JAK3 and TYK2, belong
to a family of non-receptor tyrosine kinases (28). They are phosphorylated by cytokine
and growth factor receptor signaling, which then results in
activation of STAT3 (3,29–31).
STAT3, a member of the STAT family of transcription factors, exists
in the cytoplasm and is tightly mediated under physiological
conditions. Its activation, strictly controlled in normal tissues,
contributes to tumorigenesis by driving biological processes and
cellular functions, including proliferation, survival, metastasis,
angiogenesis, immune evasion and inflammation (32–36).
The JAK/STAT3 signaling pathway has been reported to have a central
role in GC and is thus a common target of GC treatments (37,38).
For example, Kim et al (39) demonstrated that OPB-31121 exhibited
an antitumor effect on GC cells by disrupting the JAK2/STAT3
pathway. In addition, GC cell growth was reported to be reduced by
suppression of the JAK2/STAT3 pathway (38,40,41).
Another previous study demonstrated that JAK1/STAT3 signaling is
also involved in the invasion of cancer cells (42). The present study revealed that
phosphorylation of JAK1, JAK2 and STAT3 was dose-dependently
inhibited by PL in GC cells. This indicated that PL may exert its
effects on GC cells by suppression of JAK1 and JAK2 activation,
resulting in reduced STAT3 activation.
Subsequently, the effects of PL on the expression of
target genes of STAT3 relevant to proliferation (Ki-67), cell cycle
progression (Cyclin D1) and invasion (MMP-9 and Twist) were
assessed. The results showed that PL treatment significantly and
dose-dependently reduced the mRNA expression levels of all of these
genes. The present study revealed that PL suppressed the
proliferation of the MKN45 and AGS cells by inducing cell cycle
arrest at G2/M phase, while downregulation the mRNA expression of
Cyclin D1 following incubation for 24 h. Furthermore, the present
study revealed that PL inhibited the invasion of GC cells through
suppressing the expression of MMP-9 and Twist, which are target
genes of STAT3 relevant to cell invasion.
In conclusion, the present study was the first to
reveal the inhibitory effects of PL on GC cells and to assess the
underlying mechanisms. PL was demonstrated to inhibit the
proliferation, cell cycle progression as well as invasion and
migration of two GC cell lines. The underlying mechanisms were
indicated to include the suppression of the JAK1,2/STAT3 signaling
pathway as well as the inhibition of the expression of downstream
genes. These results indicated that the consumption of long pepper
is recommended for the prevention and treatment of GC, and that PL
may represent a novel chemotherapeutic drug for GC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F, Allum W, Carneiro F, Mitry E,
Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A:
Unmet needs and challenges in gastric cancer: The way forward.
Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
SR A, Cervantes A and van de Velde CJ:
Gastric cancer: Epidemiology, pathology and treatment. Ann Oncol.
14(Suppl 2): ii31–ii36. 2003.
|
|
4
|
Falcone A: Future strategies and adjuvant
treatment of gastric cancer. Ann Oncol. 14(Suppl 2): ii45–ii47.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ang SD: Gastric cancer surgery: Can east
meet west? J Surg Oncol. 102:7362010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krejs GJ: Gastric cancer: Epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Qin J, Sun YH and Liu TS:
Neoadjuvant chemotherapy for advanced gastric cancer: A
meta-analysis. World J Gastroenterol. 16:5621–5628. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ,
Kim HH, Kim WH, Lee KU and Yang HK: Changes in clinicopathological
features and survival after gastrectomy for gastric cancer over a
20-year period. Br J Surg. 98:255–260. 2011. View Article : Google Scholar
|
|
9
|
Park BS, Son DJ, Park YH, Kim TW and Lee
SE: Antiplatelet effects of acidamides isolated from the fruits of
Piper longum L. Phytomedicine. 14:853–855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YC, Lee SG, Lee HK, Kim MK, Lee SH
and Lee HS: A piperidine amide extracted from Piper longum L. fruit
shows activity against Aedes aegypti mosquito larvae. J Agr Food
Chem. 50:3765–3767. 2002. View Article : Google Scholar
|
|
11
|
Son DJ, Kim SY, Han SS, Kim CW, Kumar S,
Park BS, Lee SE, Yun YP, Jo H and Park YH: Piperlongumine inhibits
atherosclerotic plaque formation and vascular smooth muscle cell
proliferation by suppressing PDGF receptor signaling. Biochem Bioph
Res Commun. 427:349–354. 2012. View Article : Google Scholar
|
|
12
|
Bang JS, Oh da H, Choi HM, Sur BJ, Lim SJ,
Kim JY, Yang HI, Yoo MC, Hahm DH and Kim KS: Anti-inflammatory and
antiarthritic effects of piperine in human interleukin
1beta-stimulated fibroblast-like synoviocytes and in rat arthritis
models. Arthritis Res Ther. 11:R492009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fontenele JB, Leal L, Silveira ER, Felix
FH, Bezerra Felipe CF and Viana GS: Antiplatelet effects of
piplartine, an alkamide isolated from Piper tuberculatum: Possible
involvement of cyclooxygenase blockade and antioxidant activity. J
Pharm Pharmacol. 61:511–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai IL, Lee FP, Wu CC, Duh CY, Ishikawa
T, Chen JJ, Chen YC, Seki H and Chen IS: New cytotoxic
cyclobutanoid amides, a new furanoid lignan and anti-platelet
aggregation constituents from Piper arborescens. Planta Med.
71:535–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakade AS, Shah AS, Kulkarni MP and
Juvekar AR: Protective effect of Piper longum L. on oxidative
stress induced injury and cellular abnormality in adriamycin
induced cardiotoxicity in rats. Indian J Exp Biol. 46:528–533.
2008.PubMed/NCBI
|
|
16
|
Cícero Bezerra Felipe F, Trajano Sousa
Filho J, de Oliveira Souza LE, Alexandre Silveira J, Esdras de
Andrade Uchoa D, Rocha Silveira E, Deusdênia Loiola Pessoa O and de
Barros Viana GS: Piplartine, an amide alkaloid from Piper
tuberculatum, presents anxiolytic and antidepressant effects in
mice. Phytomedicine. 14:605–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vedhanayaki G, Shastri GV and Kuruvilla A:
Analgesic activity of Piper longum Linn. root. Indian J Exp Biol.
41:649–651. 2003.
|
|
18
|
Randhawa H, Kibble K, Zeng H, Moyer M and
Reindl K: Activation of ERK signaling and induction of colon cancer
cell death by piperlongumine. Toxicol In Vitro. 27:1626–1633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhillon H, Chikara S and Reindl KM:
Piperlongumine induces pancreatic cancer cell death by enhancing
reactive oxygen species and DNA damage. Toxicol Rep. 1:309–318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong LH, Chen XX, Wang H, Jiang QW, Pan
SS, Qiu JG, Mei XL, Xue YQ, Qin WM and Zheng FY: Piperlongumine
induces apoptosis and synergizes with cisplatin or paclitaxel in
human ovarian cancer cells. Oxid Med Cell Longev. 2014:9068042014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Jackson C, Judd LM, Menheniott T, Kronborg
I, Dow C, Yeomans ND, Boussioutas A, Robb L and Giraud A: Augmented
gp130-mediated cytokine signalling accompanies human gastric cancer
progression. J Pathol. 213:140–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heinrich P, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Böger C, Behrens HM and Röcken C: Ki67 -
an unsuitable marker of gastric cancer prognosis unmasks
intratumoral heterogeneity. J Surg Oncol. 113:46–54. 2016.
View Article : Google Scholar
|
|
26
|
Wang Y, Zhou X, Shan B, Han J, Wang F, Fan
X, Lv Y, Chang L and Liu W: Downregulation of microRNA-33a promotes
cyclin dependent kinase 6, cyclin D1 and PIM1 expression and
gastric cancer cell proliferation. Mol Med Rep. 12:6491–6500.
2015.PubMed/NCBI
|
|
27
|
Gao XH, Yang XQ, Wang BC, Liu SP and Wang
FB: Overexpression of twist and matrix metalloproteinase-9 with
metastasis and prognosis in gastric cancer. Asian Pac J Cancer
Prev. 14:5055–5060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jatiani SS, Baker SJ, Silverman LR and
Reddy EP: JAK/STAT pathways in cytokine signaling and
myeloproliferative disorders: Approaches for targeted therapies.
Genes Cancer. 1:979–993. 2010. View Article : Google Scholar
|
|
29
|
Wu H, Huang M, Cao P, Wang T, Shu Y and
Liu P: MiR-135a targets JAK2 and inhibits gastric cancer cell
proliferation. Cancer Biol Ther. 13:281–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,2/STAT3
signaling induces apoptosis, cell cycle arrest and reduces tumor
cell invasion in colorectal cancer cells. Neoplasia. 10:287–297.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ingley E and Klinken SP: Cross-regulation
of JAK and Src kinases. Growth Factors. 24:89–95. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akira S: Roles of STAT3 defined by
tissue-specific gene targeting. Oncogene. 19:2607–2611. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Avalle L, Pensa S, Regis G, Novelli F and
Poli V: STAT1 and STAT3 in tumorigenesis: A matter of balance.
JAKSTAT. 1:65–72. 2012.PubMed/NCBI
|
|
34
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mukhopadhyay A1, Banerjee S, Stafford LJ,
Xia C, Liu M and Aggarwal BB: Curcumin-induced suppression of cell
proliferation correlates with down-regulation of cyclin D1
expression and CDK4-mediated retinoblastoma protein
phosphorylation. Oncogene. 21:8852–8861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weinstein IB: Disorders in cell circuitry
during multistage carcinogenesis: the role of homeostasis.
Carcinogenesis. 21:857–864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu LF, Zhu YB, Qiao MM, Zhong J, Tu SP and
Wu YL: Constitutive activation and clinical significance of Stat3
in human gastric cancer tissues and cell lines. Chin Med J.
84:2064–2069. 2004.In Chinese.
|
|
38
|
Judd LM, Menheniott TR, Ling H, Jackson
CB, Howlett M, Kalantzis A, Priebe W and Giraud AS: Inhibition of
the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and
in vivo. PloS One. 9:e959932014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim
TY, Oh DY and Bang YJ: OPB–31121, a novel small molecular
inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an
antitumor activity in gastric cancer cells. Cancer Lett.
335:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khanna P, Chua PJ, Bay BH and Baeg GH: The
JAK/STAT signaling cascade in gastric carcinoma (Review). Int J
Oncol. 47:1617–1626. 2015.PubMed/NCBI
|
|
41
|
Huang W, Yu LF, Zhong J, Wu W, Zhu JY,
Jiang FX and Wu YL: Stat3 is involved in angiotensin II-induced
expression of MMP2 in gastric cancer cells. Dig Dis Sci.
54:2056–2062. 2009. View Article : Google Scholar
|
|
42
|
Tactacan CM, Phua YW, Liu L, Zhang L,
Humphrey ES, Cowley M, Pinese M, Biankin AV and Daly RJ: The
pseudokinase SgK223 promotes invasion of pancreatic ductal
epithelial cells through JAK1/Stat3 signaling. Mol Cancer.
14:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|