Introduction
Telocytes (TCs) are a type of interstitial cells
first identified by Popescu's group in 2005 and officially named in
2010. They exist in many tissues and organs, including those of
humans and other vertebrates (1–3). TCs
are characterized by a small cell body and long, slender processes
called telopodes (Tps). As a distinctive feature, Tps are extremely
long with thin prolongations, with alternation of dilations
(podoms) and thin segments (podomeres) (4–7).
In the heart, TCs have been observed in the
myocardium, epicardium, endocardium, valves and cardiac stem cell
niches (3–5,8–10).
Based on different junction types, TCs are documented to be in
close contact with virtually all types of cells in the human heart,
such as cardiac stem cells, cardiomyocyte progenitors, blood
capillaries, nerve endings and other cells found in the
interstitial space (9,11). They form the interstitial cell
network and contribute to the regulation of homeostasis, and
renovation and regeneration in the heart (12). Additionally, TCs have been
demonstrated to be important interstitial cells to guide or nurse
putative stem cells and progenitor cells in stem cell niches in a
number of tissues and organs (1,13).
Thus, TCs may serve as a new target for regenerative medicine.
Previous studies have indicated that the number of TCs reduced and
the interstitial cell network was impaired during myocardial
infarction (14,15). However, after the transplantation
of cardiac TCs in infarcted and border zones of the heart, the
infarction size decreased and myocardial function was improved
(14,15). In addition, TCs have been found in
the atrial appendages and shown to be involved in isolated atrial
amyloidosis and the pathogenesis of atrial fibrillation (9).
Although increasing numbers of studies on TCs have
emerged, the majority are limited to the investigation of their
histology and morphology. Due to numerous difficulties in cell
separation, purification and cultivation, research on their
function and the mechanisms behind their biological effects
progressing slowly. Therefore, the present study ultilized a novel
method for the separation and purification of TCs. In addition to
the distinctive morphology of TCs, their molecular markers have
additionally been identified to some extent. Although TCs have no
clear immunophenotypic markers, a number of studies have reported
that TCs are capable of binding different antibodies (16,17).
Based on several reports, it has been suggested that
double-positive immunostaining with cluster of differentiation
(CD)34/platelet-derived growth factor receptor α (PDGFRα) and
CD34/vimentin is appropriate for identifying TCs (16). Based on this approach, the present
study sorted CD34/PDGFRα double positive cells by flow cytometry to
purify TCs, and then confirmed their identity by immunofluorescence
with anti-vimentin antibodies and electron microscopy. Establishing
a successful protocol will enable the large-scale isolation and
culture of TCs.
Materials and methods
Isolation and culture of TCs from heart
tissues
This study was approved by the ethics committee of
Xinhua Hospital Affiliated to Shanghai Jiaotong University School
of Medicine (Shanghai, China; approval no. XHEC-F-2016-012).
Six-week-old female Sprague Dawley (150–200 g) rats (Shanghai
Songlian Laboratory Animal Farms, Production license:
SCXK2007-0011) were anesthetised with 3% pentobarbital sodium. The
hearts were removed under sterile conditions and placed in 50 ml
centrifuge tube with ice-cold Dulbecco's phosphate-buffered saline
(D-PBS) supplemented with 1% penicillin and streptomycin (PS).
Following rinsing with fresh D-PBS to remove blood, the hearts were
minced into millimeter-sized pieces in a sterile culture dish
containing Dulbecco's modfied Eagle's medium (DMEM)/F12 (12400-024,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 1% PS. The pieces were washed twice using a short
centrifugation at 250 × g for 1 min at room temperature and
resuspended in D-PBS to remove the blood. Subsequently, an
enzymatic digestion medium was added and the mixture was incubated
at 37°C on a shaker at 180 rpm for 40 min. The enzymatic digestion
medium was a mixture of 1.5 mg/ml collagenase type 2 (V900892;
Sigma-Aldrich, St. Louis, MO, USA), DMEM/F12 and 1% PS. The
solution was filtered through a 41 µm nylon mesh (EMD
Millipore, Billerica, MA, USA) and the collected cell suspension
was centrifuged at 300 × g for 10 min. The cells were then seeded
into sterile culture dishes containing 10 ml DMEM/F12 supplemented
with 10% fetal bovine serum (FBS; 16000-044; Gibco; Thermo Fisher
Scientific, Inc.) and 1% PS and cultured in a humidified atmosphere
at 37°C for 1 h to allow fibroblast attachment. The unattached
cells (containing TCs) were replated onto a new dish with the above
medium, and the medium was replaced every 2 days thereafter. Cell
cultures were examined using an inverted microscope and
photographed at 72 and 96 h after seeding. The cells were detached
by digestion in 0.25% trypsin/EDTA (Invitrogen; Thermo Fisher
Scientific, Inc.) for 1-2 min after they had grown to 80%
confluence, and then reseeded at a split ratio of 1:3 under the
same conditions.
Flow cytometric analysis and sorting of
isolated cardiac TCs
TC content in the enriched cultures was assessed by
double labeling with phycoerythrin-conjugated monoclonal anti-CD34
(ab187284; Abcam, Cambridge, MA, USA) and rabbit polyclonal
anti-PDGFRα (sc-338; Santa Cruz Biotechnology, Dallas, TX, USA).
The TCs were analyzed using a BD FACS CantoII cytometer (BD
Biosciences, San Jose, CA, USA). Passage-2 cells were washed with
D-PBS and harvested by trypsinization. The samples were stained
with the above antibodies for 1 h at room temperature. Cells were
then incubated with donkey anti-rabbit H&L-labeled secondary
antibodies (ab150075; Abcam) for 30 min at room temperature. As a
negative control, unstained cell aliquots were incubated with D-PBS
under the same conditions. According to the results of the
identification, the flow cytometry was adjusted to set the gate and
CD34+/PDGFRα+ cells were sorted to purify the
cardiac TCs. The sorted cells were collected in a collecting tube,
which was prefilled with complete culture medium and 2% PS, and
then centrifuged at 300 × g for 5 min. The cell density was
adjusted to 1×105/ml with DMEM/F12 medium containing 10%
FBS and 1% PS, following which the cells were seeded into sterile
culture dishes and cultured in a humidified atmosphere of 5%
CO2 at 37°C.
Immuofluorescent staining
As an independent confirmation and to visualize the
appearance of sorted TCs, immunofluorescent staining was conducted
on cells grown on coverslips. The sorted cells were fixed in 4%
formaldehyde for 15 min, washed three times in D-PBS and then
incubated in 5% bovine serum albumin for a further 20 min.
Subsequently, cells were incubated with rabbit monoclonal
anti-vimentin antibodies (ab92547; Abcam) at 4°C overnight in the
dark. After washing with PBS three times, cells were incubated with
Alexa Fluor 594 labeled anti-rabbit secondary antibodies (8889;
Cell Signaling Technology, Inc., Danvers, MA, USA) at 37°C for 1 h,
and then stained with 4′,6-diamidino-2-phenylindole (DAPI; P36935;
Thermo Fisher Scientific, Inc.). Finally, the immunolabeled samples
were observed and imaged using an Olympus IX83 fluorescence
inverted microscope (Olympus Corporation, Tokyo, Japan).
Transmission electron microscopy
(TEM)
Cell samples were processed for TEM according to
routine procedures as previously described (4,9). In
brief, cell samples were fixed with 2% glutaraldehyde in PBS for 2
h at 4°C. Following two rinses in PBS for 10 min, the samples were
postfixed in 1% phosphate-buffered osmium for 2 h at 4°C.
Subsequently, the samples were dehydrated in an increasing ethanol
series (30, 50, 70, 80, 95 and 100%), cleared in propylene oxide
and embedded in araldite. Ultrathin sections (50–100 nm) sections
were obtained and stained with electronic lead citrate. The
sections were examined with a Morgagni 286 transmission electron
microscope (FEI Company, Eindhoven, Netherlands). Digital electron
micrographs were recorded with a MegaView III charge-coupled device
using iTEM SIS software (Olympus Soft Imaging Systems, Münster,
Germany).
Statistical analysis
Data were analysed using SPSS software, version 19
(IBM SPSS, Armonk, NY, USA) and Student's two-tailed t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Culture and purification of TCs from
heart tissue
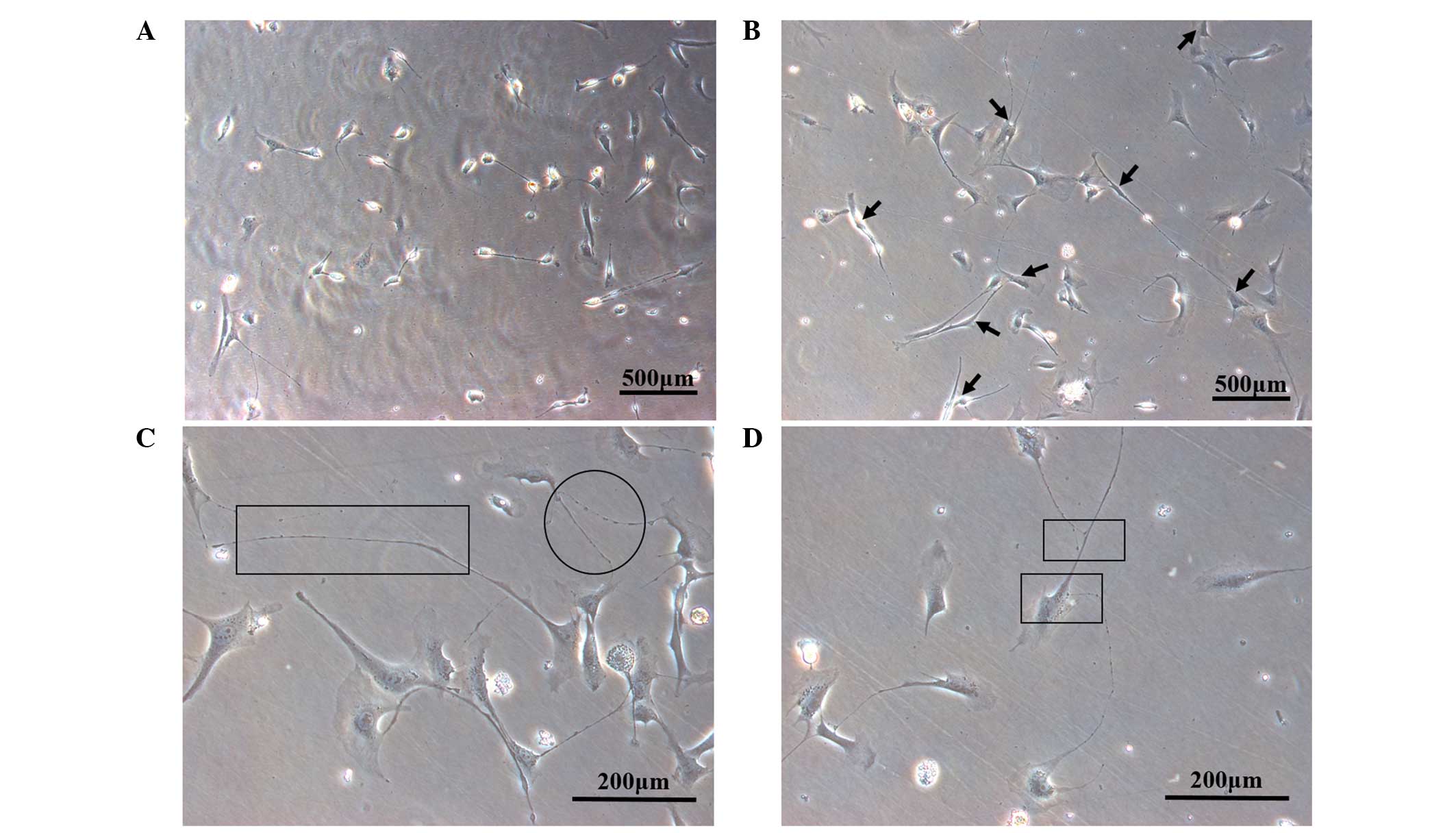
Following 72 h of primary culture, single adherent
cells were observed in a distinct area of the culture dish. These
cells were rhombic or irregular in shape, highly refractive,
inconsistent in size and shape, clear at boundaries, but with
obvious characteristic projections (Fig. 1A). After 96 h of culture, a cell
monolayer was observed, with TCs exhibiting the characteristic
outline of a small cell body and long moniliform Tps. Additionally,
connections between the cells by Tps were observed, with these
interconnected in the form of a network (Fig. 1B). TCs are characterized by a small
cell body and the presence of Tps. As a distinct feature, Tps have
extremely long and thin prolongations, with alternation of podoms
and podomeres (Fig. 1C and D).
After 7 days, the cells grew to 80% confluence in culture.
Following the initial culture of cardiac primary cells, adherent
cells were digested and then stained for the markers CD34 and
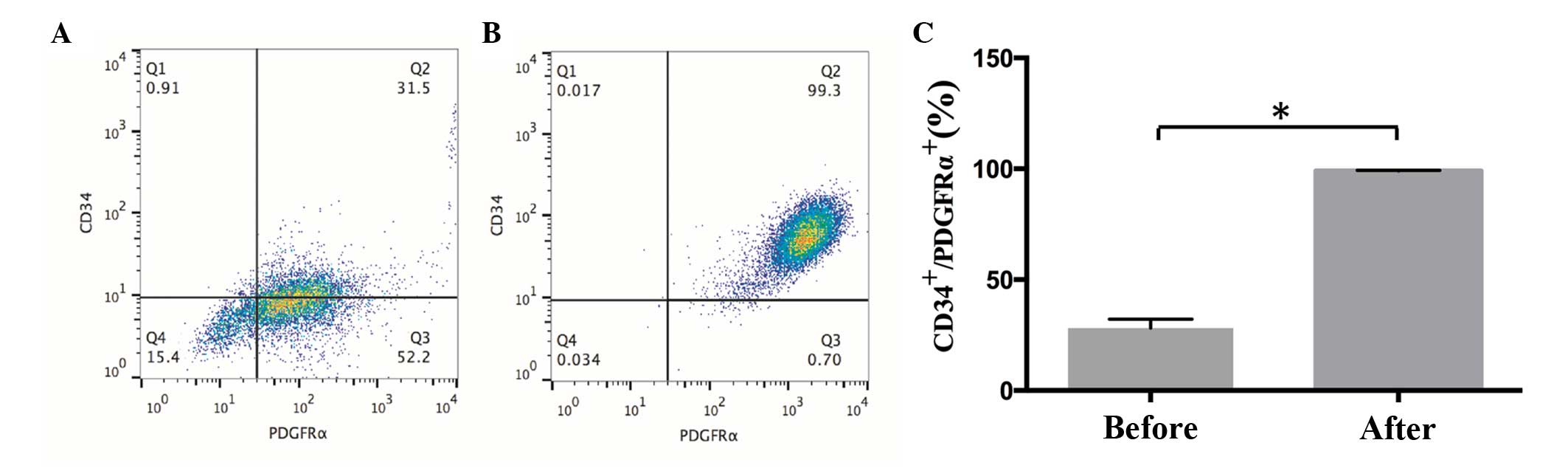
PDGFRα, with positive expression identified by flow cytometry
(Fig. 2). The proportions of cells
were as follows: CD34−/PDGFRα−, 15.4%;
CD34+/PDGFRα−, 0.91%; CD34−/PDGFRα+, 52.2%; and
CD34+/PDGFRα+, 31.5% (Fig. 2A). Following cell sorting, the
proportion of CD34+/PDGFRα+ cells reached
99.3% (Fig. 2B). The sorted cells
were plated and cultured under normoxic conditions.
Immunofluorescence
In order to further analyze and characterize the
sorted CD34+/PDGFRα+ cells, we examined them
for immunofluorescence following incubation with anti-vimentin
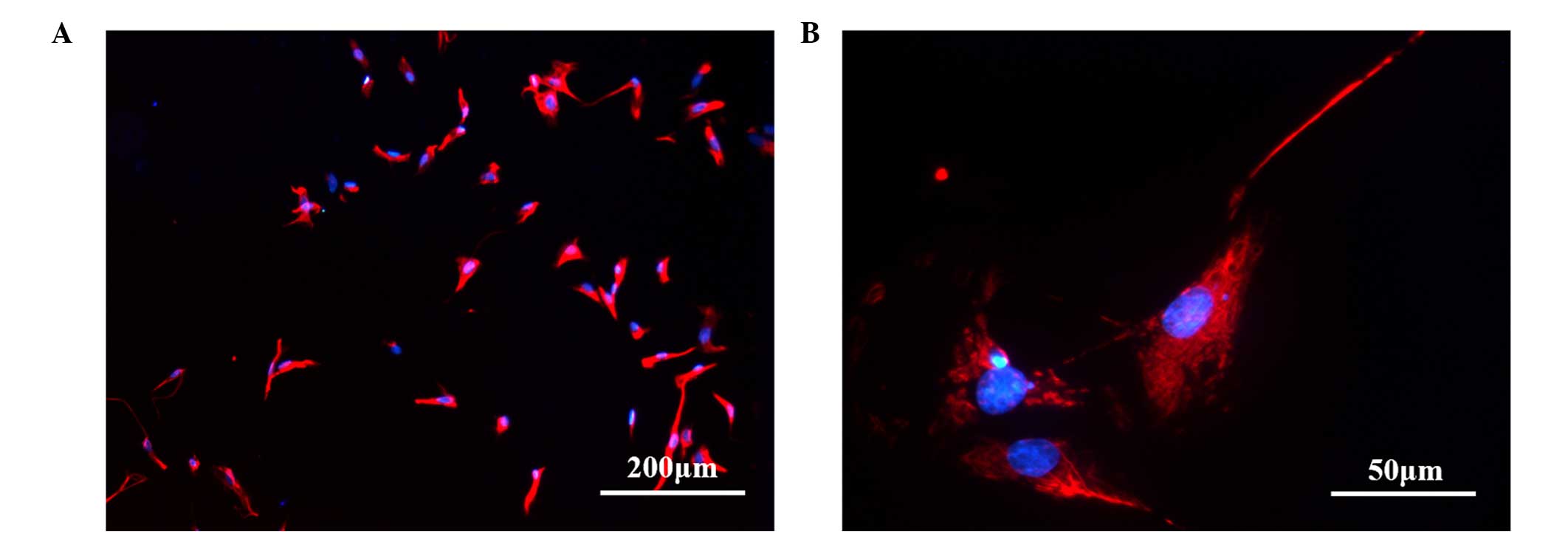
antibodies. Detection of immunofluorescence in the sorted cells
demonstrated the presence of TCs as vimentin-positive cells,
corresponding to the phenotype described previously by others
either in situ (18,19)
or in vitro (20).
The percentage of vimentin-positive cells was
quantified and the mean was determined from 5 randomly selected
magnification, ×200 fields. The results showed that 96.7% of the
sorted TCs were vimentin positive. The cells showed red
immunofluorescence, indicating that they had been labeled with
antibody and thus expressed vimentin and the nucleus was stained
blue with DAPI (Fig. 3). Flow
cytometric sorting and immunofluorescence of the isolated cells
revealed positive expression of CD34, PDGFRα and vimentin, which
are markers for TCs.
Transmission electron microscopy
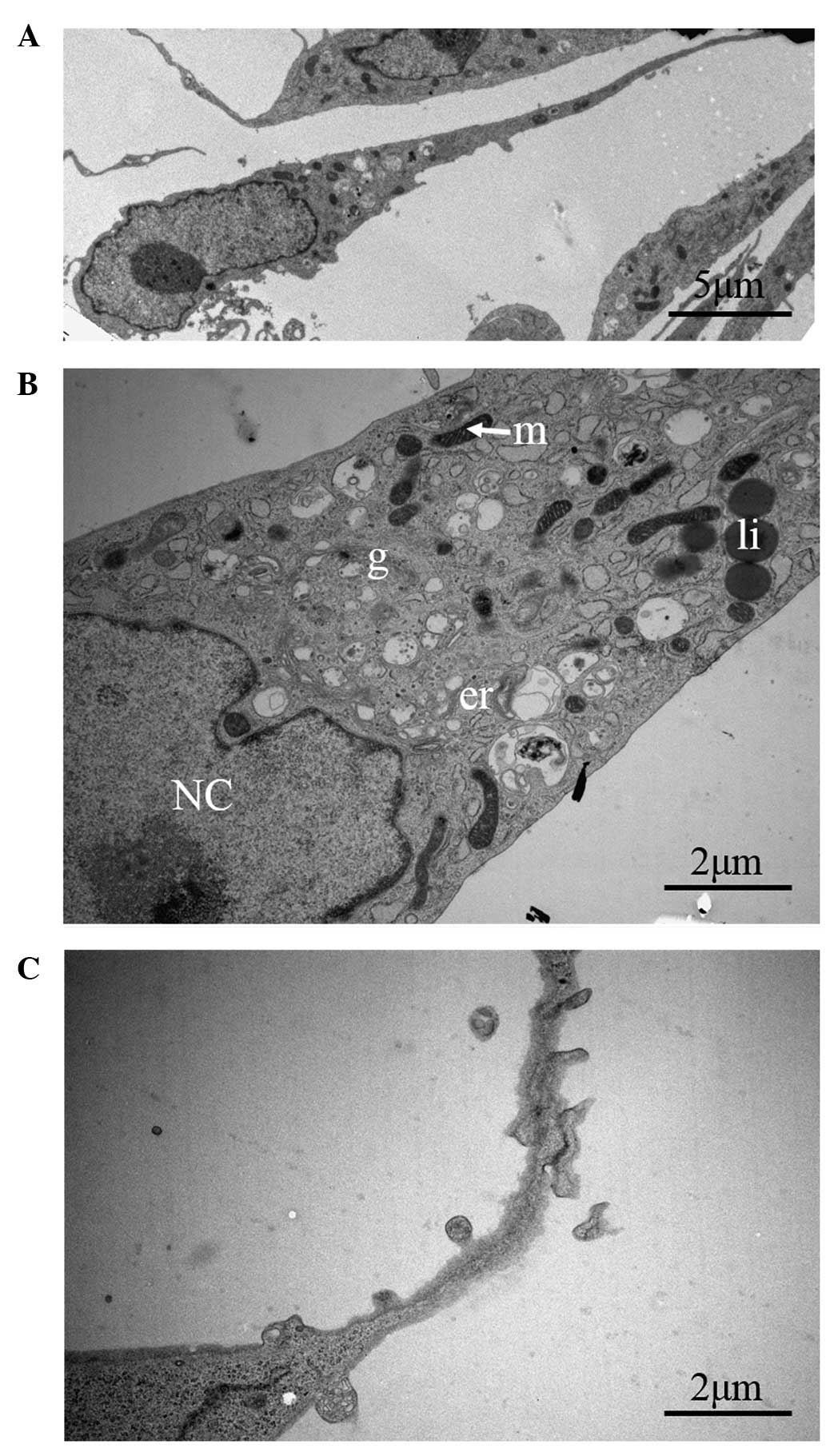
Using electron microscopy, we noted that TCs had a
small oval body, mostly occupied by the nucleus and encircled by a
small amount of cytoplasm (Fig.
4A). Additionally, we observed that the cytoplasm was filled
with mitochondria, lipid droplets, a small Golgi apparatus, in
addition to elements of smooth and rough endoplasmic reticulum
(Fig. 4B). Furthermore, higher
magnification images of Tps were obtained (Fig. 4C). As one of the most striking
features of TCs, the connections were organized through
homocellular junctions. Figure 5A
shows that tight contacts (atypical junctions) occurred between Tps
and the TC cell body. Apart from the homocellular junctions, TCs
have been demonstrated to serve an important role in cellular
communication in the heart by releasing extracellular vesicles
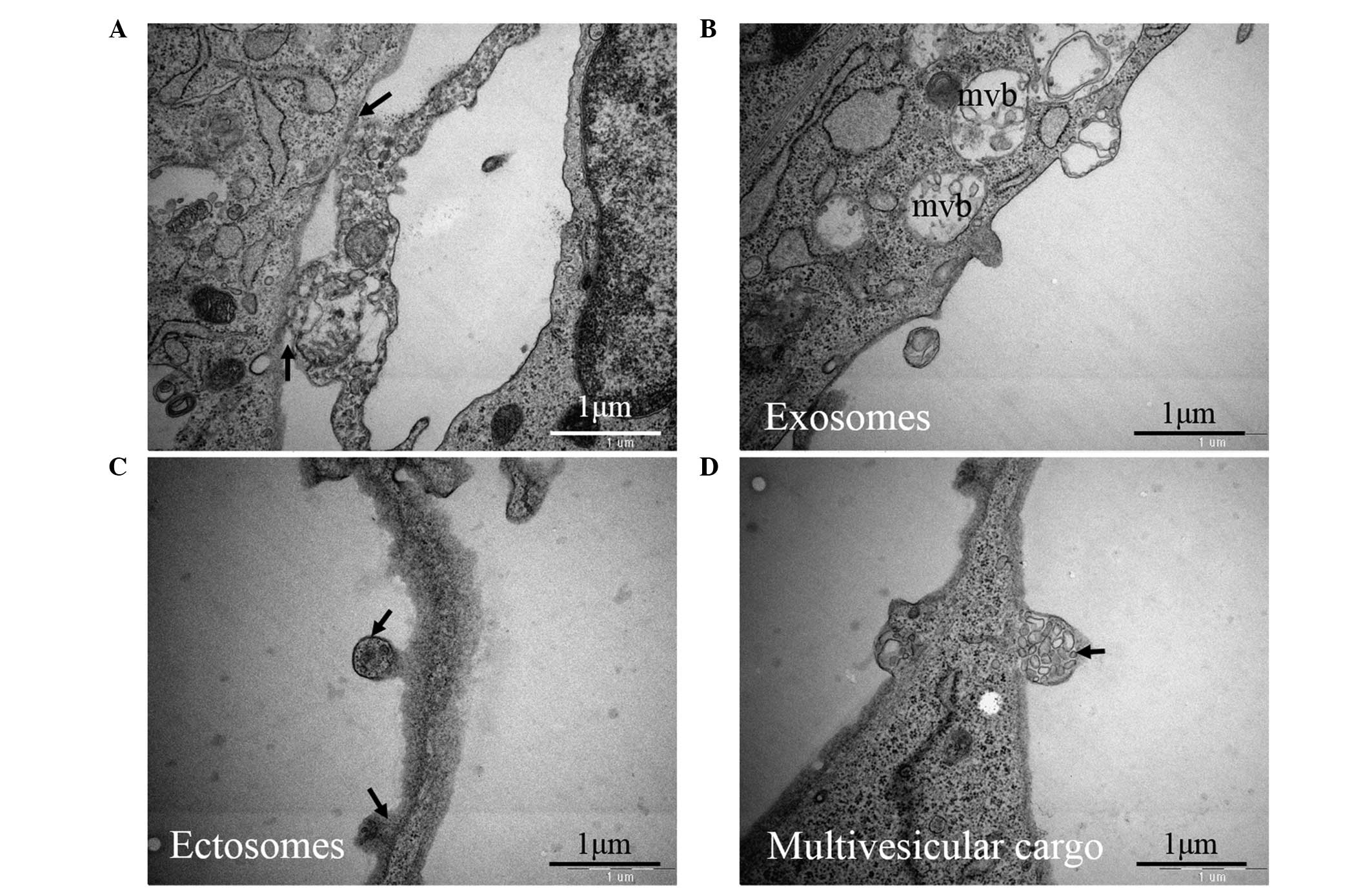
(EVs) (Fig. 5B–D). Different types
of EVs were observed: Exosomes, mainly as intraluminal vesicles
filled with multivesicular bodies (Fig. 5B); ectosomes, released from the
plasma membrane of the cell body and Tps (Fig. 5C); and clusters of endomembrane
vesicles encircled by the plasma membrane, termed multivesicular
cargo (Fig. 5D).
Discussion
Cardiac TCs are a small shape-specific cell type in
heart interstitial tissue. They can be distinguished from other
cells easily because of their characteristic morphological
structure in situ. As the positioning of cardiac TCs
gradually becomes clearer, functional research on purified TCs has
become inevitable.
In the present study, during culture it was observed
that TCs exhibited the typical characteristics of a small cell body
and Tps. We also found connections between TCs or between TCs and
other cell types interconnected in the form of a network (Fig. 1B). Different cell types have
differing adhesion abilities and require different times for
adhesion in cell suspensions obtained by enzymatic digestion. In
the present study, fibroblast attachment occurred within 2 h after
seeding, while TC attachment took 24 h (17). However, a higher proportion of TCs
could be acquired after differential adhesion for 1 h (data not
shown). Therefore, we selected differential adhesion for 1 h to
partially purify cardiac TCs in preliminary culture.
Previous studies have confirmed that TCs are able to
bind various antibodies (21,22).
Immunohistochemistry performed on a range of organs and tissues has
demonstrated phenotypical traits for TCs, and a few markers have
been verified to label them. The most reliable of these markers
seems to be CD34 (1,23). CD34 is a highly glycosylated
transmembrane glycoprotein. At present, increasing numbers of
studies have shown that CD34 serves an important role in mediating
cell adhesion, participating in the migration and positioning of
hematopoietic stem cells involved in inflammation and lymphocyte
homing. Cismasiu et al (24) observed a similar biological
function of TCs.
PDGFRα and PDGFRβ are two types of tyrosine kinases
activated by PDGF, which are important in cell survival,
proliferation, differentiation and migration (25,26).
In particular, PDGFRα is important during embryonic organogenesis
and development. PDGFRα belongs to the type III receptor tyrosine
kinase family and is an angiogenic factor (27). Recently, CD34/PDGFRα have been
proposed to be specific markers for cardiac TCs (14). Zhou et al (28) reported that PDGFRα positive cells
accounted for a large proportion of rat cardiac TC-enriched
cultures, and one-third of all cells were CD34/PDGFRα double
positive. The results of the present study confirmed the positive
expression of PDGFRα, consistent with previous studies. Therefore,
we selected CD34 and PDGFRα as the markers to identify and purify
cardiac TCs.
Currently available cell isolation techniques are
predominantly based on properties including antibody binding,
particle size, density gradient, adherence or absorbance (29–31).
Above all, antibody-binding methodology depends on antigen-antibody
binding of cell surface biomarkers, and hence provides precise
sorting, with techniques such as fluorescence-activated cell
sorting (FACS) and magnetic-activated cell sorting (MACS) (32–34).
However, the problem with MACS is that the remaining magnetic beads
on sorted cells affect further culture and testing. Density
gradient centrifugation is relatively simple, however, the purity
is relatively low. Conversely, the different media used for
separation risk cell damage. Therefore, we used FACS to sort
cardiac TCs in the present study. Our findings for double positive
expression of CD34 and PDGFRα before and after flow cytometry
sorting were 31.5 and 99.3%, respectively, which demonstrates high
efficiency.
On the basis of FACS, immunofluorescent staining for
vimentin, another TC marker, was used to confirm the sorted TCs
(2). Of the sorted TCs, 96.7% were
vimentin positive. This contributed to the aim of confirming
cardiac TCs by three markers and was consistent with the results of
immunohistochemistry to localize cardiac TCs, which co-expressed
PDGFRα, CD34 and vimentin.
As a method to identify TCs, TEM offered
high-resolution information on ultrastructural features, which aids
in understanding their function. Though the morphological
characteristics of TCs in culture were not completely in accordance
with the characteristics observed in vivo, the main
appearance was quite consistent (35,36).
The distinctive morphology of Tps is presented in Fig. 4. As TEM is a 2D assay of an
ultrathin section (~60 nm), the number of prolongations differs
depending on the position and angle of sectioning. Additionally, as
the microenvironment in culture is different to that in a normal
heart in vivo, it is understandable that the appearance of
TCs in culture was not fully typical of their morphology in
vivo.
A large number of TCs are located around the stem
pool, which exists in various organs and tissues and is crucial to
the survival, migration and differentiation of stem cells,
particularly during tissue renewal (37). Studies confirm that TCs form a
network in the myocardial interstitium and play an important role
in short- and long-distance intercellular communication by TC-TC
junctions, in addition to heterocellular junctions of TCs (11). The present study additionally
showed that tight contacts (atypical junctions) occurred between
the Tps and the TC cell body (Fig.
5B). Furthermore, Tps wrap around stem cells to supply
nutrition or contribute to signal transduction. Therefore, TCs are
also called stem cell accessory cells (38). Previous studies have reported that
intercellular connections are formed between TCs and stem cells in
the stem cell pool that participates in signal transduction by
these cells (11,39,40).
In addition, TCs can secrete extracellular vesicles in different
forms, such as exosomes, ectosomes and multivesicular cargo
(Fig. 5B–D). These vesicles may
contain small molecules or macromolecular signaling proteins
functioning as intercellular shuttles for the communication of
biological signals, which are critical to the development and
differentiation of stem cells and the collection of other accessory
cells from the blood to the stem cell pool (16). Thus, there is emerging evidence
that TCs and stem cells may act in tandem (16). A promising idea is to use stem
cells assisted by TCs to help repair damage and necrosis in future
cell-based cardiac repair strategies. Based on our hypothesis, it
is of great importance to purify cardiac TCs and the present study
contributes to that effort.
In summary, the method used in the present study is
relatively simple and effective. Furthermore, this study
demonstrates a new purification strategy for purifying TCs by flow
cytometric sorting with antibodies against CD34 and PDGFRα. The
purified cardiac TCs may become an important tool for elucidating
their biological effects, in particular in vitro studies. We
believe that this method will contribute to providing novel
approaches to understanding the regulation of homeostasis,
renovation and regeneration in the heart.
Acknowledgments
The present study was supported by the Nature
Science Foundation of China (grant no. 81170124/H0203).
References
|
1
|
Popescu LM and Faussone-Pellegrini MS:
TELOCYTES - a case of serendipity: The winding way from
Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like
Cells (ICLC) to TELOCYTES. J Cell Mol Med. 14:729–740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suciu L, Popescu LM, Gherghiceanu M,
Regalia T, Nicolescu MI, Hinescu ME and Faussone-Pellegrini MS:
Telocytes in human term placenta: Morphology and phenotype. Cells
Tissues Organs. 192:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kostin S: Myocardial telocytes: A specific
new cellular entity. J Cell Mol Med. 14:1917–1921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Popescu LM, Manole CG, Gherghiceanu M,
Ardelean A, Nicolescu MI, Hinescu ME and Kostin S: Telocytes in
human epicardium. J Cell Mol Med. 14:2085–2093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gherghiceanu M, Manole CG and Popescu LM:
Telocytes in endocardium: Electron microscope evidence. J Cell Mol
Med. 14:2330–2334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rusu MC, Jianu AM, Mirancea N, Didilescu
AC, Mănoiu VS and Păduraru D: Tracheal telocytes. J Cell Mol Med.
16:401–405. 2012. View Article : Google Scholar
|
|
7
|
Zheng Y, Zhu T, Lin M, Wu D and Wang X:
Telocytes in the urinary system. J Tranl Med. 10:1882012.
View Article : Google Scholar
|
|
8
|
Faussone-Pellegrini MS and Bani D:
Relationships between telocytes and cardiomyocytes during pre- and
post-natal life. J Cell Mol Med. 14:1061–1063. 2010.PubMed/NCBI
|
|
9
|
Mandache E, Gherghiceanu M, Macarie C,
Kostin S and Popescu LM: Telocytes in human isolated atrial
amyloidosis: Ultrastructural remodelling. J Cell Mol Med.
14:2739–2747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Sun W, Wu SM, Xiao J and Kong X:
Telocytes in human heart valves. J Cell Mol Med. 18:759–765. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gherghiceanu M and Popescu LM: Cardiac
telocytes-their junctions and functional implications. Cell Tissue
Res. 348:265–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barile L, Lionetti V, Cervio E, Matteucci
M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T and
Vassalli G: Extracellular vesicles from human cardiac progenitor
cells inhibit cardiomyocyte apoptosis and improve cardiac function
after myocardial infarction. Cardiovasc Res. 103:530–541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gherghiceanu M and Popescu LM:
Cardiomyocyte precursors and telocytes in epicardial stem cell
niche: Electron microscope images. J Cell Mol Med. 14:871–877.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Chen S, Liu J, Yuan Z, Qi X, Qin
J, Zheng X, Shen X, Yu Y, Qnin TJ, et al: Cardiac telocytes were
decreased during myocardial infarction and their therapeutic
effects for ischaemic heart in rat. J Cell Mol Med. 17:123–133.
2013. View Article : Google Scholar
|
|
15
|
Zhao B, Liao Z, Chen S, Yuan Z, Yilin C,
Lee KK, Qi X, Shen X, Zheng X, Quinn T and Cai D: Intramyocardial
transplantation of cardiac telocytes decreases myocardial
infarction and improves post-infarcted cardiac function in rats. J
Cell Mol Med. 18:780–789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cretoiu SM and Popescu LM: Telocytes
revisited. Biomo Concepts. 5:353–369. 2014. View Article : Google Scholar
|
|
17
|
Bei Y, Zhou Q, Fu S, Lv D, Chen P, Chen Y,
Wang F and Xiao J: Cardiac telocytes and fibroblasts in primary
culture: Different morphologies and immunophenotypes. PLoS One.
10:e01159912015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vannucchi MG, Traini C, Manetti M,
Ibba-Manneschi L and Faussone-Pellegrini MS: Telocytes express
PDGFRα in the human gastrointestinal tract. J Cell Mol Med.
17:1099–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Milia AF, Ruffo M, Manetti M, Rosa I,
Conte D, Fazi M, Messerini L and Ibba-Manneschi L: Telocytes in
Crohn's disease. J Cell Mol Med. 17:1525–1536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mou Y, Wang Y, Li J, Lü S, Duan C, Du Z,
Yang G, Chen W, Zhao S, Zhou J and Wang C: Immunohistochemical
characterization and functional identification of mammary gland
telocytes in the self-assembly of reconstituted breast cancer
tissue in vitro. J Cell Mol Med. 17:65–75. 2013. View Article : Google Scholar
|
|
21
|
Gangenahalli GU, Singh VK, Verma YK, Gupta
P, Sharma RK, Chandra R and Luthra PM: Hematopoietic stem cell
antigen CD34: role in adhesion or homing. Stem Cells Dev.
15:c305–c313. 2006. View Article : Google Scholar
|
|
22
|
Kim JH, Choi SC, Park CY, Park JH, Choi
JH, Joo HJ, Hong SJ and Lim DS: Transplantation of immortalized
CD34+ and CD34− adipose-derived stem cells
improve cardiac function and mitigate systemic pro-inflammatory
responses. PLoS One. 11:e01478532016. View Article : Google Scholar
|
|
23
|
Vannucchi MG, Traini C, Guasti D, Del
Popolo G and Faussone-Pellegrini MS: Telocytes subtypes in human
urinary bladder. J Cell Mol Med. 18:2000–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cismaşiu VB and Popescu LM: Telocytes
transfer extracellular vesicles loaded with microRNAs to stem
cells. J Cell Mol Med. 19:351–358. 2015. View Article : Google Scholar
|
|
25
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tallquist MD and Soriano P: Cell
autonomous requirement for PDGFRalpha in populations of cranial and
cardiac neural crest cells. Development. 130:507–518. 2003.
View Article : Google Scholar
|
|
27
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Q, Wei L, Zhong C, Fu S, Bei Y, Huică
RI, Wang F and Xiao J: Cardiac telocytes are double positive for
CD34/PDGFR-α. J Cell Mol Med. 19:2036–2042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Li P, Huang PH, Xie Y, Mai JD,
Wang L, Nguyen NT and Huang TJ: Rare cell isolation and analysis in
microfluidics. Lab Chip. 14:626–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spangrude GJ, Heimfeld S and Weissman IL:
Purification and characterization of mouse hematopoietic stem
cells. Science. 241:58–62. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tomlinson MJ, Tomlinson S, Yang XB and
Kirkham J: Cell separation: Terminology and practical
considerations. J Tissue Eng. 4:20417314124726902013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmitz B, Radbruch A, Kümmel T,
Wickenhauser C, Korb H, Hansmann ML, Thiele J and Fischer R:
Magnetic activated cell sorting (MACS)-a new immunomagnetic method
for megakaryocytic cell isolation: Comparison of different
separation techniques. Eur J Haematol. 52:267–275. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grützkau A and Radbruch A: Small but
mighty: How the MACS-technology based on nanosized
superparamagnetic particles has helped to analyze the immune system
within the last 20 years. Cytometry A. 77:643–647. 2010. View Article : Google Scholar
|
|
34
|
Wilkerson MJ: Principles and applications
of flow cytometry and cell sorting in companion animal medicine.
Vet Clin North Am Small Anim Pract. 42:53–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suciu L, Nicolescu MI and Popescu LM:
Cardiac telocytes: Serial dynamic images in cell culture. J Cell
Mol Med. 14:2687–2692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Wang Y, Zhu P, Sun H, Mou Y, Duan
C, Yao A, Lv S and Wang C: Distribution and characteristics of
telocytes as nurse cells in the architectural organization of
engineered heart tissues. Sci China Life Sci. 57:241–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galiger C, Kostin S, Golec A, Ahlbrecht K,
Becker S, Gherghiceanu M, Popescu LM, Morty RE, Seeger W and
Voswinckel R: Phenotypical and ultrastructural features of
Oct4-positive cells in the adult mouse lung. J Cell Mol Med.
18:1321–1333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kostin S and Popescu LM: A distinct type
of cell in myocardium: Interstitial Cajal-like cells (ICLCs). J
Cell Mol Med. 13:295–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cretoiu D, Hummel E, Zimmermann H,
Gherghiceanu M and Popescu LM: Human cardiac telocytes: 3D imaging
by FIB-SEM tomography. J Cell Mol Med. 18:2157–2164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fertig ET, Gherghiceanu M and Popescu LM:
Extracellular vesicles release by cardiac telocytes: Electron
microscopy and electron tomography. J Cell Mol Med. 18:1938–1943.
2014. View Article : Google Scholar : PubMed/NCBI
|