Introduction
Liver cancer is the second most common cause of
cancer-associated mortality in China. The underlying molecular
mechanisms that lead to the progression of liver cancer are
unclear. Tumor-associated gene mutation, deletion and activation
frequently lead to tumorigenesis. Previous studies have
demonstrated that p53, v-myc avian myelocytomatosis viral
oncogene homolog and hepatocyte growth factor are involved in the
genesis of liver cancer (1–6).
However, the complete process of cancer progression is far from
understood.
Replicative immortality is an important hallmark of
cancer. Cell cycle regulator dysfunction is an important event in
carcinogenesis and progression. Cyclin-dependent kinase inhibitor 3
(CDKN3) is part of the dual-specificity protein phosphatase family.
CDKN3 was identified as a cyclin-dependent kinase (CDK) inhibitor
and interacts with, and dephosphorylates, CDK2 kinase, thus
preventing its activation. CDKN3 was reported to be deleted,
mutated, or overexpressed in several types of cancer (7–10).
Previous studies have suggested that CDKN3 is expressed in
hepatocellular carcinoma tissues and the overexpression of CDKN3
promotes cell proliferation in hepatoma cells (11,12).
CDKN3 may act as an oncogene in liver cancer, as CDKN3 is negative
regulator of cell cycle via the inactivation of CDK2 (13–15).
The present study investigated the CDKN3 protein expression pattern
in liver cancer tissues and analyzed the association with
pathological stage. Small interfering RNA (siRNA) was used to
determine the importance of CDKN3 for tumor survival and cellular
resistance to therapeutic agents.
Materials and methods
Cell culture
QGY7701 hepatocellular carcinoma (HCC) cells were
used in the current study and were purchased from the Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China).
QGY7701 cells were maintained in RPMI 1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.) and 2 mM L-glutamine.
Cells were split every two days prior to reaching high confluence.
Cells were cultured in a cell incubator with 5% CO2 at
37°C.
HCC tissue sample and
immunohistochemistry (IHC)
A total of 70 samples of HCC tissues, 6
samples of liver cirrhosis tissues and 10 samples of normal liver
tissues were separately obtained following surgical resection. The
present study was approved by the ethics committee of the
Affiliated Hospital of Guangdong Medical University (Zhanjiang,
China) and written informed consent was obtained. Patients with HCC
had not received radiation/chemotherapy prior to surgery. The
characteristics of each group are presented in Table I. Cancer tissues were collected
following surgical dissection and immediately fixed in 10% formalin
overnight at room temperature. Subsequently, the fixed tissues were
dehydrated and embedded in paraffin. In order to visualize the
target proteins, the samples were deparaffinized in xylene and then
rehydrated via a graded alcohol series. The tissue sections were
then washed with PBS and heated twice in a microwave oven for 5 min
in citrate buffer (pH 6). Sections were then incubated with
CDKN3-specific antibody (Abnova Corporation, Taipei, Taiwan; cat.
no. orb100671) at a dilution of 1:200. Horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (Beyotime
Institute of Biotechnology, Haimen, China; cat. no. A0208) was used
at a dilution of 1:500. Following hematoxylin staining, the
sections were dried and sealed with a cover slip. The protein
signal was observed under a fluorescence microscope (DM2000; Leica
Microsystems GmbH, Wetzlar, Germany) and evaluated by a
pathologist. The CDKN3-positive cell number and staining degree of
each section was assessed by the same pathologist (Dr Shuo Fang,
Guangdong Medical University, Zhanjiang, China) and samples were
scored from the highest to the lowest scores (5–1 scores). The
pathological stage was evaluated by Dr Wei Dai (Guangdong Medical
University).
 | Table ICharacteristics of age, gender and HBV
infection in hepatocellular carcinoma, normal liver and liver
cirrhosis group. |
Table I
Characteristics of age, gender and HBV
infection in hepatocellular carcinoma, normal liver and liver
cirrhosis group.
| Characteristic | Cancer/normal
(n=70/10) | Odds ratio | 95% CI | Cancer/cirrhosis
(n=70/6) | Odds ratio | 95% CI |
|---|
| Age |
| <50 | 26/4 | 1.00 | | 26/2 | 1.00 | |
| >=50 | 44/6 | 1.13 | 0.29–4.370 | 44/4 | 0.85 | 0.14–4.940 |
| Gender |
| Male | 63/7 | 1.00 | | 63/5 | 1.00 | |
| Female | 7/3 | 0.26 | 0.05–1.240 | 7/1 | 0.56 | 0.05–5.460 |
| HBV |
| No | 61/2 | 1.00 | | 61/5 | 1.00 | |
| Yes | 9/8 | 27.11 | 4.95–148.440a | 9/1 | 1.36 | 0.14–12.97 |
RNA interference
A CDKN3-specific siRNA knockdown kit was purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). Lipofectamine
RNAiMAX (Thermo Fisher Scientific, Inc.) was used to transfect 20
pmol siRNA to each well of a 24-well plate with cell density of
3×105. This experiment was performed according the
manufacturer's protocol.
Cell viability assay
Cells with CDKN3 knockdown were seeded in 96-well
plates at a density of 5,000 cells/well. After 12 h culture, cells
were treated with various doses of cisplatin (Hansoh Pharmaceutical
Group Co., Ltd., Linyungang, China) and cell viability was
measured. Briefly, following cisplatin treatment, 10 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltet-razolium bromide (MTT)
solution (5 mg/ml) was added to each well. The cells were incubated
at 37°C for 2 h. The medium was removed and 100 µl dimethyl
sulfoxide was added to dissolve the formazan crystals. The
absorbance at 570 nm was determined using a spectrophotometer when
the crystals were completely dissolved.
Clonogenic survival assay
Cells were diluted with RPMI medium and seed at 500
cells/well in a 6-well plate. Cells were cultured for 7–10 days,
then the medium was removed and cells were stained with 0.1%
crystal violet for 5 min. Next, the cells were air-dried at room
temperature and the plates were imaged. The number of colonies in
each well was manually counted.
Western blotting
Cells were collected and total protein was extracted
with radioimmunoprecipitation assay buffer supplemented with 1 mM
phenylmethylsulfonyl fluoride. Protein (20 µg) was loaded
onto gels and separated by electrophoresis on a 10% SDS-PAGE gel,
the protein was then transferred to a polyvinylidene difluoride
membrane (250 mA, 2 h). The membranes containing protein blots were
incubated in blocking buffer (5% non-fat milk) for 1 h at room
temperature, prior to the addition of primary antibodies. Rabbit
primary antibodies against AKT serine/threonine kinase (AKT; cat.
no. 4685), p53 (cat. no. 2527), p21 (cat. no. 2947; Cell Signaling
Technology, Inc., Danvers, MA, USA) and CDKN3 (Abnova Corporation)
were used at a dilution of 1:500 and incubated overnight at 4°C.
The horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (EarthOx Life Sciences, Millbrae, CA, USA; cat. no.
E030120-01) was used in dilution of 1:5,000 and incubated at room
temperature for 1–2 h. Tris-buffered saline supplemented with 0.5%
Triton X-100 was used as the washing buffer. An ECL western
blotting substrate (Pierce; Thermo Fisher Scientific, Inc.) was
used to detect the bands and this was visualized with X-ray
film.
Statistical analysis
Data were collected, analyzed and presented as the
mean ± standard deviation. One-way analysis of variance and Tukey's
honest significant difference test was performed to compare
differences among multiple groups. Odds ratio and 95% confidence
interval were applied to verify the characteristic differences
between each group. Logistic regression analysis was used to
examine the association between CDKN3 expression and the tumor
clinical pathology index. Statistical analysis was conducted on
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
CDKN3 expression is downregulated in HCC
tissues
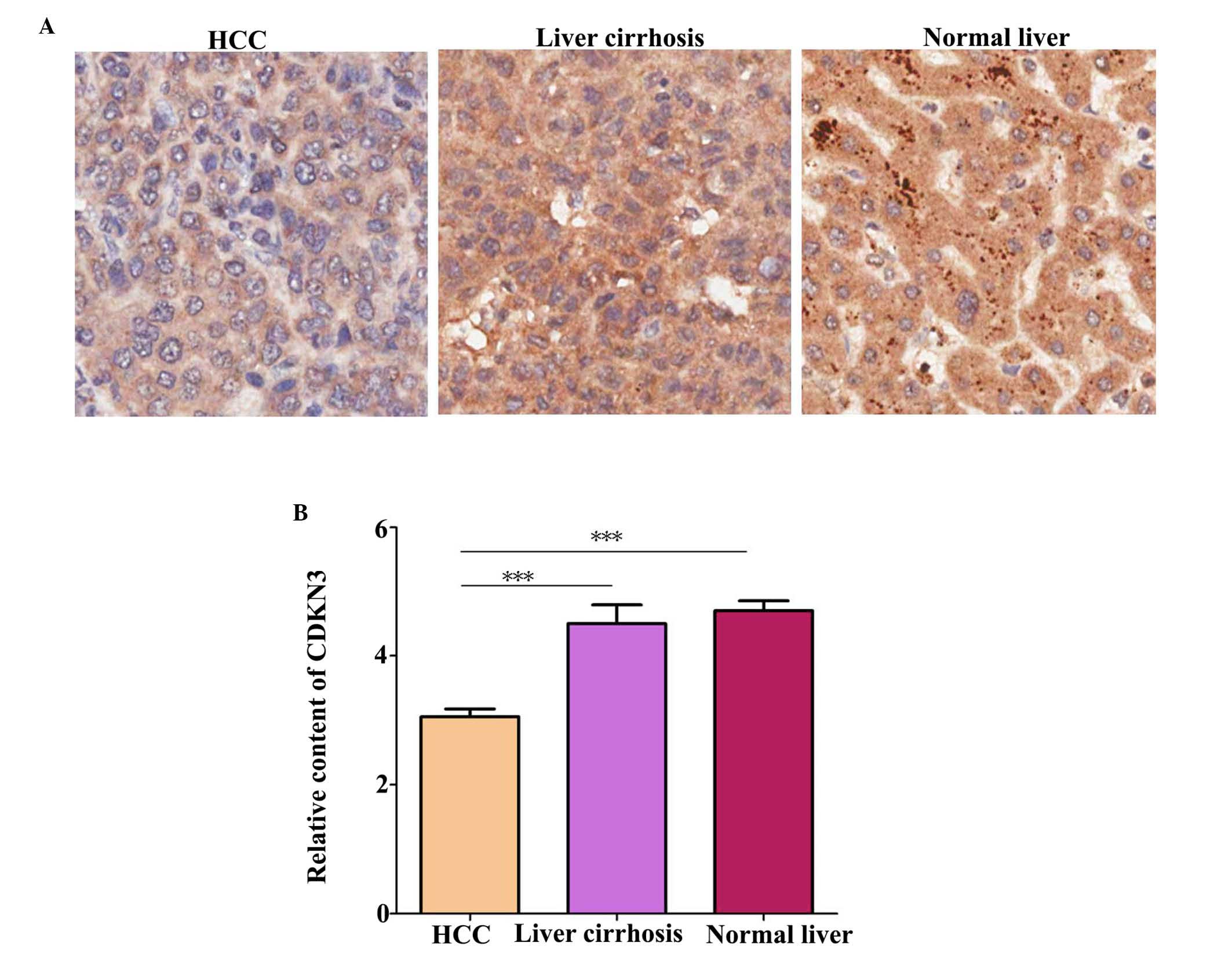
CDKN3 protein level in tissues was detected by IHC.
A total of 70 HCC tissue, 10 liver tissue and 6 liver cirrhosis
tissue samples were examined. The characteristics of the samples in
each group are presented in Table
I. Statistical analysis identified no significant difference in
age and gender among the groups. Notably, the hepatitis B virus
(HBV) infection ratio was significantly higher in HCC tissues
compared with normal liver tissues (P=0.041). Additionally, CDKN3
expression levels were higher in normal and liver cirrhosis tissues
compared with HCC tissues (Fig. 1;
P=0.00034).
CDKN3 expression is associated with tumor
stage
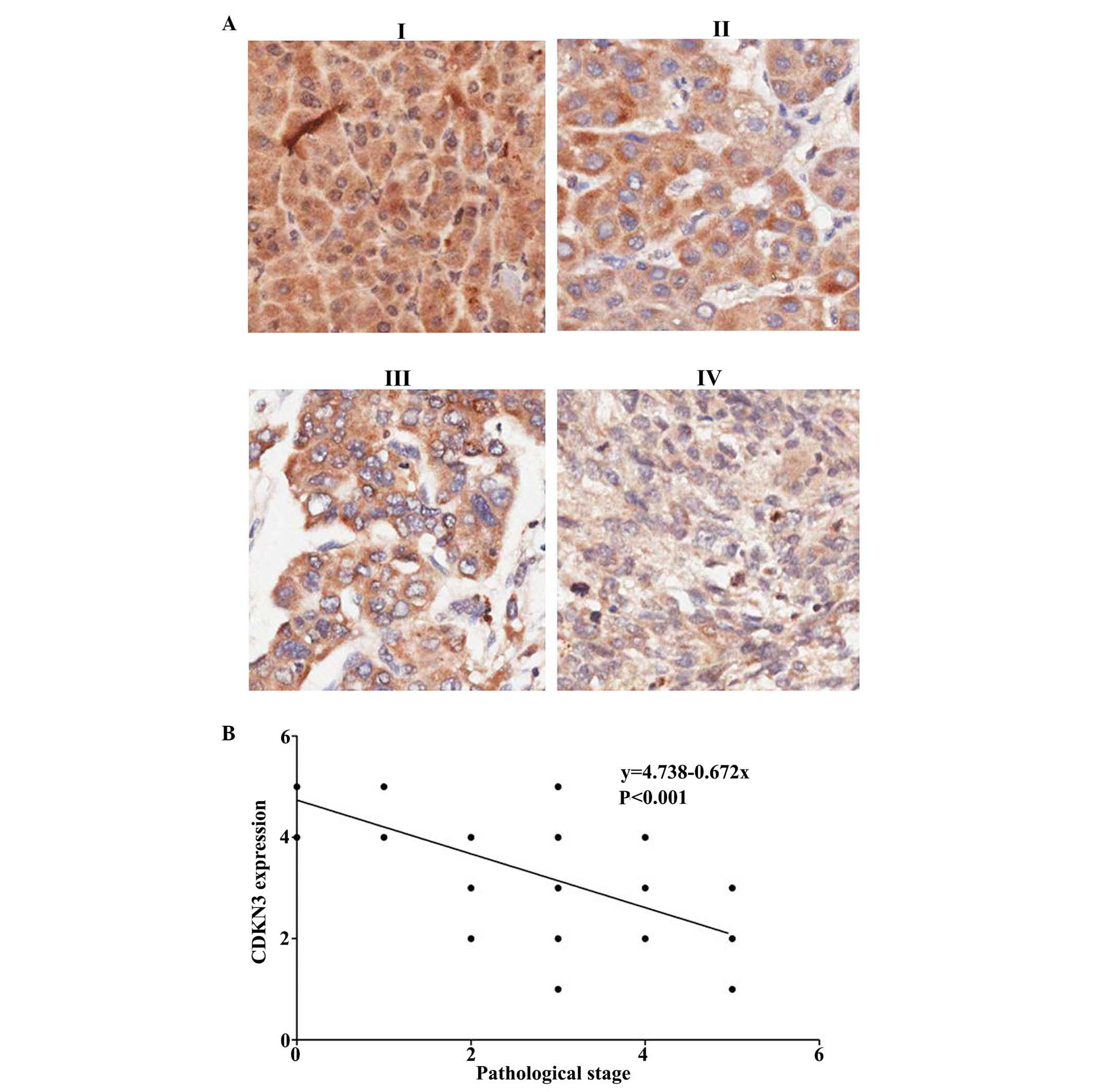
Tumor pathological stage was evaluated according to
the cell nuclear morphology and the karyoplasmic ratio. This is a
vital factor for cancer prognosis. A tumor at a high stage is
associated with poor prognosis. The HCC tumor stage was evaluated
and the association between CDKN3 expression and tumor stages was
analyzed (Fig. 2A). It was
determined that CDKN3 is negatively associated with tumor stage
(Fig. 2B; P<0.00026). Thus,
CDKN3 expression levels were downregulated in high stage cancer
with immature tumor cells.
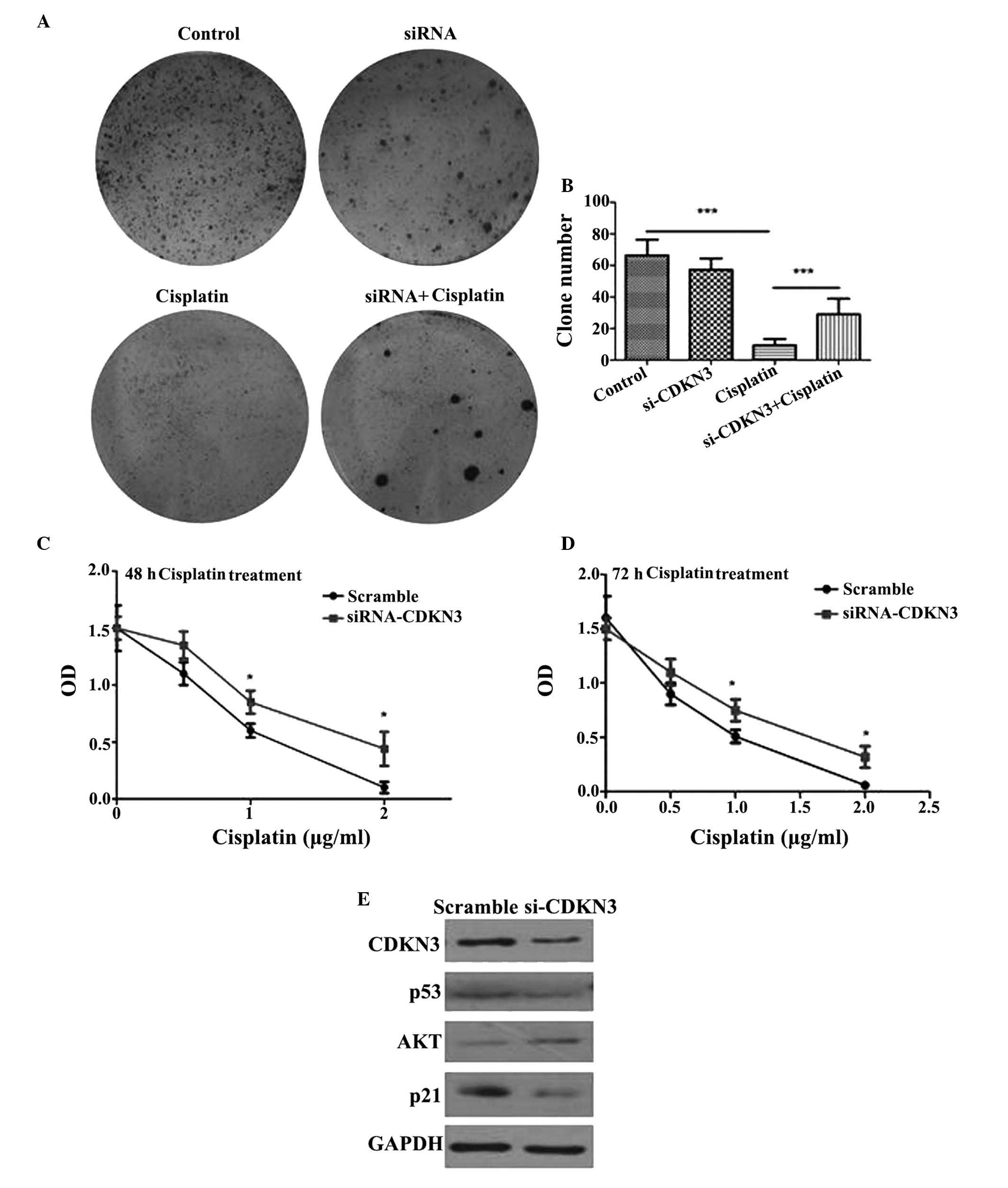
Depletion of CDKN3 increases cell
survival and cisplatin tolerance
To determinate the function of CDKN3 in QGY7701
cells, CDKN3 was knocked-down by transfection with CDKN3-specific
siRNA. The knockdown of CDKN3 enhanced the colony formation
capacity of the cells at 12 h. Larger tumor cell colonies formed
following CDKN3 knockdown compared with the control group, in
addition, the number of colonies also increased compared with the
control group (Fig. 3A and B).
Cisplatin is frequently used for the treatment of cancer. Thus,
QGY7701 cells were treated with cisplatin following CDKN3 knockdown
and cell viability was measured. QGY7701-KD cells exhibited a
higher tolerance to cisplatin compared with the control group
(P=0.024; Fig. 3C and D).
Knockdown of CDKN3 activates the
AKT/p53/p21 signaling pathway
The AKT/phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K) pathway was investigated for possible alterations
in signaling. It was demonstrated that AKT expression levels were
upregulated and the downstream proteins, including p53 and p21 were
decreased by CDKN3 siRNA compared with scramble controls (Fig. 3E). These results suggested that low
expression levels of CDKN3 promote the activation of the AKT/PI3K
signaling pathway and inhibit p53 and p21, which contributes to the
survival of tumor cells and increases their tolerance to cisplatin
cytotoxicity.
Discussion
Liver cancer is the second most common cause of
cancer-associated mortality and its incidence in China is
increasing. A previous epidemiological study demonstrated that
HBV-infection was the primary cause of liver cancer in China
(16). Further investigation of
liver carcinogenesis in Chinese patients is vital for the efficient
treatment of liver cancer.
Deregulation of the cell cycle results in
uncontrolled proliferation and apoptosis-resistant tumor cells.
Abnormal changes in cell cycle-associated proteins associated with
tumorigenesis. CDKN3 dephosphorylates threonine-161 of CDK1 during
mitosis, which is essential for normal G1/S transition. A previous
study identified that CDKN3 is downregulated in brain tumors
(15). However, previous studies
have also demonstrated high expression of CDKN3 in various types of
tumor (9,17,18).
Thus, the importance of CDKN3 in carcinogenesis has not been fully
elucidated. A previous study demonstrated overexpression of CDKN3
in liver cancer tissues, however, whether CDKN3 expression is
clinically important remains uncertain and the detailed molecular
mechanisms of CDKN3 in the development and progression of liver
cancer remain unclear.
The present study analyzed CDKN3 expression in HCC
tissues. The CDKN3 expression levels between normal and liver
cancer tissues were compared. CDKN3 expression was measured in 10
normal liver tissues, CDKN3 exhibited higher expression in normal
liver tissues compared with tumor tissues. The HBV infection ratio
was higher in patients with HCC tissues compared with controls, and
CDKN3 was consistently expressed at a higher level in normal liver
tissues compared with HCC tissues. The results of the current study
suggest that CDKN3 is a highly expressed in normal liver tissues.
Thus, downregulation of CDKN3 may enhance the progression of liver
cancer. It was also demonstrated, that CDKN3 expression was
associated with the differentiation stage of tumors, therefore,
CDKN3 may affect tumor proliferation, metamorphosis and
invasion.
To determine the function of CDKN3 in the tumor
cells, siRNA interference was performed to deplete CDKN3 levels in
liver cancer cells. To investigate the impact of CDKN3 expression
on the cell viability of tumor cells, MTT assay was performed to
determine the changes in cell viability. Inhibition of CDKN3 did
not have an effect on the cell viability; however, it did affect
the reactivity to cisplatin. It is possible that CDKN3 expression
promotes the transduction of apoptotic signals, which is important
for the elimination of unhealthy cells and prevention of malignant
transformation of cells. Colonic potential was determined by colony
formation assay and the knockdown of CDKN3 promoted the formation
of larger cell colonies. The AKT/p53/p21 signaling pathway is vital
for the survival of tumor cells, these proteins were quantified
using western blot analysis. It was demonstrated that depletion of
CDKN3 may lead to the activation of the AKT/p53/p21 signaling
pathway (19). Thus, high
expression levels of CDKN3 may be beneficial for normal cells. Low
expression of CDKN3 may be a marker for poor liver cancer
prognosis.
The results of the current study contrast with
previous studies (12,14). This may because previous studies
detected CDKN3 mRNA expression levels as opposed to protein
expression levels. Additionally, normal liver tissues were not
tested in the previous studies in order to confirm the changes of
CDKN3 expression. In the present study, CDKN3 expression was
associated with the pathological tumor stage. Therefore, the tumor
sample collection (tumor stage distribution and number of cases) is
important for correct conclusions to be reached. The current study
collected samples from 70 HCC cases with integral case information,
including tumor stage, age, gender and HBV infection status. CDKN3
protein was quantified using IHC. Characteristics, including age,
gender and HBV infection status of each group were examined using
odds ratio and 95% confidence interval. It was observed that there
was no difference in age and gender between the cancer and normal
groups, suggesting this does not result in the CDKN3 alteration.
However, the HBV infection ratio was higher in the cancer group
compared with normal people, however, further research is required
to elucidate the association between CDKN3 expression and HBV
infection. In conclusion, CDKN3 may act as a tumor suppressor in
liver tissues by modulation of the cell survival signal
transduction, monitoring carcinogenesis and elimination of abnormal
cells.
References
|
1
|
Shiraha H, Yamamoto K and Namba M: Human
hepatocyte carcinogenesis (review). Int J Oncol. 42:1133–1138.
2013.PubMed/NCBI
|
|
2
|
Zimonjic DB and Popescu NC: Role of DLC1
tumor suppressor gene and MYC oncogene in pathogenesis of human
hepatocellular carcinoma: Potential prospects for combined targeted
therapeutics (review). Int J Oncol. 41:393–406. 2012.PubMed/NCBI
|
|
3
|
Kirstein MM and Vogel A: The pathogenesis
of hepatocellular carcinoma. Dig Dis. 32:545–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meng X, Franklin DA, Dong J and Zhang Y:
MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res.
74:7161–7167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawaguchi M and Kataoka H: Mechanisms of
hepatocyte growth factor activation in cancer tissues. Cancers
(Basel). 6:1890–1904. 2014. View Article : Google Scholar
|
|
6
|
Xie B and Dong JH: HGF/c-Met and
metastasis of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za
Zhi. 13:396–398. 2005.In Chinese. PubMed/NCBI
|
|
7
|
Espinosa AM, Alfaro A, Roman-Basaure E,
Guardado-Estrada M, Palma Í, Serralde C, Medina I, Juárez E,
Bermúdez M, Márquez E, et al: Mitosis is a source of potential
markers for screening and survival and therapeutic targets in
cervical cancer. PLoS One. 8:e559752013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai MW, Chen TC, Pang ST and Yeh CT:
Overexpression of cyclin-dependent kinase-associated protein
phosphatase enhances cell proliferation in renal cancer cells. Urol
Oncol. 30:871–878. 2012. View Article : Google Scholar
|
|
9
|
Li T, Xue H, Guo Y and Guo K: CDKN3 is an
independent prognostic factor and promotes ovarian carcinoma cell
proliferation in ovarian cancer. Oncol Rep. 31:1825–1831.
2014.PubMed/NCBI
|
|
10
|
Taylor KJ, Sims AH, Liang L, Faratian D,
Muir M, Walker G, Kuske B, Dixon JM, Cameron DA, Harrison DJ and
Langdon SP: Dynamic changes in gene expression in vivo predict
prognosis of tamoxifen-treated patients with breast cancer. Breast
Cancer Res. 12:R392010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drozdov I, Bornschein J, Wex T, Valeyev
NV, Tsoka S and Malfertheiner P: Functional and topological
properties in hepa-tocellular carcinoma transcriptome. PLoS One.
7:e355102012. View Article : Google Scholar
|
|
12
|
Xing C, Xie H, Zhou L, Zhou W, Zhang W,
Ding S, Wei B, Yu X, Su R and Zheng S: Cyclin-dependent kinase
inhibitor 3 is over-expressed in hepatocellular carcinoma and
promotes tumor cell proliferation. Biochem Biophys Res Commun.
420:29–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Jiang X, Yu Y, Huang W, Xing H, Agar
NY, Yang HW, Yang B, Carroll RS and Johnson MD: KAP regulates ROCK2
and Cdk2 in an RNA-activated glioblastoma invasion pathway.
Oncogene. 34:1432–1441. 2015. View Article : Google Scholar
|
|
14
|
Yeh CT, Lu SC, Chen TC, Peng CY and Liaw
YF: Aberrant transcripts of the cyclin-dependent kinase-associated
protein phosphatase in hepatocellular carcinoma. Cancer Res.
60:4697–4700. 2000.PubMed/NCBI
|
|
15
|
Nalepa G, Barnholtz-Sloan J, Enzor R, Dey
D, He Y, Gehlhausen JR, Lehmann AS, Park SJ, Yang Y, Yang X, et al:
The tumor suppressor CDKN3 controls mitosis. J Cell Biol.
201:997–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou J, Liu Z and Gu F: Epidemiology and
prevention of hepatitis B virus infection. Int J Med Sci. 2:50–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Y, Jiang X, Schoch BS, Carroll RS,
Black PM and Johnson MD: Aberrant splicing of cyclin-dependent
kinase-associated protein phosphatase KAP increases proliferation
and migration in glioblastoma. Cancer Res. 67:130–138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SW, Reimer CL, Fang L, Iruela-Arispe
ML and Aaronson SA: Overexpression of kinase-associated phosphatase
(KAP) in breast and prostate cancer and inhibition of the
transformed phenotype by antisense KAP expression. Mol Cell Biol.
20:1723–1732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCubrey JA, Steelman LS, Franklin RA,
Abrams SL, Chappell WH, Wong EW, Lehmann BD, Terrian DM, Basecke J,
Stivala F, et al: Targeting the RAF/MEK/ERK, PI3 K/AKT and p53
pathways in hematopoietic drug resistance. Adv Enzyme Regul.
47:64–103. 2007. View Article : Google Scholar
|