Introduction
Psoriasis is a common chronic inflammatory skin
disease with a prevalence of 0.2-3%, depending on the population of
origin (1). It is well known that
psoriasis is a T-cell mediated disease, with a disturbed balance in
the interplay of keratinocytes and immune cells (2). The pathogenesis of CD4-positive
(CD4+) T helper (Th) lymphocytes is important in
psoriasis progression. It has been reported that interleukin
(IL-22), as well as IL-17, are involved in the pathogenesis of
psoriasis (3,4). These two cytokines can be secreted by
Th17 cells. In addition, a previous study identified T cells, which
produced IL-22 alone, without IL-17 or interferon γ (IFNγ), and
these were subsequently termed as Th22 cells (5). Th17 and Th22 cells are affected by
the cytokine, IL-23, which is also involved in psoriasis (6). A further study demonstrated that the
frequencies of Th17 and Th22 cells were significantly elevated in
patients with psoriasis. These studies demonstrated that Th17 and
Th22 cells are important in the pathogenesis of psoriasis (7).
An increasing number of studies have demonstrated
the vital role of genetics in the development of psoriasis, and
>30 loci have been identified as susceptibility genes in
psoriasis (8,9). In a genome-wide association study,
runt-related transcription factor 3 (RUNX3) was identified
as a genetic risk factor for psoriasis (10). In addition, a previous study
demonstrated that the expression of RUNX3 was increased in
patients with psoriasis patients, confirming this result (11). RUNX3 is one of the
runt-domain family of transcription factors, which has been
reported to be a tumor suppressor gene, and regulates cell
proliferation and apoptosis in several types of human tumor
(12). RUNX3 is commonly
expressed in the spleen, peripheral blood, spinal cord cells, bone
marrow, B cells and T cells (13).
The inactivation of RUNX3 is considered to be associated
with the occurrence and development of various human diseases,
including gastric and colon cancer (14,15)
and other epithelial diseases (16). RUNX3 is important in the
function of the immune system. Studies have reported that
RUNX3-deficient mice spontaneously develop two immunological
abnormalities: Airway hypersensitivity and colitis (17,18).
Following RUNX3 knockdown in T cells, the target mice
spontaneously developed asthma-like features, including elevated
levels of IgA, IgE and IgG1, and the infiltration of eosinophils
and lymphocytes in the lung (19).
Furthermore, RUNX3 is important in the differentiation of T
cells (20). However, its role and
underlying mechanism in the differentiation of Th17 and Th22 cells
in psoriasis have not been investigated in detail.
In the present study, the expression level of
RUNX3 and the frequencies of Th17 and Th22 cells in
psoriasis were determined. In particular, the role of RUNX3
in regulating the differentiation of CD4+ T cells and
the underlying mechanism in psoriasis were investigated. By the
forced overexpression and knockdown of RUNX3, the present study
determined the importance of RUNX3 in psoriasis through
examining its effect on regulating the levels of Th17 and Th22
cells. Taken together, the results of the present study may
identify a novel therapeutic target and provide a foundation for
gene therapy in psoriasis.
Materials and methods
Patients
The study cohort in the present study comprised 32
patients with psoriasis (20 males and 12 females), as well as 30
healthy control individuals (18 males and 12 females). The age of
the patients with psoriasis was between 20 and 48 years the healthy
controls, between 22 and 50 years old. The patients were recruited
from the Dermatological department of the First Affiliated
Hospital, Xinxiang Medical University (Weihui, China) from July
2013 to June 2014. All patients were diagnosed by clinical features
and disease activity was assessed by Psoriasis Area and Severity
Index (21), patients were
excluded if they had any of the following conditions: Diabetes
mellitus; renal failure; history of neoplasm; major cardiovascular
and cerebrovascular disease. The healthy controls were recruited as
volunteers at the First Affiliated Hospital and Xinxiang Medical
University (Weihui, China. The study was approved by the ethics
committee of Xinxiang Medical University (approval no. 2014055) and
written informed consent was provided by all participants.
Specimen collection
Fasting venous peripheral blood samples (10 ml) were
collected from the patients with psoriasis and the healthy control
individuals. Following centrifugation for 15 min at 335.4 × g and
4°C, the serum samples were stored at −80°C until further use.
CD4+ T cells were isolated from each blood sample using
a human CD4+ positive selection magnetic column
(Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany), according to
the manufacturer's protocol. The absolute counts of the
CD4+ T cells were measured using flow cytometry. The
purity of the CD4+ T cells was typically >90,
according to the protocols, and the cells were cultured in human T
cell culture AIM V Medium CTS at a density of 105 at 5%
CO2 and 37°C (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The cells were grown to 80% confluence.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction analysis
(RT-qPCR)
Total RNA was extracted from the cultured
CD4+ T cells using TRIzol reagent and chloroform
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols and the following temperature protocol:
30°C for 10 min and 42°C for 30 min. The concentration and purity
of the total RNA were detected using an ultraviolet (UV)
spectrophotometer (BioSpectrometer; Eppendorf AG, Hamburg,
Germany). cDNA was synthesized using M-MLV reverse transcriptase
(Clontech Laboratories, Palo Alto, CA, USA). The mRNA was analyzed
using an RT-qPCR mixture system containing cDNA templates, primers
synthesized by Shanghai Sangon Biological Engineering and
Technology & Services Co., Ltd. (Shanghai, China), and Absolute
SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Inc.). The
PCR primers (Shanghai Sangon Biological Engineering and Technology
& Services Co., Ltd., Shanghai, China) used were as follows:
RUNX3 F 5′-ACCTGTCACAACGGCCAGAAC-3′,R 5′-TTCCAGTGAGGACAGGCCAAG-3′;
β-actin F 5′-GGCGGCACCACCATGTACCCT-3′, R
5′-AGGGGCCGGACTCGTCATACT-3′. PCR cycling conditions were as
follows: Enzyme activation 95°C 15 min per cycle, denaturation 95°C
at 15 sec per 40 cycles and annealing/extension at 60°C for 60 sec
using a LightCycler sequence detection system (480; Roche
Diagnostics, Indianapolis, IN, USA). The gene mRNA expression
levels were normalized to those of β-actin. Data were analyzed
using the 2−ΔΔCq method (22). This experiment was performed three
times.
Western blot analysis
Total protein was extracted from the CD4+
T cells using a radioimmunoprecipitation assay lysis buffer (Thermo
Fisher Scientific, Inc.) and quantified using a UV
spectrophotometer. A total of 25 μg protein per lane
separated by 10% sodium dodecyl sulfate-polyacrylamide gel (Thermo
Fisher Scientific, Inc.) electrophoresis. Following being
transferred onto nitrocellulose membranes (Thermo Fisher
Scientific, Inc.) and blocked in Tris-buffered saline-Tween buffer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing 5%
nonfat dry milk for 30 min, the membranes were incubated for 16 h
at 4°C with polyclonal rabbit anti-RUNX3 (cat. no. sc-30197;
1:1,000) or polyclonal rabbit anti-β-actin antibodies (cat. no.
sc-7210; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), followed by incubation with the corresponding horseradish
peroxidase-conjugated secondary goat anti-rabbit antibody (cat. no.
sc-2004; 1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature and washed twice with phosphate-buffered saline. A
fluorescent Western blotting detection system (Thermo Fisher
Scientific, Inc.) was used. Densitometry analysis was performed
using MultiGauge 3.1 software (Thermo Fisher Scientific, Inc.). The
protein expression levels were normalized to those of β-actin.
Flow cytometric analysis
The percentages of Th17 and Th22 in CD4+
T cells were detected using flow cytometric analysis, according to
the manufacturer's protocols. Briefly, the cells at a density of
105 were incubated with phorbol ester, ionomycin and
monensin (all Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C.
The cells were then stained with fluorescein isothiocyanate
(FITC)-labeled mouse anti-human CD4 (cat. no. 550628; 1:100; BD
Biosciences, San Diego, CA, USA), phycoerythrin (PE)-labeled mouse
monoclonal anti-IL-17 (cat. no. 12-7178; 1:100; eBioscience, Inc.,
San Diego, CA, USA), and PE-labeled monoclonal mouse anti-IL-22
(cat. no. 12-7229-41; 1:100; eBioscience, Inc.), respectively, for
30 min at room temperature. The results were analyzed using
CellQuest Pro 5.1 software (BD Biosciences, Franklin Lakes, NJ,
USA).
CD4+ T cell transfection
For the transfection of the CD4+ T cells,
RUNX3 small interfering (si)RNA was purchased from
GenePharma (Shanghai, China), and lentiviral vectors containing the
RUNX3 gene were constructed (GeneChem Co., Ltd., Shanghai,
China). The CD4+ T cells were seeded at 106
cells/well transfected with the RUNX3 lentiviral plasmid,
empty lentiviral vector, RUNX3 siRNA and siRNA control,
respectively, according to the instructions provided with the
Lipofectamine RNAiMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h of transfection at room temperature,
the cells were collected for further analysis.
Enzyme-linked immunosorbent assay
(ELISA)
The cytokine concentrations were measured using
corresponding quantification ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocols.
Optical density values (OD values) were read at 450 nm using a
microplate reader (SynergyH1; BioTek, Winooski, VT, USA) following
the assay. The concentrations of cytokines were calculated,
according to the corresponding OD value and concentration of the
standard substance.
Statistical analysis
All data were processed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). The results are expressed as the
mean ± standard deviation. Statistical analysis was calculated
using one-way analysis of variance followed by a Bonferroni test.
P≤0.05 were considered to indicate a statistically significant
difference.
Results
Expression of RUNX3 is increased in
CD4+ T cells from patients with psoriasis
To determine the expression level of RUNX3,
CD4+ T cells were isolated from blood samples from each
patient with psoriasis and control subject. The mRNA expression of
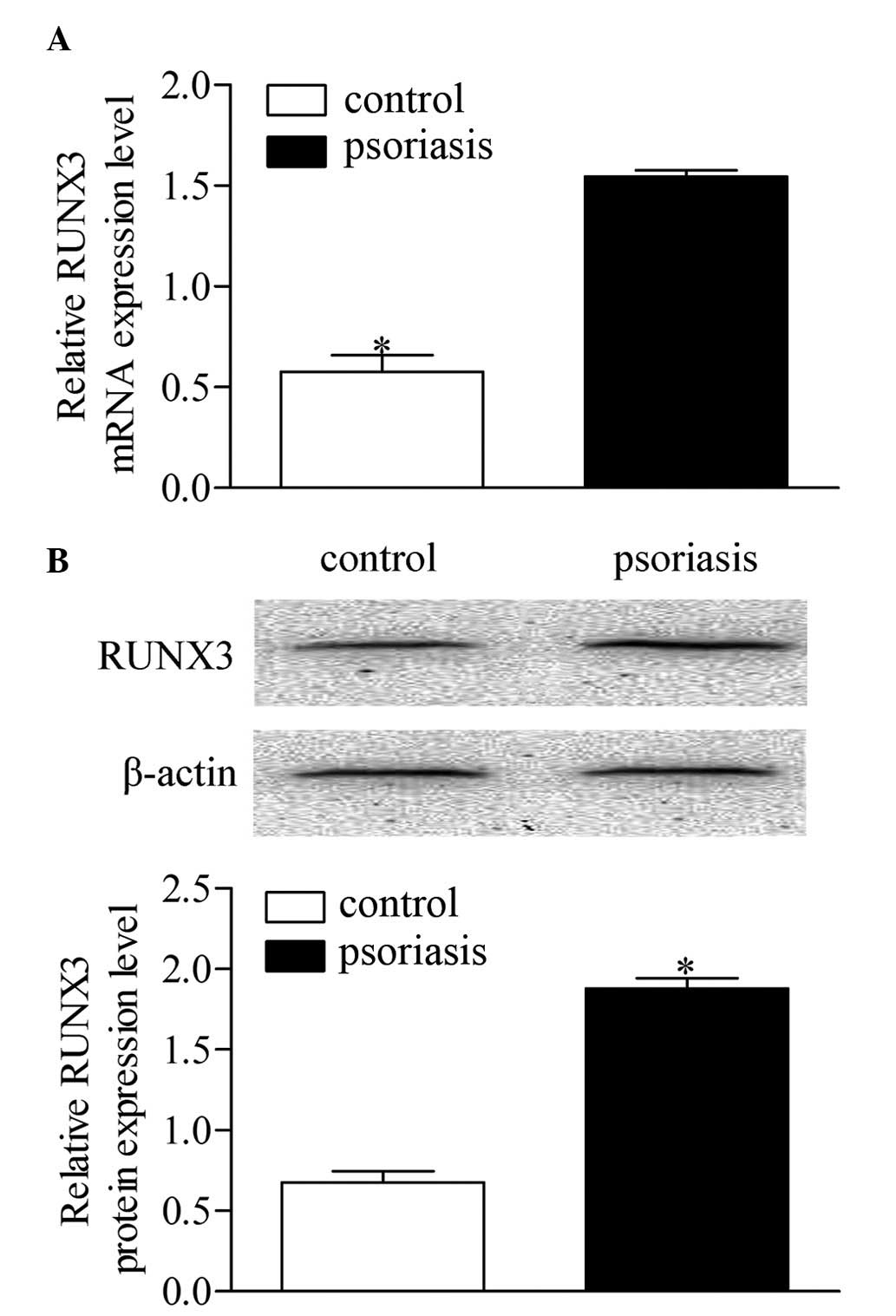
RUNX3 was measured using RT-qPCR. As shown in Fig. 1A, the mRNA expression level of
RUNX3 was significantly increased in the patients with
psoriasis patients, compared with the healthy controls. Western
blot analysis showed that the protein level of RUNX3 was also
significantly elevated in the patients with psoriasis, compared
with the healthy controls (Fig.
1B). These results revealed a notable upregulation of
RUNX3 in CD4+ T cells from patients with
psoriasis, which is relevant to psoriasis development.
Th17 and Th22 cells are dysregulated in
patients with psoriasis
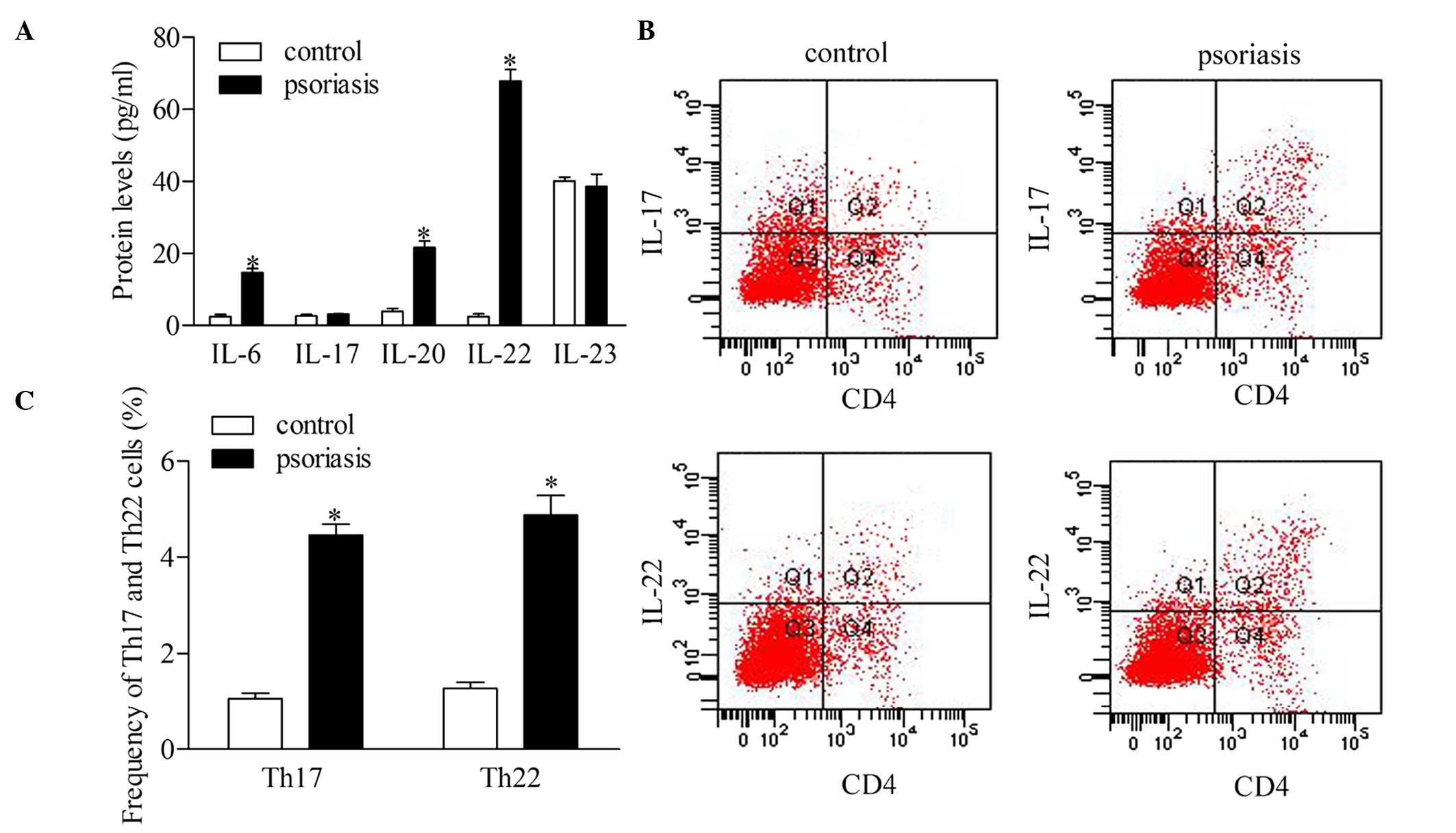
To investigate the roles of Th17 and Th22 cells, the
present study detected the serum levels of IL-6, IL-17, IL-20,
IL-22 and IL-23 in the supernatant of CD4 T cells of patients with
psoriasis. The statistical analyses revealed that the protein
expression levels of IL-6, IL-20 and IL-22 were significantly
higher in the patients with psoriasis, compared with the healthy
controls. However, no significant differences were found in the
concentrations of IL-17 and IL-23 between the psoriatic patients
and control group (Fig. 2A). To
further confirm the dysregulation of Th17 and Th22 cells, the
present study evaluated the frequency of Th17 and Th22 cells using
flow cytometric analysis (Fig. 2B
and C). The results showed that the frequencies of the Th17 and
Th22 cells increased significantly in the patients with psoriasis,
compared with the healthy controls.
RUNX3 overexpression increases
concentrations of cytokines associated with Th17 and Th22 in normal
CD4+ T cells
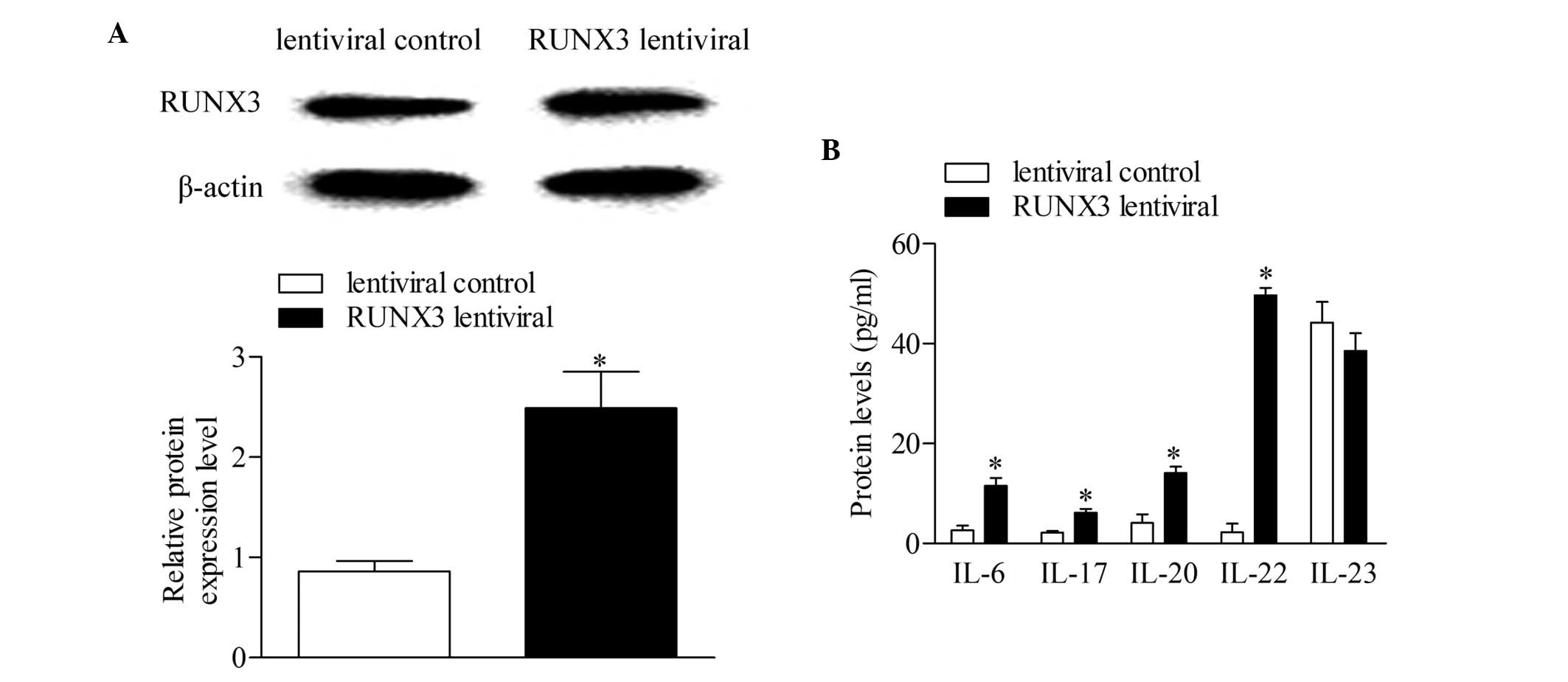
The RUNX3 lentiviral plasmid and empty
lentiviral vector were transfected into CD4+ T cells
from healthy controls to investigate the effect of RUNX3
overexpression in normal CD4+ T cells. Following 48 h of
transfection, the expression level of RUNX3 was detected using
Western blot analysis. The results showed that the expression of
RUNX3 increased significantly in the CD4+ T cells
transfected with the RUNX3 lentiviral plasmid, compared with
the empty lentiviral vector-transfected control group (Fig. 3A). In addition, the concentrations
of IL-6, IL-17, IL-20 and IL-22 increased significantly with
RUNX3 overexpression, compared with the control group. No
significant differences were observed in the concentrations of
IL-23 between groups (Fig.
3B).
RUNX3 knockdown inhibits the production
of cytokines associated with Th17 and Th22 in CD4+ T
cells from patients with psoriasis
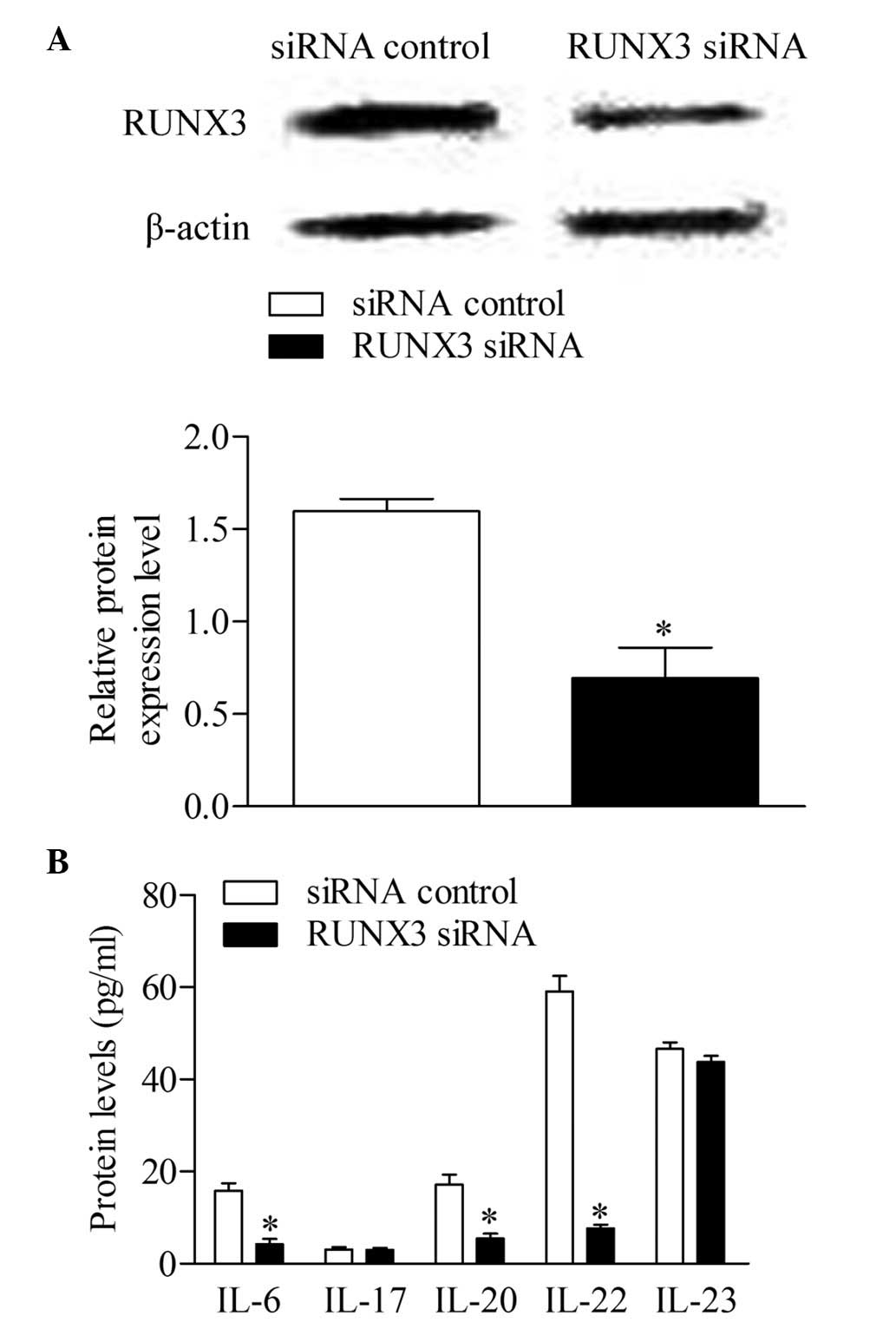
The RUNX3 siRNA and siRNA control were
transfected into CD4+ T cells from patients with
psoriasis to detect whether the inhibition of RUNX3 can
reverse immune dysfunction. The results of the Western blot
analysis showed that the expression of RUNX3 decreased
significantly in the CD4+ T cells transfected with
RUNX3 siRNA, compared with the control group (Fig. 4A). Additionally, the concentrations
of IL-6, IL-20 and IL-22 decreased significantly following
RUNX3 siRNA transfection, compared with the control group.
No significant differences were observed in the concentrations of
IL-17 and IL-23 between groups (Fig.
4B).
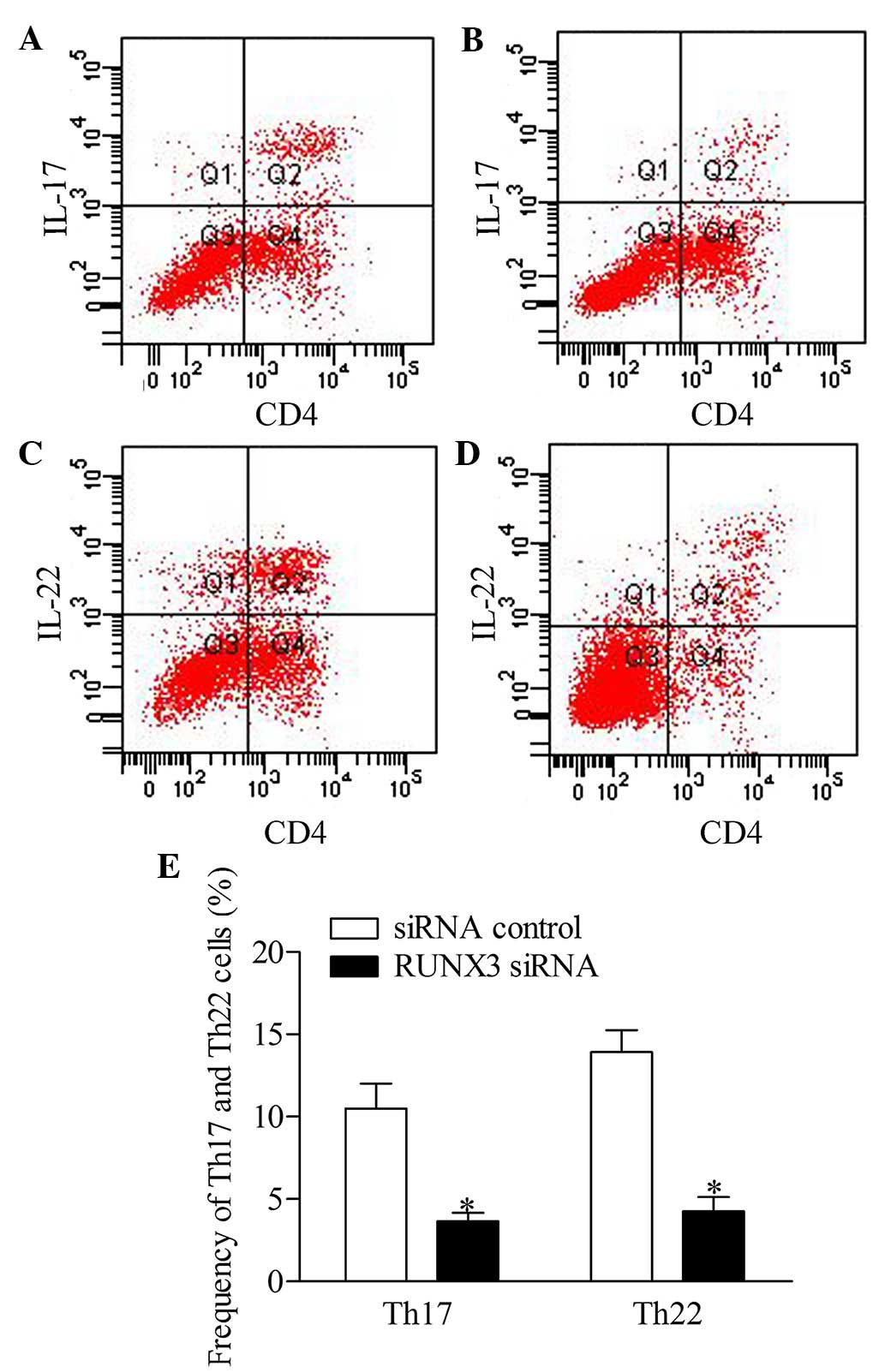
RUNX3 knockdown represses the frequency
of Th17 and Th22 cells in CD4+ T cells from patients
with psoriasis
To further investigate the effect of RUNX3
inhibition on Th17 and Th22 cells in CD4+ T cells, the
present study detected the frequencies of Th17 and Th22 cells from
patients with psoriasis transfected with RUNX3 siRNA and the
siRNA control using flow cytometry. The results showed that the
frequencies of Th17 and Th22 cells in the RUXN3 siRNA
transfection group were decreased significantly, compared with
those in the control group (Fig.
5).
Discussion
Psoriasis is well known as a T cell-dependent
autoimmune disease of the joints and skin (23). Studies have suggested that there is
a genetic contribution, and >30 risk loci for psoriasis have
been identified (9). Genetic
factors explain 68% of the variation in the susceptibility to
psoriasis (24). Tsoi et al
demonstrated that RUNX3 may be a susceptibility gene for
psoriasis (10). In addition, Apel
et al further confirmed that RUNX3 is a candidate for
involvement in psoriasis and other T cell-mediated diseases
(11). The present study showed
that the expression level of RUNX3 increased significantly
in CD4+ T cells from patients with psoriasis, compared
with the healthy controls, which confirmed that RUNX3 is a
susceptibility gene for psoriasis and was consistent with the
previous reports.
CD4+ T cells are the most important
element of adaptive systems and immune homeostasis. According to
the local cytokine environment and the nature of the encountered
stimulus itself, naïve CD4+ T cells are differentiated
into various subsets of Th or regulatory T cells (25). One of the T cell subsets, Th17
cells, is known for producing IL-17, IL-6, IL-21, IL-22 and tumor
necrosis factor (TNF) (26). Th17
cells are differentiated from naïve CD4+ T cells under
the effect of the transforming growth factor β (TGF-β), IL-6 and
IL-1, whereas their expansion and survival are controlled
predominantly by IL-23 (27,28).
Previous investigation has shown that Th17 cells can be generated
by IL-6, IL-23 and IL-1β in the absence of TGF-β (29). It has been reported that cytokines
produced by Th17 cells initiate parakeratosis and acanthosis
hyperkeratosis (30). Th17 cells
have been demonstrated to be involved in the development of T cell
migration and activation, neutrophil and monocyte chemotaxis, and
neovascularization (31).
Th17 cells were originally considered to be the
predominant source in the production of the IL-22 cytokine, however
studies have shown that certain CD4+ T cells secrete
IL-22 alone, without IL-17, and these have subsequently been termed
Th22 cells (32). Th22 cells also
produce cytokines, including IL-26 and IL-13. Studies have
demonstrated that TNF-α and IL-6 can promote the Th22 phenotype,
with the assistance of plasmacytoid dendritic cells (33,34).
The levels of Th17 and Th22 cells have been shown to be abnormal in
patients with psoriasis (7). In
the present study, it was found that the serum levels of IL-6,
IL-20 and IL-22 were elevated in patients with psoriasis patients,
compared with healthy control individuals. It is widely known that
IL-6 contributes to the inflammatory and autoimmune processes of
Th17 cells (31), and elevated
serum levels of IL-6 have been observed in patients with psoriasis
in numerous studies (35,36). IL-20 is produced by keratinocytes
in the presence of IL-22, IL-17 and TNF-α, but not IFN-γ or IL-20
itself (37). The effect of IL-20
often appears in the later phases of psoriasis pathogenesis
(38), and increased levels of
IL-20 have been noted in lesional skin, as well as in the blood, in
patients with psoriasis (39). In
addition, the present study found that the frequencies of Th17 and
Th22 cells were elevated in the patients with psoriasis, compared
with the healthy controls. No significantly differences were found
in the concentrations of IL-17 and IL-23 between the patients with
psoriasis and control group. This confirmed the involvement of Th17
and Th22 cells in the pathogenesis of psoriasis, and the increased
level of IL-22 without elevated IL-17 level suggested that Th22
cells are more important in the inflammatory process.
As has been previously reported, RUNX3 is
important in the differentiation of T cells (20). Thus, the present study investigated
the potential role and mechanism of RUNX3 in regulating Th17
and Th22 cells in psoriasis. The effect of RUNX3
overexpression on the expression levels of Th17 and Th22
cell-associated cytokines was compared with normal CD4+
T cells. The results showed that the levels of IL-6, IL-17, IL-20
and IL-22 increased significantly due to the overexpression of
RUNX3, similar to the physiological features in psoriasis.
Currently, the RUNX3 gene has become important in potential
therapeutic targets for psoriasis. Liang et al (40) demonstrated that signaling
transducer and activator of transcriptions 4, which is a central
mediators in the generation of inflammation during protective
immune responses and immune-mediated diseases, may be an effective
therapeutic target for autoimmune diseases, including psoriasis
(40). It has been reported that
RUNX3 is a therapeutic target for gastric cancer and can be
involved in a various human diseases, including psoriasis,
according to its function in immune dysfunction (41). In the present study, the effect of
RUNX3 knockdown was detected in CD4+ T cells from
patients with psoriasis. The results showed that the inhibition of
RUNX3 suppressed the production of IL-6, IL-17, IL-20 and
IL-22. In addition, the frequencies of Th17 and Th22 cells
decreased significantly following transfection with RUNX3
siRNA. These results suggested that the inhibition of RUNX3
regulated Th17 and Th22 cells, leading to an improvement in immune
dysfunction in psoriasis.
In conclusion, the present study demonstrated that
RUNX3 was upregulated in CD4+ T cells from
patients with psoriasis. In addition, the importance of Th17 and
Th22 cells in psoriasis was confirmed. Notably, the present study
found that the inhibition of RUNX3 affected the
differentiation of CD4+ T cells, which downregulated the
frequencies of Th17 and Th22 cells in psoriasis. Consequently,
targeting RUNX3 may support a promising therapeutic strategy
for the treatment of psoriasis.
Acknowledgments
This study was supported by the Science and
Technology Reasearch Key Project of The Education Department, Henan
province (grant no. 13A320852) and the Science and Technology
Research Plan in Xinxiang City (grant no. ZG13022).
Abbreviations:
|
Ps
|
psoriasis
|
|
Th
|
T helper
|
|
IL
|
interleukin
|
|
IFNγ
|
interferon γ
|
|
RUNX3
|
runt-related transcription factor
3
|
References
|
1
|
Sales R and Torres T: Psoriasis and
metabolic syndrome. Acta Dermatovenerol Croat. 22:169–174.
2014.PubMed/NCBI
|
|
2
|
Diani M, Altomare G and Reali E: T cell
responses in psoriasis and psoriatic arthritis. Autoimmun Rev.
14:286–292. 2015. View Article : Google Scholar
|
|
3
|
Leipe J, Grunke M, Dechant C, Reindl C,
Kerzendorf U, Schulze-Koops H and Skapenko A: Role of Th17 cells in
human autoimmune arthritis. Arthritis Rheum. 62:2876–2885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boniface K, Guignouard E, Pedretti N,
Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G,
Yssel H, et al: A role for T cell-derived interleukin 22 in
psoriatic skin inflammation. Clin Exp Immunol. 150:407–415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nograles KE, Zaba LC, Shemer A,
Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R,
Krueger JG and Guttman-Yassky E: IL-22-producing ῾T22᾽ T cells
account for upregulated IL-22 in atopic dermatitis despite reduced
IL-17-producing TH17 T cells. J Allergy Clin Immunol.
123:1244–1252.e2. 2009. View Article : Google Scholar
|
|
6
|
Cauli A and Mathieu A: Psoriatic
arthritis: Genetics and pathogenesis. Reumatismo. 64:71–78. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benham H, Norris P, Goodall J, Wechalekar
MD, FitzGerald O, Szentpetery A, Smith M, Thomas R and Gaston H:
Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis
Res Ther. 15:R1362013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang H, Jin X, Li Y, Jiang H, Tang X, Yang
X, Cheng H, Qiu Y, Chen G, Mei J, et al: A large-scale screen for
coding variants predisposing to psoriasis. Nat Genet. 46:45–50.
2014. View
Article : Google Scholar
|
|
9
|
Garber K: Genetics: Deep exploration.
Nature. 492(Suppl): S56–S57. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsoi LC, Spain SL, Knight J, Ellinghaus E,
Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al:
Identification of 15 new psoriasis susceptibility loci highlights
the role of innate immunity. Nat Genet. 44:1341–1348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Apel M, Uebe S, Bowes J, Giardina E,
Korendowych E, Juneblad K, Pasutto F, Ekici AB, McManus R, Ho P, et
al: Variants in RUNX3 contribute to susceptibility to psoriatic
arthritis, exhibiting further common ground with ankylosing
spondylitis. Arthritis Rheum. 65:1224–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen HX, Wang S, Wang Z, Zhang ZP and Shi
SS: Overexpression of RUNX3 inhibits malignant behaviour of Eca109
cells in vitro and vivo. Asian Pac J Cancer Prev. 15:1531–1537.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagi R, Junttila IS, Wei G, Urban JF Jr,
Zhao K, Paul WE and Zhu J: The transcription factor GATA3 actively
represses RUNX3 protein-regulated production of interferon-gamma.
Immunity. 32:507–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blyth K, Cameron ER and Neil JC: The RUNX
genes: Gain or loss of function in cancer. Nat Rev Cancer.
5:376–387. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
He SY, Jiang RF, Jiang J, Xiang YS and
Wang L: Investigation of methylation and protein expression of the
gene in colon carcinogenesis. Biomed Rep. 3:687–690.
2015.PubMed/NCBI
|
|
16
|
Ito K, Lim AC, Salto-Tellez M, Motoda L,
Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, et al: RUNX3
attenuates beta-catenin/T cell factors in intestinal tumorigenesis.
Cancer Cell. 14:226–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brenner O, Levanon D, Negreanu V, Golubkov
O, Fainaru O, Woolf E and Groner Y: Loss of Runx3 function in
leukocytes is associated with spontaneously developed colitis and
gastric mucosal hyperplasia. Proc Natl Acad Sci USA.
101:16016–16021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fainaru O, Woolf E, Lotem J, Yarmus M,
Brenner O, Goldenberg D, Negreanu V, Bernstein Y, Levanon D, Jung S
and Groner Y: Runx3 regulates mouse TGF-beta-mediated dendritic
cell function and its absence results in airway inflammation. EMBO
J. 23:969–979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naoe Y, Setoguchi R, Akiyama K, Muroi S,
Kuroda M, Hatam F, Littman DR and Taniuchi I: Repression of
interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to
the Il4 silencer. J Exp Med. 204:1749–1755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Djuretic IM, Cruz-Guilloty F and Rao A:
Regulation of gene expression in peripheral T cells by Runx
transcription factors. Adv Immunol. 104:1–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oji V and Luger TA: The skin in psoriasis:
Assessment and challenges. Clin Exp Rheumatol. 93(Suppl 5): S14–19.
2015.
|
|
22
|
Wang G, Wang L, Sun S, Wu J and Wang Q:
Quantitative measurement of serum microRNA-21 expression in
relation to breast cancer metastasis in Chinese females. Ann Lab
Med. 35:226–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghoreschi K, Weigert C and Röcken M:
Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol.
25:574–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lonnberg AS, Skov L, Skytthe A, Kyvik KO,
Pedersen OB and Thomsen SF: Heritability of psoriasis in a large
twin sample. Br J Dermatol. 169:412–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation. Immunity.
30:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miossec P: IL-17 and Th17 cells in human
inflammatory diseases. Microbes Infect. 11:625–630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van den Berg WB and McInnes IB: Th17 cells
and IL-17 a-focus on immunopathogenesis and immunotherapeutics.
Semin Arthritis Rheum. 43:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bettelli E, Korn T, Oukka M and Kuchroo
VK: Induction and effector functions of T(H)17 cells. Nature.
453:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peck A and Mellins ED: Breaking old
paradigms: Th17 cells in autoimmune arthritis. Clin Immunol.
132:295–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volpe E, Battistini L and Borsellino G:
Advances in T helper 17 cell biology: Pathogenic role and potential
therapy in multiple sclerosis. Mediators Inflamm. 2015:4751582015.
View Article : Google Scholar
|
|
31
|
Asarch A, Barak O, Loo DS and Gottlieb AB:
Th17 cells: A new paradigm for cutaneous inflammation. J Dermatolog
Treat. 19:259–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang
J, Chen H and Wu C: Memory IL-22-producing CD4+ T cells specific
for Candida albicans are present in humans. Eur J Immunol.
39:1472–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
N, Pan HF and Ye DQ: Th22 in inflammatory
and autoimmune disease: Prospects for therapeutic intervention. Mol
Cell Biochem. 353:41–46. 2011. View Article : Google Scholar
|
|
35
|
Kaur S, Zilmer K, Leping V and Zilmer M:
Comparative study of systemic inflammatory responses in psoriasis
vulgaris and mild to moderate allergic contact dermatitis.
Dermatology. 225:54–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chandran V: Soluble biomarkers may
differentiate psoriasis from psoriatic arthritis. J Rheumatol
Suppl. 89:65–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sabat R and Wolk K: Research in practice:
IL-22 and IL-20: Significance for epithelial homeostasis and
psoriasis pathogenesis. J Dtsch Dermatol Ges. 9:518–523.
2011.English, German. PubMed/NCBI
|
|
38
|
Wolk K, Haugen HS, Xu W, Witte E, Waggie
K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al:
IL-22 and IL-20 are key mediators of the epidermal alterations in
psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl).
87:523–536. 2009. View Article : Google Scholar
|
|
39
|
Wolk K, Witte E, Warszawska K,
Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Döcke WD, Asadullah
K, Volk HD, et al: The Th17 cytokine IL-22 induces IL-20 production
in keratinocytes: A novel immunological cascade with potential
relevance in psoriasis. Eur J Immunol. 39:3570–3581. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang Y, Pan HF and Ye DQ: Therapeutic
potential of STAT4 in autoimmunity. Expert Opin Ther Targets.
18:945–960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Subramaniam MM, Chan JY, Yeoh KG, Quek T,
Ito K and Salto-Tellez M: Molecular pathology of RUNX3 in human
carcinogenesis. Biochim Biophys Acta. 1796:315–331. 2009.PubMed/NCBI
|