Introduction
Glioblasoma multiforme (GBM) is one of the most
aggressive types of malignant brain tumor and has a poor prognosis,
with a median survival time of ~14.6 months and a World Health
Organization (WHO) grade IV (1–3).
According to the cancer stem cell theory, cancer stem cells (CSCs)
and stem-like side populations (SPs) are responsible for therapy
failure and tumor recurrence. These CSCs resist current
conventional treatment strategies and re-initiate cancer growth;
they are thus responsible for minimal residual disease (4–6).
Several studies reported the existence of brain cancer stem cells
(BCSCs), which have marked self-renewing properties owing to their
elevated expression of stem-cell surface markers, high
differentiation capacity, evasion of apoptosis and resistance to
chemotherapeutics (7,8). Therefore, understanding the molecular
mechanism of BCSC-mediated tumor recurrence may aid in the
development of novel cancer therapeutic drugs.
Brain cancer stem cells can be isolated using a
fluorescence-activated cells sorting (FACs)-based Hoechst 33342 dye
exclusion assay. The purified BCSCs are termed as SP cells, as they
appear as distinct populations on the side of the main cell
population in the flow cytometry dot plot (9,10).
Brain cancer SP cells possess the distinct features of BCSCs,
including resistance to chemotherapeutics, enhanced neurosphere
formation, overexpression of stem-cell surface genes, including
Nanog, Oct-4, CD133, CD44, CD34, CD29 and CD24, as well as
adenosine triphosphate-binding cassette (ABC) transporter genes,
including ABCB1 (MDR1), ABCC1 and ABCG2, and genes associated with
resistance to apoptosis (11). In
addition, Notch1 signaling was shown to be aberrantly regulated in
SP cells and Notch1 inactivation enhanced their sensitivity to
chemotherapeutic drug treatments (12,13).
Hence, elucidating the mechanism underlying Notch1
signaling-mediated drug resistance and tumor relapse will aid in
the development of novel treatments targeting BCSCs and may allow
the complete eradication of brain tumors. For this purpose, the
present study evaluated the expression of Notch1 signaling proteins
in glioblastoma SP cells isolated by FACS. Furthermore,
Notch1-specific RNA interference (RNAi) technology was used to
silence Notch1 in SP cells and the effects on the neurosphere
formation ability and chemotherapeutic resistance of SP cells were
assessed. The present study suggested that Notch1 inhibition may
represent a promising strategy for the eradication of glioblastoma
by targeting their SP cells.
Materials and methods
Patient samples and establishment of
glioblastoma cell culture
The present study was approved by the Ethics
Committee of the College of Medicine at Zhejiang University
(Hangzhou, China). Written consent was obtained from all patients.
Glioblastoma samples (stage, malignant glioblastoma) were collected
from 10 cancer patients (age, 18–39 years; 6 male and 4 female)
undergoing surgery at the Department of Neurosurgery of the First
Affiliated Hospital of Zhejiang University between January 2012 and
December 2014. The samples were cut and fixed in 4% formalin
solution (Merck-Millipore, Darmstadt, Germany). Fixed tissues were
dehydrated in ethanol (Merck-Millipore), cleared using xylene
(Merck-Millipore) and embedded in paraffin (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Tissue paraffin blocks were
sectioned into 5-µm slices using a microtome. A histological
analysis confirmed that the patients had WHO grade IV
glioblastoma.
The glioblastoma tissues harvested from the patients
were mechanically disaggregated using a scalpel in Dulbecco's
modified Eagle's medium: Nutrient mixture F-12 (DMEM/F-12;
Mediatech, Manassas, VA, USA) and passed through 100-µm
nylon mesh cell strainers (Falcon; BD Biosciences, Franklin Lakes,
NJ, USA). The resulting cell suspensions were washed two times
using DMEM/F-12, followed by culture in complete media (DMEM/F-12)
containing B27 (Invitrogen; Thermo Fisher Scientific, Inc.) plus 20
ng/ml basic fibroblast growth factor (Sigma-Aldrich, St. Louis, MO,
USA), 20 ng/ml epidermal growth factor (Sigma-Aldrich), 50 U/ml
penicillin and 50 mg/ml streptomycin (both Thermo Fisher
Scientific, Inc.).
Isolation of SP cells
Hoechst 33342 staining and FACS were used to purify
SP cells. Cultured glioblastoma cells were suspended at
106 cells/ml in DMEM containing 10% fetal bovine serum
(FBS; Sigma-Aldrich) and labeled with Hoechst 33342-bis-benzimide
(5 µl/ml; Sigma-Aldrich) alone (n=7) or in combination with
verapamil (0.8 µl/ml; Sigma-Aldrich) (n=7). Following
mixing, the cell suspensions were incubated in a water bath at 37°C
for 90 min. Cells were then centrifuged at 65 × g for 10 min at
4°C, and then re-suspended in 500 µl Hank's balanced salt
solution containing 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich).
Finally, cells were counterstained with 2 µg/ml propidium
iodide (Sigma-Aldrich) at 4°C. The cells were filtered through a
50-µm nylon mesh (BD Biosciences) to remove any cell clumps
and were then subjected to FACS (FACS Aria II; BD Biosciences) with
excitation of the Hoechst 33342 dye at 355 nm and analysis of its
dual-wavelength fluorescence at 450 nm (blue) and 675 nm (red). SP
and non-SP cells were isolated under sterile conditions and kept in
DMEM containing 10% FBS.
Glioma sphere formation assay
The gliosphere formation assay was performed
according to the procedure of a previous study (14). After seven days of culture, the
numbers of spheres generated from SP and non-Sp cells were counted.
Three independent experiments were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted and reverse-transcribed into
cDNA using a Reverse Transcriptase kit (Fermentas; Thermo Fisher
Scientific, Inc.). An iCycler IQ real-time detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using IQ Supermix
with SYBR-Green (Bio-Rad Laboratories, Inc.) was used for qPCR
analysis. The primer sequences for Notch1 and Hes-1, as well as the
reaction conditions, were designed based on a previous study
(13) and were as follows: Notch1
forward, 5′-GCGAGGTCAACACAGACGAG-3′ and reverse,
5′-CAGGCACTTGGCACCATTC-3′; and Hes-1 forward,
5′-TGGAGAGGCGGCTAAGGTGT-3′ and reverse,
5′-GCTGGTGTAGACGGGGATGAC-3′. The primer sequences for the other
genes were as follows, according to a previous study (15): ABCG2 forward,
5′-GGATGAGCCTACAACTGGCTT-3′ and reverse,
5′-CTTCCTGAGGCCAATAAGGTG-3′; Oct4 forward,
5′-TCGAGAACCGAGTGAGAGGC-3′ and reverse,
5′-CACACTCGGACCACATCCTTC-3′; Sox2 forward,
5′-CACACTGCCCCTCTCACACAT-3′ and reverse,
5′-CATTTCCCTCGTTTTTCTTTGAA-3′; Nanog forward,
5′-CCAACATCCTGAACCTCAGCTAC-3′ and reverse,
5′-GCCTTCTGCGTCACACCATT-3′; and GAPDH forward,
5′-TCTGCTCCTCCTGTTCGACA-3′ and reverse, 5′-AAAAGCAGCCCTGGTGACC-3′.
The thermocycling conditions were as follows: Initial denaturation
at 94°C for 2 min, followed by 35 cycles of denaturation at −95°C
for 15 sec, annealing at −58°C for 45 sec and extension at −60°C
for 30–45 sec. The final extension step occurred at 72°C for 5 min.
The mRNA expression levels were normalized to the GAPDH internal
reference gene and the relative expression levels were calculated
using the 2−ΔΔCq method (16). Reactions were performed in
triplicate.
Transfection of small interfering
(si)RNAs
siRNAs specific to Notch1 were purchased from
Dharmacon (Lafayette, CO, USA), and had the following sequences:
Notch1#1, 5′-GCCUGGACAAGAUCAAUGAtt-3′; and Notch1#2,
5′-GCCUGUCUGAGGUCAAUGAtt-3′. Scrambled siRNAs (Dharmacon)
containing the same nucleotide content as the Notch1 siRNAs, but in
a random sequence, were used as the control. SP cells were
transfected with 1 µg/µl siRNAs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The siRNA-transfected SP cells were subjected to the assays after
48 h of incubation.
Western blot analysis
Total protein was extracted from the SP and non-SP
cells, and the protein concentration was determined using the
Bradford Protein assay (Sigma-Aldrich). Equal quantities of protein
(20 µg/lane) were subjected to 10% SDS-PAGE and transferred
onto nitrocellulose membranes. The membranes were then blocked
using Tris-buffered saline supplemented with 0.1% Tween-20 (TBST;
50 mM Tris-HCl, pH 8.2, 150 mM NaCl) containing 5% w/v non-fat dry
milk and subsequently incubated with the rabbit anti-Notch1
monoclonal antibody (1:5,000; ab52627; Abcam, Cambridge, UK),
rabbit anti-ABCG2 polyclonal antibody (1:10,000; ab63907; Abcam),
rabbit anti-Hes-1 polyclonal antibody (1:500; ab71559; Abcam) and
rabbit anti-GAPDH polyclonal antibody (1:10,000; ab37168; Abcam)
for 3 h at room temperature. GAPDH was used as a loading control.
Following washing three times for 10 min each with 1X TBST, the
cells were treated with alkaline phosphatase-conjugated goat
anti-rabbit immunoglobulin G heavy&light chains (1:5,000;
ab97048; Abcam) for 3 h at room temperature. Antibodies were
visualized using the Pierce™ ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). Subsequently, blots were scanned and
quantitative analysis was performed using a densitometer (Bio-Rad
GS-710; Bio-Rad Laboratories, Inc.).
Cell resistance assay
Cells were seeded into 96-well plates at
1×103 cells/well and incubated at 37°C for 24 h, prior
to treatment with the following chemotherapeutic drugs for 48 h:
5-Fluorouracil (5-FU; 10 µg/ml), oxaliplatin (100 mM) and
cisplatin (5 mg/ml; all Sigma-Aldrich). Cell resistance was
determined by calculating the mean optical density (OD) at 450 nm
(OD450) for three independent experiments for each
group. The resistance of the groups to the chemotherapeutic drugs
was calculated using the following formula: Resistance rate (%) =
[OD450(experimental group)/OD450(control group)] ×100.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance and Student's t-test were performed to
assess significant differences between experimental and control
groups. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
Notch signaling is upregulated in
glioblastoma SP cells
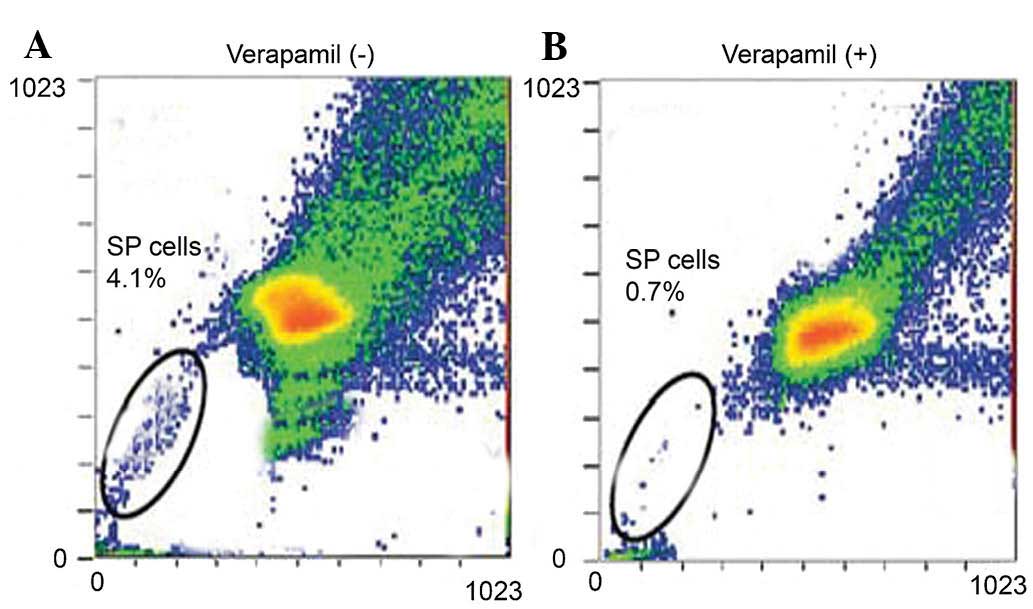
Staining with the cell-permeable DNA-binding dye
Hoechst 33342 and FACS analysis showed that untreated glioblastoma
cells contained a SP of ~4.1% (Fig.
1A). Furthermore, following treatment with the ABS transporter
inhibitor verapamil, the SP was diminished to 0.7% (Fig. 1B). Subsequently, the SP cells
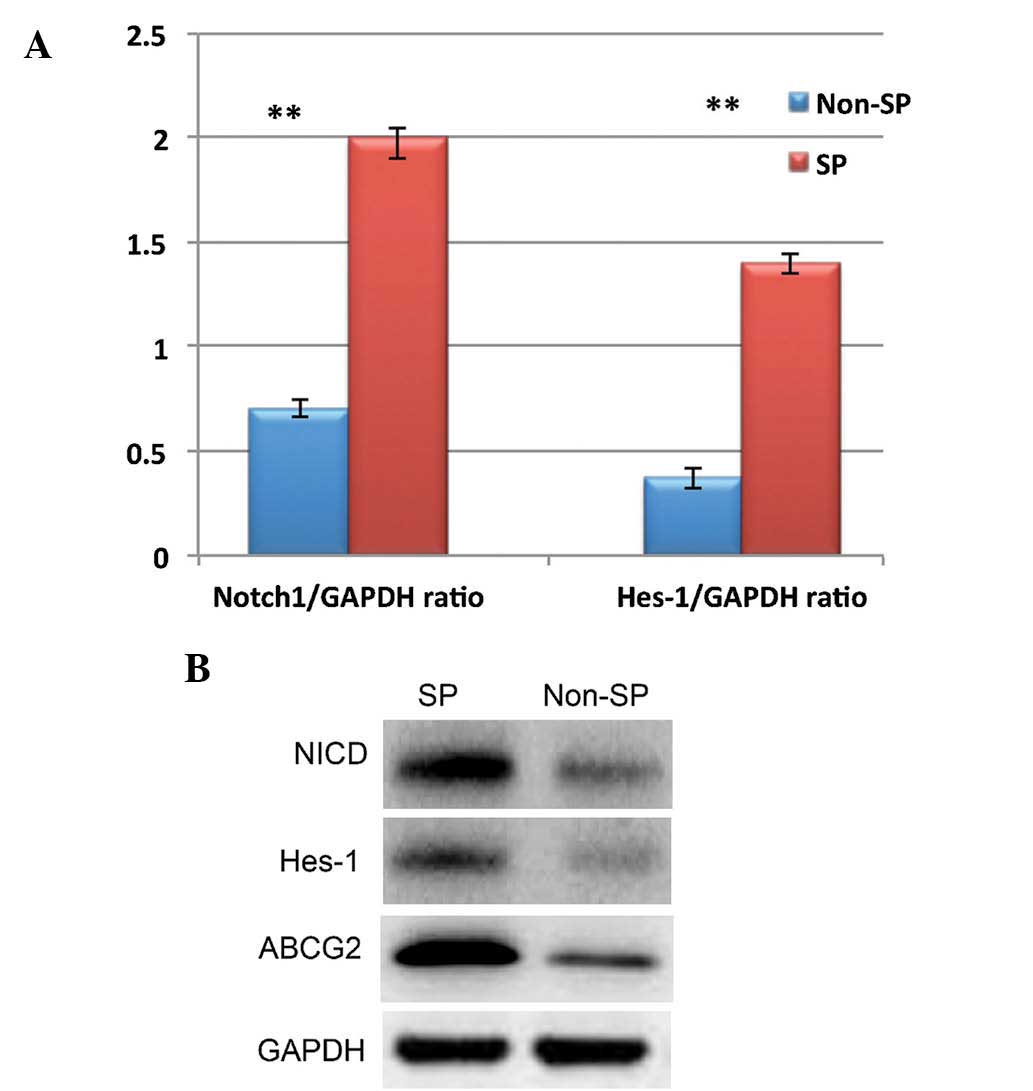
isolated by FACS were subjected western blot and RT-qPCR analysis
of proteins and mRNA involved in Notch signaling pathways,
including NICD and Hes-1. NICD and Hes-1 expression was
significantly upregulated in the SP cells when compared to that in
the non-SP cells (Fig. 2A and B).
These results confirmed the presence of SP cells in glioblastoma
and indicate that Notch signaling is upregulated in SP cells.
Elevated Notch signaling enhances the
self-renewal capacity of glioblastoma SP cells
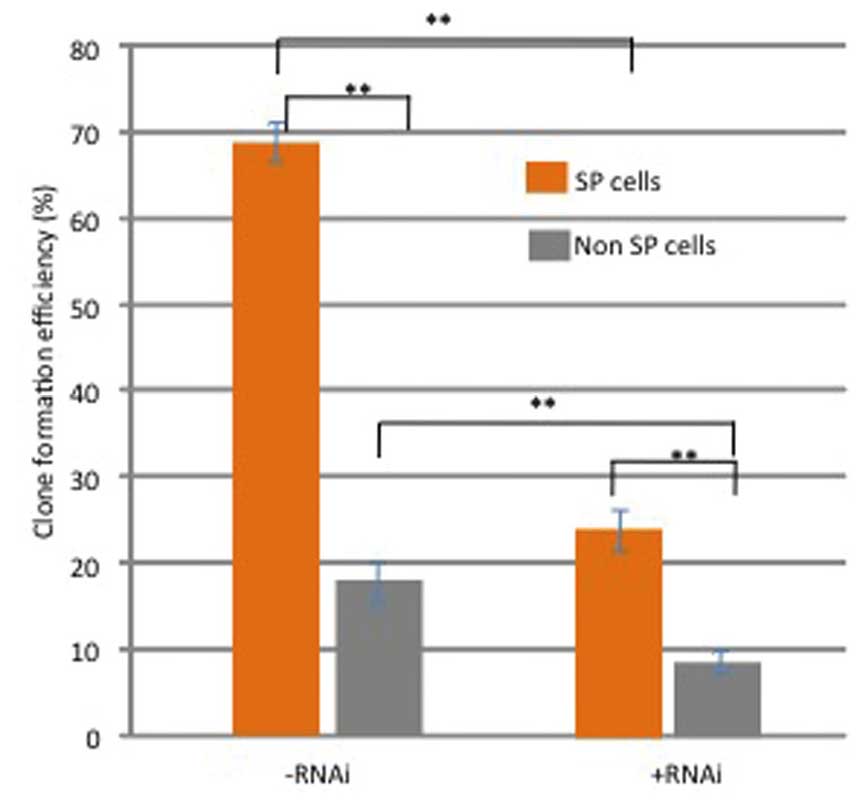
In order to investigate the role of Notch signaling
in the self-renewal of SP cells, the effects of Notch1 RNAi on
their sphere forming ability were analyzed. As shown in Fig. 3, the ability of native SP cells to
form tumor spheres was significantly enhanced, as compared with
non-SP cells (P<0.01). However, the sphere forming capacity of
SP cells was significantly compromised following RNAi of Notch1
(P<0.05; Fig. 3). In addition,
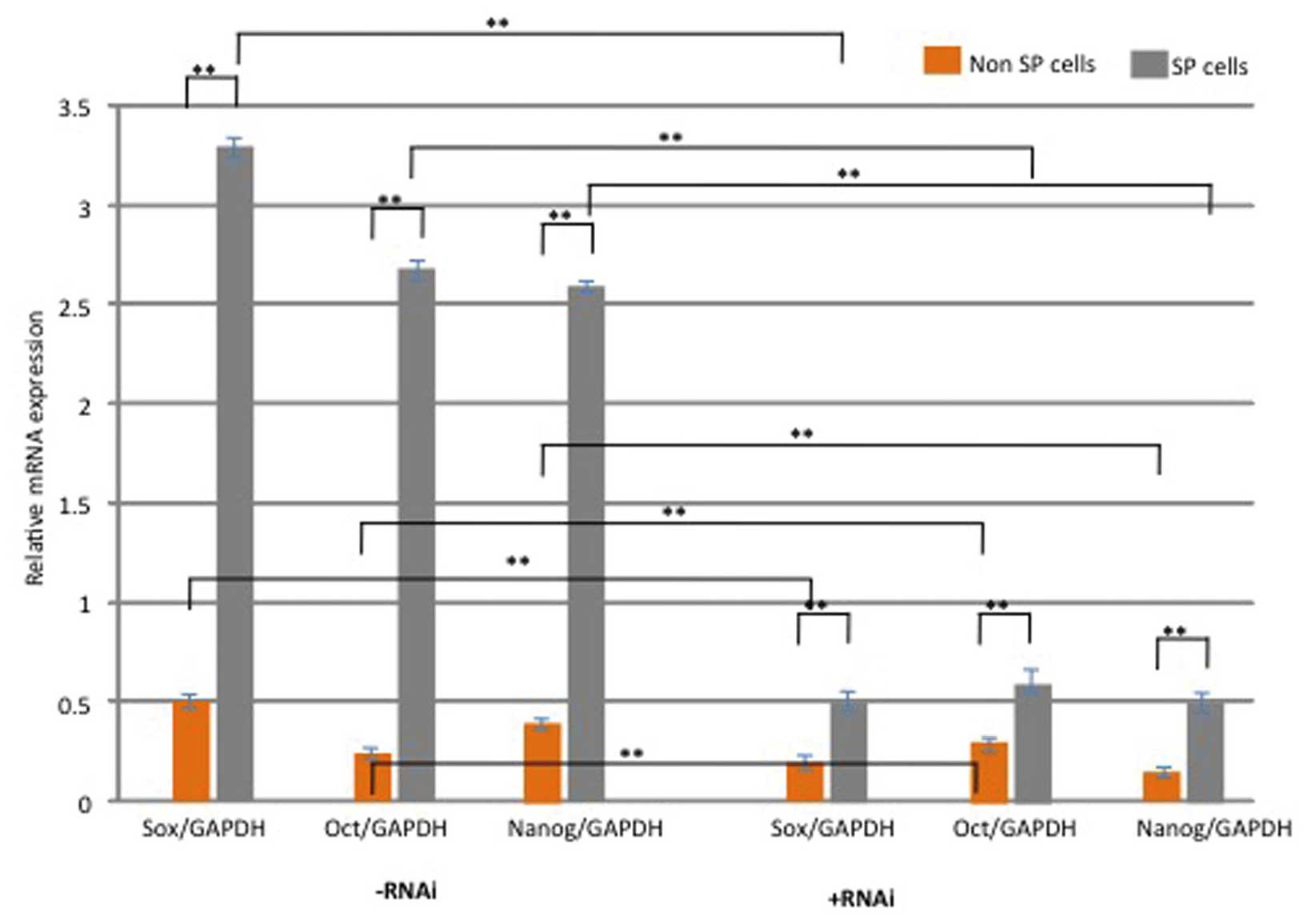
the expression levels of the stem cell-surface proteins Oct-4,
Nanog and Sox2 were assessed. As shown in Fig. 4, the relative mRNA expression
levels of these genes were significantly upregulated in SP cells,
as compared with non-SP cells (P<0.05). However, following
knockdown of Notch1, the expression of these genes was
significantly down-regulated in SP cells (P<0.05; Fig. 4). These results suggest that
activation of Notch1 in glioblastoma SP cells may have a crucial
role in the maintenance and self-renewal of SP cells.
Overexpression of Notch1 is associated
with resistance of SP cells to chemotherapy
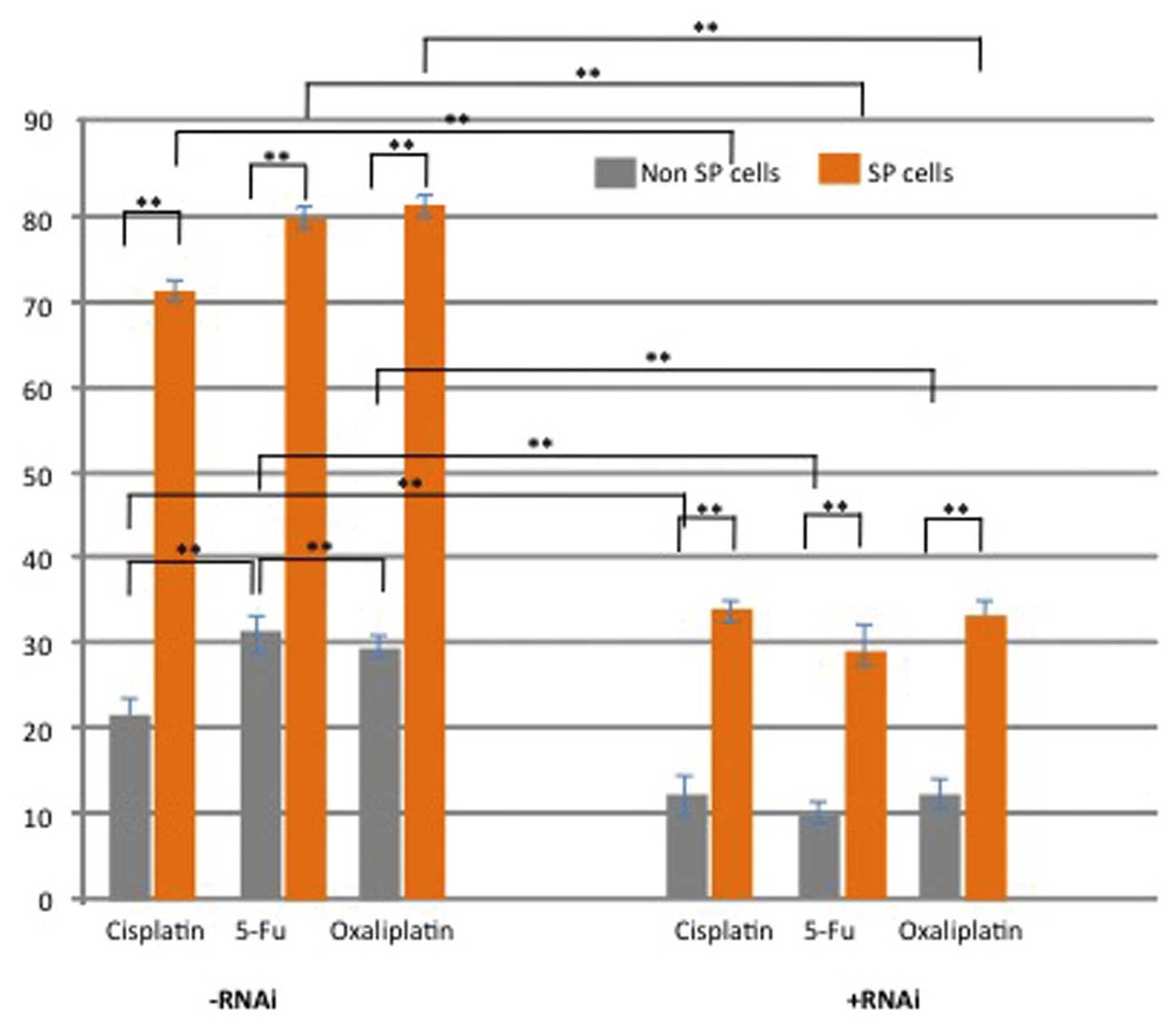
Next, the present study performed a chemotherapy
resistance assay in order to determine the survival rates of SP and
non-SP cells following treatment with the DNA-targeting drugs 5-FU,
cisplatin and oxaliplatin. The cell survival rate of SP cells
treated with the chemotherapeutics was significantly higher than
that of non-SP cells following treatment (P<0.05) (Fig. 5). However, knockdown of Notch1 in
SP cells significantly reduced the survival rate of SP cells
following incubation with the drugs (Fig. 5). These results indicated that
elevated Notch1 signaling may be associated with chemoresistance
and evasion of apoptosis of glioblastoma SP cells.
Discussion
According to the CSCs theory, the resistance of
small populations of CSCs or CSC-like SPs to existing
chemotherapeutics, and their ability to re-initiate tumors
following chemotherapy, are the main reasons for tumor recurrence
and metastasis following treatment (4–6).
Therefore, the targeting of CSCs is required in order to provide
effective cancer treatments and to improve the overall survival
rate of patients with cancer.
The present study aimed to characterize a CSC-like
SP of glioblastoma and to evaluate the role of Notch1 signaling in
their tumorigenic and chemoresistant phenotypes. Previous studies
on a glioblastoma cell line detected the existence of 1–2.5%
CSC-like SP cells, which possessed all of the characteristic
features of CSCs. (3,17). In the present study, FACS was used
to identify and isolate a CSC-like SP of ~4.1% from patient-derived
glioma cells. Upon treatment with the ABC transporter inhibitor
verapamil, the SP was markedly reduced to 0.7%. At high
concentrations, verapamil specifically inhibits ABCG2 transporter
proteins (18); thus suggesting
that the upregulation of ABCG2 transporter proteins may be involved
in the resistance of glioblastoma SP cells to therapeutic
drugs.
A previous study reported that elevated expression
of ABCG2 was involved in downstream targeting of Notch1 signaling,
which promoted the expression of stem cell-associated proteins such
as Nestin in glioma cells (19).
Notch1 signaling was shown to efficiently promote the proliferation
and self-renewal of SP cells (20,21).
Therefore, the present study analyzed Notch1 signaling in
patient-derived glioblastoma SP cells isolated by FACS. In the SP
cells, the expression of Notch1 signaling genes NICD and Hes-1 at
the protein and mRNA levels were markedly upregulated, as compared
with that in non-SP cells. Furthermore, the SP cells showed
enhanced drug resistance and elevated expression of stem-cell
surface genes Oct-4, Sox-2 and Nanog, which may be major factors in
the maintenance and self-renewal, which was also shown to be
enhanced in SP cells compared with that of non-SP cells. Of note,
silencing of Notch1 signaling reduced the self-renewal capacity,
enhanced the sensitivity of SP cells to DNA-targeting
chemotherapeutic drugs and the expression of stemness genes was
also significantly down-regulated. Consistent with these findings,
previous studies on different cancer types demonstrated that
Notch-depleted SP cells were more sensitive to chemotherapy and
apoptosis, and that their self-renewal ability and high
proliferation rate were also compromised (22–25).
All of these results suggested that Notch1 signaling has a crucial
role in drug resistance and maintenance of self-renewal of SP
cells.
In conclusion, the present study suggested that
elevated Notch1 signaling in glioblastoma SP cells is responsible
for drug resistance and recurrence of glioblastoma. However, the
link between Notch1 signaling and cell division/cell death
signaling mechanisms requires to be further elucidated in order to
facilitate the development of novel anti-cancer drugs to target
CSCs and possibly cure glioblastoma.
Acknowledgments
The authors would like to thank Dr Dong Chen
(Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin,
China) and Dr Tie-Jun Wang (Department of Radiation Oncology, The
Second Hospital of Jilin University, Changchun, China) for sharing
their experimental protocols.
References
|
1
|
Holland EC: Gliomagenesis: Genetic
alterations and mouse models. Nat Rev Genet. 2:120–129. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleihues P, Louis DN, Scheithauer BW,
Rorke LB, Reifenberger G, Burger PC and Cavenee WK: The WHO
classification of tumors of the nervous system. J Neuropathol Exp
Neurol. 61:215–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukaya R, Ohta S, Yamaguchi M, Fujii H,
Kawakami Y, Kawase T and Toda M: Isolation of cancer stem-like
cells from a side population of a human glioblastoma cell line,
SK-MG-1. Cancer Lett. 28:150–157. 2010. View Article : Google Scholar
|
|
4
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Fujii H, Kawakami Y, Kawase T and Toda M: A distinct ʻside
populationʼ of cells with high drug efflux capacity in human tumor
cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004. View Article : Google Scholar
|
|
5
|
Challen GA and Little MH: A side order of
stem cells: The SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramachandran C and Melnick SJ: Multidrug
resistance in human tumors-molecular diagnosis and clinical
significance. Mol Diagn. 4:81–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
8
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris MA, Yang H, Low BE, Mukherjee J,
Guha A, Bronson RT, Shultz LD, Israel MA and Yun K: Cancer stem
cells are enriched in the side population cells in a mouse model of
glioma. Cancer Res. 68:10051–10059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robey RW, Shukla S, Finley EM, Oldham RK,
Barnett D, Ambudkar SV, Fojo T and Bates SE: Inhibition of
P-glycoprotein (ABCB1) and multidrug resistance associated
protein-1 (ABCC1)-mediate transport by the orally administered
inhibitor CBT-1 (R). Biochemical Pharmacol. 75:1302–1312. 2008.
View Article : Google Scholar
|
|
12
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24 (−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu S, Zhang R, Liu F, Wang H, Wu J and
Wang Y: Notch inhibition suppresses nasopharyngeal carcinoma by
depleting cancer stem like side population cells. Oncol Rep.
28:561–566. 2012.PubMed/NCBI
|
|
14
|
Chearwae W and Bright JJ: PPARγ agonists
inhibit growth and expansion of CD133+ brain tumour stem
cells. Br J Cancer. 99:2044–2053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo D, Xu BL, Zhang XH and Dong MM: Cancer
stem-like side population cells in the human nasopharyngeal
carcinoma cell line cne-2 possess epithelial mesenchymal transition
properties in association with metastasis. Oncol Rep. 28:241–247.
2012.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2-cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozvegy-Laczka C, Hegedus T, Várady G,
Ujhelly O, Schuetz JD, Váradi A, Kéri G, Orfi L, Német K and
Sarkadi B: High-affinity interaction of tyrosine kinase inhibitors
with the ABCG2 multidrug transporter. Mol Pharmacol. 65:1485–1495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar
|
|
20
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lino MM, Merlo A and Boulay JL: Notch
signaling in glioblastoma: A developmental drug target? BMC Med.
8:722010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XP, Zheng G, Zou L, Liu HL, Hou LH,
Zhou P, Yin DD, Zheng QJ, Liang L, Zhang SZ, et al: Notch
activation promotes cell proliferation and the formation of neural
stem cell-like colonies in human glioma cells. Mol Cell Biochem.
307:101–108. 2008. View Article : Google Scholar
|
|
23
|
Fan X, Khaki L, Zhu TS, Soules ME, Talsma
CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, et al: NOTCH pathway
blockade depletes CD133-positive glioblastoma cells and inhibits
growth of tumor neurospheres and xenografts. Stem Cells. 28:5–16.
2010.
|
|
24
|
Sikandar SS, Pate KT, Anderson S, Dizon D,
Edwards RA, Waterman ML and Lipkin SM: NOTCH signaling is required
for formation and self-renewal of tumor-initiating cells and for
repression of secretory cell differentiation in colon cancer.
Cancer Res. 70:1469–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhen Y, Zhao S, Li Q, Li Y and Kawamoto K:
Arsenic trioxide-mediated Notch pathway inhibition depletes the
cancer stem-like cell population in gliomas. Cancer Lett.
292:64–72. 2010. View Article : Google Scholar
|