Introduction
Osteosarcoma is a primary malignant bone tumor with
high morbidity in juveniles and adults, accounting for ~60% of
malignant bone tumors in the first two decades of life (1–3).
Despite advances in diagnostic techniques and therapeutic
management, the long-term survival of patients with osteosarcoma
remains extremely low. The molecular mechanisms underlying the
disease progression remain poorly understood (4,5).
Therefore, an improved understanding of the molecular mechanisms
associated with the development and progression of human
osteosarcoma is essential to find effective therapeutic
interventions.
MicroRNAs (miRNAs) are small non-coding RNAs of
21–25 nucleotides in length that regulate the expression of target
genes post-transcriptionally and serve important roles in various
biological processes, including differentiation, development,
proliferation and apoptosis (6–8).
Emerging evidence shows that miRNAs are involved in the
pathogenesis of the majority of types of cancer. The aberrant
expression of miRNAs has been observed in a number of types of
cancer, with this tightly associated with cell proliferation,
migration, invasion and apoptosis. Previous studies have indicated
that the expression of miR-125a-5p was significantly reduced in
numerous types of tumors, including lung cancer (9), myeloma (10), colorectal adenomas and
adenocarcinomas (11), and breast
cancer (12). However, the
biological role and the molecular mechanisms associated with
miR-125a-5p in osteosarcoma remain unknown.
In the present study, the expression of miR-125a-5p
was observed to be reduced in osteosarcoma tissues and cell lines
compared with paired adjacent normal bone tissues and osteoblastic
cells. The overexpression of miR-125a-5p inhibited cell migration,
invasion and restrained epithelial-mesenchymal transition (EMT). In
addition, at the molecular level, matrix metalloproteinase-11
(MMP-11) was a indicated to be a direct target of miR-125a-5p in
osteosarcoma.
Materials and methods
Patient samples
A total of 28 paired osteosarcoma tissue specimens
and adjacent matched normal osteosarcoma tissues were obtained from
the People's Hospital of Xinjiang Uygur Autonomous Region. All
samples were immediately snap-frozen in liquid nitrogen and stored
at −80°C until use. All patients (or patients' parents on behalf of
the children) agreed to participate in the study and gave written
informed consent. The study was approved by the Ethics Committee of
People's Hospital of Xinjiang Uygur Autonomous Region.
Cell culture and transfection
Human osteosarcoma cell lines (HOS, Saos-2, MG-63
and U2OS) and the normal osteoblast cells were obtained from
American Type Culture Collection (Manassas, VA, USA). All cells
were cultured in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.). Cultures were maintained at 37°C in a humidified atmosphere
with 5% CO2.
miR-125a-5p mimics and negative control were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
sequences were as follows: Mimics, 5′-AGACCCUUUAACCUGUGAGGAC-3′;
negative control, 5′-CAGUACUUUUGUGUAGUACAA-3′. A total of
5×106 MG-63 or U2OS cells were transfected with
miR-125a-5p mimics or negative control using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following
manufacturer's protocol.
Analysis of miR-125a-5p expression using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA from tissue samples and cell lines was
harvested using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). A total of 1 µg of total RNA was
reverse transcribed to cDNA using the miScript II RT kit (Qiagen
GmbH, Hilden, Germany) according to the manufacturer's instruction.
The RT reaction components were as follows: 5X miScript HiSpec
Buffer (4 µl), 10X Nucleics Mix (2 µl), miScript
Reverse Transcriptase Mix (2 µl), Template RNA (3 µl)
and RNase-free water (9 µl). The RT protocol was follows: 60
min at 37°C, 5 min at 95°C, then stored at −20°C. RT-qPCR was
performed using an Applied Biosystems 7500 Real-time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following cycling conditions: 95°C for 20 min; 95°C for 10 sec; and
60°C for 20 sec for 40 cycles. The relative expression ratio of
miR-125a-5p in tissues and cells was quantified using the
2−ΔΔCq method (13).
Small nuclear U6 snRNA was used as an internal control. The primers
used for the amplification were as follows: miR-125a-5p forward
primer, 5′-AGGACATCCAGGGTCACAGGTGA-3′, reverse primer, miScript
SYBR Green PCR kit Universal Primer; U6 forward primer,
5′-CTCGCTTCGGCAGCACA-3′, reverse primer,
5′-ACGCTTCACGAATTTGCGT-3′.
Cell migration and invasion assays
A wound-healing assay was used to assess the effect
of miR-125a-5p on cell migration ability in MG-63 and U2OS cells.
An artificial wound was created 6 h following transfection using a
pipette tip on the confluent cell monolayer, and to visualize
migrated cells and wound healing, images were captured from five
randomly selected fields in each sample taken at 0 and 12 h. The
effect of miR-125a-5p on cell invasion ability was examined using a
Transwell assay. A total of 2×105 cells were plated in
the top chamber of the insert pre-coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). Cells were plated in medium
without serum, and medium supplemented with 10% FBS was added as a
chemoattractant in the lower chamber. The cells were incubated for
48 h, and the cells that migrated through the matrix to the other
side of the insert were fixed with 100% methanol for 2 min at room
temperature, stained with hematoxylin, and counted in 10 random
fields per well (magnification, ×100).
Bioinformatics
TargetScan (http://www.targetscan.org/), miRanda (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/) were used to predict the
potential targets of miR-125a-5p in osteosarcoma.
Dual luciferase assays
Luciferase reporters were successfully constructed
using molecular cloning technology. The target sequence was
inserted into psiCHECK-2 luciferase reporter vectors (Promega
Corporation, Madison, WI, USA) to obtain
psiCHECK-2-MMP-11-WT-3′-untranslated region (UTR), which contains
the miR-125a-5p binding sequence (MMP-11-3′-UTR sequence). The
MMP-11 3′-UTRs contained sequences with mutations
(psiCHECK-2-MMP-11-MUT-3′-UTR) in the putative binding site of
MMP-11 3′-UTRs was chemically synthesized by Shanghai GenePharma
Co., Ltd. MG-63 cells (4×105) were seeded in 24-well
plates and transfected with the psiCHECK-2-MMP-11-WT-3′-UTR or
psiCHECK-2-MMP-11-MUT-3′-UTR recombinant plasmids using
Lipofectamine 2000, and miR-125a-5p mimics or negative control for
24 h. Luciferase values were determined using the Dual-Luciferase
Reporter Assay System (Promega Corporation). The firefly luciferase
gene was used as an internal control to normalize the transfection
efficiency.
Western blotting
MG-63 cells were lysed with radioimmunoprecipitation
assay lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.).
The lysate was centrifuged at 13,000 × g for 20 min at 4°C and the
supernatant was collected as total proteins. Protein concentration
was determined using a bicinhoninic acid assay kit (Thermo Fisher
Scientific, Inc.). Sodium dodecyl sulfate polyacrylamide gel
electrophoresis (12%; Invitrogen; Thermo Fisher Scientific, Inc.)
was used to separate the proteins (40 µg) prior to transfer
to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat milk for 2 h, membranes
were incubated with the following primary antibodies overnight at
4°C: Mouse monoclonal anti-E-cadherin (1:500; sc-8426), mouse
monoclonal anti-N-cadherin (1:1,000; sc-271386), mouse monoclonal
anti-vimentin (1:500; sc-373717), rabbit polyclonal anti-MMP-11
(1:200; sc-8836-R), and mouse monoclonal anti-GAPDH antibody
(1:2,000; sc-365062; all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Subsequently, membranes were incubated for 1 h at
room temperature with rabbit anti-mouse IgG (1:5,000 ab6728; Abcam,
Cambridge, UK) and goat anti-mouse IgG (1:5,000; ab97051; Abcam).
The protein bands detected by enhanced chemiluminescence (GE
Healthcare Life Sciences, Little Chalfont, UK). The levels of GAPDH
were used as loading controls. Western blot bands were obtained
using Imaging System, and the protein density was quantified using
Odyssey software, version 1.2 (LI-COR Biosciences, Lincoln, NE,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA) and data are
presented as the mean ± standard error. The miR-125a-5p expression
differences between osteosarcoma tissues and normal bone tissues
were analyzed using a paired samples t-test. The miR-125a-5p
expression in osteosarcoma cell lines was analysed using one-way
analysis of variance and dual luciferase assay was analyzed using
two-way analysis of variance and Dunnett's test. The other data was
analyzed using an independent-samples t-test. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were repeated a minimum of three times.
Results
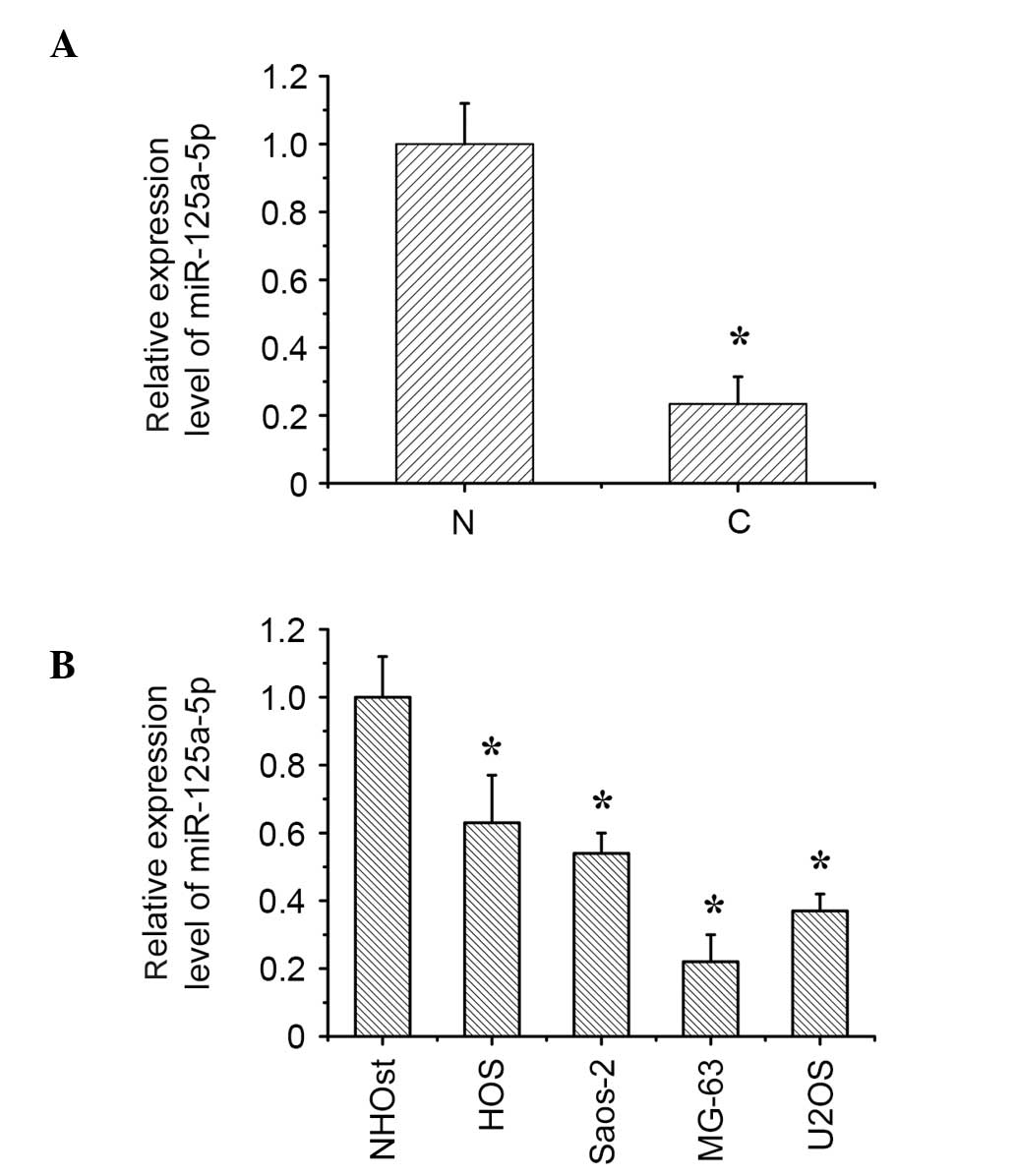
The expression of miR-125a-5p is
downregulated in osteosarcoma tissues and cell lines
The expression levels of miR-125a-5p were
downregulated in osteosarcoma tissues compared with the matched
normal bone tissues, as measured by RT-qPCR (Fig. 1A). The miR-125a-5p expression
levels in osteosarcoma cell lines (HOS, Saos-2, MG-63, and U2OS)
were additionally downregulated compared with the normal osteoblast
cells (Fig. 1B).
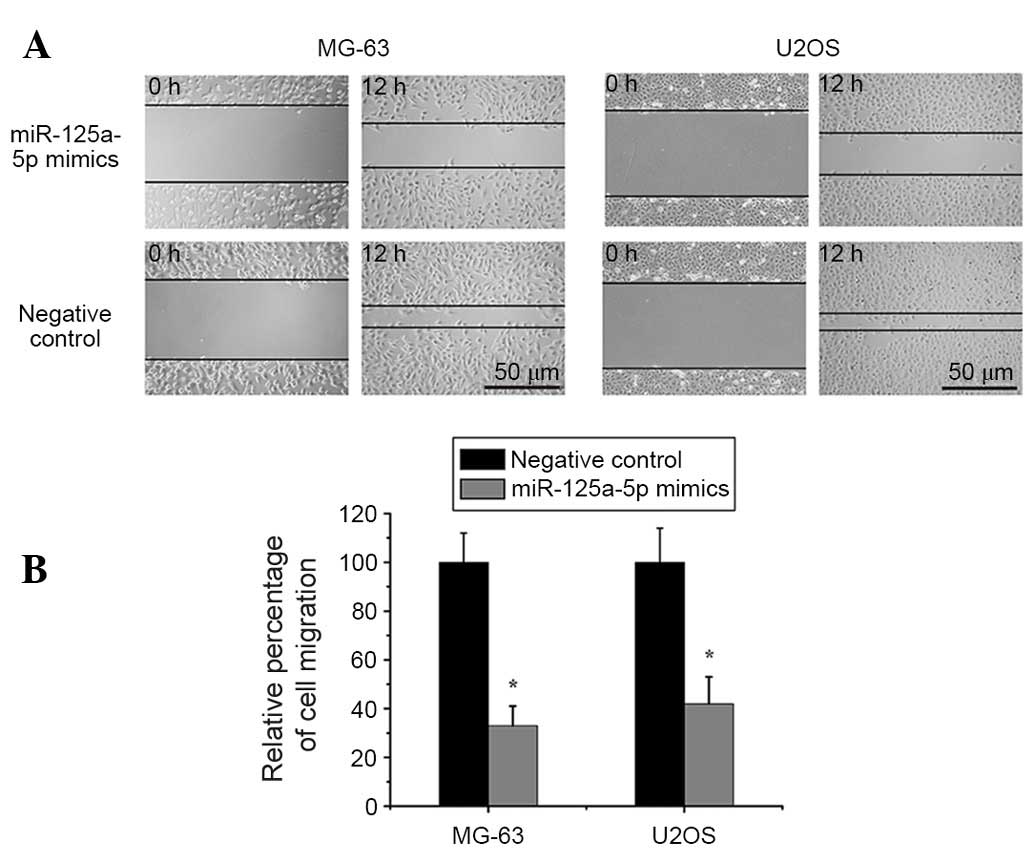
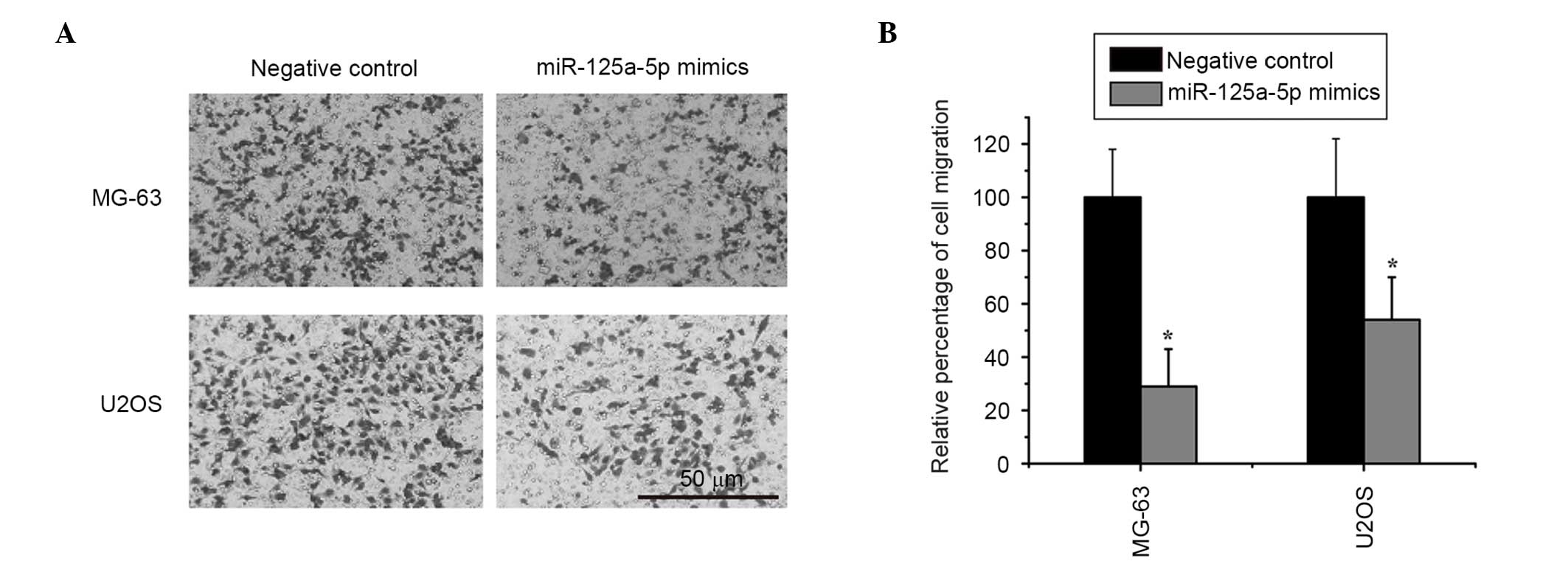
Restoration of miR-125a-5p expression
inhibits cell migration and invasion in osteosarcoma
In order to assess the effects of miR-125a-5p on
osteosarcoma cell migration and invasion, miR-125a-5p mimics or
negative controls were transfected into MG-63 and U2OS cells.
Wound-healing and Transwell assays indicated that enhancing the
expression levels of miR-125a-5p markedly inhibited migration and
invasion compared with the control group in MG-63 and U2OS cells
(Figs. 2 and 3).
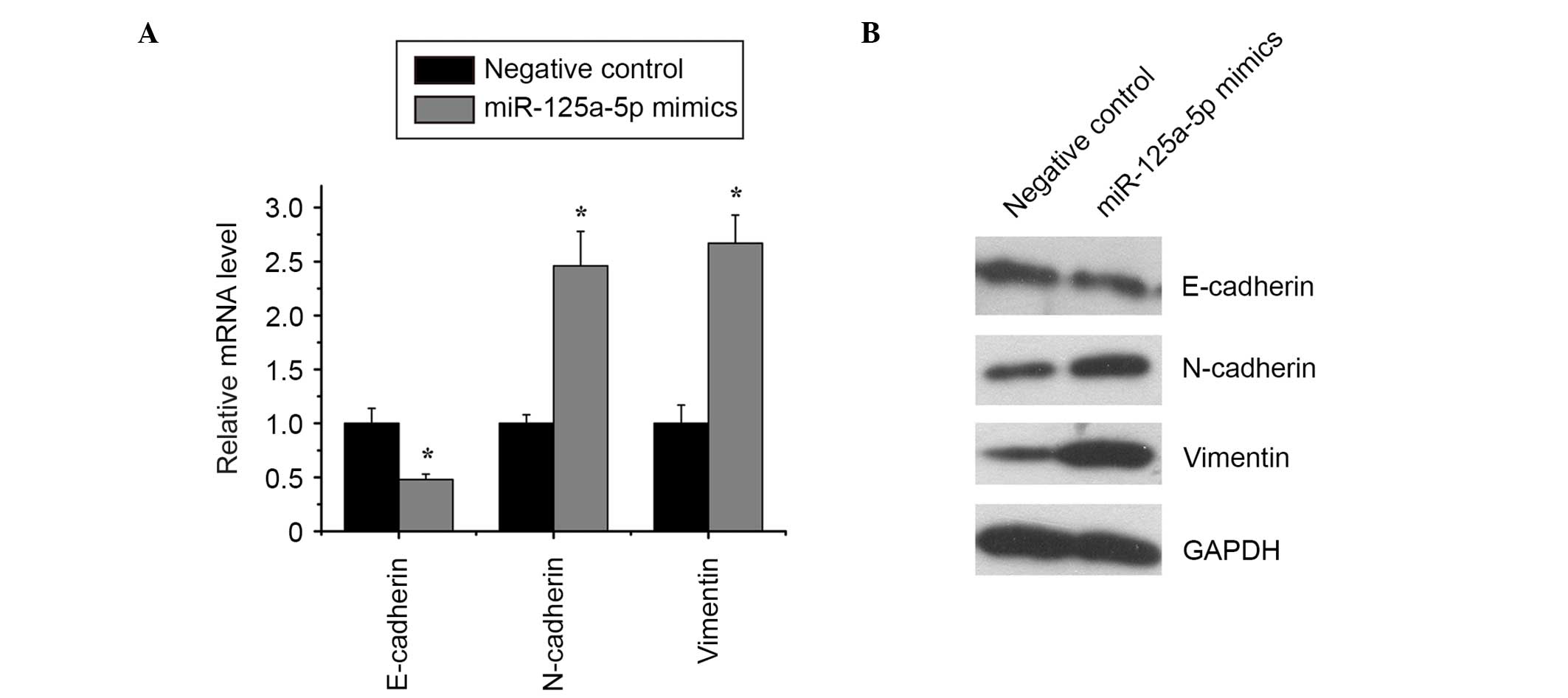
Restoration of miR-125a-5p expression
reduced EMT in osteosarcoma
As indicated by RT-qPCR and western blotting, the
overexpression of miR-125a-5p in MG-63 cells reduced the expression
of E-cadherin and increased the expression of N-cadherin and
vimentin (Fig. 4), which suggested
that miR-125a-5p inhibits EMT in osteosarcoma.
MMP-11 is a direct target of miR-125a-5p
in osteosarcoma
To explore the downstream targets of miR-125a-5p,
bioinformatics was performed to identify potential targets. MMP-11
was identified as a putative gene by the bioinformatics prediction,
with its 3′-UTR containing a complementary site for the seed region
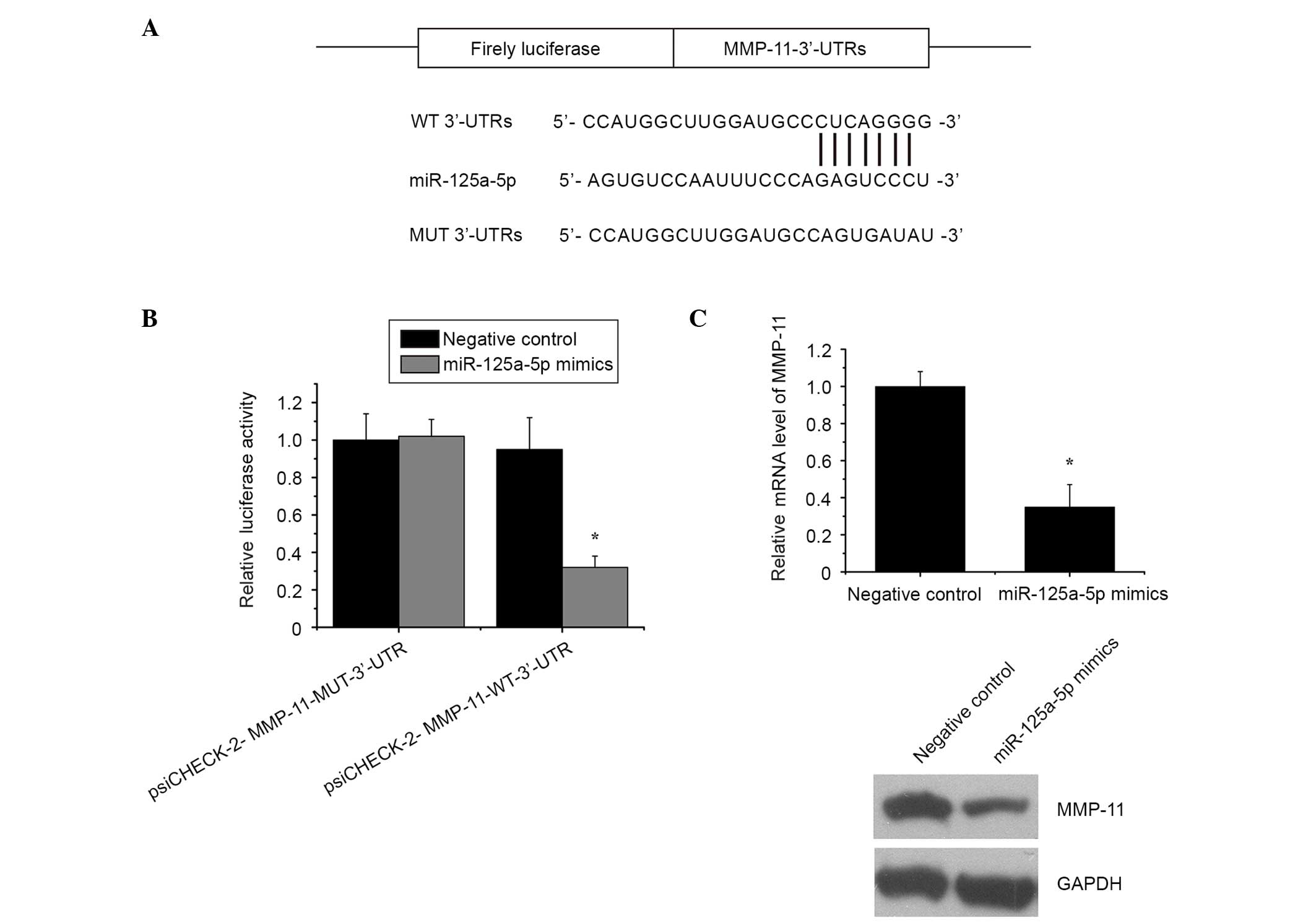
of miR-125a-5p (Fig. 5A).
In order to obtain further direct evidence that
MMP-11 was a target of miR-125a-5p, the binding site of miR-125a-5p
in the 3′UTR of MMP-11 mRNA was characterized. The results
indicated that miR-125a-5p significantly reduced the luciferase
activity, while the mutant reporter co-transfected with miR-125a-5p
did not result in a significant reduction in the relative
luciferase activity (Fig. 5B).
Subsequently, RT-qPCR and western blot analysis was conducted to
examine the inhibitory effect of miR-125a-5p on endogenous MMP-11
expression in MG-63 cells. As expected, inducing miR-125a-5p
expression markedly inhibited the expression of MMP-11 in MG-63
cells (Fig. 5C).
Discussion
Increasingly, studies have demonstrated that
miR-125a-5p is frequently abnormally expressed in a variety of
human malignancies, including glioblastoma (14), gastric cancer (15), non-small cell lung cancer (16), oral squamous cell carcinoma
(17) and hepatocellular carcinoma
(18). Downregulation of
miR-125a-5p inhibits the cell cycle, proliferation and induces
apoptosis by directly targeting the ErbB pathway in acute myeloid
leukemia (19). miRNA-125a-5p
inhibits glioblastoma cell proliferation and promotes cell
differentiation by targeting transcriptional coactivator with
PDZ-binding motif (20). In
addition, miR-125a-5p has been demonstrated to suppress breast
tumorigenesis by targeting histone deacetylase 4 (21). In the present study, miR-125a-5p
was observed to be downregulated in osteosarcoma tissues and cell
lines. Additionally, restoration of miR-125a-5p expression resulted
in reductions in osteosarcoma cell migration, invasion and the
inhibition of cellular EMT. These results suggest that miR-125a-5p
acts as a tumor suppressor gene, and may contribute to the
development and metastasis of osteosarcoma.
It has been previously reported that MMPs contribute
to cell migration and invasion in various types of cancer (22). MMPs are a family of zinc-dependent
extracellular endoproteinases, which have the ability to degrade
the cell extracellular matrix and basement membrane (23). To date, greater than 24 MMPs have
been identified in humans, and are classified into five groups
based on substrate specificity: Interstitial collagenases,
gelatinases, stromelysins, matrilysins and membrane-type MMPs
(24,25). MMP-11, also termed stromelysin-3,
is encoded by the MPP-11 gene located on chromosome 22 at q11.23 in
the human genome (26).
Increasingly, studies have demonstrated that MMP-11 is frequently
highly expressed in numerous types of cancer, including breast,
brain, gastric and lung cancer (27). A previous study reported that
MMP-11 deficiency resulted in an increased tumor-free survival rate
and modulated the distant invasion ability of tumors (28). The results from the present study
suggest that MMP-11 is an important downstream target of
miR-125a-5p in osteosarcoma. miR-125a-5p directly binds to the
3′-UTR of MMP-11, which was verified by the dual-luciferase
reporter assay. In addition, upregulation of miR-125a-5p markedly
reduced the MMP-11 protein expression levels in osteosarcoma cells.
Together, these data suggest that miR-125a-5p suppresses migration,
invasion and EMT through direct targeting of MMP-11 in
osteosarcoma.
In conclusion, the present study demonstrated that
miR-125a-5p was downregulated in osteosarcoma tissues and cell
lines. Restoration of miR-125a-5p expression inhibited cell
migration, invasion and EMT of osteosarcoma through the direct
targeting of MMP-11. To the best of our knowledge, the present
study is the first to demonstrate that the miR-125a-5p/MMP-11 axis
regulates the migration, invasion and EMT of osteosarcoma cells.
These observations indicate that miR-125a-5p may be a potential
novel target for gene therapy of osteosarcoma.
References
|
1
|
Tang J, Shen L, Yang Q and Zhang C:
Overexpression of metadherin mediates metastasis of osteosarcoma by
regulating epithelial-mesenchymal transition. Cell Prolif.
47:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montanaro L, Mazzini G, Barbieri S, Vici
M, Nardi-Pantoli A, Govoni M, Donati G, Treré D and Derenzini M:
Different effects of ribosome biogenesis inhibition on cell
proliferation in retinoblastoma protein- and p53-deficient and
proficient human osteosarcoma cell lines. Cell Prolif. 40:532–549.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li G, Cai M, Fu D, Chen K, Sun M, Cai Z
and Cheng B: Heat shock protein 90B1 plays an oncogenic role and is
a target of microRNA-223 in human osteosarcoma. Cell Physiol
Biochem. 30:1481–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: Implications for therapy
in osteosarcoma. Expert Rev Anticancer Ther. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu CL, Tsai HC, Chen ZW, Wu CM, Li TM,
Fong YC and Tang CH: Ras activation mediates WISP-1-induced
increases in cell motility and matrix metalloproteinase expression
in human osteosarcoma. Cell Signal. 25:2812–2822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonazzi VF, Stark MS and Hayward NK:
MicroRNA regulation of melanoma progression. Melanoma Res.
22:101–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martello G, Rosato A, Ferrari F, Manfrin
A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T,
et al: A MicroRNA targeting dicer for metastasis control. Cell.
141:1195–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mo YY: MicroRNA regulatory networks and
human disease. Cell Mol Life Sci. 69:3529–3531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang L, Huang Q, Chang J, Wang E and Qiu
X: MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in
lung cancer cells. Exp Lung Res. 37:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leotta M, Biamonte L, Raimondi L,
Ronchetti D, Di Martino MT, Botta C, Leone E, Pitari MR, Neri A,
Giordano A, et al: A p53-dependent tumor suppressor network is
induced by selective miR-125a-5p inhibition in multiple myeloma
cells. J Cell Physiol. 229:2106–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gattolliat CH, Uguen A, Pesson M, Trillet
K, Simon B, Doucet L, Robaszkiewicz M and Corcos L: MicroRNA and
targeted mRNA expression profiling analysis in human colorectal
adenomas and adenocarcinomas. Eur J Cancer. 51:409–420. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh TH, Hsu CY, Tsai CF, Long CY, Wu CH,
Wu DC, Lee JN, Chang WC and Tsai EM: HDAC inhibitors target HDAC5,
upregulate microRNA-125a-5p, and induce apoptosis in breast cancer
cells. Mol Ther. 23:656–666. 2015. View Article : Google Scholar :
|
|
13
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cortez MA, Nicoloso MS, Shimizu M, Rossi
S, Gopisetty G, Molina JR, Carlotti C Jr, Tirapelli D, Neder L,
Brassesco MS, et al: miR-29b and miR-125a regulate podoplanin and
suppress invasion in glioblastoma. Genes Chromosomes Cancer.
49:981–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashiguchi Y, Nishida N, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Mochizuki H, Hase K, Doki Y and Mori
M: Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
16
|
Jiang L, Huang Q, Zhang S, Zhang Q, Chang
J, Qiu X and Wang E: Hsa-miR-125a-3p and hsa-miR-125a-5p are
downregulated in non-small cell lung cancer and have inverse
effects on invasion and migration of lung cancer cells. BMC Cancer.
10:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tiwari A, Shivananda S, Gopinath KS and
Kumar A: MicroRNA-125a reduces proliferation and invasion of oral
squamous cell carcinoma cells by targeting estrogen-related
receptor α: Implications for cancer therapeutics. J Biol Chem.
289:32276–32290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ,
Kim MG, Chang YG, Shen Q, Park WS, Lee JY, et al: Sirtuin7
oncogenic potential in human hepatocellular carcinoma and its
regulation by the tumor suppressors MiR-125a-5p and MiR-125b.
Hepatology. 57:1055–1067. 2013. View Article : Google Scholar
|
|
19
|
Ufkin ML, Peterson S, Yang X, Driscoll H,
Duarte C and Sathyanarayana P: miR-125a regulates cell cycle,
proliferation, and apoptosis by targeting the ErbB pathway in acute
myeloid leukemia. Leuk Res. 38:402–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan J, Xiao G, Peng G, Liu D, Wang Z,
Liao Y, Liu Q, Wu M and Yuan X: MiRNA-125a-5p inhibits glioblastoma
cell proliferation and promotes cell differentiation by targeting
TAZ. Biochem Biophys Res Commun. 457:171–176. 2015. View Article : Google Scholar
|
|
21
|
Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai
CY, Hou MF, Lee JN, Wu DC, Wang SC and Tsai EM: miR-125a-5p is a
prognostic biomarker that targets HDAC4 to suppress breast
tumorigenesis. Oncotarget. 6:494–509. 2015.
|
|
22
|
King SE: Matrix metalloproteinases: New
directions toward inhibition in the fight against cancers. Future
Med Chem. 8:297–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan D, Dai H and Liu JW: Serum levels of
MMP-11 correlate with clinical outcome in Chinese patients with
advanced gastric adenocarcinoma. BMC Cancer. 11:1512011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kähäri VM and Saarialho-Kere U: Matrix
metalloproteinases and their inhibitors in tumour growth and
invasion. Ann Med. 31:34–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark AF: New discoveries on the roles of
matrix metalloproteinases in ocular cell biology and pathology.
Invest Ophthalmol Vis Sci. 39:2514–2516. 1998.PubMed/NCBI
|
|
26
|
Valdivia A, Peralta R, Matute-González M,
García Cebada JM, Casasola I, Jiménez-Medrano C, Aguado-Pérez R,
Villegas V, González-Bonilla C, Manuel-Apolinar L, et al:
Co-expression of metalloproteinases 11 and 12 in cervical scrapes
cells from cervical precursor lesions. Int J Clin Exp Pathol.
4:674–682. 2011.PubMed/NCBI
|
|
27
|
Wu D, Li M, Wang L, Zhou Y, Zhou J, Pan H
and Qu P: microRNA145 inhibits cell proliferation, migration and
invasion by targeting matrix metallopeptidase-11 in renal cell
carcinoma. Mol Med Rep. 10:393–398. 2014.PubMed/NCBI
|
|
28
|
Peruzzi D, Mori F, Conforti A, Lazzaro D,
De Rinaldis E, Ciliberto G, La Monica N and Aurisicchio L: MMP11: A
novel target antigen for cancer immunotherapy. Clin Cancer Res.
15:4104–4113. 2009. View Article : Google Scholar : PubMed/NCBI
|