Introduction
Cisplatin (CDDP) is a common broad-spectrum
antitumor therapeutic agent used in clinical settings. It is
recommended in the treatment of a number of types of cancer,
including melanoma, cancer of the head and neck, non-small cell
lung cancer, liver cancer, cervical cancer and nasopharyngeal
carcinoma (1). However, as with
numerous cytotoxic anti-tumor therapeutic agents, CDDP results in
toxic side effects, as it also affects non-cancerous cells. CDDP is
also unstable in the body, further limiting its efficacy in
clinical use (2). In order to
increase the curative effect and reduce the toxic side effects of
CDDP, targeted drug delivery aims to selectively treat a tumor area
or enable therapeutic agents to enter tumor cells, thus reducing
the therapeutic agent concentration within healthy tissue. Previous
studies regarding anticancer targeted drug-delivery systems have
aimed to establish methods of targeting tumor tissue; however, the
present study investigates how to deliver a greater concentration
of therapeutic agent into tumor tissue upon reaching the tumor
location.
Tumor characteristics, such as rapid metabolism and
cell division, result in a slightly acidic environment (3). The pH-sensitive magnetic
nanoparticles (MNPs) deliver the therapeutic agent to the tumor
using the difference in physiological pH between the healthy tissue
and tumor tissue. This increases the concentration of therapeutic
agent in the tumor and improves the bioavailability (3). The use of pH-sensitive, targeted
therapeutic agent release in the treatment of malignant tumors has
progressed, however, it has been observed that certain pH-sensitive
MNPs induce therapeutic agent release outside of tumor cells
(3). The therapeutic agents
released outside of cell then spreads to healthy tissue and reduces
the target specificity of the therapeutic agent. Under normal
conditions, the use of a receptor and a ligand allows specific
positioning of the ligand, and separation by normal biological
function is non-toxic and non-immunogenic (3). Low molecular weight ligands,
including folic acid (FA), vitamin B1 (thiamin) and sugars,
attached to or coating the MNP facilitate active targeting
(4). Furthermore, peptides,
proteins and antibodies improve the MNP efficacy.
The method of combining a pH-sensitive carrier with
pH-sensitive chemical bonds was adopted in the present study,
according to a previous study (5).
The majority of malignant tumor cells exhibit positive FA
expression, therefore, a novel pH-sensitive FA-modified MNP drug
delivery system may target cancer cells and deliver the therapeutic
agent by combining highly efficient endocytosis, targeted drug
delivery and pH-sensitive release of the therapeutic agent.
The aim of the present study was to introduce pH
sensitivity to a drug delivery system, optimize its preparation and
characterize its physicochemical properties, in order to elucidate
the mechanism of targeting by assessing CDDP uptake by tumor
cells.
Materials and methods
Reagents
Sodium alginate (molecular weight, ~60,000;
viscosity, 2 cps) was obtained from Qingdao Crystal Rock Biology
Development, Co., Ltd (Qingdao, China). Analytically pure
poly(ethylene glycol) (PEG; molecular weight, 2,000) and
triethylamine was sourced from Shanghai Crystal Pure Reagent Co.,
Ltd. (Shanghai, China). Analytically pure FA was obtained from
Guangdong Guanghua Chemicals Factory Co., Ltd. (Guangdong, China).
Nitrophenyl chloroformate hydroxylamine hydrochloride, acetic acid,
dimethylsulfoxide (DMSO), polymer film-lined copper net,
paraformaldehyde, Prussian blue, Neutral Red, Gluteraldehyde was
sourced from Southern Medical University (Guangzhou, China). Acros
Organics™ dicyclohexylcarbodiimide (DCC) and
N-hydroxysuccinimide (NHS) were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). , sodium periodate,
FeCl3·6H2O and
FeSO4·4H2O were purchased from Guangzhou
Chemical Reagent Factory. CDDP was obtained from Shandong Qilu
Pharmaceutical Factory (Shandong, China). Phenanthroline was
obtained from Tianjin No. 1 Chemical Reagent Factory (Tianjin,
China), and o-phenylene-diamine from Tianjin Kemiou Chemical
Reagent Co., Ltd. (Tianjin, China). N,N-dimethylformamide was
obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China). CNE-2 and HNE-1 were obtained from Shanghai Institutes for
Biological Sciences (Shanghai, China). RPMI-1640 and fetal bovine
serum (FBS) were obtained from Thermo Fisher Scientific Inc.
(Shanghai, China). Phosphate-buffered saline (PBS) was obtained
from Wuhan Boster Biotechnology Co., Ltd. (Wuhan, China). MTS kits
were obtained from Promega Corporation (Beijing, China). ELISA kits
were obtained from Takara Biotechnology Co., Ltd (Dalian,
China).
Instruments and equipment
Transmission electron microscopy (TEM) was performed
using a TecnalTM G2 Polara (J&R Scientific instruments Co.,
Ltd., Guangzhou, China) and a ZetaPALS ζ-potential analyzer
(Brookhaven Instruments, Corporation, Holtsville, NY, USA). An MPMS
XL-7VSM magnetometer (Quantum Design, Inc., San Diego, CA, USA),
Alpha 1-2LD vacuum freeze drier (Martin Christ
Gefriertrocknungsanlagen, GmbH, Osterode am Harz, Germany),
DZF-6201 vacuum drying oven (Shanghai Yiheng Technology Co., Ltd.,
Shanghai, China), THZ-100 constant temperature shaking incubator
(Shanghai Yiheng Technology Co., Ltd.) and a UVIKON923 ultraviolet
(UV)-visible spectrophotometer (Bio-Tek Instruments, Inc.,
Winooski, VT, USA) were used during the present study.
Preparation of PEG with hydrazine and FA
conjugation
The method of PEG attachment to basic hydrazine was
conducted as described in a previous study (6). Dried PEG (20 g) was dissolved in 60
ml anhydrous methylene chloride, and added into 40 ml anhydrous
methylene chloride solution containing 6.05 g nitrophenyl
chloroformate and 12.5 ml anhydrous triethylamine. This was
performed drop by drop in an ice-water bath, the resulting molar
ratio of PEG, nitrophenyl chloroformate and triethylamine was
1:3:9. Spin steaming was used to remove the majority of the
dichloromethane solvent following a 12-h reaction in the dark.
Anhydrous ether was added to precipitate the PEG activated by
nitrophenyl chloroformate, this was then vacuum dried.
The PEG activated by nitrophenyl chloroformate was
dissolved in 100 ml dichloromethane, and 29.1 ml water and 29.1 ml
hydrazine solution was added, the final molar ratio of PEG and
hydrazine was 1:30. Spin steaming was performed to remove the
majority of the dichloromethane solvent following the reaction at
room temperature. Anhydrous ether was added to precipitate the PEG
modified by hydrazine and this was vacuum dried.
FA-PEG-NHNH2 was prepared as previously
described (7). FA (0.88 g) was
dissolved in anhydrous DMSO,0.84 g DCC and 0.44 g NHS were added,
and the reaction proceeded at room temperature for 4 h with
continuous mixing. Double hydrazine (4 g) was added to the PEG
[PEG2000(NHNH2)2], continuously mixed and the
reaction proceeded for 8 h. Following the reaction, 120 ml
double-distilled water (ddH2O) was added, the
undissolved solid was removed by filtering and the product
(FA-PEG-NHNH2) was freeze-dried.
Preparation of aldehyde sodium alginate
(ASA)-modified CDDP-loaded Fe3O4 MNPs
ASA was prepared according to a previous study by
Laurienzo et al (8) and
coprecipitation was used to prepare the ASA-modified CDDP-loaded
Fe3O4 MNPs, as described previously (9). The product (molecular weight,
~14,000) was then dialyzed (TXDJZ-60; Solarbio Technology Co.,
Ltd., Beijing, China) for three days in double distilled water to
remove unattached CDDP.
Preparation of ASA-CDDP-modified MNPs,
decorated with FA and PEG-hydrazine
FA-PEG-NHNH2 (0.3 g) was dissolved in 10
ml ddH2O and added to 30 ml ferrofluid (Wenzhou
JingCheng Chemical Co., Ltd., Wenzhou, China) containing 0.5 g
ASA-modified CDDP-loaded MNPs. According to the previously
described method of hydrazone bond formation (10), anhydrous acetic acid was used to
create an acidic environment and obtain a pH value of 5. The
reaction was conducted with continuous mixing, at room temperature
under the protection of nitrogen for 48 h. The solution was
dialyzed in distilled water for three days (molecular weight,
5,000–14,000) and subsequently freeze-dried.
Detection of particle size, shape and
dispersity of the magnetic core
A sample of the drug delivery system,
FA-PEG-NH-N=MNPs-CDDP was prepared by dilution to 6–300 mg/ml, and
the sample was dropped on a polymer film-lined copper net.
Subsequent to slow drying, the sample was deposited with a layer of
carbon film (thickness, 10–20 nm) and observed by TEM.
Detection of saturation magnetization and
evaluation of magnetic responsiveness
At 25°C, a hysteresis curve of the sample was
determined between −10 and 10kOe. Samples of FA-PEG-NH-N=MNPs-CDDP
(4 ml) were added to two 5-ml centrifuge tubes, one was placed into
a constant magnetic field and the distribution of the MNPs was
observed following 2 h.
Detection of hydrodynamic diameter and
ζ-potential
FA-PEG-N H-N=M N Ps-CDDPs, ASA-M N Ps and CDDP-MNPs
were diluted to a certain concentration prior to analysis (iron
concentration, 0.02 mg/ml prior to testing). The analysis was
conducted on the ZetaPALS ζ-potential and laser particle size
analyzer. A scattering angle of 90° and temperature of 25°C was
used; the analysis was repeated to obtain an mean value of three
experiments. The aim was to evaluate the change in MNP size during
preparation.
Observing MNP stability
The CDDP-loaded MNPs were placed in a 4°C
refrigerator for 2 months, and their shape and any changes in CDDP
loading capacity were subsequently observed.
Detection of iron content and CDDP
loading capacity
The present study used the phenanthroline method, as
previously described (11), to
detect the iron content in the FA-PEG-NH-N=MNPs-CDDP. The
o-phenylenediamine colorimetric method (12) was used to detect the CDDP content
in FA-PEG-NH-N=MNPs-CDDP (13).
Observation of cell targeting by iron
staining
Prussian blue staining can be conducted to
demonstrate the MNPs taken up into the cell via formation of ferric
ferrocyanide, by the reaction of Fe3+ with
(K4Fe(CN)6), where iron is present. Two
nasopharyngeal carcinoma cell lines were used in the present study,
CNE-2, which is folate receptor-negative and HNE-1, which is folate
receptor-positive. RPMI-1640 culture medium was used to prepare a
single-cell suspension and, following digestion and centrifugation
at 1,000 × g, the concentration of the cells was adjusted to
2×104 cells/ml. The solution was inoculated in a 24-well
plate (well volume, 500 µl) and the HNE-1 cells were
cultured for 24 h in RPMI-1640 complete medium without FA.
Following 24 h of adherent growth, the medium was removed and
FA-PEG-NH-N=MNPs-CDDP and MNPs were diluted with RPMI-1640 without
FA and cultured for 6 h; the concentration of CDDP was 5, 10 and 20
µg/ml according to the iron content. The culture media was
removed from the wells, the MNPs were collected and washed with
PBS. The cells were fixed in 4% paraformaldehyde solution for 30
min at room temperature and washed with ddH2O. Prussian
blue dye was added and the samples were placed in the dark at room
temperature for 30 min. Neutral red dye was added as a contrast dye
following washing with ddH2O and an inverted microscope
was used for observation and to obtain images.
Observation of cell targeting by TEM
Following culture of the HNE-1 cells with MNPs and
FA-PEG-NH-N=MNPs-CDDP, the condition of the cells following the
uptake of MNPs was observed by TEM. A single-cell suspension of
HNE-1 cells was produced with RPMI-1640 culture medium without FA.
Following digestion and centrifugation, cells (5×104
cells/ml) were inoculated in 6-well plates (well volume, 2 ml). The
cells were cultured for 24 h, subsequent to this,
FA-CDDP-FA-PEG-NH-N=MNPs-CDDP (5, 10 and 20 µg/ml depending
on iron content) and MNPs diluted in RPMI-1640 complete medium
without FA were added and cultured for 6 h. The medium was
collected and washed with PBS to remove any remaining MNPs. The
cells were trypsinized and the cell suspension was centrifuged for
5 min at 1,000 × g, following which the supernatant was discarded.
Gluteraldehyde (2.5%) was added to the cell pellet and the cells
were fixed at 4°C. Centrifugation at 1,000 × g was repeated and the
supernatant discarded, 1% osmic acid was added and dehydration was
conducted using a gradient of acetone concentrations. The cells
were embedded in epoxy resin and thin sections (40–50 nm) were cut
and observed by TEM following staining with uranyl acetate and lead
citrate to observe the uptake of MNPs by the cells.
Detection of cytotoxicity of
FA-PEG-NH-N=MNPs-CDDP with the MTS assay
Pancreatin was used to digest HNE-1 cells in the
logarithmic phase and RPMI-1640 medium was used to prepare a
single-cell suspension without FA, containing 10% FBS. The
suspension (200 µl) was inoculated on a 96-well plate at a
density of 3×104 cells/ml. The plates were incubated
overnight at 37°C in an atmosphere of 5% CO2.
Each culture plate was comprised of the treatment
[pure CDDP, FA-PEG-NH-N=MNPs-CDDP (pH 6.5 and 7.4), and the MNPs
group], control (culture medium without any treatment) and blank
control (culture medium without cells) wells. The treatment
concentrations were 0.5, 1, 2, 4, 8 and 25 µg/ml CDDP (three
wells per concentration), and the concentration in the MNP group
was determined by the iron content. The culture plates were
incubated for 24 and 48 h at 37℃ in 5% CO2 with
saturated humidity. Following incubation of the cells, 20 µl
MTS/phenazine methosulfate was added to the wells and incubated for
3–4 h. Following 10 sec of agitation to mix the color, ELISA was
used to detect absorbance at a wavelength of 490 nm.
The half maximal inhibitory concentration was
calculated for the different concentrations administered to HNE-1
cells for 24 and 48 h using probit analysis. In addition, the
inhibition ratio at each concentration was calculated as follows:
Inhibition ratio (%) = control group A490−treatment
group A490/control group A490. Where all
groups had been normalized to zero.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used to
perform statistical analysis. Factorial analysis, one way analysis
of variance, least significant difference, Student-Newman-Keuls and
Dunnett's method were used to analyze the data from the present
study. P<0.05 was considered to indicate a statistically
significant difference.
Results
Confirmation of product
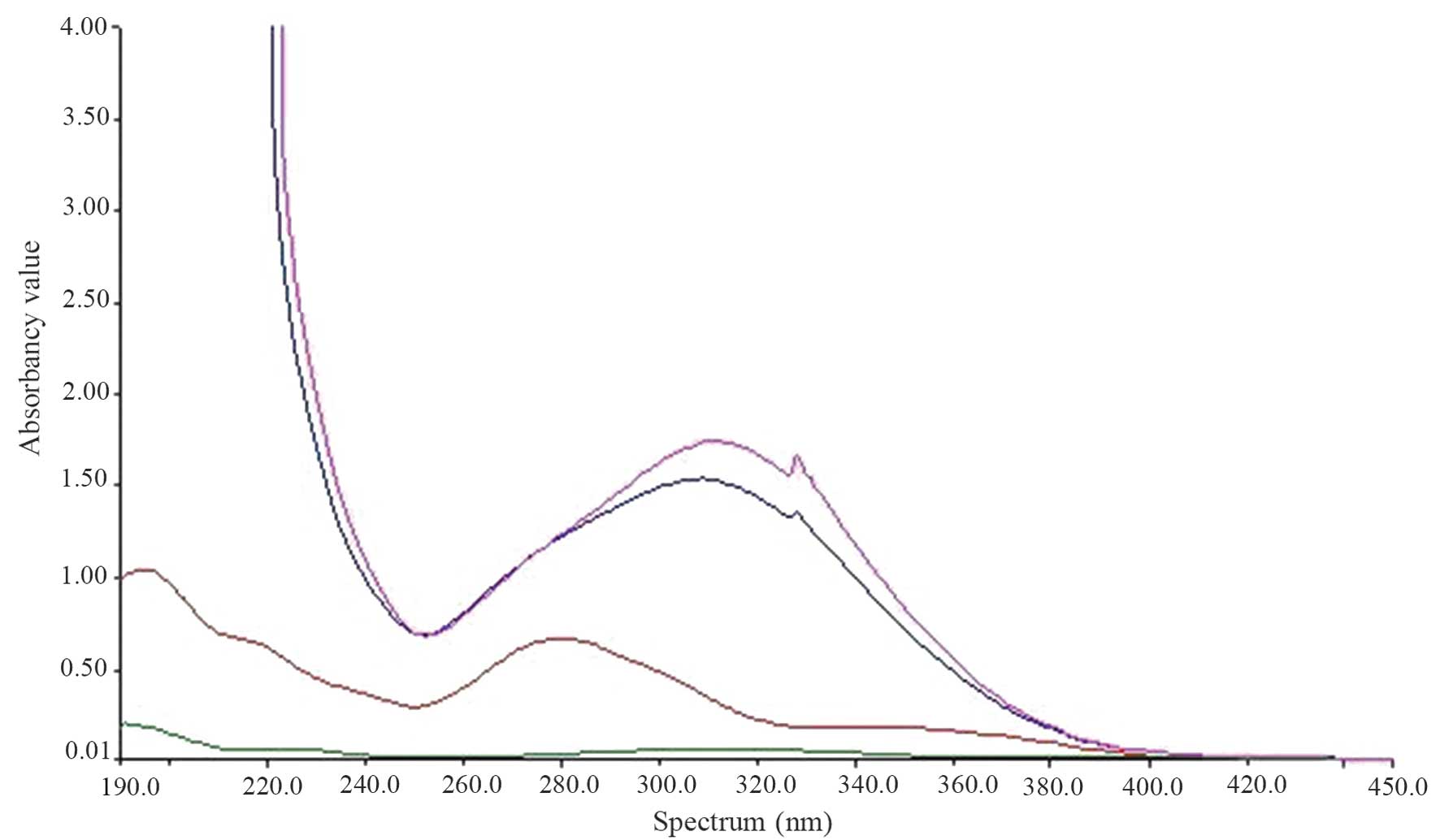
UV-visible spectroscopy was conducted (Fig. 1). PEG(NHNH2)2
demonstrated no marked absorption peak and FA exhibited an
absorption peak at 280 nm. FA-NH2NH-PEG-NHNH2
demonstrated a conjugate absorption peak at 310 nm demonstrating
the bond between FA and PEG (NHNH2)2. However
two of the absorption curves are the same, demonstrating that the
method of forming the product is reproducible and practical.
In the UV mapping of FA-PEG-NH-N=MNPs-CDDP, the
absorption curves were disordered (causing MNPs to appear black),
which was caused by interference between 200 and 300 nm.
CDDP and iron content detection in the
CDDP carrier
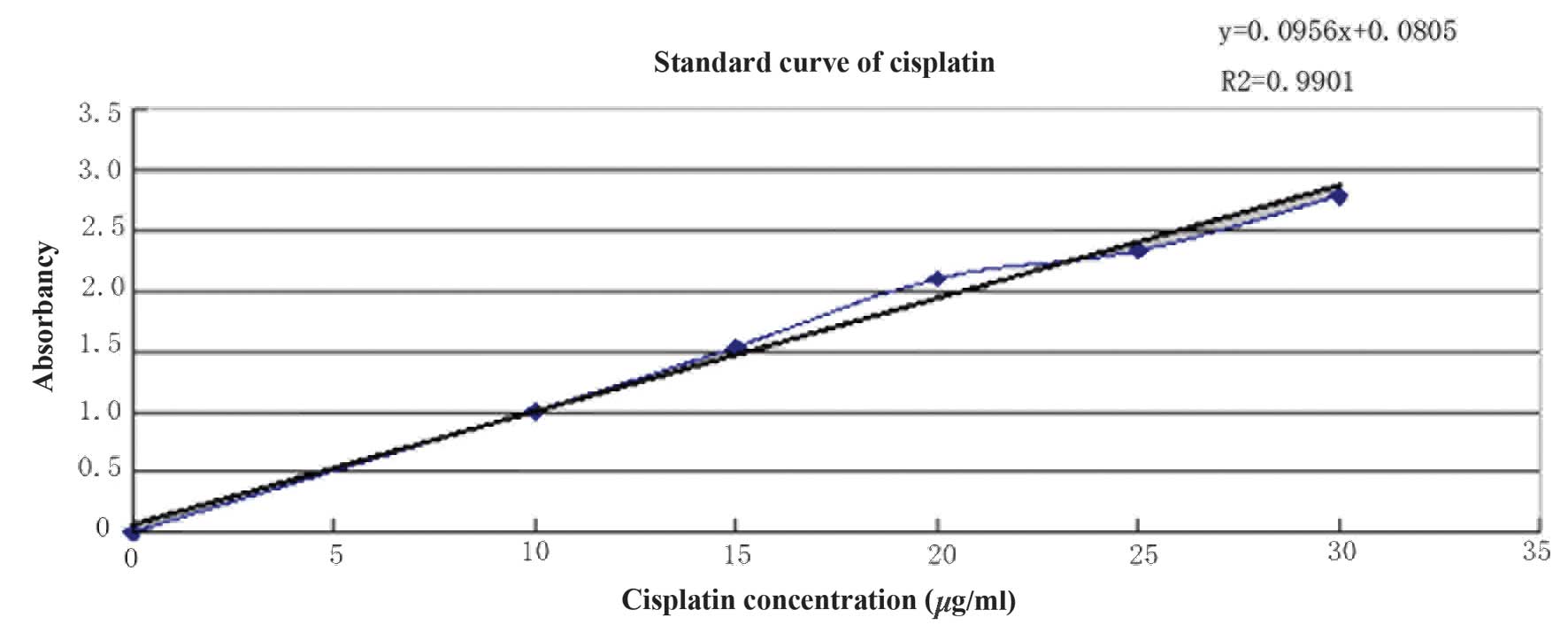
CDDP concentration was determined using the
o-phenylendiamine method and the standard curve is presented
in Fig. 2. The regression equation
is as follows: y=0.0956x+0.0805, R2 =0.9901 and the CDDP
concentration was calculated as 0.773 mg/ml. The concentration of
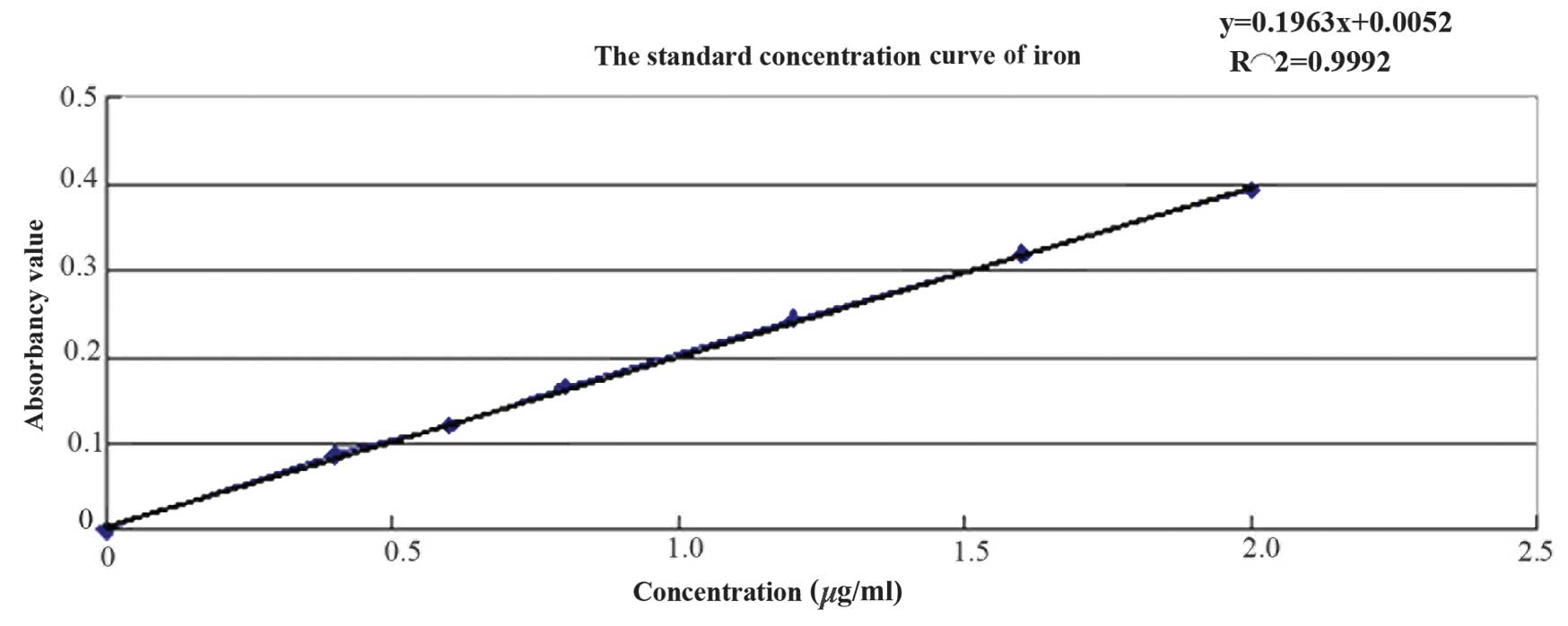
iron was determined using the phenanthroline method and the
standard curve is presented in Fig.
3. The regression equation is as follows: y=0.1963x+0.0052,
R2 =0.9992, the iron concentration was calculated as
1.908 mg/ml.
Shape and structure of MNPs
The freeze-dried powder of the pH-sensitive
FA-modified CDDP-loaded MNPs is uniformly black; when scattered
into distilled water ultrasound processing produced a uniform
colloidal solution.
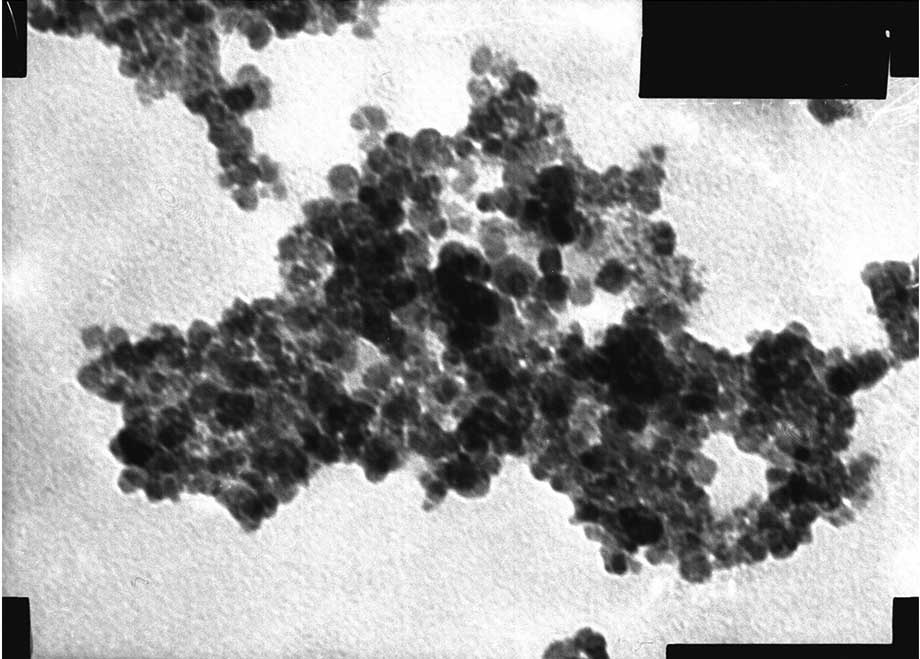
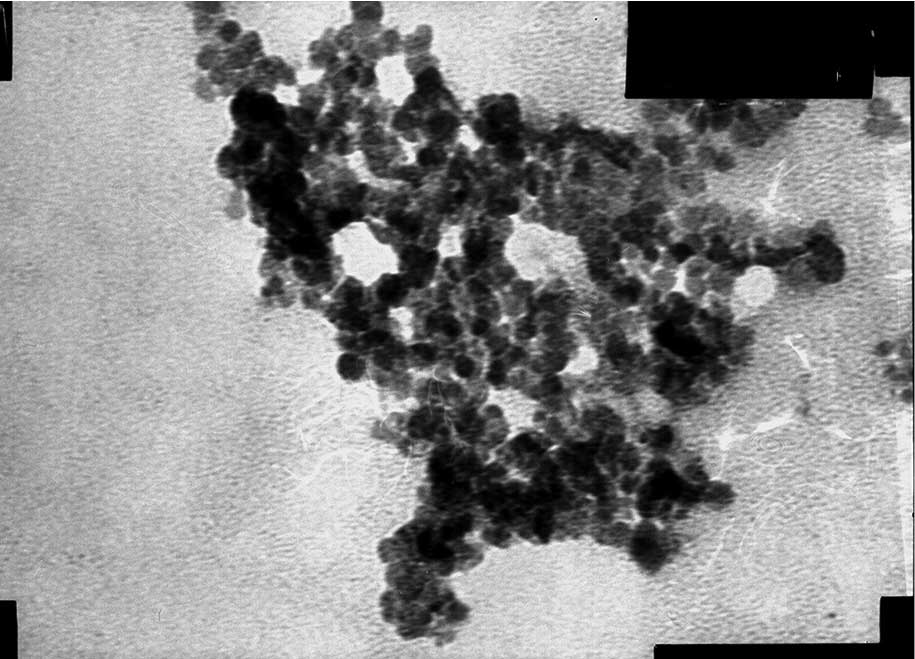
The shape of the MNPs was observed by TEM (Figs. 4 and 5). The two MNPs were round with a mean
particle size of 10.2±1.5 nm.
Saturation magnetization
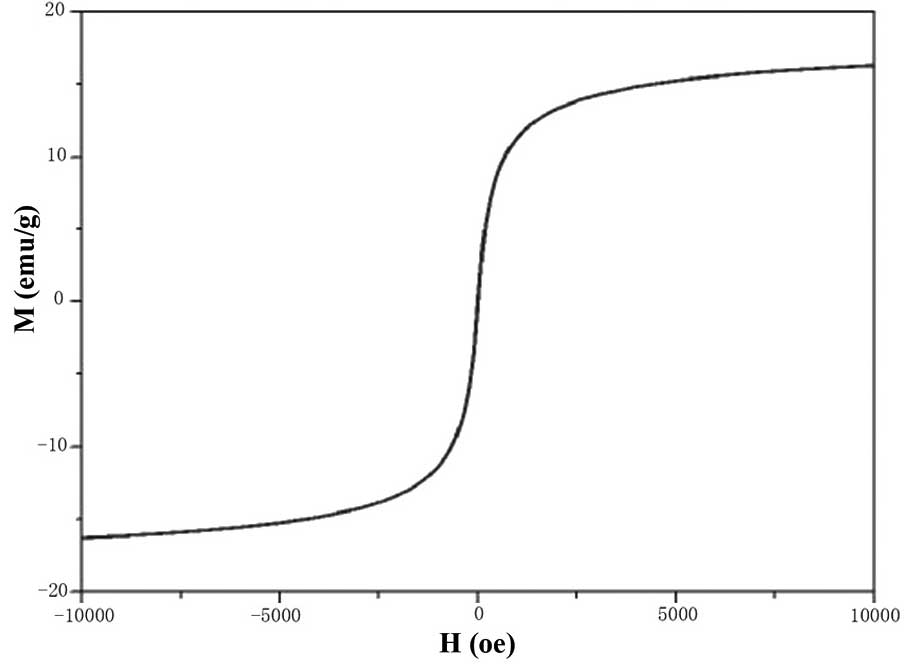
The maximum saturation magnetization of
FA-PEG-NH-N=MNPs-CDDP was 16.3±0.2 emu/g. The coercive force was
zero (Fig. 6), indicating that the
MNPs have good superparamagnetism and magnetic responsiveness.
Fig. 7 presents the
two centrifuge tubes containing pH-sensitive FA-modified
CDDP-loaded MNPs. The MNPs located to the tube wall closest to the
magnet, with the solution becoming light and transparent; this
transparency increased over time. However, in the centrifuge tube
away from the magnet, the MNPs remained evenly scattered indicated
by the black solution. When the centrifuge tubes were removed from
the magnet, the MNPs rapidly scattered throughout the solution
becoming black within 5 sec. This further the good magnetic
responsiveness and superparamagnetism of the MNPs.
Particle size and ζ-potential
A laser particle analyzer was used to detect dynamic
light scattering and ascertain the particle size of
FA-PEG-NH-N=MNPs-CDDP. The products produced in the preparation
process, ASA-MNPs and CDDP-MNPs, served as a comparison. The
results demonstrate that the mean hydrodynamic diameter of
FA-PEG-NH-N=MNPs-CDDP was 176.6±1.1 nm and the ζ-potential was
−20.91±1.76 mV. The hydrodynamic diameter of the ASA-MNPs was
198.9±3.4 nm and the ζ-potential was −29.04±2.17 mV. The
hydrodynamic diameter of CDDP-MNPs was 153.4±1.8 nm and the
ζ-potential was −25.08±0.96 mV. All the results demonstrate
logarithmic distribution.
Stability
Sediment was observed at the bottom of the tubes
following removal of the solution, which increased with the
concentration of pH-sensitive FA-modified CDDP-loaded MNPs in the
solution. However, following ultrasound processing, the sediment
was evenly scattered in the liquid.
The stability of the MNPs was analyzed two months
following production by assessing the concentration of CDDP and
iron in the solution that the MNPs were stored in (Table I). No significant changes in
concentration were observed (P>0.05), suggesting high
stability.
 | Table IStability of
FA-PEG-NH-N=MNPs-CDDP. |
Table I
Stability of
FA-PEG-NH-N=MNPs-CDDP.
| Component | Concentration
(µg/ml)
| T-value | P-value |
|---|
| 0 months | 2 months |
|---|
| CDDP | 0.191±0.010 | 0.190±0.002 | 0.234 | 0.826 |
| Iron | 0.366±0.008 | 0.376±0.013 | −0.906 | 0.416 |
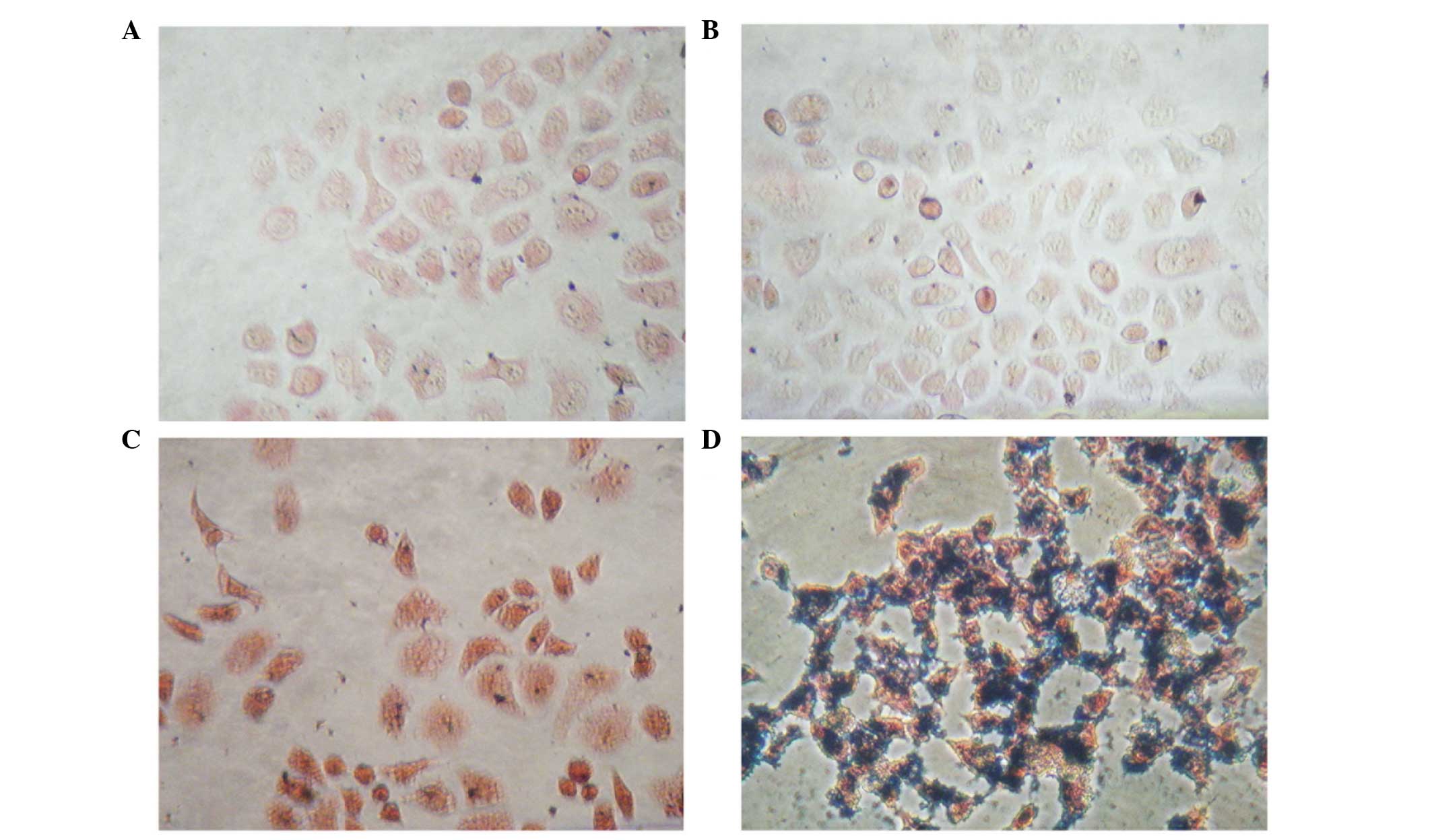
Prussian blue staining
The PEG-NH-N=MNPs-CDDP and MNPs were cultured at
different concentrations with CNE-2 (folate receptor-negative) and
HNE-1 (folate receptor-positive) cells for 6 h. Prussian blue was
added and following culture with FA-PEG-NH-N=MNPs-CDDP, only the
HNE-1 cells demonstrated a positive result (blue staining; Fig. 8)
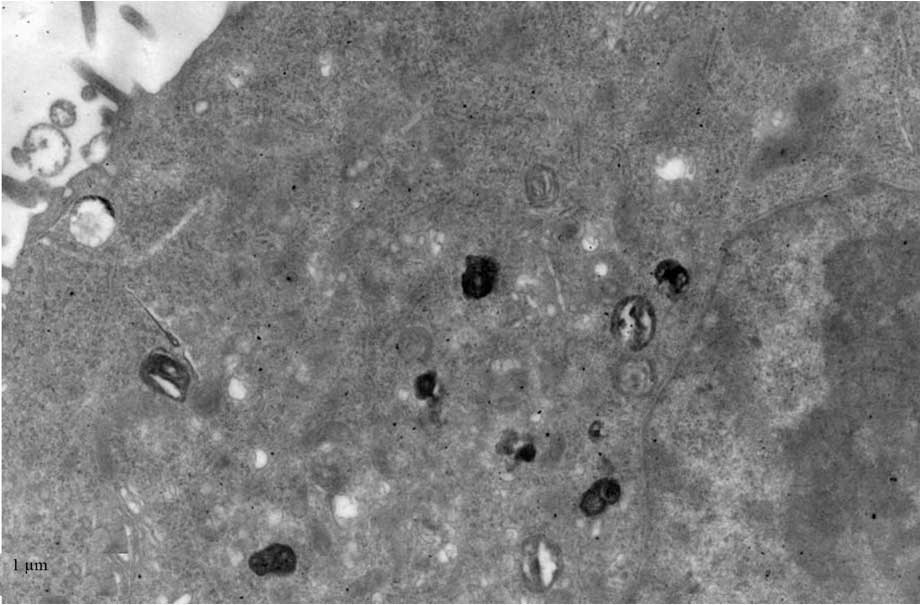
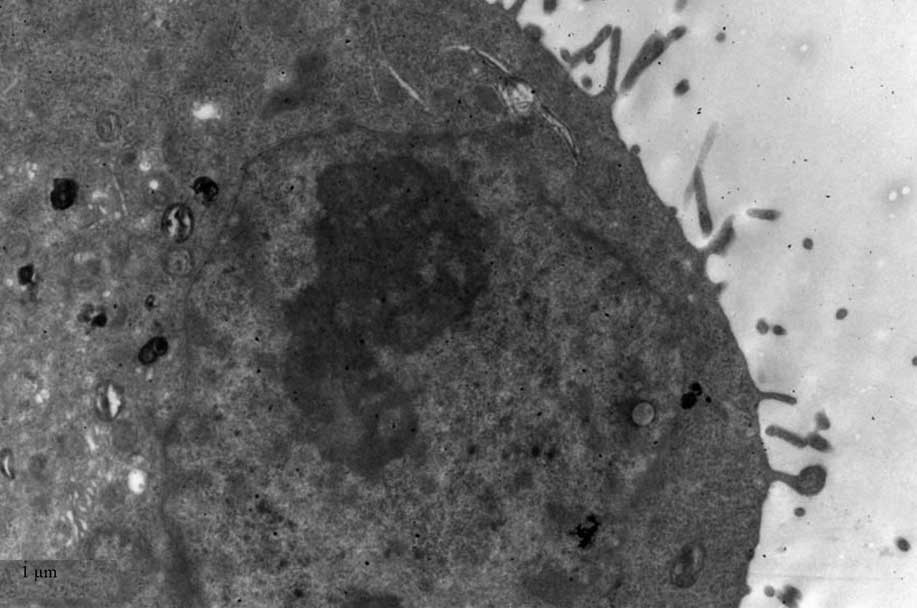
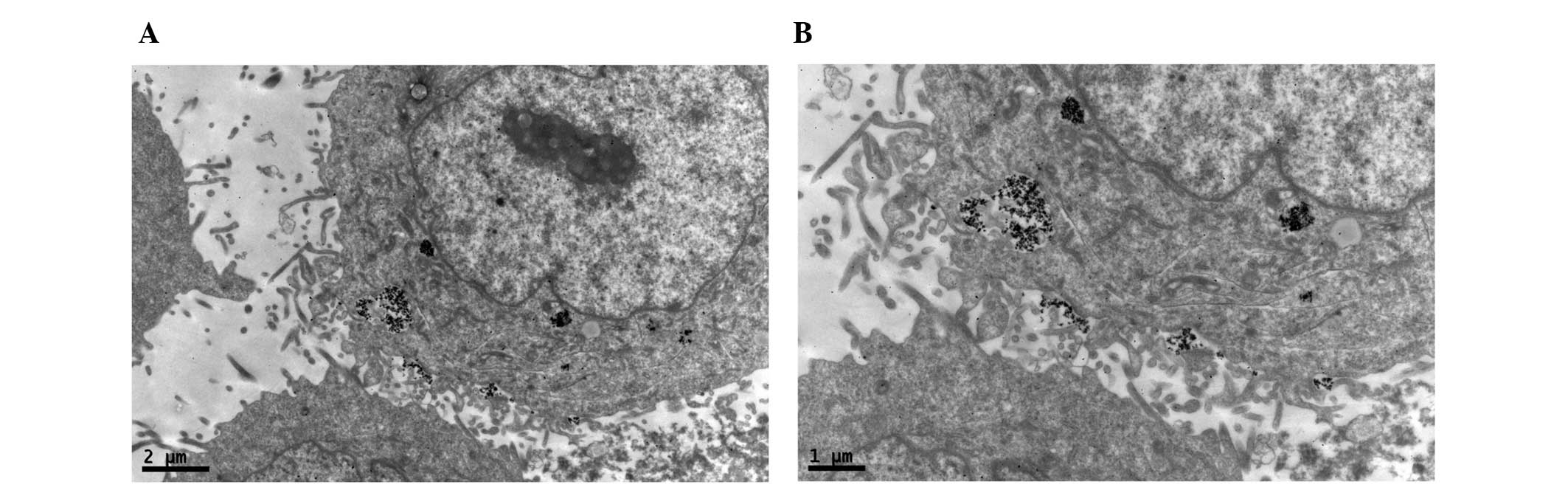
TEM
TEM demonstrated black high density MNPs located
within HNE-1 cells following a 6-h culture with
FA-PEC-NH-N=MNPs-CDDP. A high density shadow is located inside the
pinocytotic vesicle; furthermore a pseudopod extending from the
surface of the cell membrane to take up MNPs. This was not observed
in the normal cells (Figs.
9Figure 10–11).
Fig. 11
demonstrates two magnification strengths, but this phenomenon is
apparent in the normal cell, however above the high density
particle also can not be seen in HNE-1 cell following co-culture
MNPs and HNE-1 for 6 h.
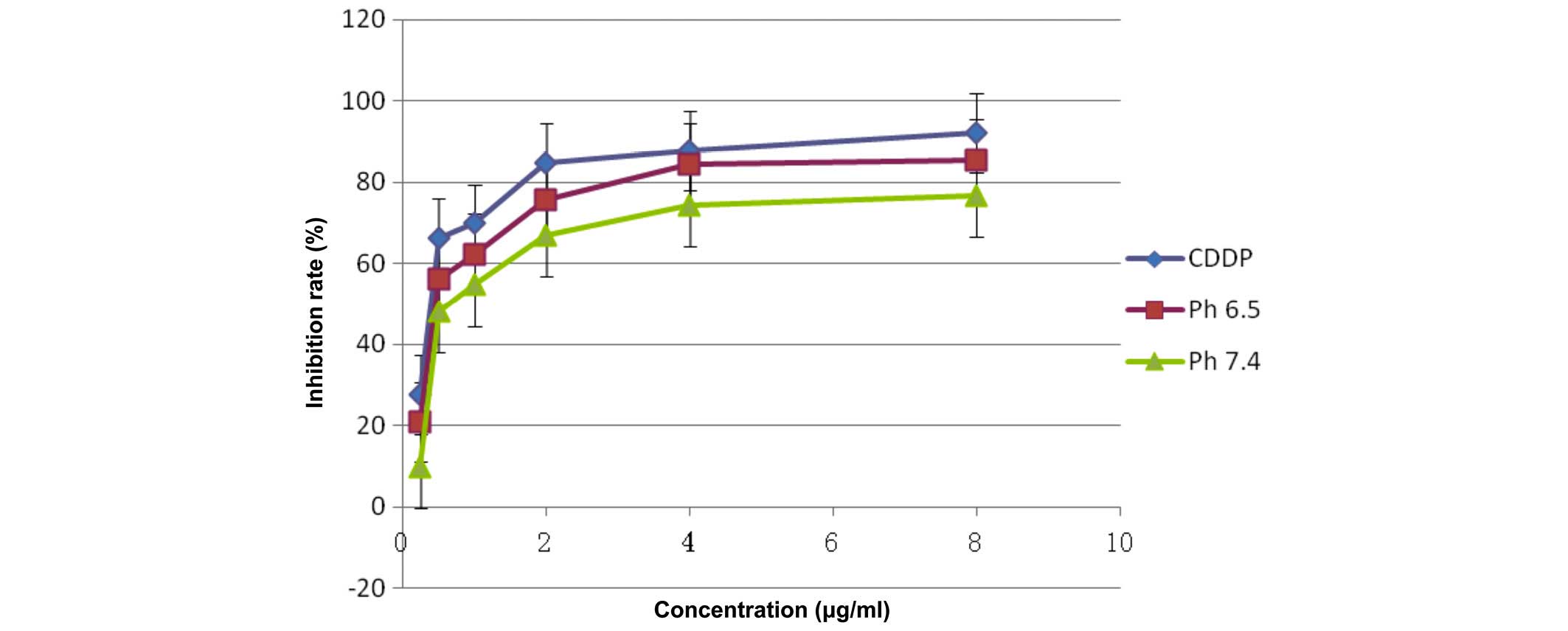
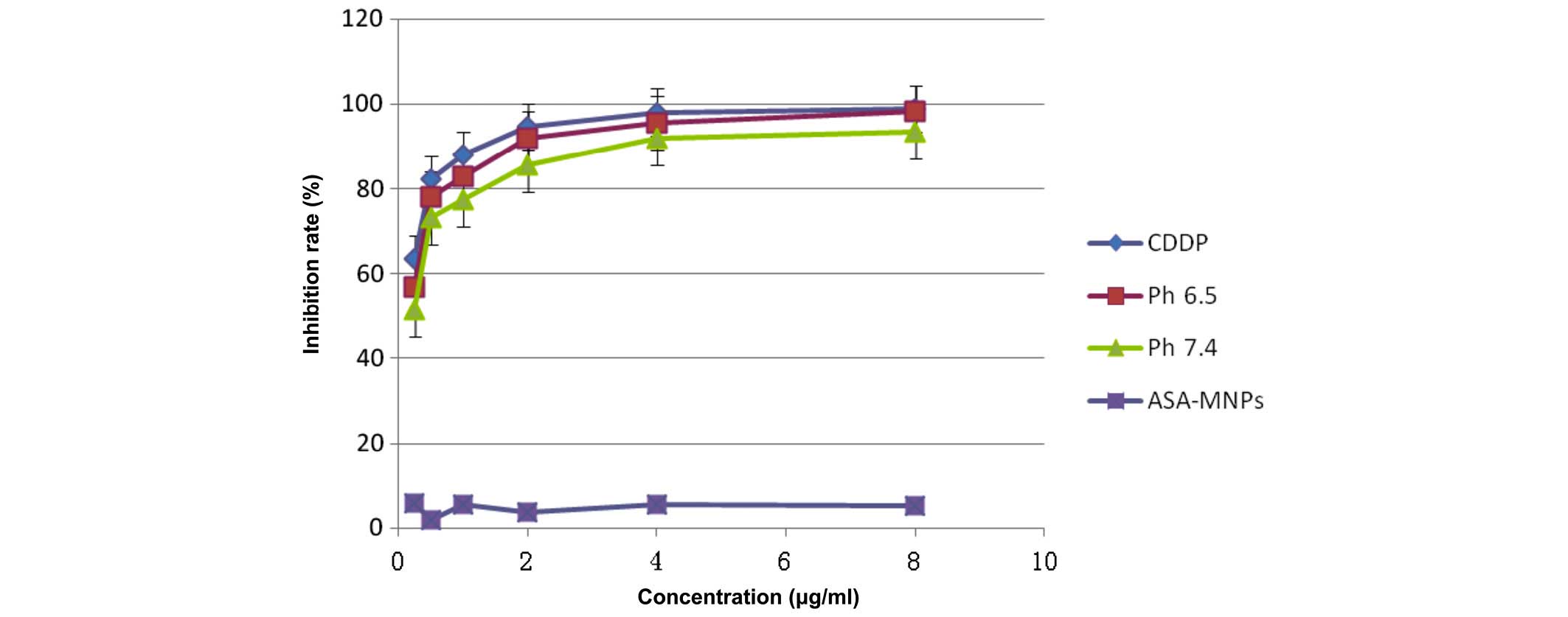
Detection of cell inhibition by MTS
assay
The pure CDDP group, and the treatment groups
treated with pH 6.5 or 7.4 FA-PEG-NH-N=MNPs-CDDP demonstrate
markedly different inhibition ratios. The differences are dose
dependent (F=11,325.296; P<0.0001; Figs. 12 and 13). Inhibition of HNE-1 cells occurred
regardless of whether the pH value of FA-PEG-NH-N=MNPs-CDDP was 7.4
or 6.5, or whether the culture period was 24 or 48 h; however,
inhibition was dose- and time-dependent (P<0.05). The empty
carrier, ASA-modified MNPs, exerted little influence on the
inhibition of HNE-1 cells (P>0.05). Furthermore, following 24-h
culture, the inhibition ratio for cells treated with pH 6.5 or 7.4
MNPs were lower when compared with pure CDDP, with greater
inhibition in the pH 6.5 group when compared with the pH 7.4
MNP-treated cells (Fig. 12).
However, following culture for 48 h, Figure 13 demonstrates that differences
in inhibition ratios in the pH 7.4 and 6.5 groups reduce although
remain statistically significant.
Discussion
CDDP is one of the most effective antitumor
chemotherapeutic agents for head and neck cancer. However, CDDP has
numerous limitations, including, rapid clearance from the blood,
formation of irreversible binding to plasma proteins, it readily
hydrolyzes, transforming into reverse CDDP, and causing marked
toxicity, loss of antitumor activity and drug resistance.
The present study used a carrier MNP loaded with
CDDP to attempt to overcome these limitations and improve the
efficacy of treatment with CDDP. pH-sensitive FA-modified
CDDP-loaded MNPs were produced. The current study characterized
various properties of the drug-delivery system. The freeze-dried
powder is black and uniform and when scattered in double distilled
water, generates a uniform colloidal solution following ultrasound
processing. TEM was used to observe the MNP, which appeared uniform
and regular with a mean particle size of 10.2±1.5 nm; dynamic light
scattering was used and indicated that the hydrodynamic diameter
was 176±1.1 nm, these results indicate the MNPs would be suitable
as an active targeting nano-drug-delivery system. The ζ-potential
of FA-PEG-NH-N=MNPS-CDDP was −20.22±1.36 mV indicating good
stability. A solution containing the CDDP-loaded MNPs also
demonstrated good stability, with no marked sedimentation or
flocculation occurring over the 2-month observation period at a
constant temperature. The CDDP loading capacity and iron content
did not significantly change, which further indicated the stability
of the product. In addition, it exhibited good superparamagnetism
and magnetic responsiveness under a constant magnetic field. MNPs
were observed on the tube wall closest to a magnetic field, however
became evenly scattered following removal from the magnetic field.
Its maximum saturation magnetization, as detected by magnetic
curve, was 16.3±0.2 emu/g and the residual magnetism was 0. The
concentration of CDDP being transported was 0.773 mg/ml and the
iron concentration was 1.908 mg/ml. In addition, the MNPs could be
filtered from water by ultrafiltration, enabling acquisition of a
high concentration of CDDP.
The dynamic light scattering of pH-sensitive
FA-modified CDDP-loaded MNPs measured by laser particle analyzer
was 176.6±1.1 nm and the ζ-potential was −20.91±1.76 mV. The
particle size as measured by TEM was 10.2±1.5 nm. The particle size
obtained with the dynamic light scattering test was greater than
that observed by TEM. There are two possible factors resulting in
this, firstly dynamic light scattering is more sensitive in
measuring larger particles, and, secondly when TEM samples are
prepared, due to the volatilization of solvent, the nanometer
polymer particle may have collapsed while those undergoing dynamic
light scattering analysis are in a solution maintaining their shape
(12,14). The dynamic light scattering results
of particle size demonstrate that FA-PEG-NH2N=H-CHO-MNPs-CDDP
particles had a mean hydrodynamic diameter of 176.6±1.1 nm, the
ASA-MNPs had a mean of 198.9±3.4 nm, but that of the CDDP-loaded
MNPs (CDDP-MNPs) was 153.4±1.8 nm. These are produced during
preparation of the final product. The size of CDDP-MNPs is markedly
smaller than ASA-MNPs, possibly due to CDDP complexing with sodium
alginate, which results in the linear molecule that was stretched
becoming coiled, thus, reducing the hydrodynamic diameter. The
pH-sensitive FA-modified CDDP-loaded MNP is formed by wrapping
FA-PEG-NHNH2 around the surface of CDDP-MNPs resulting in a larger
particle size, although it remains smaller than ASA-MNPs. The
ζ-potential of the pH-sensitive FA-modified CDDP-loaded MNPs was
−20.91±1.76 mV. The particles in the solution are mutually rejected
due to their negative charge, indicating a good colloid stability,
however, the negative potential is lower than ASA-MNPs (−29.04±2.17
mV) and CDDP-MNPs (−25.08±0.96 mV). This may be due to the wrapping
of the outer layer by FA-PEG-NHNH2 decreasing the carboxyl groups.
Therefore, it is not as stable as CDDP-MNPs, but has good
stability.
The present study aimed to elucidate the
distribution of MNPs within cells following uptake. Only the HNE-1
cells (folate receptor-positive) cultured with
FA-PEG-NH-N=MNPs-CDDP demonstrated staining with Prussian blue dye.
As the blue stain is due to ferrous ferricyanide precipitation
formed by ferric acid and potassium ferrocyanide under acidic
conditions, this result indicates that FA-PEG-NH-N=MNPs-CDDP
culture is the only method by which MNPs are able to enter the
nasopharyngeal carcinoma cell lines. The entry to the cell is
mediated by the FA and the FA receptor. The FA-modified MNPs were
observed in the HNE-1 cells under TEM following culture for 6 h.
The particle diameter is comparable with those prepared above. But
above phenomenon are all not seen in normal cells following culture
of pure MNPs and HNE-1 for 6 h.
The results of the present study demonstrate that a
pH-sensitive FA-modified CDDP-loaded MNP can enter HNE-1 cells due
to their upregulated expression of folate receptors on the cell
surface. Entering the cell allows CDDP to be released in the low pH
environment within the cell.
A pH-sensitive therapeutic agent release system has
been demonstrated to be effective in cancer therapy. As the
therapeutic agent-loaded MNPs are not released in surrounding
tissue, side effects of the therapeutic agent on normal tissue are
reduced. Upon reaching the tumor tissue and entering cells by
targeting the folate receptors, the MNPs are taken up by endosomes
with a pH value between 4.5 and 6.5, this releases CDDP from the
MNP. The hydrazone bond between the dianzyl PEG and sodium alginate
is hydrolyzed in acidic conditions resulting in the FA-dianzyl
detaching from the MNP, which exposes CDDP. This also breaks the
bond between sodium alginate and CDDP, releasing CDDP and allowing
it to target the DNA. However, when the MNPs are in the blood and
normal tissue with a higher pH of 7.4, the hydrazone bond remains
stable and CDDP is not released.
The MTS assay demonstrated that administration of
CDDP, either directly or via the FA-modified CDDP-loaded MNPs,
inhibited the HNE-1 cell proliferation. Following a 24-h culture,
the inhibition ratio with each treatment group
[FA-PEG-NH-N=MNPs-CDDP (pH 6.5) and FA-PEG-NH-N=MNPs-CDDP (pH 7.4)]
increased in a dose- and time-dependent manner. The pH 6.5 delivery
group exerted greater inhibition effects than the pH 7.4 group,
however, CDDP alone produced greater inhibitory ratio. This
suggests that the MNPs require longer to allow the therapeutic
agent to dissociate inside the cell and do not reach the
concentration achieved by CDDP alone. As MNP (pH 6.5) exerts a
greater inhibitory effect, the results suggest the importance of
the hyrazone bond in pH sensitivity. However, following 48 h, the
inhibition ratio of pure CDDP is similar to the experimental MNP
groups, pH 6.5 and 7.4. The longer incubation time may be important
in this. The similar values for the different pH groups may be due
to the fall in pH following endocytosis, despite the environment
being a higher pH in the pH 7.4 group, releasing CDDP to affect the
cell. The present study demonstrated that the blank MNPs, at the
same concentration, did not influence the growth of HNE-1 cells at
24 or 48 h indicating that MNPs alone were not cytotoxic.
In conclusion, the CDDP content of the pH-sensitive,
FA-modified CDDP-loaded MNPs was 0.773 mg/ml, the iron content was
1.908 mg/ml; the mean particle diameter of magnetic nuclei, as
observed by TEM, was ~10.2±1.5 nm, the mean hydrodynamic diameter
detected using a laser particle analyzer was 176.6±1.1 nm. The
ζ-potential was −20.91±1.76 mV and the maximum saturation
magnetization intensity was 16.3±0.2 emu/g. These results suggest
that the pH-sensitive FA-modified CDDP-loaded MNPs have good
stability, magnetic responsiveness and superparamagnetism The
hydrazone bond can be hydrolyzed at a low pH, exposing and
releasing CDDP. The present study provides a foundation for further
investigation into targeted treatment of malignant tumors,
particularly nasopharyngeal cancer.
Acknowledgments
The present study was supported by grants from the
Ministry of Education Research Fund for the Doctoral Program (grant
no. 20114433110001), the Guangdong Natural Science Foundation
Project (grant no. s2013010016730) and the National Natural Science
Foundation of China (grant nos. 81372477, 81260406 and
81573000).
References
|
1
|
Kuang Y, Liu J, Liu Z and Zhuo R:
Cholesterol-based anionic long-circulating cisplatin liposomes with
reduced renal toxicity. Biomaterials. 33:1596–1606. 2012.
View Article : Google Scholar
|
|
2
|
Rath KS, Naidu SK, Lata P, Bid HK, Rivera
BK, McCann GA, Tierney BJ, Elnaggar AC, Bravo V, Leone G, et al:
HO-3867, a safe STAT3 inhibitor, is selectively cytotoxic to
ovarian cancer. Cancer Res. 74:2316–2327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu S, Wu G, Gu X, Wang J, Wang Y, Gao H
and Ma J: Magnetic and pH-sensitive nanoparticles for antitumor
drug delivery. Colloids Surf B Biointerfaces. 103:15–22. 2013.
View Article : Google Scholar
|
|
4
|
Kolhatkar R, Lote A and Khambati H: Active
tumor targeting of nanomaterials using folic acid, transferrin and
integrin receptors. Curr Drug Discov Technol. 8:197–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie M, Chen S, Xu X, et al: Preparation of
two kinds of super-paramagnetic carriers-supported cis-platinum
complexes and the comparison of their characteristics. Chinese
Science Bulletin. 51:151–157. 2006. View Article : Google Scholar
|
|
6
|
Hu X, Liu S, Huang Y, Chen X and Jing X:
Biodegradable block copolymer-doxorubicin conjugates via different
linkages: Preparation, characterization, and in vitro evaluation.
Biomacromolecules. 11:2094–2102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohguchi Y, Kawano K, Hattori Y and Maitani
Y: Selective delivery of folate-PEG-linked, nanoemulsion-loaded
aclacinomycin A to KB nasopharyngeal cells and xenograft: Effect of
chain length and amount of folate-PEG linker. J Drug Target.
16:660–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laurienzo P, Malinconico M, Motta A and
Vicinanza A: Synthesis and characterization of a novel
alginate-poly(ethylene glycol) graft copolymer. Carbohydr Polym.
62:274–28. 2005. View Article : Google Scholar
|
|
9
|
Liu J, Xie MQ, Zhang T, Zhang HZ and Xu
YM: The preparation and characterization of cisplatin magnetic
nanomedicine targeted by folic acid molecular. Zhongguo Zuzhi
Gongcheng YanjiuYu Linchuang Kangfu. 25:4631–4637. 2011.
|
|
10
|
Nguyen R and Huc I: Optimizing the
reversibility of hydrazone formation for dynamic combinatorial
chemistry. Chem Commun (Camb). 9:942–943. 2003. View Article : Google Scholar
|
|
11
|
Qiu XX: Determination of ferrous by
spectrophotometry in water. Journal of Southwest University for
Nationalities (Natural Science Edition). 1:111–113. 2011.In
Chinese.
|
|
12
|
Azzam T and Eisenberg A: Control of
vesicular morphologies through hydrophobic block length. Angew Chem
Int Ed Engl. 45:7443–7447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Avichezer D, Schechter B and Arnon R:
Functional polymers in drug delivery: Carrier-supported CDDP
(cis-platin) complexes of polycarboxylates - effect on human
ovarian carcinoma. React Funct Polym. 36:59–69. 1998. View Article : Google Scholar
|
|
14
|
Jiang M, Eisenberg A, Liu GJ and Zhang X:
Macromolecular Self-Assembly. Science Press; Beijing: 2006, In
Chinese.
|