Introduction
Leukemia is a hematopoietic malignancy caused by
acquired somatic mutation and ranks among the ten types of
malignant neoplasm with the highest incidence rate. Chronic myeloid
leukemia is a common hematopoietic malignancy, accounting for 20%
of all types of leukemia (1).
Cancer fusion protein p210bcr/abl generated by Ph chromosome has an
important role in these malignant phenotypes, as it has been
demonstrated to induce leukemia-like syndrome in cancerous mice, to
promote tumor-cell proliferation and survival in vitro as
well as to be implicated in the evasion of apoptosis of tumor cells
(2).
Autophagy is a conserved self-degradation system in
eukaryotic cells involved in numerous physiological and
pathological processes. Autophagosomes are a typical feature of
autophagy, and the control of their formation and degradation is
the main regulatory factor in autophagy (3). Autophagy has a dual nature of
promoting cell survival and death, and is also closely associated
with the development, metastasis and drug resistance of cancer
(4). Targeting autophagy may be a
novel strategy to treat cancer and address drug resistance.
In-depth study of autophagy in leukemia will lead to the
elucidation of the induction and regulatory mechanisms of
leukemia-cell autophagy and provide novel targets and strategies
for leukemia treatment and possible cures (5).
The mechanisms of action of the promising
anti-cancer drug candidate alisertib are complex and have remained
to be fully elucidated. As alisertib has been demonstrated to
induce apoptosis (6), the present
study assessed the underlying molecular mechanisms. A recent study
showed that alisertib exerts pro-autophagic effects on human
osteosarcoma cell lines through the activation of a
mitochondria-mediated pathway and inhibition of the p38
mitogen-activated protein kinase (MAPK)/phosphoinositide-3 kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway
(7). To assess whether alisertib
exerts its effects against leukemia cells via similar mechanisms,
its ability to inhibit proliferation, induce autophagy and affect
MAPK/PI3K/Akt/mTOR signaling were assessed in the REH leukemia cell
line.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from Mediatech Inc. (Manassas, VA,
USA). Alisertib (chemical structure shown in Fig. 1) was purchased from Selleckchem
Inc. (Houston, TX, USA). Acid phosphatase assay (AP) and
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI) were obtained from BD Biosciences (San Diego, CA, USA).
All antibodies used for western blot analysis were from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell lines and culture
The REH human acute lymphocytic leukemia cell line
was purchased from Central South University (Hunan, China). REH
cells were cultured in DMEM medium supplemented with 2 mM
l-glutamine and 1% of
antibiotic/antimycotic mixture (Sigma-Aldrich, St. Louis, MO, USA).
Cells were maintained in a humidified atmosphere of 5%
CO2/95% air at 37°C.
Cell viability assay
Cells were seeded into 96-well plates at a density
1.0–2.0×104 cells/well and treated with alisertib (0,
0.1, 1 or 5 µM) for 24 h. For the PA assay, the cells were
subsequently incubated for 2 h at 37°C with 100 ml p-nitrophenyl
phosphate solution (5 mM) and 0.1% Triton X-100. The reaction was
terminated by addition of 10 ml NaOH (1 M) and the number of viable
cells was measured at 405 nm using a Multiskan Spectrum microplate
reader (Thermo Fisher Scientific, Inc.). For the MTT assay, 20
µl MTT was added to the cells, which were then cultured for
an additional 4 h. Following aspiration of the MTT solution,
dimethylsulfoxide (Nanjing Chemical Reagent Co., Ltd., Nanjing,
China) was added to each well and plates were agitated for 20 min.
The absorbance was then measured with Multiskan Spectrum microplate
reader at 490 nm.
Apoptosis assay
Cells were seeded into six-well plates at a density
1.0–2.0×106 cells/well and treated with alisertib (0,
0.1, 1 and 5 µM) for 24 h. Following harvesting, cells were
washed twice with ice-cold phosphate-buffered saline (PBS),
re-suspended in binding buffer and then incubated with 10 µl
Annexin V-FITC and 5 µl PI in the dark for 30 min. The
samples were then analyzed by flow cytometry (FACSAria III; BD
Biosciences).
Immunofluorescence microscopy
Cells were seeded into six-well plates at a density
1.0–2.0×106 cells/well and treated with alisertib (0,
0.1, 1 and 5 µM) for 24 h. Cells were then fixed with 4%
paraformaldehyde (Solarbio Science and Technology, Ltd., Beijing,
China) for 30 min at room temperature and permeabilized using
pre-cooled methanol on ice for 10 min. Binding sites were then
blocked with 5% normal goat serum (Thermo Fisher Scientific, Inc.)
and 0.3% Triton X-100 (Thermo Fisher Scientific, Inc.) for 1 h.
Cells were then incubated with mouse monoclonal anti-light chain
(LC)3B (cat. no. sc-376404; 1:300; Santa Cruz Biotechnology, Inc.)
followed by 1 h of incubation with Alexa Fluor 488 goat anti-mouse
immunoglobulin G (cat. no. 8878; 1:1,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA) as a secondary antibody. After
incubation, cells were washed with ice-cold PBS, mounted onto
microscopic slides with Flouromount-G (Southern Biotech,
Birmingham, AL, USA) and observed using a confocal laser scanning
microscope (TCS SP5; Leica Microsystems GmbH, Wetzlar,
Germany).
Western blot analysis
Cells was seeded into six-well plates at a density
1.0–2.0×106 cells/well and treated with alisertib (0,
0.1, 1 and 5 µM) for 24 h. Cells were then lysed with
ice-cold lysis buffer (1 mM phenylmethylsulfonylfluoride, 30 mM
Tris-HCl pH 8.0, 150 mM NaCl, protease/phosphatase inhibitor
cocktail and 1% nonidet P-40) for 30 min. The lysate was then
centrifuged at 12,000 × g for 10 min at 4°C. Equal amounts of
protein (40–50 µg) were separated by sodium dodecyl
sulfate-polyacrylamide gel (Sangon Biotech Co., Ltd., Shanghai,
China) electrophoresis and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Following incubation with mouse monoclonal anti-phosphorylated
(p)-mTOR (cat. no. sc-293132; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc.), mouse monoclonal p-Akt (cat. no. sc-293125;
1:1,000 dilution; Santa Cruz Biotechnology, Inc.), rabbit
polyclonal anti-p-adenosine monophosphate-activated kinase (AMPK)
(cat. no. 4186; 1:2,000 dilution; Cell Signaling Technology, Inc.)
and rabbit polyclonal anti-β-actin (cat. no. R1207-1; 1:800
dilution; HangZhou HuaAn Biotechnology Co., Ltd., Hangzhou, China).
Subsequently, membranes were incubated with goat anti-rabbit (cat.
no. A0208) and goat anti-mouse (cat. no. A0216) horseradish
peroxidase-conjugated secondary antibodies from Beyotime Institute
of Biotechnology (Haimen, China) at 1:5,000 dilution for 2 h at
room temperature. Proteins were visualized using an enhanced
chemiluminescence western blot detection system (EMD Millipore) and
visualization was performed using Image J (version 1.31; National
Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Values are expressed as the mean ± standard error.
Statistical significance of the differences was analyzed by one-way
analysis of variance followed by the Student-Newman-Keuls test. All
statistical analysis was performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). All experiments were performed three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Alisertib reduces the viability of REH
cells
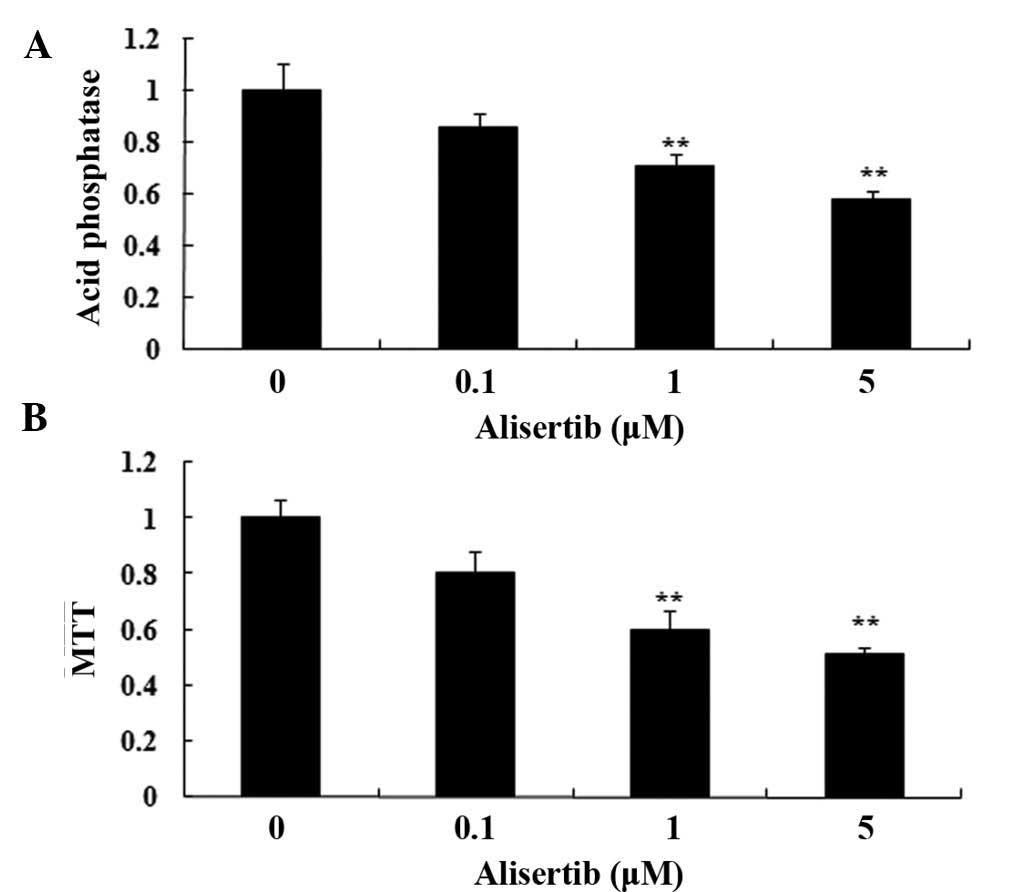
The present study assessed the effects of alisertib
on viability of REH cells using AP and MTT assays. Incubation of
REH cells with alisertib at 1 and 5 µM for 24 h
significantly decreased the cell viability (Fig. 2). These results showed that
alisertib potently decreases the viability of REH cells.
Alisertib induces apoptotic death of REH
cells
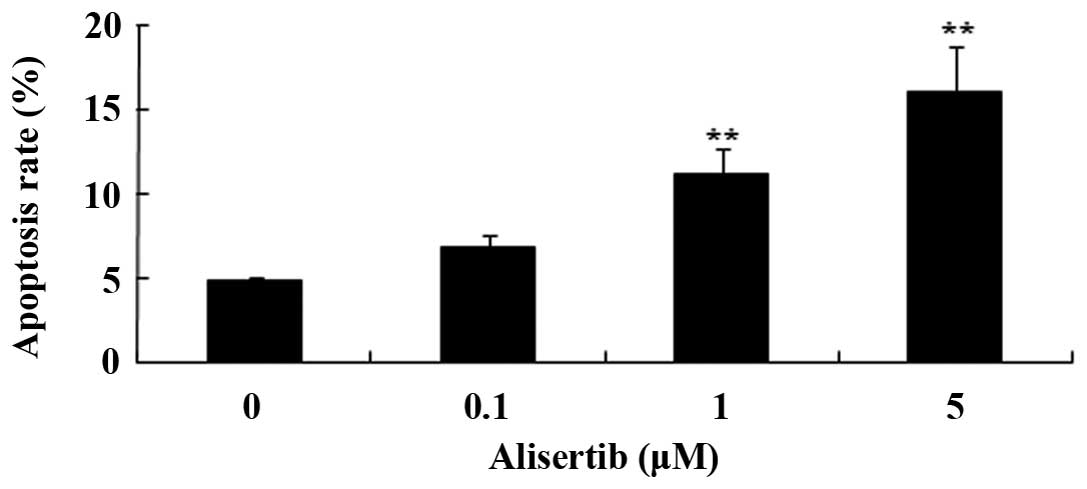
Next, the present study explored the effects of
alisertib on apoptosis of REH cells using flow cytometry following
Annexin V-FITC and PI staining. As shown in Fig. 3, treatment with alisertib at 1
µM for 24 h caused a two-fold increase of the apoptotic rate
of REH cells, while 5 µM alisertib increased the apoptotic
rate by three-fold. These results demonstrated that alisertib
exerts its anti-cancer effects on REH cells by inducing
apoptosis.
Alisertib induces autophagy in REH
cells
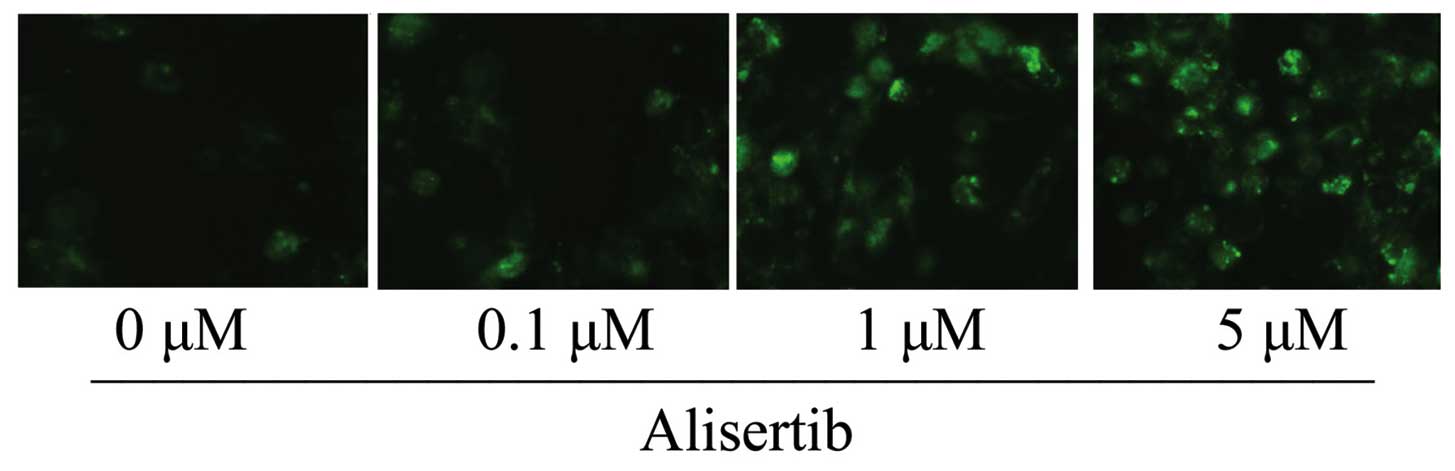
Since a previous study reported on the ability of
alisertib to induce authophagy in osteosarcoma cells (7), the present study assessed whether
alisertib induces autophagy in REH leukemia cells. As demonstrated
by immunofluorescence microscopy, incubation of REH cells with
alisertib at 1 and 5 µM for 24 h resulted in marked
expression of the autophagic marker LC3B (Fig. 4). These results indicated that
alisertib may exert its anti-cancer effects via inducing autophagy
of REH cells.
The mechanism of action of alisertib on
leukemia cells may be mediated via deactivation of the
MAPK/PI3K/Akt/mTOR pathway and activation of AMPK
To elucidate the possible molecular mechanisms via
which alisertib induces apoptosis and autophagy in leukemic cells,
its effects on the levels of p-mTOR, p-Akt, p-AMPK and p-p38 were
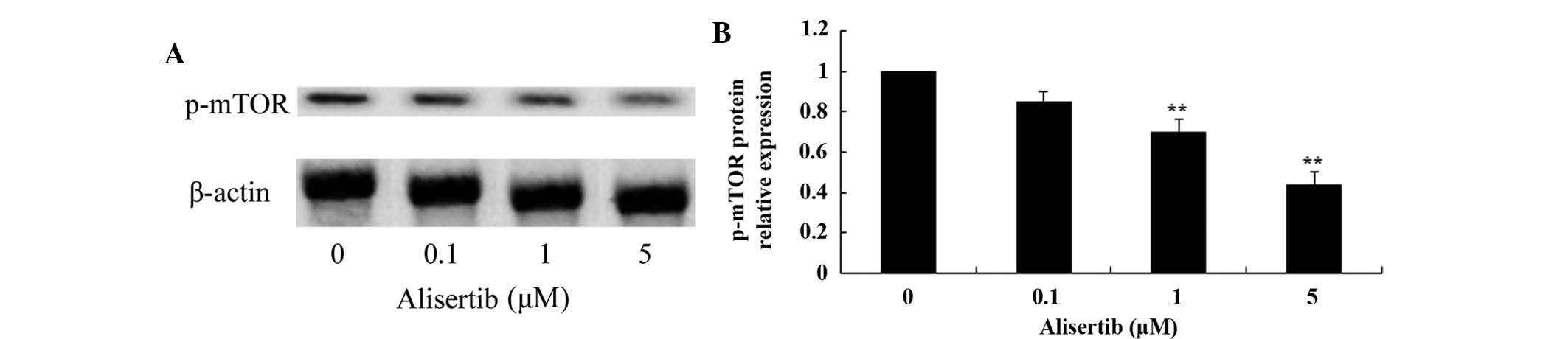
assessed by western blot analysis. As shown in Fig. 5, treatment with 1 and 5 µM
alisertib significantly reduced the levels of p-mTOR protein in REH
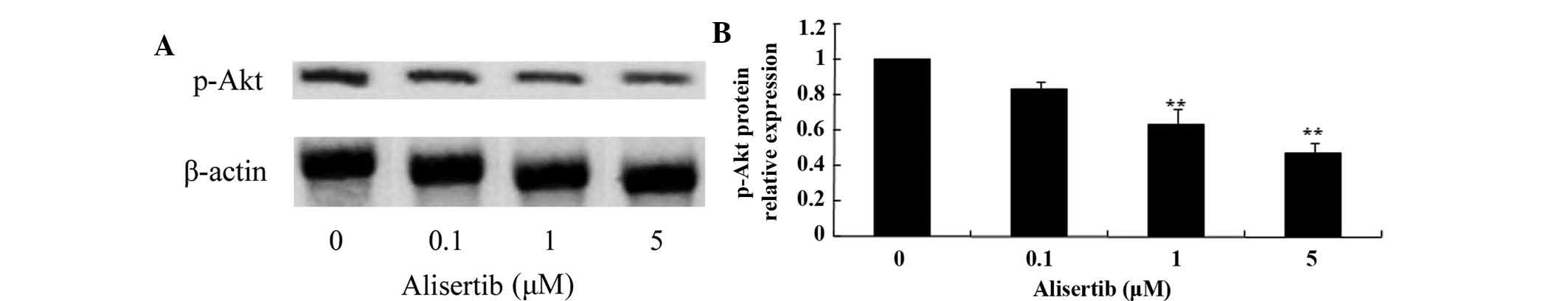
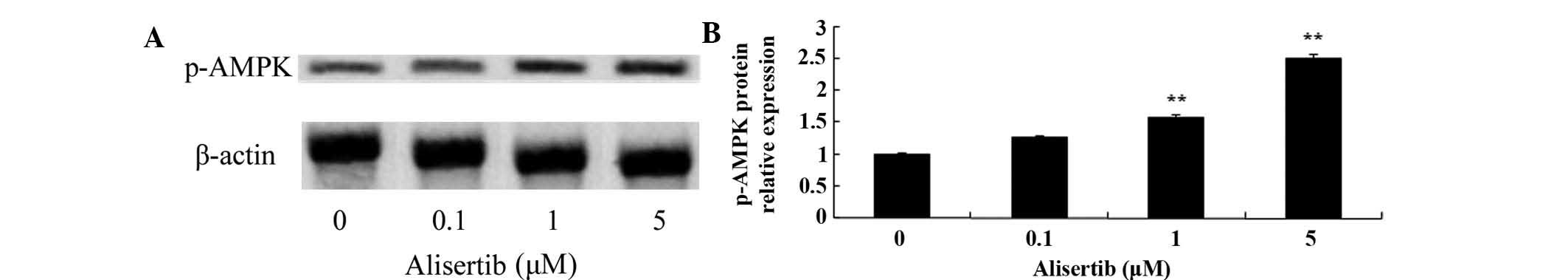
cells; furthermore, the levels of p-Akt were reduced (Fig. 6), the levels of p-AMPK were
increased (Fig. 7) and the levels
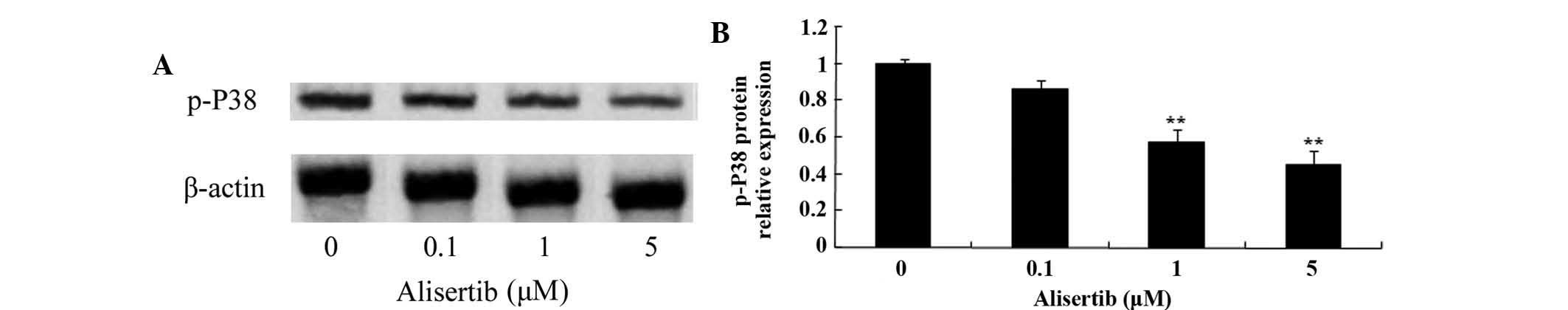
of p-p38 (Fig. 8) were decreased
(P<0.01 for all). These results suggested that deactivation of
MAPK/PI3K/Akt/mTOR signaling and activation of AMPK may be involved
in the mechanism of action of alisertib in leukemia cells.
Discussion
Studies have indicated a significant link between
autophagy and neoplastic transformations of blood cells. Autophagy
facilitates the removal of the mitochondria in red blood cells,
which has an important regulatory role in early differentiation and
maturation of red blood cells as well as in the development of
myelodysplastic syndrome (8). In
addition, autophagy may be the main cause of megakaryocyte
dysfunction in myelodysplastic syndrome and idiopathic
thrombocytopenic purpura (9). A
variety of bioactive compounds and irradiation are known to induce
autophagic death of leukemia cells. The results of the present
study indicated that treatment with alisertib significantly reduced
the viability of REH cells and promoted apoptotic cell death in a
dose-dependent manner. Qi et al (10) indicated that alisertib inhibits the
proliferation and induces apoptosis of T-Hodgkin lymphoma cells.
Melichar et al (11)
revealed that alisertib suppressed the grow of small-cell lung
cancer, non-small-cell lung cancer, gastro-oesophageal
adenocarcinoma, breast cancer and head and neck squamous-cell
carcinoma. Furthermore, the results of the present study indicated
that alisertib possesses activity against leukemia cells.
Autophagy is an important intracellular
self-degradation mechanism, during which macromolecules and
organelles within the cell are transported to the lysosomes for
digestion and degradation by double-membrane vesicles, releasing
free small molecules for recycling in the cell (12). Almost all types of eukaryotic cell
have the capacity to undergo autophagy (13). It is known that this mechanism has
an important role in cell physiological processes, including cell
development, differentiation, senescence and death, while the
specific mechanisms have remained to be fully elucidated. Autophagy
can be divided into macro-autophagy, micro-autophagy and
chaperone-mediated autophagy (14). In contrast to the ubiquitin protein
degradation system, autophagy can avert pathogenic attacks as well
as cell damage caused by physical and chemical factors, apart from
acting as a energy retrieval by self digestion (15). Autophagy also acts in a power
switch-like manner to change the threshold level of the regulation
of cell survival in response to apoptotic stimuli. In the embryonic
development period as well as in adult organisms, excessive levels
of autophagy lead to autophagic death (16). The findings of the present study
showed that alisertib dose-dependently induced autophagy in REH
cells. Wang et al (17)
reported that alisertib induces cell cycle arrest and autophagy of
human pancreatic cancer cells through PI3K/Akt/mTOR. Furthermore,
Ding et al (18) also
reported that alisertib induced apoptosis and autophagy of human
epithelial ovarian cancer cells. These results suggested that
induction of autophagy represents a major mechanism of action of
alisertib, which is a desired mechanism of cancer treatments.
mTOR is an important factor regulating cell growth
and proliferation, and influences processes including
nutrition-associated molecules in signal transduction, regulation
of protein translation and cell cycle progression; furthermore,
activated mTOR is an inhibitor of autophagy (19). The initiation of autophagy is
mainly inhibited by molecules targeting mTOR, including TOR complex
1 (TORC1) and type I PI3K (20).
Signaling of growth factors within the cell and information
regarding the nutrient status and energy levels are conveyed to
TORC1 through Type I PI3K and Akt/protein kinase B pathways,
resulting in the activation of TORC1 at an appropriate level,
thereby activating mTOR to inhibit autophagy (21). Therefore, the activity of mTOR is
influenced by the activity of TORC1 activity to regulate the level
of autophagy (22). The results of
the present study revealed that alisertib decreased the
phosphorylation/activation of mTOR as well as that of Akt and p38,
thereby inducing autophagy in REH cells, which was in line with the
findings of a previous study (17). Furthermore, alisertib significantly
activated AMPK in REH cells. Yuan et al (23) indicated that alisertib induces
apoptosis and autophagy via activation of AMPK and suppression of
p38 MAPK in human gastric cancer. These results indicated that
alisertib induces autophagy in REH cells and other cancer cell
types via activation of AMPK and suppression of the PI3K/Akt/mTOR
pathway.
In conclusion, the present study reported that
alisertib exerted marked anti-cancer effects against the REH
leukemia cell line by suppressing cell grow, promoting apoptosis
and inducing autophagy. The underlying molecular mechanism of the
autophagy-inducing effects of alisertib in REH cells were indicated
to include the deactivation of the PI3K/Akt/mTOR pathway and
activation of AMPK.
References
|
1
|
Yi B, Zhang M, Schwartz-Albiez R and Cao
Y: Mechanisms of the apoptosis induced by CD176 antibody in human
leukemic cells. Int J Oncol. 38:1565–1573. 2011.PubMed/NCBI
|
|
2
|
Manzotti G, Mariani SA, Corradini F,
Bussolari R, Cesi V, Vergalli J, Ferrari-Amorotti G, Fragliasso V,
Soliera AR, Cattelani S, et al: Expression of p89 (c-Mybex9b), an
alternatively spliced form of c-Myb, is required for proliferation
and survival of p210BCR/ABL-expressing cells. Blood Cancer J.
2:e712012. View Article : Google Scholar
|
|
3
|
Thapalia BA, Zhou Z and Lin X: Autophagy,
a process within reperfusion injury: An update. Int J Clin Exp
Pathol. 7:8322–8341. 2014.
|
|
4
|
Huang Z, Ye B, Dai Z, Wu X, Lu Z, Shan P
and Huang W: Curcumin inhibits autophagy and apoptosis in
hypoxia/reoxygenation-induced myocytes. Mol Med Rep. 11:4678–4684.
2015.PubMed/NCBI
|
|
5
|
Su X, Wang X, Liu Q, Wang P, Xu C and
Leung AW: The role of Beclin 1 in SDT-induced apoptosis and
autophagy in human leukemia cells. Int J Radiat Biol. 91:472–479.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manfredi MG, Ecsedy JA, Chakravarty A,
Silverman L, Zhang M, Hoar KM, Stroud SG, Chen W, Shinde V, Huck
JJ, et al: Characterization of Alisertib (MLN8237), an
investigational small-molecule inhibitor of aurora A kinase using
novel in vivo pharmacodynamic assays. Clin Cancer Res.
17:7614–7624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH,
He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, et al: Pro-apoptotic and
pro-autophagic effects of the Aurora kinase A inhibitor alisertib
(MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the
activation of mitochondria-mediated pathway and inhibition of p38
MAPK/PI3 K/Akt/mTOR signaling pathway. Drug Des Devel Ther.
9:1555–1584. 2015.
|
|
8
|
Bunaciu RP, Tang T and Mao CD:
Differential expression of Wnt13 isoforms during leukemic cell
differentiation. Oncol Rep. 20:195–201. 2008.PubMed/NCBI
|
|
9
|
Wang Y, Lin Z, Huang H, He H, Yang L, Chen
T, Yang T, Ren N, Jiang Y, Xu W, et al: AMPK is required for
PM2.5-induced autophagy in human lung epithelial A549 cells. Int J
Clin Exp Med. 8:58–72. 2015.PubMed/NCBI
|
|
10
|
Qi W, Spier C, Liu X, Agarwal A, Cooke LS,
Persky DO, Chen D, Miller TP and Mahadevan D: Alisertib (MLN8237)
an investigational agent suppresses Aurora A and B activity,
inhibits proliferation, promotes endo-reduplication and induces
apoptosis in T-NHL cell lines supporting its importance in PTCL
treatment. Leuk Res. 37:434–439. 2013. View Article : Google Scholar
|
|
11
|
Melichar B, Adenis A, Lockhart AC,
Bennouna J, Dees C, Kayaleh O, Obermannova R, DeMichele A,
Zatloukal P, Zhang B, et al: Safety and activity of alisertib, an
investigational aurora kinase A inhibitor, in patients with breast
cancer, small-cell lung cancer, non-small-cell lung cancer, head
and neck squamous-cell carcinoma, and gastro-oesophageal
adenocarcinoma: A five-arm phase 2 study. Lancet Oncol. 16:395–405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wu K, Xiao X, Liao J, Hu Q, Chen
H, Liu J and An X: Autophagy as a regulatory component of
erythropoiesis. Int J Mol Sci. 16:4083–4094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moon HS, Kim B, Gwak H, Suh DH and Song
YS: Autophagy and protein kinase RNA-like endoplasmic reticulum
kinase (PERK)/eukaryotic initiation factor 2 alpha kinase (eIF2α)
pathway protect ovarian cancer cells from metformin-induced
apoptosis. Mol Carcinog. Feb 7–2015.Epub ahead of print.
|
|
14
|
Condello M, Caraglia M, Castellano M,
Arancia G and Meschini S: Structural and functional alterations of
cellular components as revealed by electron microscopy. Microsc Res
Tech. 76:1057–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krampe S and Boles E: Starvation-induced
degradation of yeast hexose transporter Hxt7p is dependent on
endocytosis, autophagy and the terminal sequences of the permease.
FEBS Lett. 513:193–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamark T and Johansen T: Autophagy: Links
with the proteasome. Curr Opin Cell Biol. 22:192–198. 2010.
View Article : Google Scholar
|
|
17
|
Wang F, Li H, Yan XG, Zhou ZW, Yi ZG, He
ZX, Pan ST, Yang YX, Wang ZZ, Zhang X, et al: Alisertib induces
cell cycle arrest and autophagy and suppresses
epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and
sirtuin 1-mediated signaling pathways in human pancreatic cancer
cells. Drug Des Devel Ther. 9:575–601. 2015.PubMed/NCBI
|
|
18
|
Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST,
He ZX, Edelman JL, Wang D, Yang YX, Zhang X, et al: Alisertib, an
Aurora kinase A inhibitor, induces apoptosis and autophagy but
inhibits epithelial to mesenchymal transition in human epithelial
ovarian cancer cells. Drug Des Devel Ther. 9:425–464.
2015.PubMed/NCBI
|
|
19
|
Jung CH, Kim H, Ahn J, Jung SK, Um MY, Son
KH, Kim TW and Ha TY: Anthricin Isolated from Anthriscus sylvestris
(L.) Hoffm. Inhibits the growth of breast cancer cells by
inhibiting Akt/mTOR signaling and its apoptotic effects are
enhanced by autophagy inhibition. Evid Based Complement Alternat
Med. 2013:3852192013. View Article : Google Scholar
|
|
20
|
Badura S, Tesanovic T, Pfeifer H, Wystub
S, Nijmeijer BA, Liebermann M, Falkenburg JH, Ruthardt M and
Ottmann OG: Differential effects of selective inhibitors targeting
the PI3K/AKT/mTOR pathway in acute lymphoblastic leukemia. PLoS
One. 8:e800702013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vakana E, Sassano A and Platanias LC:
Induction of autophagy by dual mTORC1-mTORC2 inhibition in
BCR-ABL-expressing leukemic cells. Autophagy. 6:966–967. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X,
Wang D, Yang T, Wang NJ, Zhao RJ and Zhou SF: Inhibition of mitotic
Aurora kinase A by alisertib induces apoptosis and autophagy of
human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther.
9:487–508. 2015.PubMed/NCBI
|