Introduction
Multiple myeloma is a clonal malignancy of plasma
cells characterized by bone destruction, monoclonal proteins,
hypercalcemia, excess bone marrow plasma, renal damage and
immunodeficiency (1,2). The incidence of multiple myeloma
varies globally, from 1/100,00 individuals in China to ~4/100,000
individuals in developed countries (3). Patients with multiple myeloma will
often develop recurrence or an increased susceptibility to fungal,
viral and bacterial infections, which are the major cause of
multiple myeloma-associated mortality (4,5). The
survival rates of patients with multiple myeloma can now exceed 10
years as a result of therapy comprising hematopoietic stem cell
transplantation in combination with novel chemotherapeutic agents,
including thalidomide, lenalidomide and bortezomib (6–8).
However, chemotherapy often produces drug resistance and high
levels of toxicity, therefore, developing a more effective agent
remains a priority in the treatment of multiple myeloma.
Over previous decades, several natural products
derived from plants have shown promising structures for the
development of novel agents for use in cancer treatment.
Ginsenosides, a traditional Chinese medicine, have been reported to
exhibit antitumor properties (9,10).
Ginsenoside Rg3 (Rg3), a monomer derived from heat-processed
ginseng, has been found to have potent antitumor effects (11,12).
Although Rg3 has been reported to inhibit cancer cell proliferation
and induce cell death in melanoma (13), breast cancer (14), acute leukemia (15), glioma (16) and hepatocellular carcinoma
(17), the activity of Rg3 against
cell growth in multiple myeloma and its functional targets remain
to be fully elucidated.
The objectives of the present study were to
investigate the activity of Rg3 in inhibiting the growth of
multiple myeloma cell lines, and to elucidate the underlying
mechanisms. The results demonstrated the antiproliferative effect
of Rg3 against multiple myeloma cells. In addition, the mechanism
underlying the action of Rg3 was correlated with the inhibition of
secretion of insulin-like growth factor (IGF)-1 and inactivation of
the AKT/mammalian target of rapamycin (mTOR) signaling pathway. The
present study indicates that Rg3 may be a potential clinical
therapeutic agent for multiple myeloma.
Materials and methods
Materials and reagents
Ginsenoside Rg3 (Rg3) was purchased from Yatai
Pharmaceuticals Co., Ltd (Jilin, China) with 98% purity, assayed
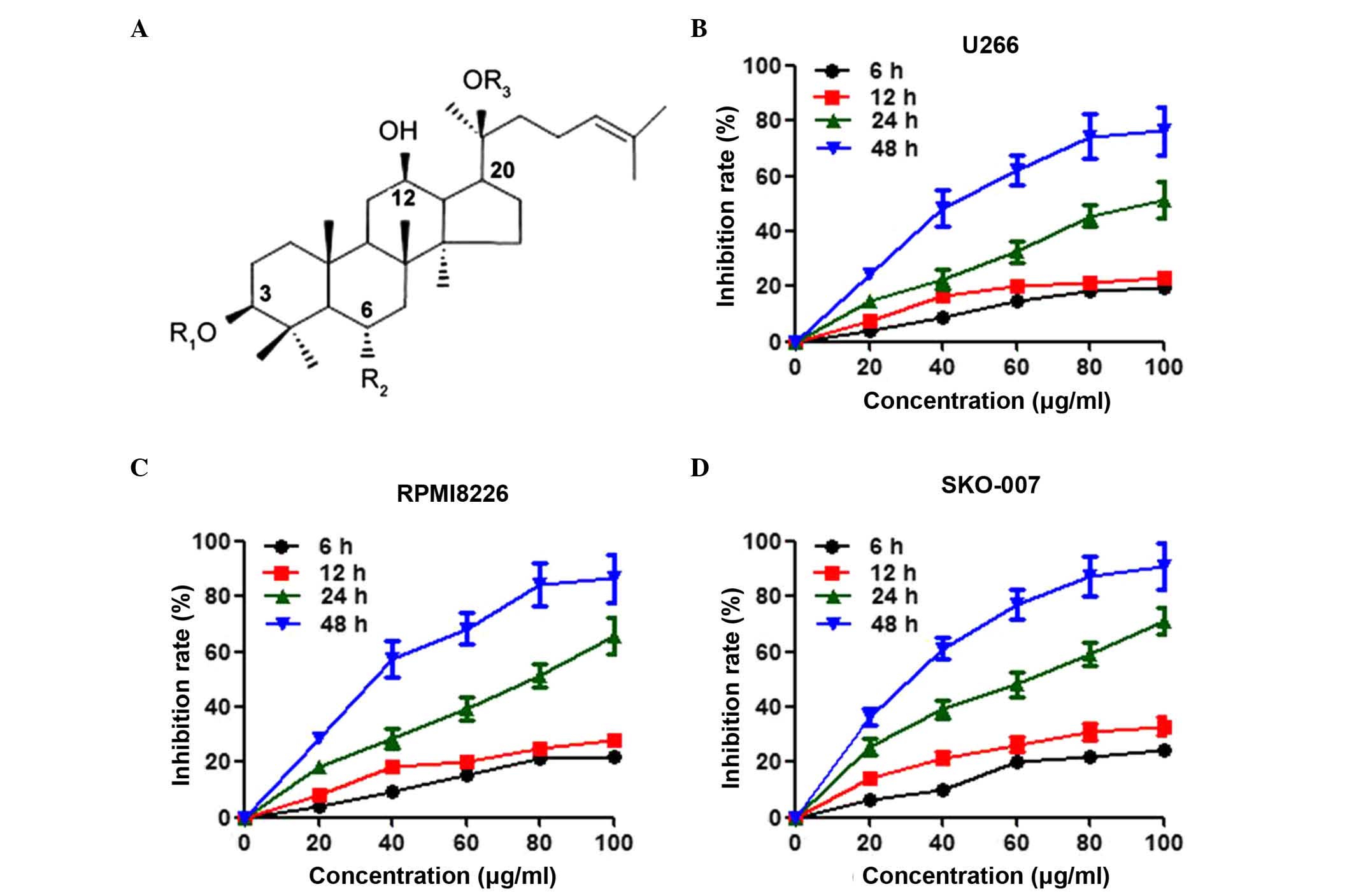
using high-performance liquid chromatography (HPLC; Fig. 1A). The powder was dissolved in
dimethyl sulfoxide (DMSO) in a stock concentration of 100 mg/ml.
The final concentration of DMSO in the culture medium was ≤0.1%.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin, streptomycin and phosphate-buffered saline (PBS)
were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Antibodies obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) were as follows, all used at 1:500 dilution:
Mouse anti-cyclin D1 monoclonal antibody (cat. no. sc-8396), mouse
anti-p27 monoclonal antibody (cat. no. sc-393380), mouse
anti-phosphorylated (phospho)-extracellular signal regulated kinase
(Erk)1/2 monoclonal antibody (cat. no. sc-7383), rabbit anti-Erk1/2
polyclonal antibody (cat. no. sc-292838), mouse anti-phospho-c-Jun
N-terminal kinase (JNK) monoclonal antibody (cat. no. sc-6254),
mouse anti-JNK monoclonal antibody (cat. no. sc-7345), rabbit
anti-phophos-p38 polyclonal antibody (cat. no. sc-17852-R), mouse
anti-p38 monoclonal antibody (cat. no. sc-81621) and rabbit
anti-IGF-1 polyclonal antibody (cat. no. sc-9013). Antibodies
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA)
were as follows, all used at 1:1,000 dilution: Mouse anti-B cell
lymphoma-2 (Bcl-2) monoclonal antibody (cat. no. 15071), rabbit
anti-Bcl-2-associated X protein (Bax) polyclonal antibody (cat. no.
5023), mouse anti-cytochrome C monoclonal antibody (cat. no.
12963), rabbit anti-cytochrome c oxidase (Cox) IV polyclonal
antibody (cat. no. 4850), mouse anti-caspase-9 monoclonal antibody
(cat. no. 9508), mouse anti-caspase-8 monoclonal antibody (cat. no.
9746), rabbit anti-caspase-3 polyclonal antibody (cat. no. 9665),
rabbit anti-phospho-AKT polyclonal antibody (cat. no. 5012), mouse
anti-AKT monoclonal antibody (cat. no. 2920), rabbit
anti-phospho-mTOR polyclonal antibody (cat. no. 2976) and mouse
anti-mTOR monoclonal antibody (cat. no. 2983). IGF-1, rabbit
polyclonal anti-retinoblastoma (Rb; cat. no. SAB4502589), mouse
monoclonal anti-phospho-Rb (cat. no. R6878) and rabbit polyclonal
anti-GAPDH (cat. no. G8795) were purchased from Sigma-Aldrich (St.
Louis, MO, USA) and used at 1:1,000 dilution. The Cell Counting
kit-8 assay (CCK-8), radioimmunoprecipitation assay (RIPA) lysis
buffer, bicinchoninic acid (BCA) kit, enhanced chemiluminescence
(ECL) system and Fluorescein isothiocyanate (FITC)-Annexin V
Apoptosis Detection kit were obtained from Beyotime Institute of
Biotechnology (Jiangsu, China).
Cell culture
The U266, RPMI8226 and SKO-007 human multiple
myeloma cell lines, were obtained from America Type Culture
Collection (Rockville, MD, USA) and maintained in DMEM supplemented
with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin at 37°C
in a 5% CO2 atmosphere.
Cell viability assay
Multiple myeloma cells were seeded in 96-well plates
at a density of 5×103 cells/well overnight and then
treated with Rg3 at different concentrations (0, 20, 40, 60, 80 and
100 µg/ml), and for different durations (6, 12, 24 and 48
h), as indicated. Subsequently, fresh medium containing 10
µl CCK-8 reagent was added, followed by incubation at 37°C
for 2 h. The absorbance of each well was measured at 450 nm on a
plate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Western blot analysis
The cells were washed with cold PBS and lysed with
lysis buffer. The protein concentration was determined using the
BCA kit. Equal quantities (40 µg) of protein were separated
on 12% SDS-PAGE gels (GenScript, Piscataway, NJ, USA) and then
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk for 1 h, and then probed with appropriate primary antibodies
overnight at 4°C, followed by blotting with horseradish peroxidase
(HRP)-labeled goat anti-mouse IgG (cat. no. A0216) or HRP-labeled
goat anti-rabbit IgG (cat. no. A0208) secondary antibodies
(1:1,000; Beyotime Institute of Biotechnology) at room temperature
for 1 h. The target bands were detected using the ECL system, and
the band intensity was determined using ImageJ software (version
1.41; NIH, Bethesda, MD, USA).
Cell cycle analysis
The cells were harvested by centrifugation and
processed for cell cycle analysis, as described previously
(3). Briefly, the cells were
digested with 0.25 g/l trypsin and harvested by centrifugation at
1,000 × g for 10 min at room temperature. Following incubation with
70% ethanol at −20°C for 15 min, the cells were stained with 20
mg/ml propidium iodide (PI) and incubated for 30 min at room
temperature. The cells were analyzed for DNA content using
FACScalibur flow cytometry (BD Biosciences, San Jose, CA, USA). The
percentages of cells containing different DNA contents were
quantified using CellQuest software (version 5.1; BD
Biosciences).
Apoptosis detection
The cellular apoptotic ratios were detected with the
FITC-Annexin V Apoptosis Detection kit using flow cytometry.
Briefly, the cells were trypsinized and harvested by
centrifugation. The cell pellets were re-suspended in a binding
buffer of Annexin V-FITC and PI at room temperature in the dark for
15 min. The apoptotic cells were counted using flow cytometry (BD
Biosciences), with the percentage of apoptotic cells expressed as
the FITC/PI ratio.
Isolation of mitochondria
The isolation of mitochondrial and cytoplasmic
proteins was performed using a Mitochondria Isolation kit (Thermo
Fisher Scientific Inc.), according to the manufacturer's protocol.
The cytosolic and mitochondrial fractions were analyzed using
western blot analysis. Cox IV was used as an internal control for
the mitochondrial fraction.
ELISA assay
The concentrations of IGF-1 were determined using a
Human IGF-I Quantikine ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
The absorbance was measured at a wavelength of 450 nm using a
microplate reader (Bio-Tek Instruments, Inc.).
Statistical analysis
All data are presented as the mean ± standard error
of the mean, and the n value indicates the number of independent
experiments. Data were statistically analyzed using Student's
t-test on GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Rg3 on the viability of
multiple myeloma cell lines
The uncontrolled cell proliferation of cancer cells
is vital in the progression of cancer, therefore, the present study
evaluated the effects of Rg3 on the viability of multiple myeloma
cells. As shown in Fig. 1B,
treatment with Rg3 inhibited the viability of the U266 cells in a
time- and dose-dependent manner. The inhibition of cell viability
in the U266 cells following treatment with Rg3 for 48 h reached the
maximal level at 80 µg/ml, and the half maximal inhibitory
concentration (IC50) value was 47.5 mg/l. In addition to U266, two
other multiple myeloma cell lines, RPMI8226 and SKO-007, were also
included in the present study to examine these effects. Rg3 had a
more potent effect in inhibiting the viability of the RPMI8226
(IC50=36.8 µg/ml) and SKO-007 (IC50=31.5 µg/ml)
cells, compared with the U266 cells (Fig. 1C and D).
Rg3 arrests the cell cycle of multiple
myeloma cells
Flow cytometric cell cycle analysis was performed
using PI staining to determine the effect of Rg3 on cell cycle
progression. Treatment of the U266 cells with 20, 40 and 80
µg/ml Rg3 increased the percentage of the cell population in
the G1 phase and decreased the percentage in the S
phase. However, Rg3 had no effect on the G2/M phase (Fig. 2A and B). The effect of Rg3 on cell
cycle progression was also examined in the SKO-007 cells (Fig. 2C and D). Similarly, the cell cycle
of the SKO-007 cells was arrested in the G1 phase by
Rg3, indicating that Rg3 may inhibit multiple myeloma cell
proliferation through suppressing the cell cycle transition from
the G1 to the S phase. The cell cycle transition between
the G1 and S phase is strictly regulated by the balance
of cyclins and cyclin-dependent kinase inhibitors (18,19).
Therefore, to further investigate the mechanisms underlying how Rg3
arrested G1/S transition, the expression levels of
proteins regulating cell cycle progression in U266 cells, including
cyclin D1, p27 and phospho-Rb, were determined. The results of the
western blot analysis showed that Rg3 decreased the protein
expression of cyclin D1 and phosphorylation of Rb, and increased
the expression of p27 in a dose-dependent manner (Fig. 2E and F).
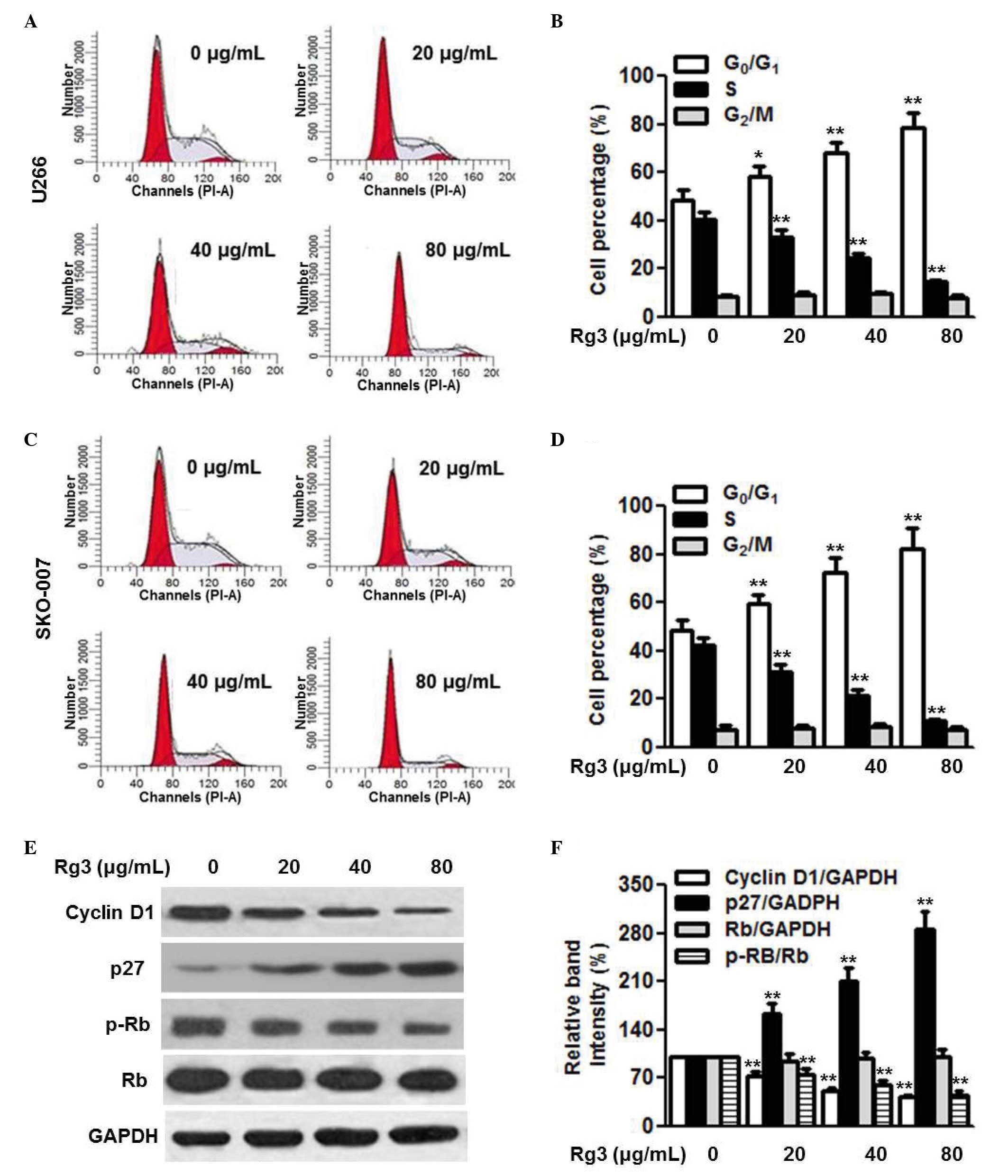 | Figure 2Rg3 arrests cell cycle at the G1/S
transition. Multiple myeloma cells were incubated with different
concentrations of Rg3 (20, 40 and 80 µg/ml) for 48 h. Cell
cycle was analyzed using flow cytometry. (A) Representative images
of cell cycle distribution in the U266 cells, with (B) statistical
analysis of the percentages of cells in the G0/G1, S and G2/M
phases. (C) Cell cycle distribution of the SKO-007 cells with (D)
statistical analysis of the percentages of cells in the G0/G1, S
and G2/M phases Expression levels of cycle-associated proteins were
examined using western blot analysis in U266 cells. (E)
Representative images of the western blot are shown, with the
results of (F) densitometric analysis. Data are expressed as the
mean ± standard error of the mean (n=6). 0 µg/ml indicates
the dimethyl sulfoxide vehicle, *P<0.05 and
**P<0.01, vs. 0 µg/ml group. Rg3, ginsenoside
Rg3; Rb, retinoblastoma; p-, phosphorylated. |
Rg3 induces multiple myeloma cells
apoptosis via the mitochondria-dependent pathway
To examine the effect of Rg3 on cell survival, its
effects on cell apoptosis were determined. Annexin V-FITC/PI
staining followed by flow cytometric analysis revealed that
treatment of the U266 cells with 20, 40 and 80 µg/ml Rg3 for
48 h significantly increased the percentage of apoptotic cells in
the in U266 cell population (Fig. 3A
and B). Similar results were observed in the RPMI8226 and
SKO-007 cells (data not shown). Bcl-2 and Bax are anti-apoptotic
and pro-apoptotic proteins, and the ratio of Bcl-2/Bax appears to
be a determinant of cell survival and death. To understand the
mechanism by which Rg3 induces cell apoptosis, the expression
levels of Bcl-2 and Bax, and the ratio of Bcl-2 to Bax were
measured. The results of the western blot analysis showed that Rg3
treatment markedly decreased the protein expression of Bcl-2 and
increased the protein expression of Bax in the U266 cells,
resulting in a further decrease in the Bcl-2/Bax ratio (Fig. 3C and D). The mitochondria-dependent
signaling pathway is critical for cell apoptosis. The release of
cytochrome C from the mitochondria into the cytoplasm triggers the
downstream apoptotic signal, consequently resulting in cell
apoptosis (20). Therefore, the
present study examined the release of cytochrome C. As expected,
Rg3 treatment caused a significant decrease in the protein
expression of cytochrome C in the mitochondria, and an increase in
the cytoplasm (Fig. 3E and F).
Cytochrome C release sequentially activates downstream
apoptosis-associated proteins, including caspases. Western blot
analysis demonstrated that Rg3 increased the protein expression
levels of cleaved caspase-9, caspase-8 and caspase-3 (Fig. 3G and H). Collectively, these data
suggested that mitochondrial dysfunction may underlie, at least
partially, the enhanced effect of Rg3 on myeloma cell
apoptosis.
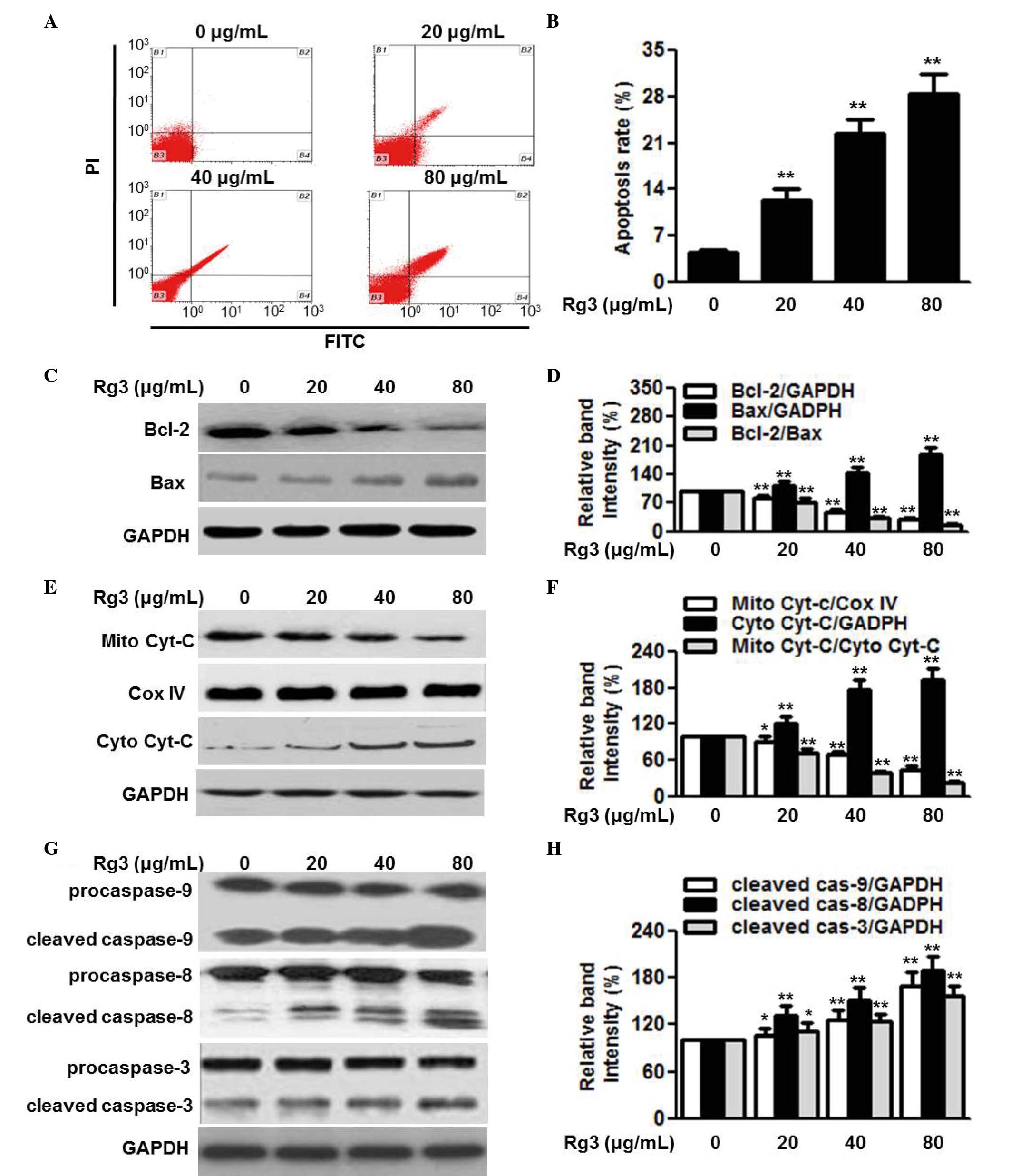 | Figure 3Rg3 induces cell apoptosis via the
mitochondria-dependent pathway. (A) U266 cells were treated with
different concentrations of Rg3 (20, 40 and 80 µg/ml) for 48
h. Cell apoptosis was determined using Annexin V/PI staining
followed by flow cytometry. (B) Quantitative analysis of the
percentage of apoptotic cells. (C) Expression levels of Bcl-2 and
Bax were examined using western blot analysis. Representative
western blot images are shown. (D) Densitometric analysis of Bcl-2,
Bax and the Bcl-2/Bax ratio. (E) Protein expression levels of Cyt-C
in the mitochondria (Mito) and cytoplasm (Cyto) were determined
using western analysis. Cox IV was used as a loading control for
mitochondrial protein. (F) Densitometric analysis of the
mitochondrial expression of cytochrome C and cytosol cytochrome C,
and release of cyt C from the mitochondria into the cytoplasm. (G)
Cleaved caspase-9, -8 and -3 were measured by western blotting and
(H) analyzed by densitometry. Data are expressed as the mean ±
standard error of the mean (n-6). 0 µg/ml indicates dimethyl
sulfoxide vehicle, *P<0.05 and
**P<0.01, vs. 0 µg/ml group. Cyt C, cytochrome
C; Rg3, ginsenoside Rg3; Bcl-2, B cell lymphoma-2; Bax,
Bcl-2-associated X protein; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
AKT/mTOR, but not MAP kinase, signaling
is involved in the action of Rg3 on multiple myeloma cell
proliferation and survival
The phosphorylation of AKT and downstream mTOR has
been reported to be involved in the progression of several types of
malignancy, including multiple myeloma (3,21).
To clarify the signal transduction pathways by which Rg3 exerts its
antitumor effects, the present study first examined the activation
of the AKT/mTOR pathway. As shown in Fig. 4A and B, the phosphorylation of AKT
and its downstream protein, mTOR, was attenuated by Rg3 treatment
in a dose-dependent manner. MAP kinase signaling is also a
regulator of cell cycle progression and tumorigenesis (22,23).
However, Rg3 treatment did not alter the phosphorylation of Erk1/2,
JNK or p38 (Fig. 4C and D). These
data excluded the possibility that MAP kinase signaling was
involved in the effects of Rg3 on multiple myeloma cell
proliferation and survival.
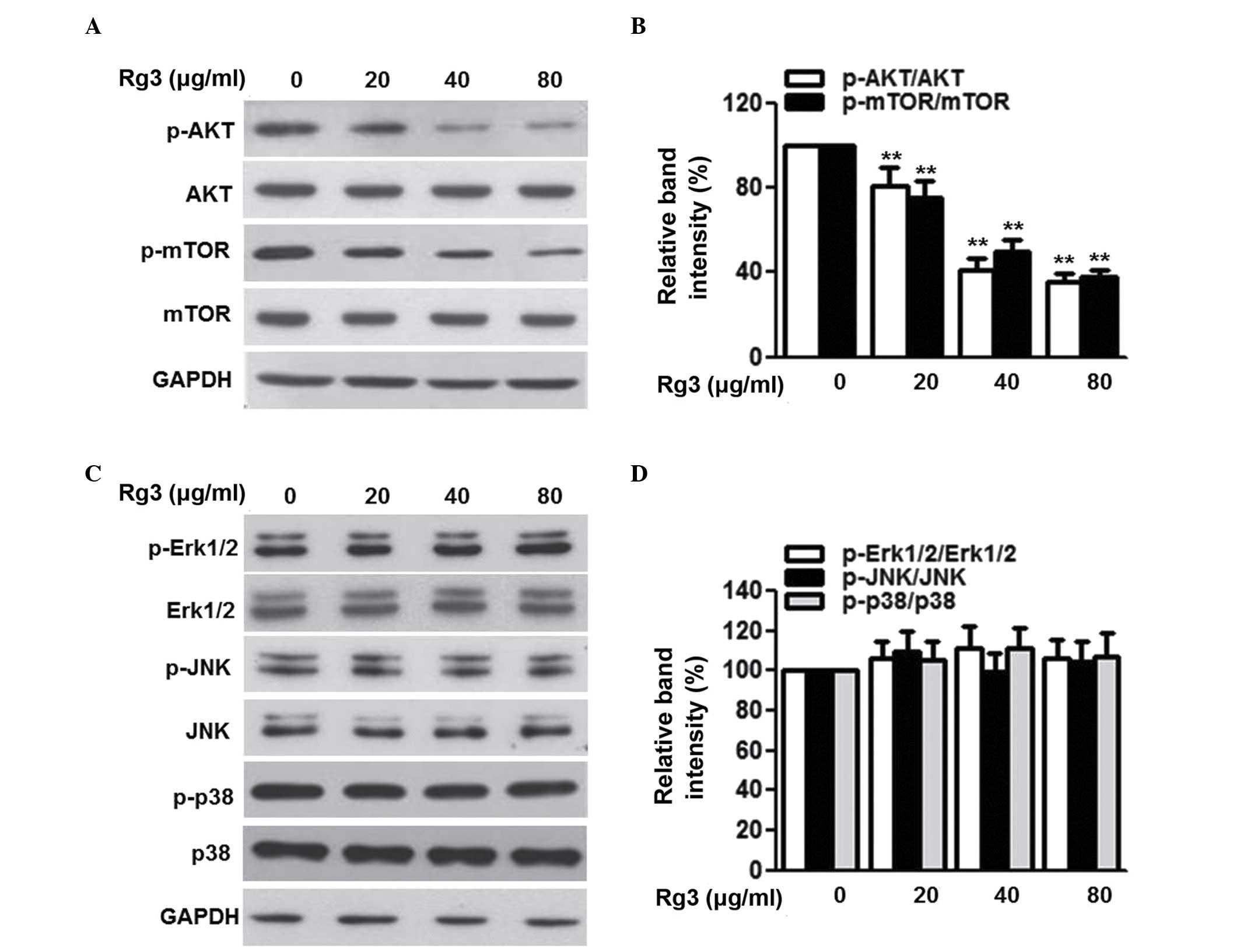 | Figure 4Rg3 inhibits the phosphorylation of
AKT and mTOR, but not mitogen-activated protein kinases, in U266
cells. The cells were treated with different concentrations of Rg3
(20, 40 and 80 µg/ml) for 48 h. (A) Expression and
phosphorylation of AKT and mTOR were examined using western blot
analysis, and (B) densitometric analysis was performed. (C)
Expression levels and phosphorylation of Erk1/2, JNK and p38 were
determined using western blot analysis, and (D) densitometric
analysis was performed. Data are expressed as the mean ± standard
error of the mean (n-6). 0 µg/ml indicates the dimethyl
sulfoxide vehicle, **P<0.01, vs. 0 µg/ml
group. Rg3, ginsenoside Rg3, mTOR, mammalian target of rapamycin;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; p-, phosphorylated. |
Rg3 attenuates AKT/mTOR activation via
inhibiting the secretion of IGF-1
IGF-1 is an important pathway of AKT/mTOR (24). Therefore, the present study
investigated whether Rg3 affects this pathway. The results of the
western blot analysis showed that Rg3 had no effect on the protein
expression of IGF-1 (Fig. 5A).
However, the secretion of IGF-1 was markedly decreased following
Rg3 treatment (Fig. 5B). In
addition, treatment with IGF-1 reversed the Rg3-induced
inactivation of the AKT/mTOR pathway, as evidenced by the
significant restoration in AKT and mTOR phosphorylation (Fig. 5C and D). The present study further
examined whether IGF-1 was involved in the effects of Rg3 on cell
proliferation and survival. The results of the CCK-8 assay revealed
that the reduction in cell viability induced following Rg3
treatment was gradually inhibited by IGF-1 in a dose-dependent
manner (Fig. 5E). As expected,
Rg3-induced cell apoptosis was almost eliminated by the addition of
IGF-1 (Fig. 5F).
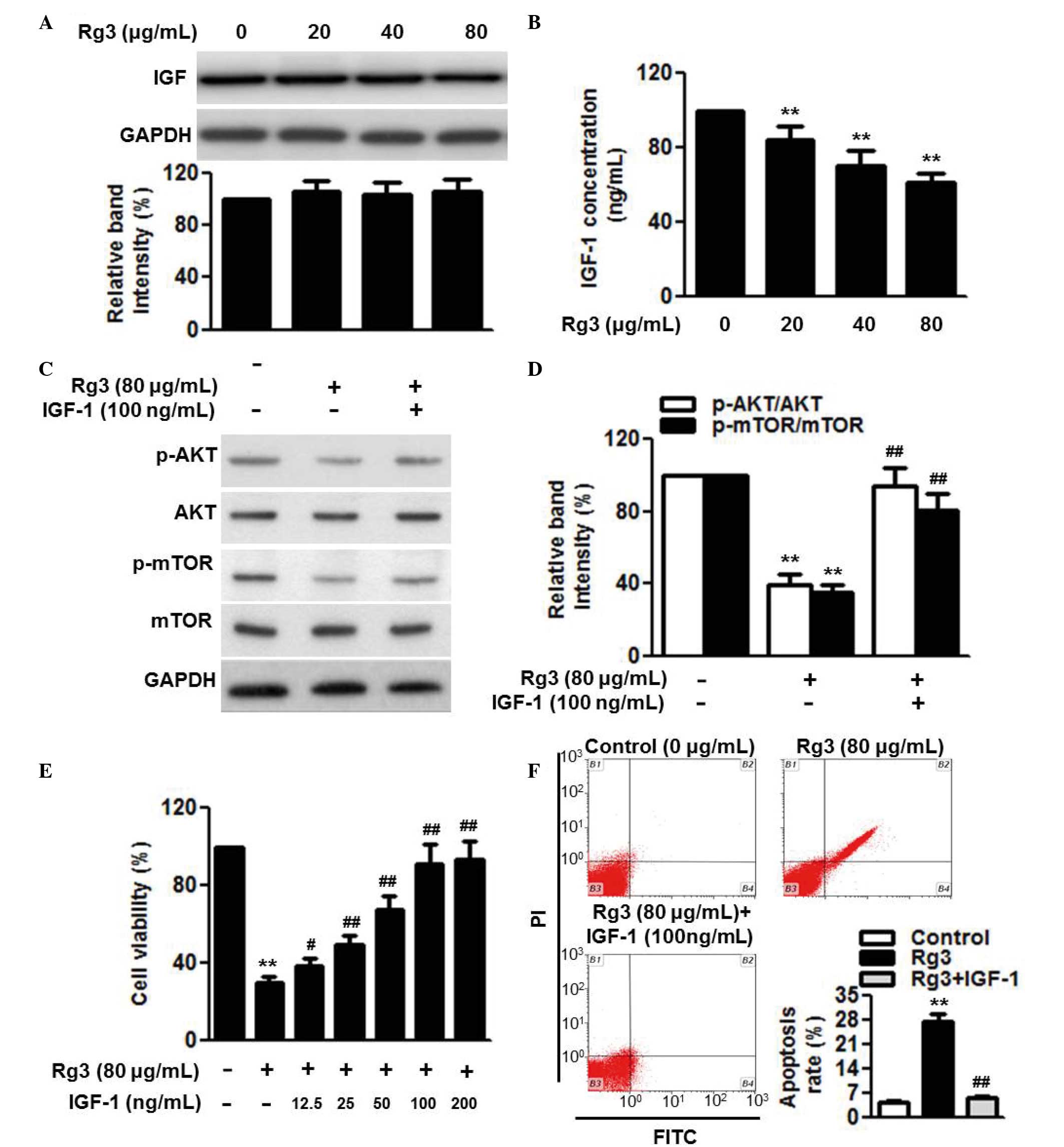 | Figure 5IGF-1 secretion is involved in the
effects of Rg3 on cell proliferation and apoptosis. U266 cells were
treated with different concentrations of Rg3 (20, 40 and 80
µg/ml) for 48 h. (A) Expression and (B) secretion of IGF-1
were measured using western blot analysis and an ELISA assay,
respectively. Cells were incubated with Rg3 (80 µg/ml) for
48 h in the presence or absence of IGF-1 (100 ng/ml). (C)
Expression levlels and the phosphorylation of AKT and mTOR were
examined using western blot analysis, and (D) densitometric
analysis was performed (E) Effects of IGF-1 (12.5, 25, 50, 100 and
200 µg/ml) on the regulation of cell proliferation by Rg3
treatment (80 µg/ml) were examined. (F) Effects of IGF-1
(100 µg/ml) on the regulation of cell apoptosis by Rg3
treatment (80 µg/ml) were examined. Data are expressed as
the mean ± standard error of the mean (n=6). 0 µg/ml
indicates the dimethyl sulfoxide vehicle, **P<0.01,
vs. 0 µg/ml group; #P<0.05,
##P<0.01, vs. 80 µg/ml group. Rg3, ginsenoside
Rg3, IGF-1, insulin-like growth factor-1; mTOR, mammalian target of
rapamycin; p-, phosphorylated; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
Several experimental and clinical studies have been
performed on the effects of Rg3 on cancer. These studies have
identified Rg3 as being effective in the treatment of certain types
of cancer. Shen Yi capsule has been approved by the China Food and
Drug Administration, with Rg3 as the active pharmaceutical
ingredient, for clinical use in cancer treatment (25). However, the specific functions of
Rg3 in the treatment of multiple myeloma remain to be fully
elucidated. The present study provided evidence of the
anti-multiple myeloma activity of Rg3 in cultured cells. The data
revealed that Rg3 inhibited the proliferation of the U266, RPMI8226
and SKO-007 human multiple myeloma cell lines, and induced cellular
apoptosis, which were consistent with previous studies (26,27).
The data obtained in the present study revealed that
the anti-proliferative effect of Rg3 in multiple myeloma cells was
due to inhibition of the cell cycle transition between the
G1 and S phases. Cell cycle progression is strictly
regulated by cyclin-dependent kinases 4 and 6, which are activated
by cyclin D1, but attenuated by p27 (28). In addition, the activation of
cyclin-dependent kinases 4 and 6 can lead to the phosphorylation of
Rb and consequently promote the transition between the
G1 and S phase (3). In
the present study, it was found that the expression of cyclin D1
and the phosphorylation of Rb were attenuated by Rg3, whereas the
expression of p27 was elevated. The apoptotic process is usually
associated with the imbalance between the levels of Bcl-2 and Bax
(29). During apoptotic
stimulation, the increased expression of Bax enhances membrane
permeability, which results in the release of cytochrome C from the
mitochondria into cytoplasm, and activates a family of proteases,
including caspase-9, caspase-8 and caspase-3, driving the cell
toward apoptosis (30,31). The present study showed that Rg3
treatment induced the release of mitochondrial cytochrome C into
the cytoplasm, increased the protein expression levels of cleaved
caspase-9, caspase-8 and caspase-3, and decreased the Bcl-2/Bax
ratio. These data indicated that Rg3-induced apoptosis in multiple
myeloma cells was mitochondria-dependent.
A previous study demonstrated that Rg3 induced U266
human multiple myeloma cell apoptosis through activation of the Bax
protein (26). In addition, Song
et al (27) reported that
inhibition of the secretion of vascular endothelial growth factor
may contribute to the anti-proliferative effects of Rg3. However,
apart from these reports, there is no more information regarding
the mechanisms underlying the functions of Rg3 in inhibiting the
growth of multiple myeloma cells. Deregulation of the Akt/mTOR and
MAPK pathway is a common event in human cancer, and is crucial for
tumor cell proliferation, cell cycle transition and apoptosis
(32,33). Notably, the results of the present
study provided the first evidence, to the best of our knowledge,
that Rg3 affected cell proliferation and survival, predominantly
via Akt/mTOR pathway, and less via the MAPK pathway. The detailed
mechanisms to explain why Rg3 is linked less with MAPK and more
with Akt/mTOR remain to be elucidated, and further investigations
are required. In addition, the abnormal secretion of IGF-1 has been
documented to be a tumorigenic factor (21). In the present study, it was found
that Rg3 inhibited the secretion of IGF-1, but did not alter its
expression. IFG-1 is essential for the inhibitory effect of Rg3 on
activation of the AKT/mTOR pathway, suggesting that Rg3 mediated
cell proliferation and survival through IGF-1/AKT/mTOR
signaling.
In conclusion, the present study demonstrated that
Rg3 acted on the IGF-1/AKT/mTOR signal transduction pathway to
inhibit multiple myeloma cell proliferation. These results provided
evidence to support further investigation for the development of
Rg3 as a clinical drug candidate in the treatment of multiple
myeloma.
References
|
1
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Martino MT, Gullà A, Cantafio ME,
Lionetti M, Leone E, Amodio N, Guzzi PH, Foresta U, Conforti F,
Cannataro M, et al: In vitro and in vivo anti-tumor activity of
miR-221/222 inhibitors in multiple myeloma. Oncotarget. 4:242–255.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang EW, Xue SJ, Li XY, Xu SW, Cheng JD,
Zheng JX, Shi H, Lv GL, Li ZG, Li Y, et al: EEN regulates the
proliferation and survival of multiple myeloma cells by
potentiating IGF-1 secretion. Biochem Biophys Res Commun.
447:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar S and Rajkumar SV: Thalidomide and
lenalidomide in the treatment of multiple myeloma. Eur J Cancer.
42:1612–1622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson PG, Mitsiades C, Schlossman R,
Ghobrial I, Hideshima T, Munshi N and Anderson KC: Bortezomib in
the front-line treatment of multiple myeloma. Expert Rev Anticancer
Ther. 8:1053–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hideshima T, Chauhan D, Shima Y, Raje N,
Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, et
al: Thalidomide and its analogs overcome drug resistance of human
multiple myeloma cells to conventional therapy. Blood.
96:2943–2950. 2000.PubMed/NCBI
|
|
8
|
Brown RE, Stern S, Dhanasiri S and Schey
S: Lenalidomide for multiple myeloma: Cost-effectiveness in
patients with one prior therapy in England and Wales. Eur J Health
Econ. 14:507–514. 2013. View Article : Google Scholar
|
|
9
|
Lee JY, Jung KH, Morgan MJ, Kang YR, Lee
HS, Koo GB, Hong SS, Kwon SW and Kim YS: Sensitization of
TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated
DR5 upregulation in human hepatocellular carcinoma cells. Mol
Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
10
|
Park EH, Kim YJ, Yamabe N, Park SH, Kim
HK, Jang HJ, Kim JH, Cheon GJ, Ham J and Kang KS: Stereospecific
anticancer effects of ginsenoside Rg3 epimers isolated from
heat-processed American ginseng on human gastric cancer cell. J
Ginseng Res. 38:22–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.
|
|
12
|
Min JK, Kim JH, Cho YL, Maeng YS, Lee SJ,
Pyun BJ, Kim YM, Park JH and Kwon YG: 20(S)-Ginsenoside Rg3
prevents endothelial cell apoptosis via inhibition of a
mitochondrial caspase pathway. Biochem Biophys Res Commun.
349:987–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shan X, Fu YS, Aziz F, Wang XQ, Yan Q and
Liu JW: Ginsenoside Rg3 inhibits melanoma cell proliferation
through down-regulation of histone deacetylase 3 (HDAC3) and
increase of p53 acetylation. PLoS One. 9:e1154012014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim BM, Kim DH, Park JH, Surh YJ and Na
HK: Ginsenoside Rg3 inhibits constitutive activation of NF-kB
signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as
potential upstream targets. J Cancer Prev. 19:23–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng D, Wang J, Kong P, Chang C and Li J
and Li J: Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in
patient with acute leukemia via inhibiting the activation of
PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 7:2172–2178.
2014.
|
|
16
|
Sin S, Kim SY and Kim SS: Chronic
treatment with ginsenoside Rg3 induces Akt-dependent senescence in
human glioma cells. Int J Oncol. 41:1669–1674. 2012.PubMed/NCBI
|
|
17
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sedding DG, Hermsen J, Seay U, Eickelberg
O, Kummer W, Schwencke C, Strasser RH, Tillmanns H and
Braun-Dullaeus RC: Caveolin-1 facilitates mechanosensitive protein
kinase B (Akt) signaling in vitro and in vivo. Circ Res.
96:635–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Héron-Milhavet L, Franckhauser C, Rana V,
Berthenet C, Fisher D, Hemmings BA, Fernandez A and Lamb NJ: Only
Akt1 is required for proliferation, while Akt2 promotes cell cycle
exit through p21 binding. Mol Cell Biol. 26:8267–8280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuwabara I, Kuwabara Y, Yang RY, Schuler
M, Green DR, Zuraw BL, Hsu DK and Liu FT: Galectin-7 (PIG1)
exhibits pro-apoptotic function through JNK activation and
mitochondrial cytochrome c release. J Biol Chem. 277:3487–3497.
2002. View Article : Google Scholar
|
|
21
|
Cea M, Cagnetta A, Fulciniti M, Tai YT,
Hideshima T, Chauhan D, Roccaro A, Sacco A, Calimeri T, Cottini F,
et al: Targeting NAD+ salvage pathway induces autophagy in multiple
myeloma cells via mTORC1 and extracellular signal-regulated kinase
(ERK1/2) inhibition. Blood. 120:3519–3529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su F, Viros A, Milagre C, Trunzer K,
Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT,
et al: RAS mutations in cutaneous squamous-cell carcinomas in
patients treated with BRAF inhibitors. N Engl J Med. 366:207–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wajapeyee N, Serra RW, Zhu X, Mahalingam M
and Green MR: Oncogenic BRAF induces senescence and apoptosis
through pathways mediated by the secreted protein IGFBP7. Cell.
132:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palazzolo I, Stack C, Kong L, Musaro A,
Adachi H, Katsuno M, Sobue G, Taylor JP, Sumner CJ, Fischbeck KH
and Pennuto M: Overexpression of IGF-1 in muscle attenuates disease
in a mouse model of spinal and bulbar muscular atrophy. Neuron.
63:316–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Lin H, Zhu Y, Feng J, Chen Z, Li G,
Zhang X, Zhang Z, Tang J, Shi M, et al: A randomized, prospective,
multi-centre clinical trial of NP regimen (vinorelbine+cisplatin)
plus Gensing Rg3 in the treatment of advanced non-small cell lung
cancer patients. Zhongguo Fei Ai Za Zhi. 9:254–258. 2006.In
Chinese. PubMed/NCBI
|
|
26
|
Luo Y, Zhang P, Zeng HQ, Lou SF and Wang
DX: Ginsenoside Rg3 induces apoptosis in human multiple myeloma
cells via the activation of Bcl-2-associated X protein. Mol Med
Rep. 12:3557–3562. 2015.PubMed/NCBI
|
|
27
|
Song Y, Hou J, Kang L and Gao S: Effect of
20 (S)-ginsenoside Rg3 on the proliferation inhibition and
secretion of vascular endothelial growth factor of multiple myeloma
cell line U266. Zhonghua Xue Ye Xue Za Zhi. 35:519–523. 2014.In
Chinese. PubMed/NCBI
|
|
28
|
Woo RA and Poon RY: Cyclin-dependent
kinases and S phase control in mammalian cells. Cell Cycle.
2:316–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Misiti F, Orsini F, Clementi ME, Lattanzi
W, Giardina B and Michetti F: Mitochondrial oxygen consumption
inhibition importance for TMT-dependent cell death in
undifferentiated PC12 cells. Neurochem Int. 52:1092–1099. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mignotte B and Vayssiere JL: Mitochondria
and apoptosis. Eur J Biochem. 252:1–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharp ZD and Bartke A: Evidence for
down-regulation of phosphoinositide 3-kinase/Akt/mammalian target
of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory
signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med
Sci. 60:293–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|