Introduction
Renal fibroblast proliferation induces
tubulointerstitial fibrosis resulting in renal filtration
dysfunction (1) and chronic kidney
disease (CKD) (2,3), thus, the inhibition of fibroblast
proliferation to prevent CKD is an important area. Transforming
growth factor-β1 (TGF-β1) is important in the induction of
proliferation in human renal fibroblasts (4,5).
Previous studies have suggested that induction of renal fibrosis by
TGF-β1 is associated with p53 (6,7),
reactive oxygen species (8,9), the
Smad signaling pathway (10,11),
mitogen activated protein kinase (MAPK) signaling pathways
(12,13), and RhoA/Rho kinase (9,14).
These studies indicated that TGF-β1 is a critical factor in
activating numerous signal transduction pathways that result in
proliferation in renal fibroblasts. Thus, TGF-β1 was used in the
present study as a cell model for investigating anti-proliferative
effects on renal fibroblasts by gefitinib and vitamin E treatment
alone and in combination. Results from the current study
demonstrated that 0.2 nM TGF-β1 promoted renal fibroblast
proliferation.
The epidermal growth factor receptor (EGFR)
signaling pathway induces cell proliferation in various cells
(15–18). Previous studies have demonstrated
that the EGFR signaling pathway mediates renal fibroblast
proliferation and renal fibrogenesis (19,20).
Gefitinib, an EGFR tyrosine kinase inhibitor, inhibits EGFR
signaling activation resulting in cell growth arrest (21,22).
Thus, gefitinib has generally been used for clinical tumor
treatment (23–25). As EGFR mediates renal fibroblast
proliferation and EGFR is blocked by gefitinib, a previous study
has successfully used gefitinib to inhibit renal fibroblast
proliferation (26). The present
study demonstrated that gefitinib attenuates fibroblast
proliferation by blocking the EGFR signaling pathway and by
inhibiting the TGF-β1-mediated pathway. In addition, previous
studies have suggested that the EGFR signaling pathway is
associated with the TGF-β1-mediated pathway (8,27).
Similar to these studies, experimental data from the present study
also demonstrated that gefitinib inhibits TGF-β1-induced fibroblast
proliferation. Although gefitinib effectively inhibits fibroblast
proliferation to prevent renal fibrosis, the side effects as a
result of gefitinib are also clinically important (28–31).
Vitamin E exerts an anti-oxidative and protective
effect against various oxidative stress-associated diseases,
including hypertension, cardiovascular disease, hemorrhagic
liposis, and obesity-associated diseases (32–35).
However, vitamin E exerts an anti-fibrotic effect on the renal
cell-mediated TGF-β1 signaling pathway (36–38).
A previous study indicated that vitamin E reduces progression of
fibrosis in obstructed kidneys (36). Other previous studies have
demonstrated that vitamin E in combination with pentoxifylline or
Fuzheng Huayu recipe, a traditional Chinese medicine, inhibits
TGF-β1-induced fibrosis (37,38).
Results from the present study also demonstrated that vitamin E
inhibits cell proliferation in TGF-β1-treated renal fibroblasts.
Furthermore, the present study indicates that a combination
treatment of low-dose vitamin E and low-dose gefitinib has a more
marked anti-proliferative effect on TGF-β1-treated renal
fibroblasts than high-dose vitamin E treatment or high-dose
gefitinib treatment alone. This suggests that combination treatment
with low-dose vitamin E and low-dose gefitinib is a potential
therapeutic strategy to inhibit fibroblast proliferation and
prevent high-dose gefitinib treatment-induced side effects.
Three major MAPK signaling pathways contain
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase, and p38 mitogen-activated protein kinase (39,40).
Previous studies have suggested that renal fibroblast proliferation
is mediated by MAPK signaling pathways (41,42).
The present study demonstrates that ERK phosphorylation is
increased in TGF-β1-treated renal fibroblasts, suggesting
TGF-β1-induced proliferation is mediated by the ERK signaling
pathway. In addition, the present study demonstrated that
combination treatment of low-dose vitamin E and low-dose gefitinib
reduces TGF-β1-induced increases in ERK phosphorylation levels. The
present study indicates that combination treatment with low-dose
gefitinib and low-dose vitamin E has synergistic effects to inhibit
TGF-β1-induced renal fibroblast proliferation mediated by the ERK
phosphorylation signaling pathway.
Materials and methods
Materials
TGF-β1 was obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). Anti-ERK (1:400; cat. no. BS3627),
anti-p-ERK (1:400; cat. no. BS5016), anti-p38 (1:400; cat. no.
BS3567) and anti-p-p38 (1:400; cat. no. BS4766) primary rabbit
polyclonal antibodies were purchased from Bioworld (Louis Park, MN,
USA). Horseradish peroxidae-conjugated goat anti-rabbit IgG,
secondary antibody (1:2,000, cat. no. 7074) was purchased from Cell
Signaling Technology (Danvers, MA, USA).
5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay kit was
bought from Bio Basic Canada, Inc. (Markham, ON, Canada). Fetal
bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM),
non-essential amino acids, L-glutamine, and penicillin/streptomycin
were purchased from Hyclone (GE Healthcare Life Sciences, Logan,
UT, USA). Vitamin E and dimethyl sulfoxide (DMSO) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Gefitinib was purchased from
AstraZeneca UK Limited (London, UK).
Cell line and cell culture
The NRK-49F rat renal fibroblast cell line was
obtained from Bioresource Collection and Research Center (Hsinchu,
Taiwan). The cell line was cultured in DMEM supplemented with 10%
FBS, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin, and 0.1
mM non-essential amino acids, and maintained in a humidified
atmosphere with 5% CO2 at 37°C.
Cell survival rate assay
Survival rates of NRK-49F cells were measured with
the MTT assay method as described in previous studies (43,44).
Briefly, cells were cultured in 96-well plates. On the second day,
cells were divided into control group and experimental groups and
MTT assays (with DMSO treatment). were determined at 24 and 72 h
according to the manufacturer's protocols. Absorbance was
determined under a multi-well ELISA reader (SpectraMax Paradigm
Multi-Mode Microplate Reader; Molecular Devices, Sunnyvale, CA,
USA) at a wavelength of 570 nm. Survival rates were indicated using
the following formula: A570 experimental group / A570 control
group.
Cell cycle analysis
Cell cycle analysis was conducted using
fluorescence-activated cell sorting as described previously
(45,46). Briefly, NRK-49F cells from the
control and experimental groups were collected and washed with
phosphate-buffered saline (PBS; containing 140 mM NaCl, 2.5 mM KCl,
15 mM Na2HPO4 and 1.6 mM
KH2PO4), then fixed with 70% ethanol (Echo
Chemical Co., Ltd., Miaoli, Taiwan) at 4°C for 1 h. The fixed cells
were washed with PBS and then treated with 1 ml propidium iodide
(PI) solution (50 μg/ml PI, 100 μg/ml RNase A, and
0.1% Triton X-100) for 30 min at 37°C. Following this, cells were
washed with PBS and analyzed by flow cytometry (Partec
CyFlow® SL; Sysmex Partec GmbH, Görlitz, Germany). The
resulting data was analyzed with WinMDI version 2.8 software
(http://winmdi.software.informer.com/2.8/).
Sodium dodecyl sulfate (SDS)
electrophoresis and western blotting
Gel electrophoresis and western blotting were
performed as previously described (47,48).
Briefly, cells were treated with lysis buffer (containing 50 mM
Tris-HCl, 120 mM NaCl, 1 mM EDTA and 1% NP-40) and centrifuged at
16,000 × g for 10 min at 4°C. The supernatant layer containing
proteins was collected and the protein level was determined using a
Bicinchoninic Acid Protein Assay Reagent kit (Pierce Biotechnology,
Rockford, IL, USA) with a DU 530 spectrophotometer (OD 562 nm;
Beckman Coulter, Inc., Brea, CA, USA). Equal quantities of protein
(60 μg) were loaded and run on an SDS-PAGE for 45 min and
transferred to a PVDF membrane. The membranes were blocked with 5%
milk for 2 h and washed three times with PBS. The membranes were
incubated with primary antibodies in 5% milk for 2 h. The membranes
were then washed with PBS three times and incubated with secondary
antibodies for 1 h. Protein levels were analyzed with Western
Lightning® Chemiluminescence Plus reagent (PerkinElmer,
Inc., Waltham, MA, USA) and were observed with a Luminescence Image
Analysis system (LAS-4000, FUJIFILM Electronic Materials Taiwan
Co., Ltd., Tainan, Taiwan).
Statistical analysis
Data were measured from four independent experiments
and are presented as the mean ± standard deviation. The data was
analyzed using a Student's t-test with Excel 2010 (http://microsoft-excel-2010.updatestar.com/zh-tw).
P<0.05 was considered to indicate a statistically significant
difference between two groups.
Results
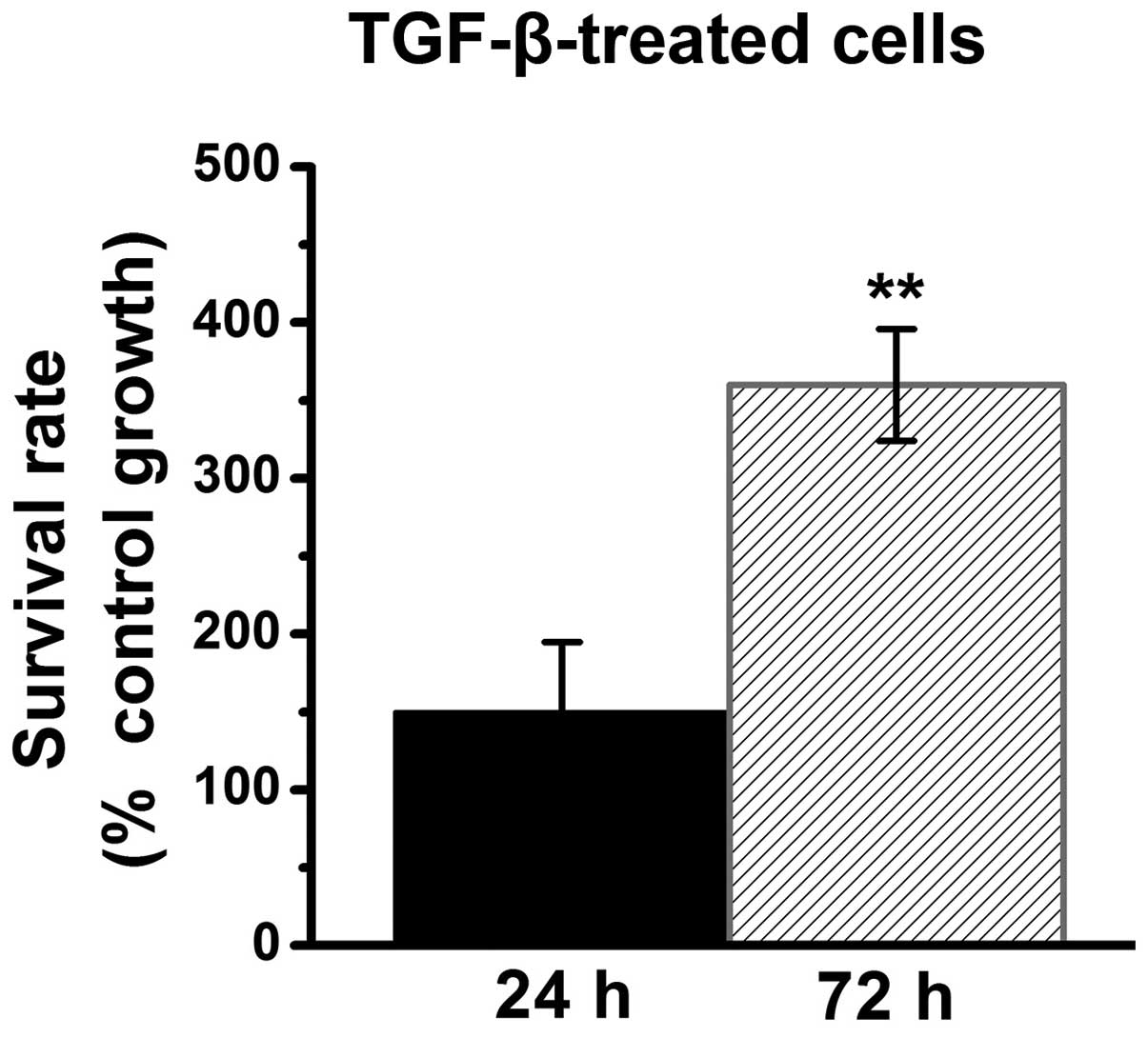
TGF-β1 induces renal fibroblast
proliferation in a time-dependent manner
Consistent with data from previous studies (4,5), the
data from the present study demonstrates that TGF-β1 induces
proliferation in renal fibroblasts. Compared with growth in the
control cells (without TGF-β1 treatment), the survival rate is
~150% in TGF-β1-treated cells at 24 h. However, the survival rate
is significantly increased by >360% in TGF-β1-treated cells at
72 h (P<0.01; Fig. 1). The
present study suggested that TGF-β1 induces cell proliferation in
renal fibroblasts in a time-dependent manner. The present study
then used TGF-β1-induced cell proliferation as an experimental
model to investigate the antiproliferative effects of gefitinib
treatment, vitamin E treatment, and combination treatment of
gefitinib and vitamin E on renal fibroblasts.
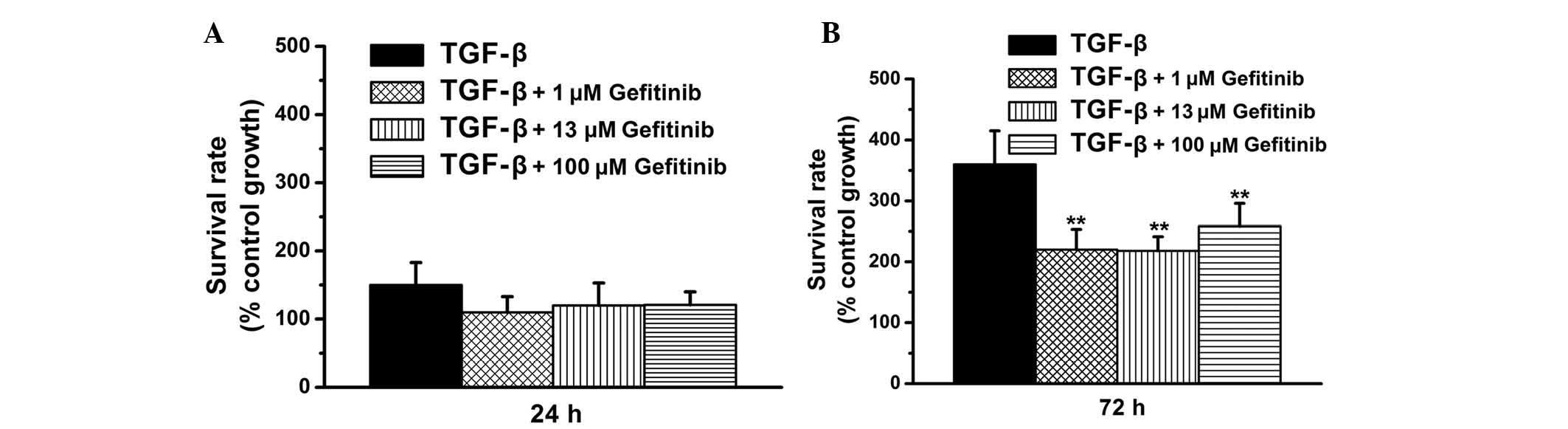
Gefitinib exerts antiproliferative
effects on TGF-β1-treated renal fibroblasts
The present study aimed to investigate whether
gefitinib inhibits TGF-β1-induced cell proliferation. The
anti-proliferative effects of gefitinib (high dose, 100 μM;
low dose for clinical tumor treatment, 13 μM; and low dose,
1 μM) were examined in TGF-β1-treated renal fibroblasts.
Compared with the control group, the 24-h survival rates in the
present study were ~150% in the TGF-β1-treated group and ~100% in
the TGF-β1 + gefitinib-treated groups (Fig. 2A). In addition, the 72-h survival
rates were >360% in the TGF-β1-treated group and <250% in the
TGF-β1 + gefitinib-treated group (P<0.01; Fig. 2B). Results from the present study
demonstrated that gefitinib reduces TGF-β1-induced cell
proliferation. Furthermore, as shown in Fig. 2, there is no marked difference in
survival rates among the TGF-β1 + gefitinib (100, 13 and 1
μM)-treated groups at 24 and 72 h. The data from the present
study suggested that high- and low-dose gefitinib (100, 13 and 1
μM) are equally effective at inhibiting TGF-β1-induced cell
proliferation (as shown in Fig.
2B).
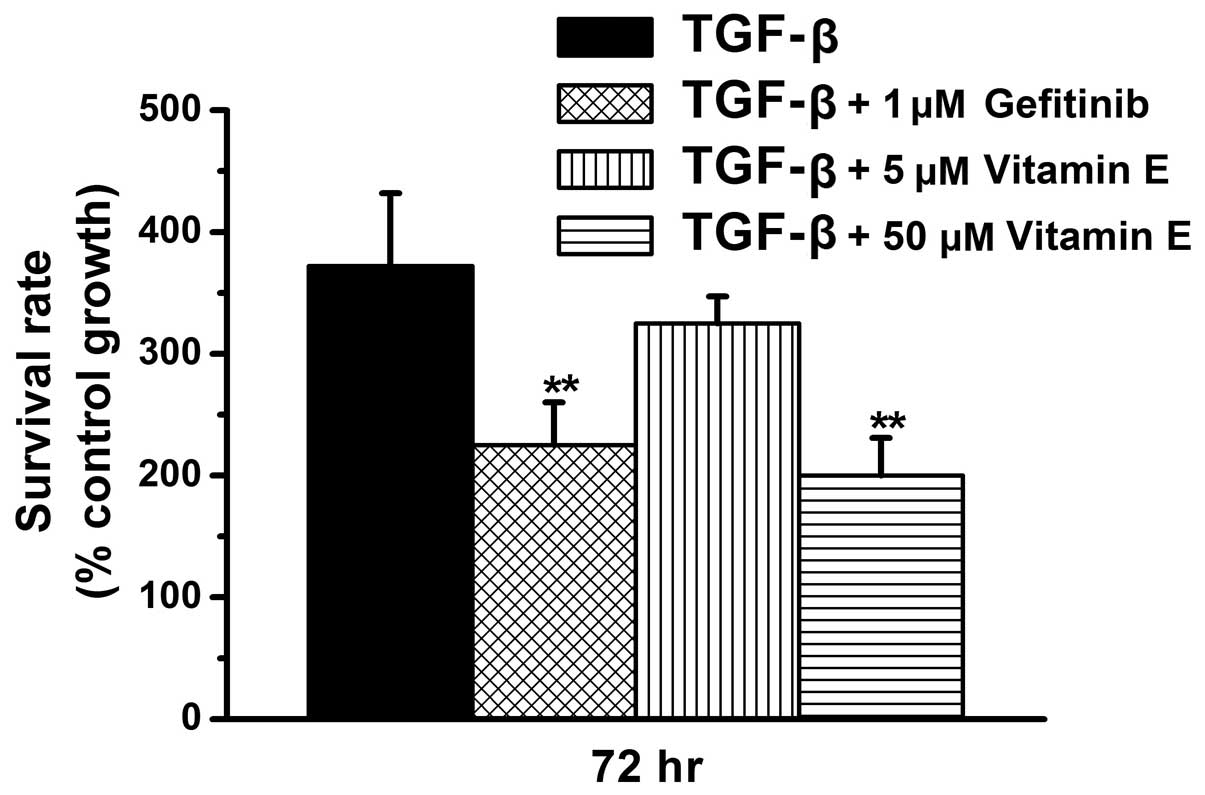
Vitamin E reduces cell proliferation in
TGF-β1-treated renal fibroblasts in a dose-dependent manner
A previous study indicated that vitamin inhibits the
progression of fibrosis in obstructed kidneys (36). Numerous other studies have
demonstrated that epithelial-mesenchymal transition (EMT) and
fibroblast proliferation induce renal fibrosis (49–52).
The present study further determined whether vitamin E inhibits
fibroblast proliferation directly to prevent progression of
fibrosis. As presented in Fig. 3,
the 72-h survival rate is >360% in the TGF-β1-treated group,
~330% in the TGF-β1 + 5 μM vitamin E-treated group, and
<230% in the TGF-β1 + gefitinib-treated group and the TGF-β1 +
50 μM vitamin E-treated group. The present study
demonstrated that high-dose vitamin E (50 μM) treatment
reduces TGF-β1-induced cell proliferation (P<0.01 vs. the TGF β1
group, Fig. 3) similarly to
gefitinib treatment. However, low-dose vitamin E (5 μM)
treatment did not markedly reduce TGF-β1-induced cell
proliferation. Thus, the results from the present study suggest
that vitamin E reduces TGF-β1-induced renal cell proliferation in a
dose-dependent manner.
Gefitinib enhances the antiproliferative
effects of vitamin E on TGF-β1-treated renal fibroblasts
The present study aimed to investigate whether
gefitinib promotes the antiproliferative effects of vitamin E on
TGF-β1-treated cells. The 72-h survival rate was >360% in the
TGF-β1-treated group, ~330% in the TGF-β1 + 5 μM vitamin
E-treated group, and ~160% in TGF-β1 + 5 μM vitamin E with
various concentrations of gefitinib-treated groups (Fig. 4A). In addition, the 72-h survival
rate was >360% in the TGF-β1-treated group, ~220% in the TGF-β1
+ 50 μM vitamin E-treated group, and ~150% in the TGF-β1 +
50 μM vitamin E with various concentrations of
gefitinib-treated groups (Fig.
4B). These data suggest that gefitinib enhances the
antiproliferative effects of vitamin E on TGF-β1-treated cells.
However, the antiproliferative effects were not markedly different
among those treated with vitamin E + various concentrations (1, 13
and 100 μM) of gefitinib. Furthermore, the data demonstrated
that although low-dose vitamin E does not have notable
antiproliferative effects, combination treatment of low-dose
vitamin E and gefitinib effectively reduces TGF-β1-induced cell
proliferation (P<0.01 vs. the TGF-β1 group, Fig. 4A).
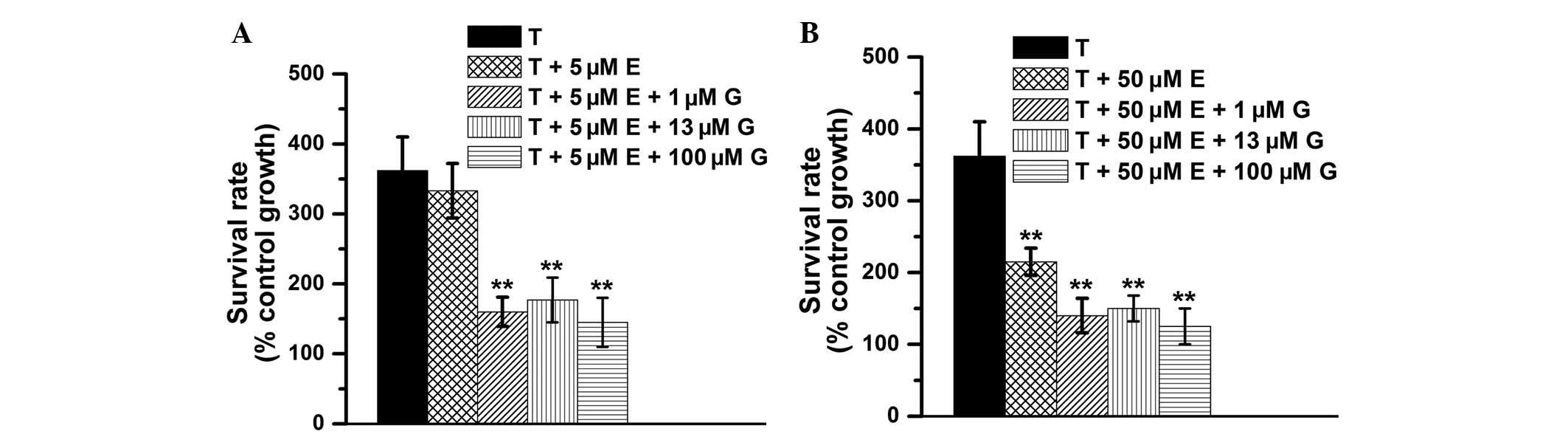 | Figure 4Cell survival rates. (A) The 72 h
survival rates of NRK49-F cells were calculated in the 0.2 nM
TGF-β1-treated, 0.2 nM TGF-β1 with 5 μM vitamin E-treated,
0.2 nM TGF-β1 with 5 μM vitamin E + 1 μM
gefitinib-treated, 0.2 nM TGF-β1 with 5 μM vitamin E + 13
μM gefitinib-treated and 0.2 nM TGF-β1 with 5 μM
vitamin E + 100 μM gefitinib-treated groups. (B) The 72 h
survival rates of NRK49-F cells were calculated in the 0.2 nM
TGF-β1-treated, 0.2 nM TGF-β1 with 50 μM vitamin E-treated,
0.2 nM TGF-β1 with 50 μM vitamin E + 1 μM
gefitinib-treated, 0.2 nM TGF-β1 with 50 μM vitamin E + 13
μM gefitinib-treated, and 0.2 nM TGF-β1 with 50 μM
vitamin E + 100 μM gefitinib-treated group. The data was
analyzed from four independent experiments and presented as the
mean ± standard deviation. **P<0.01 vs. the TGF-β1
group. TGF-β1 (T), transforming growth factor-β1; E, vitamin E; G,
gefitinib. |
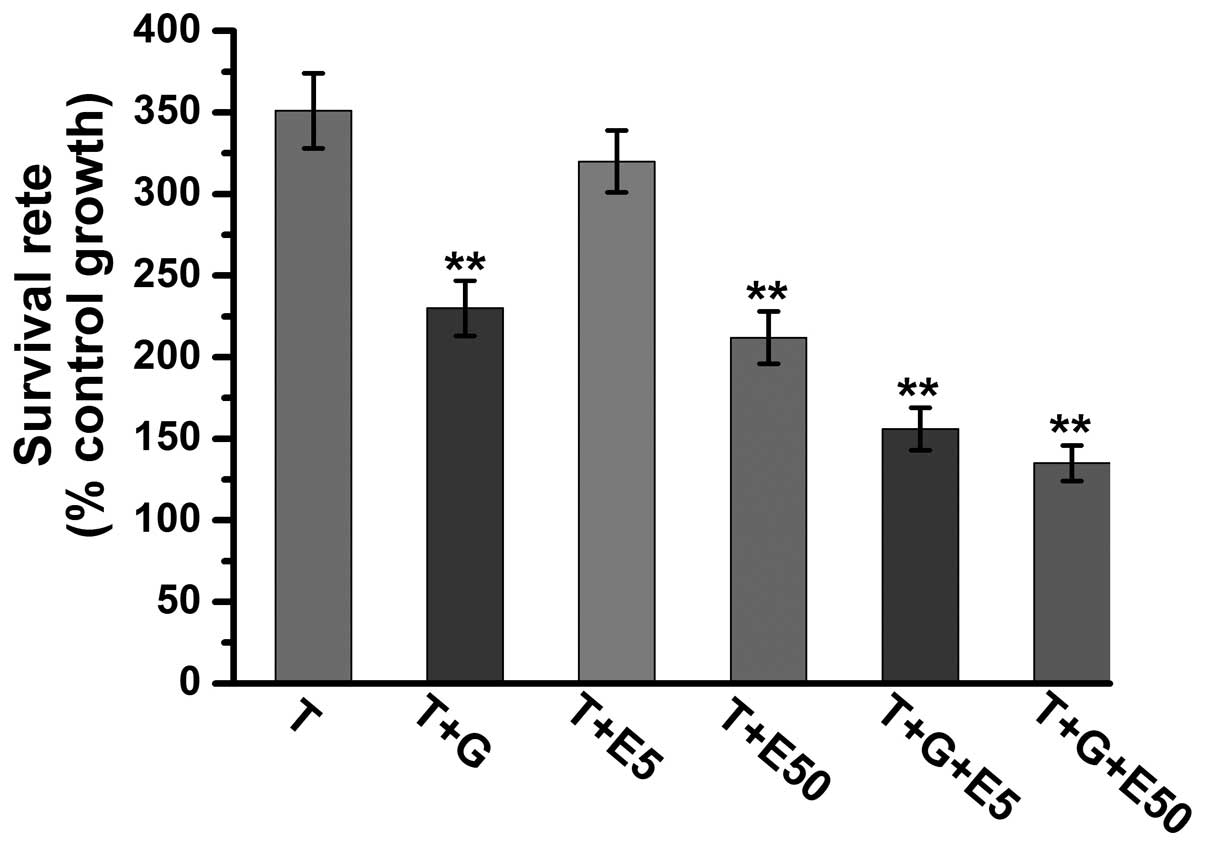
Combination treatment of low-dose
gefitinib and low-dose vitamin E has synergistic effects to reduce
TGF-β1-induced renal fibroblast proliferation
As presented in Figs.
2 and 3, gefitinib and vitamin
E have been demonstrated to exert anti-proliferative effects on
TGF-β1-treated cells. The current study further analyzed the
anti-proliferative effects on TGF-β1-induced cell proliferation in
the gefitinib-treated group, the vitamin E-treated group, and the
gefitinib + vitamin E-treated group. As presented in Fig. 5, the 72-h survival rate was
>360% in TGF-β1-treated cells, ~340% in TGF-β1 with low-dose
vitamin E-treated group, and ~250% in TGF-β1 with high-dose vitamin
E-treated or gefitinib groups. These data indicate that low-dose
vitamin E does not have marked anti-proliferative effects on
TGF-β1-induced cell proliferation; however, high-dose vitamin E and
low-dose gefitinib have similar anti-proliferative effects on
TGF-β1-induced cell proliferation. Furthermore, the present study
demonstrated that the 72-h survival rates are ~160% in
TGF-β1-induced cells with low-dose gefitinib + high-dose or
low-dose vitamin E-treated groups. These data indicate that the
anti-proliferative effects in combination treatment with low-dose
gefitinib and low-dose vitamin E is similar to combination
treatment with low-dose gefitinib and high-dose vitamin E.
Furthermore, the combination treatment with gefitinib and vitamin E
has stronger anti-proliferative effects than gefitinib treatment
alone or vitamin E treatment alone. Thus, the results of the
present study suggest that combination treatment of low-dose
gefitinib and low-dose vitamin E reduces TGF-β1-induced cell
proliferation (P<0.01 vs. the TGF-β1 group, Fig. 5).
Combination treatment with gefitinib and
vitamin E reduces TGF-β1-induced cell proliferation associated with
the cell cycle and ERK signaling pathway
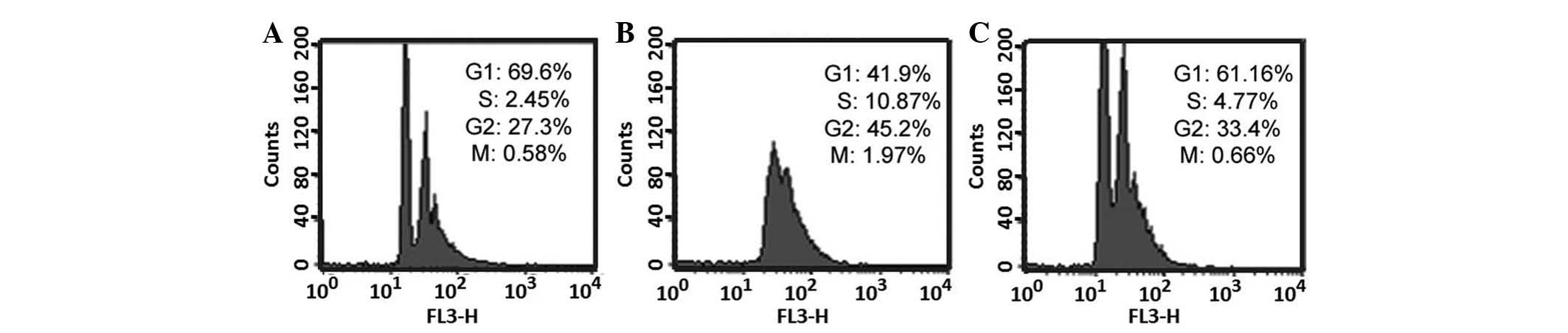
The cell cycle was analyzed in the control group,
the TGF-β1-treated group, and the TGF-β1 with gefitinib + vitamin
E-treated group. As presented in Fig.
6A, the G1 phase was ~69.6% and the S-phase is
~2.45% in the control group. As presented in Fig. 6B, the G1 phase was
~41.9% and the S-phase was ~10.87% in TGF-β1-treated group. As
presented in Fig. 6C, the
G1 phase was ~61.16% and the S-phase was ~4.77% in
TGF-β1 with gefitinib + vitamin E-treated group. All data obtained
from flow cytometry were analyzed using Student's t-test. The
S-phase percentage was significantly increased in the
TGF-β1-treated group compared with the control (P<0.05, as
determined from four independent flow cytometry experiments, data
not shown), this indicates that TGF-β1 accelerate entry to S-phase,
resulting in cell proliferation. Furthermore, S-phase percentage is
significantly increased in the TGF-β1-treated group compared with
the TGF-β1 with gefitinib + vitamin E-treated group. The result
suggested that combination treatment with gefitinib and vitamin E
may ameliorate the increase in cells entering the S-phase in
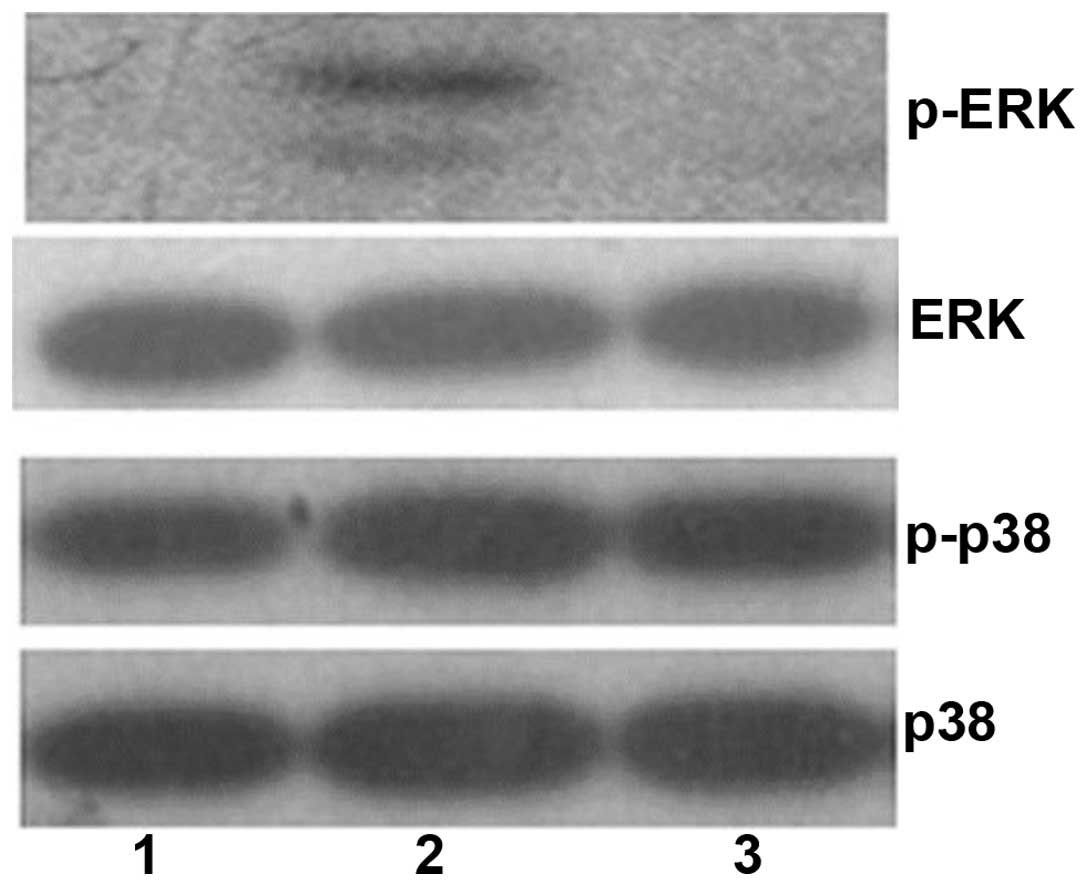
TGF-β1-treated cells. Furthermore, previous studies have
demonstrated MAPK signaling pathways, including ERK and p38
phosphorylation, are associated with renal fibroblast proliferation
(41,42). Thus, ERK and p38 phosphorylation,
p-ERK and p-p38, were analyzed in the control group, the
TGF-β1-treated group, and the TGF-β1 with gefitinib + vitamin
E-treated group (Fig. 7). Results
from the present study demonstrated that p-ERK was not observed in
the control group (Fig. 7, lane
1), but is evident in the TGF-β1-treated group (Fig. 7, lane 2). This suggests TGF-β1 may
induce renal cell proliferation may be via ERK phosphorylation.
However, p-ERK was also not observed in the TGF-β1 with gefitinib +
vitamin E-treated group (Fig. 7,
lane 3). The data indicated gefitinib + vitamin E treatment
inhibits ERK phosphorylation. However, the p-p38 levels were not
significantly different among the three groups. The results from
the current study suggest that combination treatment with gefitinib
+ vitamin E reduces TGF-β1-induced cell proliferation associated
with ERK phosphorylation.
Discussion
Gefitinib, an EGFR tyrosine kinase inhibitor,
inhibits cell growth (21,22) and has been used for various tumor
treatments, including, lung, esophageal and breast cancer (25,29,53).
Numerous studies have demonstrated that therapeutic doses of
gefitinib for clinical tumor treatment result in side effects,
including severe hepatotoxicity (29), acneiform eruption, severe xerosis
of skin, paronychia (30), and
empyema (31). However, in the
present study, the results indicated that there are similar
anti-proliferative effects on TGF-β1-treated renal fibroblasts
among high-dose, therapeutic dose, and low-dose gefitinib
treatments (Fig. 2). The results
of the present study indicate that gefitinib, a conventional
therapeutic agent for tumor treatment, may be useful for the
treatment of renal fibrosis at a low-dose.
Multiple studies have demonstrated that renal
fibrosis is induced via the EMT process and renal fibroblast
proliferation (49–52). In addition, it has been reported
that EMT and fibroblast proliferation are induced by activation of
the TGF-β1 signaling pathway (54–57).
Similar to these studies, data from the present study also showed
that TGF-β1 induces renal fibroblast proliferation. Previous
research has indicated that vitamin E in combination with other
therapeutic agents reduces progression of TGF-β1-induced fibrosis
(37,38). The current study further
demonstrated that vitamin E alone inhibits TGF-β1-induced
fibroblast proliferation (Fig. 3).
In addition, high-dose vitamin E, like gefitinib, has a more marked
anti-proliferative effect than low-dose vitamin E. Although the
anti-fibrotic effects exerted by vitamin E remain to be elucidated,
the present study demonstrated that vitamin E reduces proliferation
in TGF-β1-treated fibroblasts.
Results from the present study demonstrate that
combination treatment with gefitinib and vitamin E has an increased
anti-proliferative effect on TGF-β1-treated cells compared with
gefitinib or vitamin E treatment alone. Furthermore, these results
also demonstrated that combination treatment with low-dose
gefitinib and low-dose vitamin E has anti-proliferative effects
similar to combination treatment with high-dose gefitinib and
high-dose vitamin E. The results of the current study demonstrate
that low-dose gefitinib and low-dose vitamin E treatment may be a
potential therapeutic strategy for renal fibrosis and an effective
alternative to avoid high-dose gefitinib-induced side effects.
Previous studies have suggested that TGF-β1 induces
cell cycle-associated protein expression (4,58)
and activates MAPK signaling pathways (59,60).
Similar to these studies, the results from the present study have
also demonstrated that TGF-β1 promotes cells to enter the S-phase
and activate ERK phosphorylation. In addition, the present study
also demonstrated that combination treatment with low-dose
gefitinib and vitamin E induces G1 arrest and reduces
ERK phosphorylation levels to inhibit TGF-β1-induced
proliferation.
In conclusion, the present study demonstrated that
combination treatment with low-dose gefitinib and vitamin E has
anti-proliferative effects on TGF-β1-treated fibroblasts via cell
cycle arrest and inactivation of the ERK signaling pathway.
Acknowledgments
The present study was supported by the Taipei Tzu
Chi Hospital (grant. nos. TCRD-TPE-103-48, TCRD-TPE-104-34 and
TCRD-105-20).
References
|
1
|
Hundae A and McCullough PA: Cardiac and
renal fibrosis in chronic cardiorenal syndromes. Nephron Clin
Pract. 127:106–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YX, Zhang W, Wang WM, Yu XL, Wang YM,
Zhang MJ and Chen N: Role of moesin in renal fibrosis. PloS One.
9:e1129362014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eddy AA: Overview of the cellular and
molecular basis of kidney fibrosis. Kidney Int Suppl (2011). 4:2–8.
2014. View Article : Google Scholar
|
|
4
|
Strutz F, Zeisberg M, Renziehausen A,
Raschke B, Becker V, van Kooten C and Müller G: TGF-beta 1 induces
proliferation in human renal fibroblasts via induction of basic
fibroblast growth factor (FGF-2). Kidney Int. 59:579–592. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oujo B, Muñoz-Félix JM, Arévalo M,
Núñez-Gómez E, Pérez-Roque L, Pericacho M, González-Núñez M, Langa
C, Martínez-Salgado C, Perez-Barriocanal F, et al: L-endoglin
overexpression increases renal fibrosis after unilateral ureteral
obstruction. PloS One. 9:e1103652014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deshpande SD, Putta S, Wang M, Lai JY,
Bitzer M, Nelson RG, Lanting LL, Kato M and Natarajan R:
Transforming growth factor-β-induced cross talk between p53 and a
microRNA in the pathogenesis of diabetic nephropathy. Diabetes.
62:3151–3162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Overstreet JM, Samarakoon R, Meldrum KK
and Higgins PJ: Redox control of p53 in the transcriptional
regulation of TGF-β1 target genes through SMAD cooperativity. Cell
Signal. 26:1427–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samarakoon R, Dobberfuhl AD, Cooley C,
Overstreet JM, Patel S, Goldschmeding R, Meldrum KK and Higgins PJ:
Induction of renal fibrotic genes by TGF-β1 requires EGFR
activation, p53 and reactive oxygen species. Cell Signal.
25:2198–2209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manickam N, Patel M, Griendling KK, Gorin
Y and Barnes JL: RhoA/Rho kinase mediates TGF-β1-induced kidney
myofibroblast activation through Poldip2/Nox4-derived reactive
oxygen species. Am J Physiol Renal Physiol. 307:F159–F171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim D, Lee AS, Jung YJ, Yang KH, Lee S,
Park SK, Kim W and Kang KP: Tamoxifen ameliorates renal
tubulointerstitial fibrosis by modulation of estrogen receptor
α-mediated transforming growth factor-β1/Smad signaling pathway.
Nephrol Dial Transplant. 29:2043–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014. View Article : Google Scholar
|
|
12
|
Park SH, Cho HJ, Jeong YJ, Shin JM, Kang
JH, Park KK, Choe JY, Park YY, Bae YS, Han SM, et al: Melittin
inhibits TGF-β-induced pro-fibrotic gene expression through the
suppression of the TGFβRII-Smad, ERK1/2 and JNK-mediated signaling
pathway. Am J Chin Med. 42:1139–1152. 2014. View Article : Google Scholar
|
|
13
|
Zhang L, Zhang J, Liu X, Liu S and Tian J:
Tribbles 3 regulates the fibrosis cytokine TGF-β 1 through
ERK1/2-MAPK signaling pathway in diabetic nephropathy. J Immunol
Res. 2014:2403962014. View Article : Google Scholar
|
|
14
|
Chen G, Chen X, Sukumar A, Gao B, Curley
J, Schnaper HW, Ingram AJ and Krepinsky JC: TGFβ receptor I
transactivation mediates stretch-induced Pak1 activation and CTGF
upregulation in mesangial cells. J Cell Sci. 126:3697–3712. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lanaya H, Natarajan A, Komposch K, Li L,
Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic
M, et al: EGFR has a tumour-promoting role in liver macrophages
during hepatocellular carcinoma formation. Nat Cell Biol.
16:972–981. 2014. View
Article : Google Scholar :
|
|
16
|
Salazar N, Muñoz D, Kallifatidis G, Singh
RK, Jordà M and Lokeshwar BL: The chemokine receptor CXCR7
interacts with EGFR to promote breast cancer cell proliferation.
Mol Cancer. 13:1982014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo YH, Kim YR, Kim MS, Lee KJ, Park KH
and Hahn JH: YAC tripeptide of epidermal growth factor promotes the
proliferation of HaCaT keratinocytes through activation of EGFR.
BMB Rep. 47:581–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kundu S, Sengupta S and Bhattacharyya A:
NF-κB acts downstream of EGFR in regulating low dose cadmium
induced primary lung cell proliferation. Biometals. 26:897–911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu N, Guo JK, Pang M, Tolbert E,
Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H and Zhuang S:
Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis.
J Am Soc Nephrol. 23:854–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponnusamy M, Zhou X, Yan Y, Tang J,
Tolbert E, Zhao TC, Gong R and Zhuang S: Blocking sirtuin 1 and 2
inhibits renal interstitial fibroblast activation and attenuates
renal interstitial fibrosis in obstructive nephropathy. J Pharmacol
Exp Ther. 350:243–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han SY, Ding HR, Zhao W, Teng F and Li PP:
Enhancement of gefitinib-induced growth inhibition by Marsdenia
tenacissima extract in non-small cell lung cancer cells expressing
wild or mutant EGFR. BMC Complement Altern Med. 14:1652014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakurai MA, Ozaki Y, Okuzaki D, Naito Y,
Sasakura T, Okamoto A, Tabara H, Inoue T, Hagiyama M, Ito A, et al:
Gefitinib and luteolin cause growth arrest of human prostate cancer
PC-3 cells via inhibition of cyclin G-associated kinase and
induction of miR-630. PloS One. 9:e1001242014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bersanelli M, Tiseo M, Artioli F, Lucchi L
and Ardizzoni A: Gefitinib and afatinib treatment in an advanced
non-small cell lung cancer (NSCLC) patient undergoing hemodialysis.
Anticancer Res. 34:3185–3188. 2014.PubMed/NCBI
|
|
24
|
Deangelo DJ, Neuberg D, Amrein PC,
Berchuck J, Wadleigh M, Sirulnik LA, Galinsky I, Golub T, Stegmaier
K and Stone RM: A phase II study of the EGFR inhibitor gefitinib in
patients with acute myeloid leukemia. Leuk Res. 38:430–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalykaki A, Agelaki S, Kallergi G, Xyrafas
A, Mavroudis D and Georgoulias V: Elimination of EGFR-expressing
circulating tumor cells in patients with metastatic breast cancer
treated with gefitinib. Cancer Chemother Pharmacol. 73:685–693.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SC, Guh JY, Lin TD, Chiou SJ, Hwang
CC, Ko YM and Chuang LY: Gefitinib attenuates transforming growth
factor-beta1-activated mitogen-activated protein kinases and
mitogenesis in NRK-49F cells. Transl Res. 158:214–224. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Midgley AC, Rogers M, Hallett MB, Clayton
A, Bowen T, Phillips AO and Steadman R: Transforming growth
factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast
differentiation is mediated by hyaluronan (HA)-facilitated
epidermal growth factor receptor (EGFR) and CD44 co-localization in
lipid rafts. J Biol Chem. 288:14824–14838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao C, Chen J, Yu B, Wu X, Dai C, Zhou C
and Chen X: Effect of modified taohongsiwu decoction on patients
with chemotherapy-induced hand-foot syndrome. J Tradit Chin Med.
34:10–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yonesaka K, Suzumura T, Tsukuda H,
Hasegawa Y, Ozaki T, Sugiura T and Fukuoka M: Erlotinib is a
well-tolerated alternate treatment for non-small cell lung cancer
in cases of gefitinib-induced hepatotoxicity. Anticancer Res.
34:5211–5215. 2014.PubMed/NCBI
|
|
30
|
Madke B, Gole P, Kumar P and Khopkar U:
Dermatological side effects of epidermal growth factor receptor
inhibitors: 'PRIDE' complex. Indian J Dermatol. 59:271–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Funakoshi Y, Takeuchi Y and Maeda H:
Pneumonectomy after response to gefitinib treatment for lung
adenocarcinoma. Asian Cardiovasc Thorac Ann. 21:482–484. 2013.
View Article : Google Scholar
|
|
32
|
Kuwabara A, Nakade M, Tamai H,
Tsuboyama-Kasaoka N and Tanaka K: The association between vitamin E
intake and hypertension: Results from the re-analysis of the
national health and nutrition survey. J Nutr Sci Vitaminol (Tokyo).
60:239–245. 2014. View Article : Google Scholar
|
|
33
|
Ahmadi A, Mazooji N, Roozbeh J, Mazloom Z
and Hasanzade J: Effect of alpha-lipoic acid and vitamin E
supplementation on oxidative stress, inflammation and malnutrition
in hemodialysis patients. Iran J Kidney Dis. 7:461–467.
2013.PubMed/NCBI
|
|
34
|
Hsieh CL, Chen KC, Lin PX, Peng CC and
Peng RY: Resveratrol and vitamin E rescue valproic acid-induced
teratogenicity: The mechanism of action. Clin Exp Pharmacol
Physiol. 41:210–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen X, Tang Q, Wu J, Feng Y, Huang J and
Cai W: Effect of vitamin E supplementation on oxidative stress in a
rat model of diet-induced obesity. Int J Vitam Nutr Res.
79:255–263. 2009. View Article : Google Scholar
|
|
36
|
Tasanarong A, Kongkham S, Thitiarchakul S
and Eiam-Ong S: Vitamin E ameliorates renal fibrosis in ureteral
obstruction: Role of maintaining BMP-7 during
epithelial-to-mesenchymal transition. J Med Assoc Thai. 94(Suppl
7): S10–S18. 2011.
|
|
37
|
Hamama S, Gilbert-Sirieix M, Vozenin MC
and Delanian S: Radiation-induced enteropathy: Molecular basis of
pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1
cascade inhibition. Radiother Oncol. 105:305–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang QL, Yuan JL, Tao YY, Zhang Y, Liu P
and Liu CH: Fuzheng Huayu recipe and vitamin E reverse renal
interstitial fibrosis through counteracting TGF-beta1-induced
epithelial-to-mesenchymal transition. J Ethnopharmacol.
127:631–640. 2010. View Article : Google Scholar
|
|
39
|
Knebel B, Lehr S, Hartwig S, Haas J, Kaber
G, Dicken HD, Susanto F, Bohne L, Jacob S, Nitzgen U, et al:
Phosphorylation of sterol regulatory element-binding protein
(SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism
and the secretome of human liver cell line HepG2. Arch Physiol
Biochem. 120:216–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He W, Wang Z, Luo Z, Yu Q, Jiang Y, Zhang
Y, Zhou Z, Smith AJ and Cooper PR: LPS promote the odontoblastic
differentiation of human dental pulp stem cells via MAPK signaling
pathway. J Cell Physiol. 230:554–561. 2015. View Article : Google Scholar
|
|
41
|
Wang D, Warner GM, Yin P, Knudsen BE,
Cheng J, Butters KA, Lien KR, Gray CE, Garovic VD, Lerman LO, et
al: Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in
a murine renal artery stenosis model. Am J Physiol Renal Physiol.
304:F938–F947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao HB, Liu RH, Ling GH, Xiao L, Xia YC,
Liu FY, Li J, Liu YH, Chen QK, Lv JL, et al: HSP47 regulates ECM
accumulation in renal proximal tubular cells induced by TGF-beta1
through ERK1/2 and JNK MAPK pathways. Am J Physiol Renal Physiol.
303:F757–F765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yiang GT, Chou PL, Hung YT, Chen JN, Chang
WJ, Yu YL and Wei CW: Vitamin C enhances anticancer activity in
methotrexatetreated Hep3B hepatocellular carcinoma cells. Oncol
Rep. 32:1057–1063. 2014.PubMed/NCBI
|
|
44
|
Yu YL, Yiang GT, Chou PL, Tseng HH, Wu TK,
Hung YT, Lin PS, Lin SY, Liu HC, Chang WJ and Wei CW: Dual role of
acetaminophen in promoting hepatoma cell apoptosis and kidney
fibroblast proliferation. Mol Med Rep. 9:2077–2084. 2014.PubMed/NCBI
|
|
45
|
Nho KJ, Chun JM and Kim HK: Agrimonia
pilosa ethanol extract induces apoptotic cell death in HepG2 cells.
J Ethnopharmacol. 138:358–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu YL, Yu SL, Su KJ, Wei CW, Jian MH, Lin
PC, Tseng IH, Lin CC, Su CC, Chan DC, et al: Extended
O6-methylguanine methyltransferase promoter hypermethylation
following n-butylidenephthalide combined with 1,3-bis
(2-chloroethyl)-1-nitrosourea (BCNU) on inhibition of human
hepatocellular carcinoma cell growth. J Agric Food Chem.
58:1630–1638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei CW, Lin CC, Yu YL, Lin CY, Lin PC, Wu
MT, Chen CJ, Chang W, Lin SZ, Chen YL and Harn HJ:
n-Butylidenephthalide induced apoptosis in the A549 human lung
adenocarcinoma cell line by coupled down-regulation of AP-2alpha
and telomerase activity. Acta Pharmacol Sin. 30:1297–1306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu YL, Su KJ, Chen CJ, Wei CW, Lin CJ,
Yiang GT, Lin SZ, Harn HJ and Chen YL: Synergistic anti-tumor
activity of isochaihulactone and paclitaxel on human lung cancer
cells. J Cell Physiol. 227:213–222. 2012. View Article : Google Scholar
|
|
49
|
Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W
and Wang Y: Parathyroid hormone induces epithelial-to-mesenchymal
transition via the Wnt/β-catenin signaling pathway in human renal
proximal tubular cells. Int J Clin Exp Pathol. 7:5978–5987.
2014.
|
|
50
|
Wei J, Li Z and Yuan F: Evodiamine might
inhibit TGF-beta1-induced epithelial-mesenchymal transition in
NRK52E cells via Smad and PPAR-gamma pathway. Cell Biol Int.
38:875–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun YB, Qu X, Li X, Nikolic-Paterson DJ
and Li J: Endothelial dysfunction exacerbates renal interstitial
fibrosis through enhancing fibroblast Smad3 linker phosphorylation
in the mouse obstructed kidney. PloS One. 8:e840632013. View Article : Google Scholar
|
|
52
|
Qu X, Zhang X, Yao J, Song J,
Nikolic-Paterson DJ and Li J: Resolvins E1 and D1 inhibit
interstitial fibrosis in the obstructed kidney via inhibition of
local fibroblast proliferation. J Pathol. 228:506–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dutton SJ, Ferry DR, Blazeby JM, Abbas H,
Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A,
Falk S, et al: Gefitinib for oesophageal cancer progressing after
chemotherapy (COG): A phase 3, multicentre, double-blind,
placebo-controlled randomised trial. Lancet Oncol. 15:894–904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liao XH, Zhang L, Chen GT, Yan RY, Sun H,
Guo H and Liu Q: Augmenter of liver regeneration inhibits
TGF-β1-induced renal tubular epithelial-to-mesenchymal transition
via suppressing TβR II expression in vitro. Exp Cell Res.
327:287–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lan A, Qi Y and Du J: Akt2 mediates
TGF-β1-induced epithelial to mesenchymal transition by deactivating
GSK3β/snail signaling pathway in renal tubular epithelial cells.
Cell Physiol Biochem. 34:368–382. 2014. View Article : Google Scholar
|
|
56
|
Qi R, Li W and Yu S: FK506 inhibits the
mice glomerular mesangial cells proliferation by affecting the
transforming growth factor-β and Smads signal pathways. Renal Fail.
36:589–592. 2014. View Article : Google Scholar
|
|
57
|
Guo W, Xu H, Chen J, Yang Y, Jin JW, Fu R,
Liu HM, Zha XL, Zhang ZG and Huang WY: Prohibitin suppresses renal
interstitial fibroblasts proliferation and phenotypic change
induced by transforming growth factor-beta1. Mol Cell Biochem.
295:167–177. 2007. View Article : Google Scholar
|
|
58
|
Zhu B, Jin Y, Han L, Chen H, Zhong F, Wang
W and Chen N: Proteasome inhibitor inhibits proliferation and
induces apoptosis in renal interstitial fibroblasts. Pharmacol Rep.
65:1357–1365. 2013. View Article : Google Scholar
|
|
59
|
Rodríguez-Barbero A, Dorado F, Velasco S,
Pandiella A, Banas B and Lopez-Novoa JM: TGF-beta1 induces COX-2
expression and PGE2 synthesis through MAPK and PI3K pathways in
human mesangial cells. Kidney Int. 70:901–909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Loeffler I, Hopfer U, Koczan D and Wolf G:
Type VIII collagen modulates TGF-β1-induced proliferation of
mesangial cells. J Am Soc Nephrol. 22:649–663. 2011. View Article : Google Scholar : PubMed/NCBI
|