Introduction
The development of obesity is an international
problem. Abnormal secretion of adipokines, excessive deposition of
adipose cells in addition to a significant increase in
intracellular lipid serve causative roles in obesity; adipokines
are currently regarded as important factors in this process. As
bioactive peptides, adipokines are critically involved in the
energy metabolism and systemic insulin sensitivity. Various
adipokines have been demonstrated to be associated with insulin
resistance, obesity, type 2 diabetes and thrombotic diseases
(1), for example, leptin and
adiponectin, which possess numerous beneficial functions on
obesity, in particular leptin, which is widely regarded as an
anti-obesity hormone (2).
Adiponectin on the other hand is an insulin-sensitizing adipokine,
with direct antidiabetic, anti-atherogenic and anti-inflammatory
functions (3).
Angiopoietin-like protein 4 (Angptl4) is an
adipokine and it is predominantly expressed in the liver and
adipose tissue, and is also termed peroxisome
proliferator-activated receptor γ (PPARγ) angiopoietin-related
protein, fasting-induced adipose factor, or hepatic
fibrinogen-related protein (4).
Previous studies on Angptl4 have researched lipid and glucose
metabolism. For example, the expression of Angptl4 was observed to
be increased in a fasted state, when compared with the fed state,
implying that Angptl4 protein is associated with the to lipid
metabolism (5). In addition, it
has been reported that the blood lipid levels were reduced in
Angptl4 knockout mice, and significantly increased with ectopic
Angptl4 overexpression (6,7). Furthermore, in white adipose tissue,
Angptl4 additionally promotes the expression of genes involved in
triglyceride (TG) hydrolysis and the lipolytic release of free
fatty acids (FFA), in addition to inhibiting the expression of
lipoprotein lipase (8,9). The effects of Angptl4 on the glucose
metabolism have also been reported. Overexpression of Angptl4
contributes to high hepatic glycogen content in mice, suggesting
that Angptl4 may enhance insulin sensitivity in the liver tissues
(10). In addition, Le Jan et
al (11) demonstrated that
Angptl4 is able to significantly enhance the ability of insulin to
suppress the output of hepatic glucose, and they suggested that
this may be one of the numerous mechanisms by which Angptl4 reduces
blood glucose. Similarly, Xu et al (12) indicated that Angptl4 can reduce
blood glucose and improve glucose tolerance, thus suggesting that
Angptl4 is able to increase insulin sensitivity and improve insulin
resistance.
Regardless of the previous studies on Angptl4, the
long-term effects of this protein on the energy metabolism and
insulin sensitivity remain to fully elucidated. In the current
study, an adenovirus-mediated expression system was used to
investigate the metabolic effects of Angptl4 on high-fat-diet
(HFD)-induced obese mice. The results provided evidence that
Angptl4 is able to improve glucose tolerance in the HFD-induced
obese mice, however promotes hepatic steatosis and lipolysis, which
are in agreement with previous studies (12,13).
In addition, with normal diet mice as a control, the
phosphorylation levels of several insulin signaling pathway-related
genes were observed to be significantly downregulated in
HFD-induced obese mice, whereas were upregulated in HFD-induced
obese mice with adenovirus-mediated expression of Angptl4.
Materials and methods
Construction of adenoviral vector for
overexpression of Angptl4
The adenovirus expression vector was generated using
the AdMax™ Expression System (Microbix Biosystems, Inc.,
Mississauga, ON, Canada). The shuttle and adenoviral backbone
plasmid pDC315 carrying a loxP expression cassette in the
E1 region was purchased from Addgene, Inc. (Cambridge,
MA, USA). The recombinant plasmid pDC315-Angptl4 was constructed by
restriction enzyme (EcoRI and BamHI) cleavage and
connection. The plasmid pBHG lox ΔE1,3 Cre containing
the Cre/loxP element was purchased from Microbix Biosystems, Inc.
The recombinant adenoviral-Angptl4 (adv-Angptl4) virus was
packaged, amplified in HEK293 cells and then purified by CsCl
density gradient centrifugation at 100,000 × g for 16 h at 4°C.
Grouping of animals
A total of 24 male C57BL/6 healthy mice between 8
and 10 weeks old (~20 g) were obtained from the Shanghai
Experimental Animal Center, Chinese Academy of Sciences (Shanghai,
China) and were housed in a room under controlled temperature
(23±1°C) with free access to water. Mice were fed standard chow for
the first week to allow them to adjust to the new environment.
Subsequently, mice were divided into three groups randomly. As a
control, mice in the first group (normal control) were fed with
standard mouse chow. The other two groups were provided with a HFD
for 6 weeks, and then adenoviral (adv) and adv-Angptl4 virus were
injected via the tail vein in the second (model control) and third
(Angptl4+) groups of mice, respectively. The normal
control group continued to be fed with standard chow, whereas the
model control and Angptl4+ groups were fed with HFD for
an additional two weeks. The mice in the three groups were weighed
once a week subsequent to injection with the virus. Two weeks after
viral injection, a series of experiments were performed. All of the
experiments were conducted under institutional guidelines for the
humane treatment of laboratory animals, and the study was approved
by the ethics committee of Dahua Hospital in Shanghai Xuhui
(Shanghai, China).
Measurement of Angptl4 in mouse
serum
Serum Angptl4 concentrations of the three groups of
mice were measured using a sandwich enzyme-linked immunosorbent
assay, with the experimental kit purchased from JRDUN
Biotechnology, Co., Ltd. (Shanghai, China).
Oral glucose tolerance test
All mice were placed into other clean cages with
free access to water, however no food, for 6 h. Subsequent to
fasting, the mice were weighed, the tip of the tails were clipped
in order to obtain blood, and the fasting blood glucose levels were
measured with the OneTouch small glucometer. Mice were then rapidly
administered with an intraperitoneal injection of glucose (1 g/kg
of body weight). Blood was drawn from the mice tail tip at various
time points: T15, T30, T60, T90, T120 and T150 min for measurement
of glucose concentration.
Analysis of TG, total cholesterol (TC),
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
FFA and fasting insulin (FIN) in mice serum
The serum levels of TG and TC were detected by an
enzyme-coupled assay, the ALT and AST levels were detected by the
Reitman-Frankel method (14), FFA
levels were detected using the Copper Color Method with a
semi-automatic or fully-automatic spectrophotometer. All of the
serum indices were measured using the kit from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). The serum levels of
fasting FIN were detected using the 125I-insulin radio
immunoassay kit from the North Biotechnology Institute (Beijing,
China). The homeostasis model assessment of insulin resistance
(HOMA-IR) was calculated using the following formula: Fasting blood
glucose (FBG; mmol/l) x FIN (µIU/ml)/22.5. Subsequently, the
data were logarithmically transformed in order to obtain a more
accurate value.
Hematoxylin/eosin (HE) staining of liver
paraffin-embedded sections
Tissue blocks of livers were resected and fixed in
10% formalin for 48 h, then were embedded in paraffin.
Subsequently, they were sectioned and stained with HE. Finally, the
degree of steatosis and inflammation were analyzed under a light
microscope (magnification, ×100).
Western blot analysis
Mice were sacrificed by CO2 inhalation,
then fresh liver tissue samples were obtained from the mice in the
process of dissecting the mice. The liver tissues (~100 mg) were
rapidly weighed, and the tissues were pulverized in a mortar using
liquid nitrogen. Radioimmunoprecipitation assay (RIPA) lysis buffer
was then added (3 ml RIPA/gram of tissue) premixed with proteinase
and phosphatase inhibitors. The concentration of liver tissue
protein was quantified using the Bicinchoninic Acid kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). A total of 80 µg
protein for each sample was loaded and underwent 10/15% sodium
dodecyl sulfate-polyacrylimide gel electrophoresis for separation.
The blots were blocked with 5% non-fat milk at room temperature for
1 h and were incubated overnight at 4°C with the following primary
antibodies: Polyclonal rabbit Angptl4 (bs-1087R; 1:100; Bioss,
Inc., Woburn, MA, USA), monoclonal rabbit janus kinase 2 (JAK2)
(#3230; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), rabbit monoclonal phosphorylated (P-) JAK2 (#8082; 1:1,000;
Cell Signaling Technology, Inc.), rabbit polyclonal insulin
receptor substrate 1 (IRS-1) (#2382; 1:1,000; Cell Signaling
Technology, Inc.), rabbit polyclonal P-IRS-1 (#2381; 1:1,000; Cell
Signaling Technology, Inc.), mouse monoclonal signal transducer and
activator of transcription 3 (STAT3) (ab119352; 1:5,000; Abcam,
Cambridge, MA, USA); rabbit monoclonal P-STAT3 (ab76315, 1:200,000;
Abcam), rabbit polyclonal protein kinase B (AKT) (#9272; 1:1,000;
Cell Signaling Technology, Inc.); rabbit polyclonal P-AKT (#9271;
1:1,000; Cell Signaling Technology, Inc.), rabbit monoclonal
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (#5174; 1:1,000;
Cell Signaling Technology, Inc.). In order to ensure equal amount
of protein loaded, levels were normalized to that of GAPDH. The
blots were incubated for 1 h at 37°C temperature with the goat
anti-rabbit (A0208) and goat anti-mouse (A0216) poly-clonal
secondary antibodies (conjugated with horseradish peroxidase;
1:1,000; Beyotime Institute of Biotechnology, Shanghai, China)
following three washes with Tris-buffered saline with Tween-20
(TBST) buffer. The signals were visualized using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) following an
additional wash with TBST. Intensities were measured using ImageJ
software, version 1.4 (National Institutes of Health, Bethesda, MD,
USA) and were reported as the relative pixel normalized to that of
GAPDH.
Statistical analysis
Experiments were performed with eight mice per group
with values expressed as the mean ± standard deviation. Statistical
analysis was determined using one-way analysis of variance with
Graphpad Prism software, version 6.0. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of Angptl4 protein in HFD C57
mice following tail-vein injection with adv-Angptl4
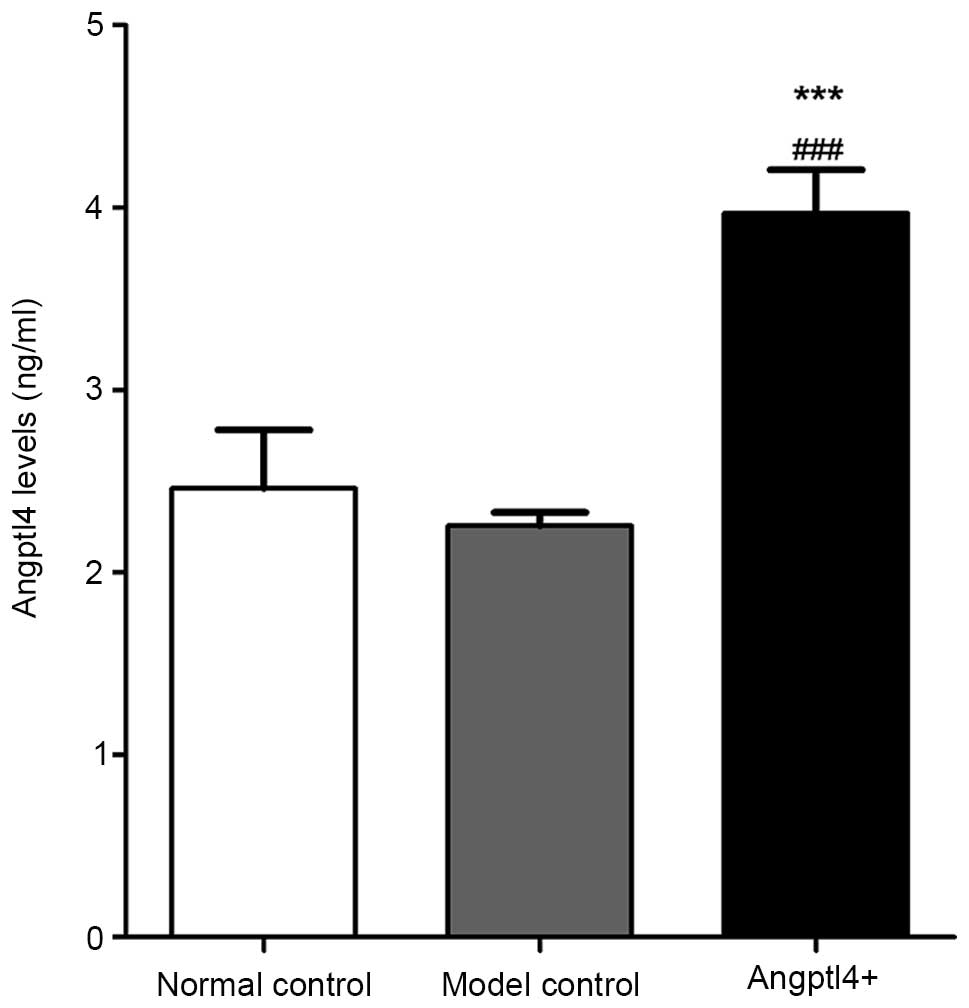
In order to verify the role of Angptl4 as a
circulating hormone in mice subsequent to viral injection, a
sandwich enzyme-linked immunoassay was conducted for measurement of
the protein in mouse serum. Serum Angptl4 concentrations in the
normal and model control groups were not significantly different,
with values of 2.44±0.32 ng/ml and 2.24±0.21 ng/ml, respectively.
The serum concentration in the Angptl4+ group was
increased compared with that of the control groups, with a
concentration of 3.86±0.24 ng/ml (Fig.
1). These results suggested that the recombinant adv-Angptl4
virus had been constructed successfully, and that the Angptl4 gene
was highly expressed in HFD mice.
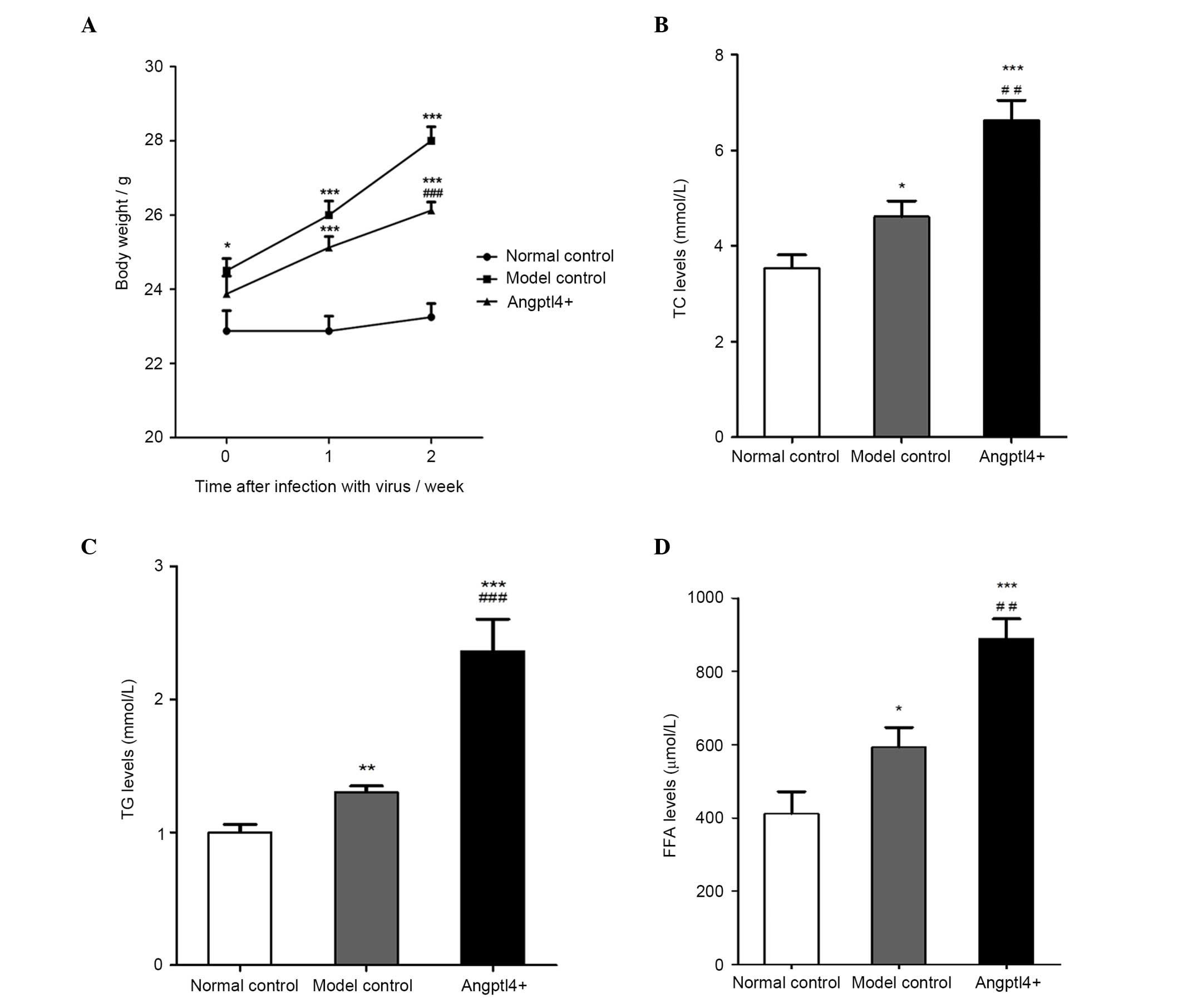
Angptl4 overexpression reduces body
weight and markedly elevates the serum levels of TC, TG and FFA in
HFD mice
A week subsequent to the injection of the virus, no
significant differences were observed between the model control and
Angptl4+ groups. After two weeks, in the model control
group, a significant increase in body weight was observed, with the
mice weighing 28±1.07 g at the end of the experiment, with an
increase ratio of 20.4%, markedly higher than that of the normal
control group. However, Angptl4 reduced the weight growth rate in
HFD mice, with an increase of 10.5%, weighing 26.13±0.64 g
(Fig. 2A). The levels of serum
indices TG, TC and FFA associated with blood lipids were detected,
and the results indicated that all index levels in the
Angptl4+ group were significantly increased compared
with that of the control groups. The levels in the model control
were significantly higher than that of the normal control group
(Fig. 2B–D).
Angptl4 overexpression reduced the weight growth
rate almost by half in HFD mice, and significantly increased the
levels of the major serum indices in the serum of HFD mice,
suggesting that subsequent to infection with Angptl4 in HFD mice,
the blood lipid levels and lipolysis were increased, which may have
directly induced hyperlipidemia.
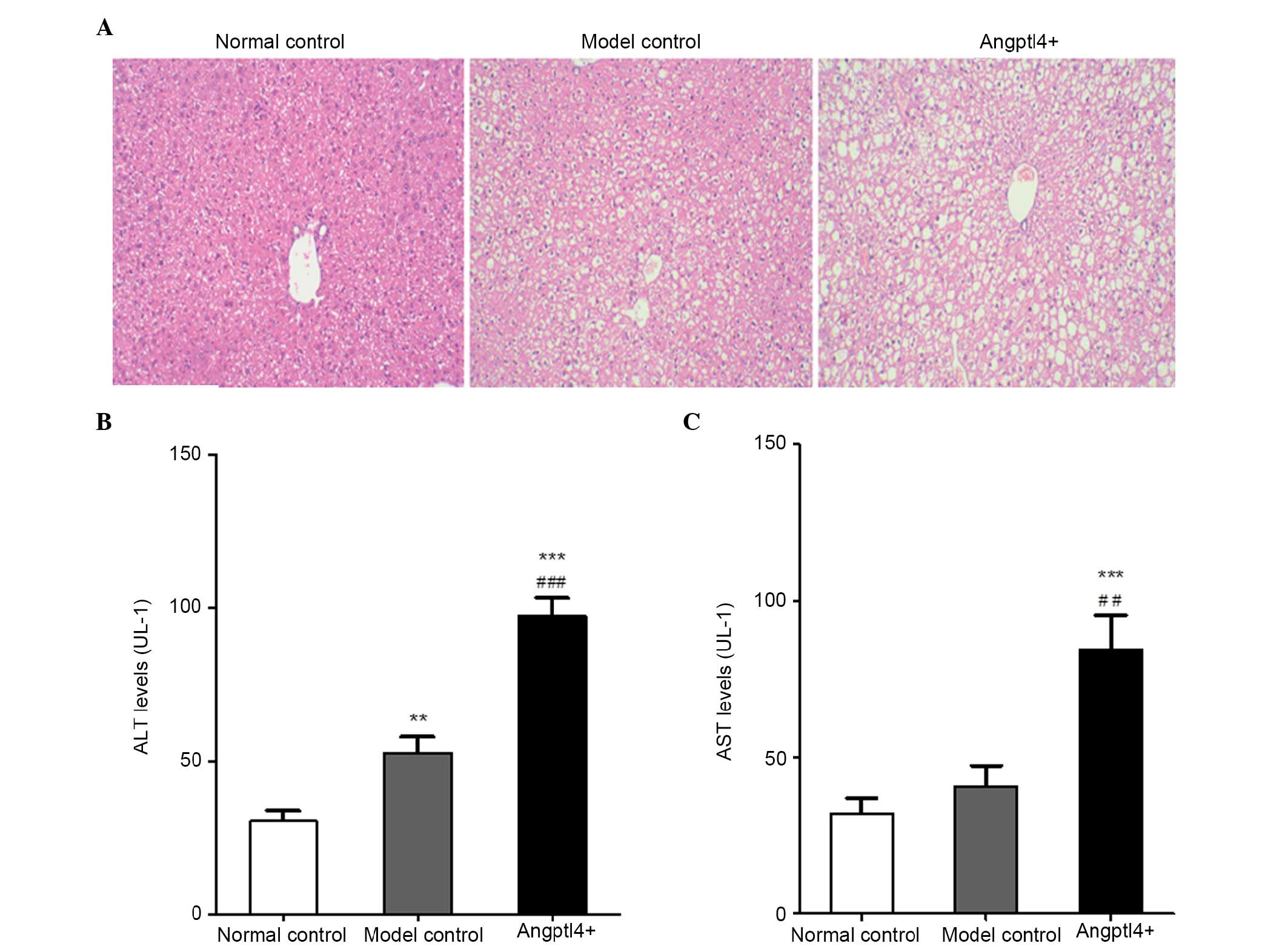
Angptl4 overexpression promotes liver
steatosis and markedly elevates the serum levels of ALT and AST in
HFD mice
In order to investigate the influence of Angptl4 on
liver function in HFD mice, HE staining was conducted in
paraffin-embedded liver tissue sections from the three groups. The
levels of ALT and AST, which are associated with liver function,
were detected. The results indicated that hepatocyte swelling
occurred in the livers of model control mice, together with
increased vacuolization in the liver cytoplasm. It is notable that
the swelling was more marked with larger fat vacuoles visible in
the liver cytoplasm in the Angptl4+ group (Fig. 3A). The ALT and AST levels in the
model control group were marginally upregulated, whereas were
significantly increased in the Angptl4+ group, with the
normal control group as the control (Fig. 3B and C).
All results indicated that overexpression of Angptl4
serves a negative role in liver steatosis in HFD, and resulted in
damage to liver function of HFD mice.
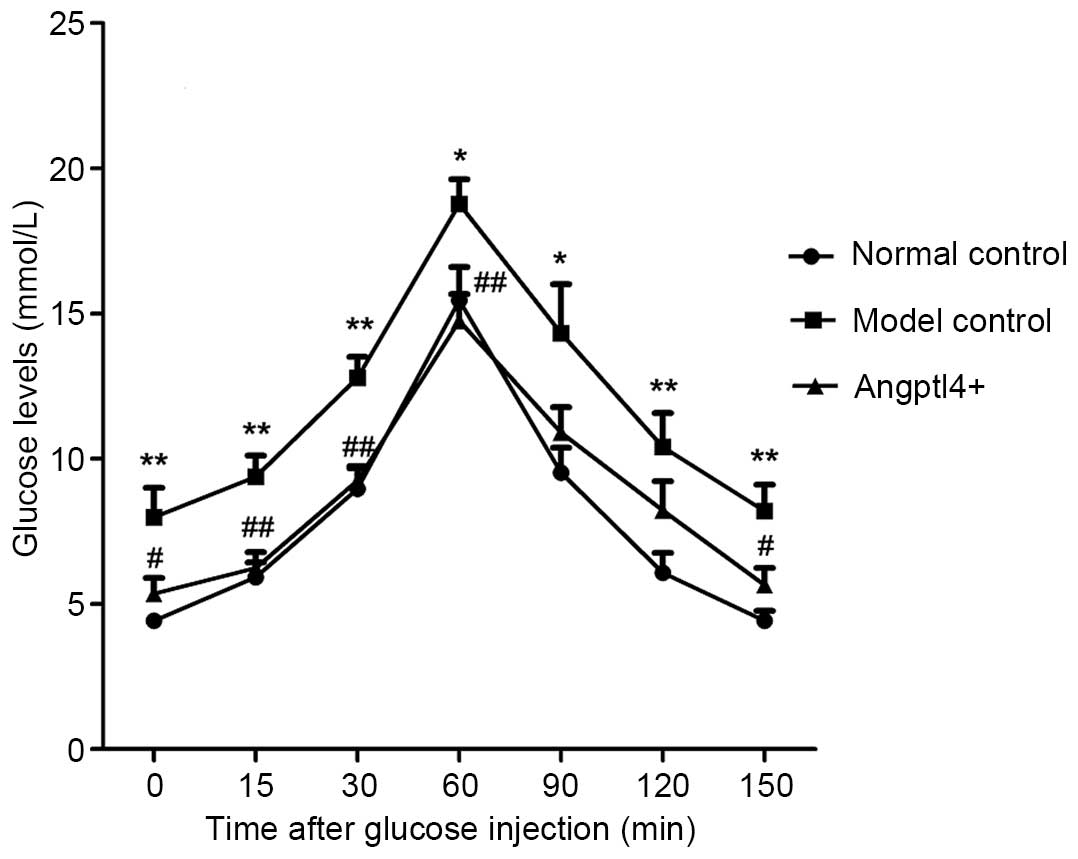
Angptl4 overexpression potently improves
glucose tolerance in HFD mice
To validate the effect of Angptl4 on blood glucose
levels, the glucose tolerance of the three groups was investigated.
With the normal control group mice as a control, it was identified
that the model control group mice exhibited a rise in blood glucose
levels throughout the glucose tolerance curve and a maximum glucose
concentration at 60 min. This increase was present, but less marked
in the Angptl4+ group, with results similar to thos of
the normal control. These results indicated that Angptl4
overexpression can improve glucose tolerance in HFD mice (Fig. 4).
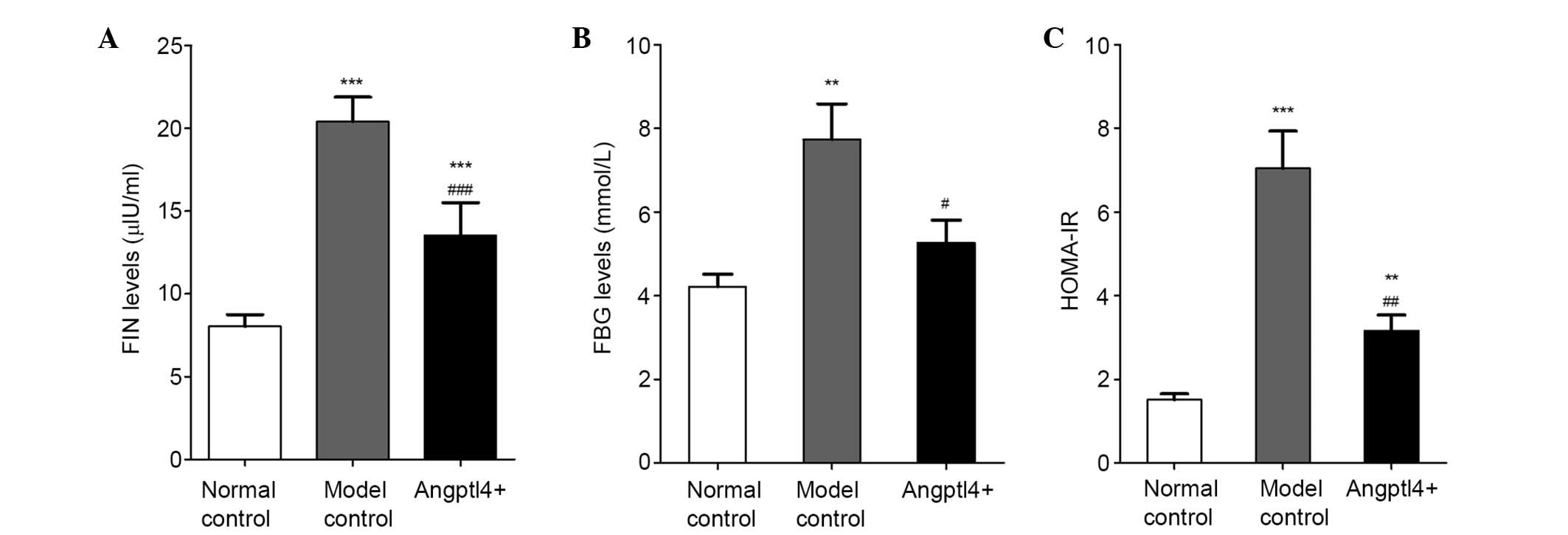
Angptl4 overexpression alleviates the
HOMA-IR levels in HFD mice
The effects of Angptl4 on FIN levels were observed,
and insulin resistance was assessed in HFD mice. The results
indicated that overexpression of Angptl4 markedly reduced FIN
(Fig. 5A) and FBG levels (Fig. 5B). HOMA-IR was increased in the
model control group, together with a lower insulin sensitivity
compared with the normal control group, while these levels were
reduced back to levels similar to the normal control in the
Angptl4+ group (Fig.
5C), suggesting that Angptl4 can alleviate insulin resistance
and enhance insulin sensitivity in HFD mice.
Angptl4 overexpression results in the
downregulation of several insulin signaling pathway-associated
genes in HFD mice
IRS-1 links the insulin receptor kinase and its
downstream serine kinases, including phosphoinositide 3-kinase
(PI3K) and AKT phosphorylation. Under the insulin resistant state,
the effects of the PI3K-AKT signal transduction pathway on insulin
stimulation are reduced (15). The
JAK2-STAT3 signaling pathway is involved in the lipid metabolism
through mediation by leptin (16).
In the current study, western blot analysis was used to detected
the phosphorylation levels of IRS-1, AKT, JAK2 and STAT3, in
addition to expression of Angptl4, in the liver samples. The
results indicated that with the normal control group as the
control, the phosphorylated proteins and Angptl4 were downregulated
in the model control group, however were upregulated in the in
Angptl4+ group to levels similar to those of the normal
control group (Fig. 6).
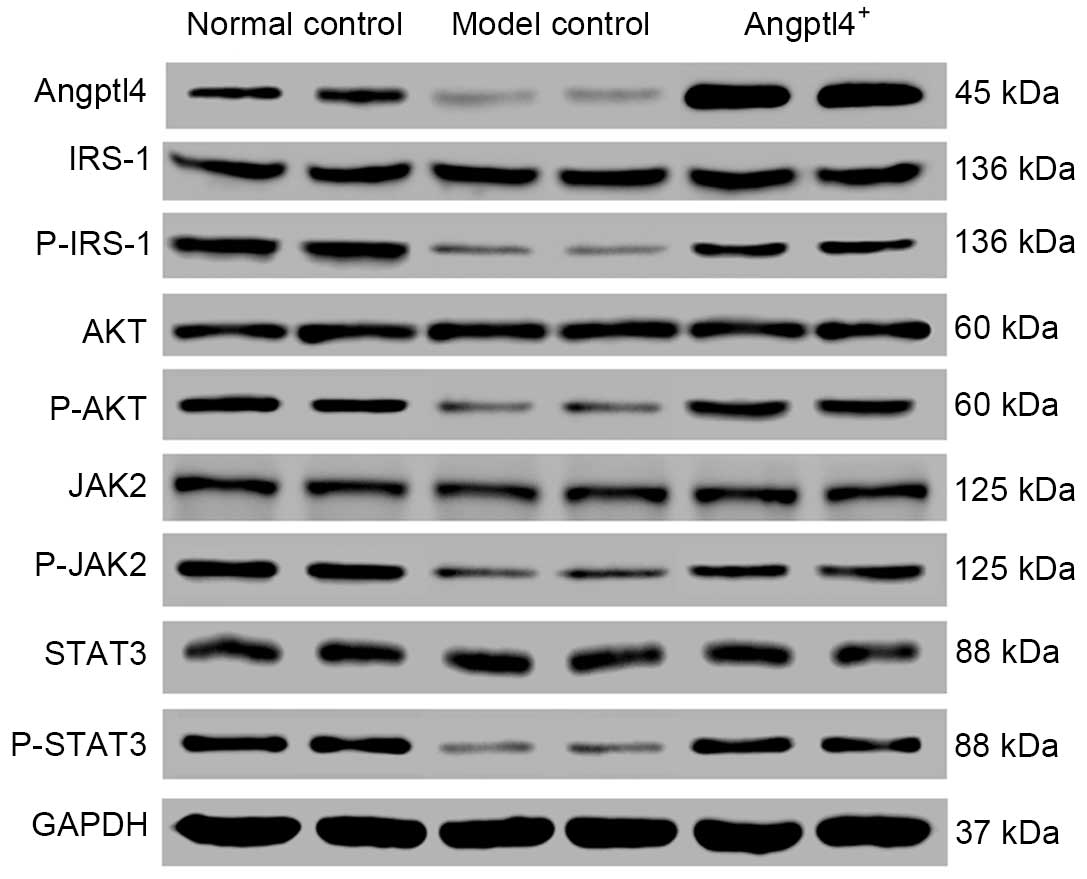 | Figure 6Angptl4 overexpression causes
alterations in the levels of certain associated genes involved in
the insulin signaling pathway in high-fat-diet mice. The
phosphorylation levels of the four selected genes: IRS-1, AKT, JAK2
and STAT3 in the liver were observed to be downregulated in the
model control group, whereas were upregulated to levels close to
the normal levels in the Angptl4+ group, with the normal
control group as the control. Angptl4, angiopoietin-like protein 4;
IRS-1, insulin receptor substrate 1; AKT, protein kinase B; JAK2,
janus kinase 2; STAT3, signal transducer and activator of
transcription 3; P-, phosphorylated; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
Angptl4, which was originally identified as a
downstream target gene of PPARs, and has been identified as an
adipokine, is associated with insulin resistance, dyslipidemia,
obesity, type 2 diabetes and thrombotic diseases (3–5,17).
The current study identified that in HFD mice, Angptl4 is a
circulating hormone involved in regulating lipid and glucose
homeostasis, and this protein also has an effect on systemic
insulin sensitivity and the insulin signal pathway.
Overexpression of Angptl4 in HFD mice reduced the
body weight of mice and stimulated the intracellular hydrolysis of
TG and TC, together with the release of FFA, which result from
adipolysis and may lead to dyslipidemia. The data of the current
study were consistent with previous studies that indicated that
Angptl4 is able to inhibit lipoprotein lipase activity, resulting
in hypertriglyceridemia and further fat hydrolysis (18,19).
In addition, given that the adipokine Angptl4 is a downstream
target gene of PPARs, it would be hypothesized to benefit from the
functions of PPARs, including potent lipid-lowering, fatty acid
oxidation enhancing and lipogenesis inhibiting abilities in the
liver tissues (20). Lipolysis
occurs to produce energy, and generally occurs in the fasted state,
when there is a shortage of blood glucose. In patients with obesity
and insulin resistance, lipolysis by white adipocytes is no longer
restricted to the fasted state, and occurs in the fed state when
insulin normally suppresses this process (21,22),
suggesting that HFD may induce lipolysis. Therefore, in the current
study, the serum levels of TC, TG and FFA in the model control
group were all increased compared with that of the normal
control.
During the investigation of the effects of Angptl4
on glycometabolism, liver function, glucose tolerance and insulin
resistance were additionally assessed. Gray et al (23) identified that, when purified human
Angptl4 was added to cultured murine adipocytes, intracellular
cyclic adenosine monophosphate levels were markedly increased, with
the lipolytic impairment reversed in Angptl4-deficient cells,
suggesting that it may be associated with glycometabolism. The
observation that the beneficial effects of Angptl4 on glucose
homeostasis and insulin resistance are associated with liver
steatosis and hyperlipidemia is unexpected. However, previous
studies using animal models suggest that certain signaling pathways
that improve glucose homeostasis and enhance insulin sensitivity
can simultaneously induce hyperlipidemia and liver steatosis. For
example, the hepatic activation of AKT, a key signaling molecule
(24), and liver-specific
depletion of phosphatase and tensin homolog, a negative regulator
of the PI3K/AKT pathway (25), are
able to improve systemic glucose tolerance, however concurrently
induce hypertriglyceridemia and liver steatosis.
The observation that HFD leads to high FIN levels,
however low Angptl4 in vivo expression, is supported by
previous studies demonstrating that insulin can repress the
expression of Angptl4, an effect reciprocal to that of
glucocorticoids (26,27). By contrast, considering the fact
that abnormalities in the insulin signaling pathway are critical
for the occurrence of insulin resistance (28,29),
the current study aimed to investigate the alterations in certain
associated genes involved in the insulin signaling pathway in HFD
mice. The results indicated that with the normal control group as
the control, the investigated genes were downregulated in the model
control group, however were upregulated in the Angptl4+
group close to the levels observed in the normal control group.
This suggested that Angptl4 affects not only insulin resistance,
however also the insulin signaling pathway, as hypothesized. A
previous study by Hou et al (30) indicated that Angptl4 is able to
induce the activation of the extracellular signal-related kinase
1/2 and PI3K/AKT pathways. In addition, Angptl4 has been reported
to activate JAK1/STAT3 signaling to regulate inducible nitric oxide
synthase expression in keratinocytes (31). These studies are in agreement with
the results of the current study. Furthermore, a recent study
(32) demonstrated that obesity
leads to low phosphorylation levels of JAK1 and STAT3.
In summary, Angptl4 induces obesity-associated
metabolic disorders. The present study suggested that Angptl4
promotes liver steatosis and lipolysis, in addition to impairing
liver function; while Angptl4 improves glucose tolerance and
insulin resistance, in addition to causing the downregulation of
various insulin signaling pathway-associated genes. The specific
mechanisms involved in the mediation of insulin signaling pathways
to affect lipid and glucose metabolism by Angptl4 remains to be
fully elucidated. It is suggested that Angptl4 may be a novel
therapeutic target in hyperlipidemia, diabetes, metabolic syndrome
and additional diseases.
Acknowledgments
The current study was supported by grants from the
projects of Shanghai Health Bureau Scientific Research (grant no.
20134126).
References
|
1
|
Kershaw EE and Flier JS: Adipose tissue as
an endocrine organ. J Clin Endocrinol Metab. 89:2548–2556. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuzawa Y, Funahashi T, Kihara S and
Shimomura I: Adiponectin and metabolic syndrome. Arterioscler
Thromb Vasc Biol. 24:29–33. 2004. View Article : Google Scholar
|
|
4
|
Yoon JC, Chickering TW, Rosen ED, Dussault
B, Qin Y, Soukas A, Friedman JM, Holmes WE and Spiegelman BM:
Peroxisome proliferator-activated receptor gamma target genen
encoding a novel angiopoietin-related protein associated with
adipose differentiation. Mol Cell Biol. 20:5343–5349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kersten S, Mandard S, Tan NS, Escher P,
Metzger D, Chambon P, Gonzalez FJ, Desvergne B and Wahli W:
Characterization of the fasting-induced adipose factor FIAF, a
novel peroxisome proliferator activated receptor target gene. J
Biol Chem. 275:28488–28493. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koster A, Chao YB, Mosior M, Ford A,
Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, et
al: Transgenic angiopoietin-like (angptl)4 overexpression and
targeted disrupt ion of angptl4 and angptl3: Regulation of
triglyceride metabolism. Endocrinology. 146:4943–4950. 2005.
View Article : Google Scholar
|
|
7
|
Ge H, Cha JY, Gopal H, Harp C, Yu X, Repa
JJ and Li C: Differential regulation and properties of
angiopoietin-like proteins 3 and 4. J Lipid Res. 46:1484–1490.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanderson LM, Degenhardt T, Koppen A,
Kalkhoven E, Desvergne B, Müller M and Kersten S: Peroxisome
proliferator-activated receptor beta/delta (PPARbeta/delta) but not
PPARalpha serves as a plasma free fatty acid sensor in liver. Mol
Cell Biol. 29:6257–6267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattijssen F and Kersten S: Regulation of
triglyceride metabolism by Angiopoietin-like proteins. Biochim
Biophys Acta. 1821:782–789. 2012. View Article : Google Scholar
|
|
10
|
Merkel M, Eckel RH and Goldberg IJ:
Lipoprotein lipase: Genetics, lipid uptake, and regulation. J Lipid
Res. 43:1997–2006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Jan S, Amy C, Cazes A, Monnot C,
Lamandé N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P and
Germain S: Angiopoietin-like 4 is a proangiogenic factor produced
during ischemia and in conventional renal cell carcinoma. Am J
Pathol. 162:1521–1528. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu A, Lam MC, Chan KW, Wang Y, Zhang J,
Hoo RL, Xu JY, Chen B, Chow WS, Tso AW and Lam KS:
Angiopoietin-like protein4 decreases blood glucose and improves
glucose tolerance but induces hyperlipidemia and hepatic steatosis
in mice. Proc Natl Acad Sci USA. 102:6086–6091. 2005. View Article : Google Scholar
|
|
13
|
Mandard S, Zandbergen F, van Straten E,
Wahli W, Kuipers F, Müller M and Kersten S: The fasting-induced
adipose factor/angiopoietin-like protein 4 is physically associated
with lipoproteins and governs plasma lipid levels and adiposity. J
Biol Chem. 281:934–944. 2006. View Article : Google Scholar
|
|
14
|
Reitman S and Frankel S: A colorimetric
method for the determination of serum glutamic oxalacetic and
glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cusi K, Maezono K, Oaman A, Pendergrass M,
Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR and Mandarino LJ:
Insulin resistance differentially affects the pi3-kinase- and MAP
kinase-mediated signaling in human muscle. J Clin Invest.
105:311–320. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WZ, Zhao SM and Gao SZ: Research
progress on leptin-mediated JAK/STAT signaling pathway in lipid
metabolism. Chin J Cell Biol. 33:584–589. 2011.
|
|
17
|
Kim HK, Youn BS, Shin MS, Namkoong C, Park
KH, Baik JH, Kim JB, Park JY, Lee KU, Kim YB and Kim MS:
Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and
body weight. Diabetes. 59:2772–2780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida K, Shimizugawa T, Ono M and
Furukawa H: Angiopoietin-like protein 4 is a potent
hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein
lipase. J Lipid Res. 43:1770–1772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oike Y, Akao M, Kubota Y and Suda T:
Angiopoietin-like proteins: Potential new targets for metabolic
syndrome therapy. Trends Mol Med. 11:473–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee CH, Olson P and Evans RM: Minireview:
Lipid metabolism, metabolic diseases, and peroxisome
proliferator-activated receptors. Endocrinology. 144:2201–2207.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samuel VT, Petersen KF and Shulman GI:
Lipid-induced insulin resistance: Unravelling the mechanism.
Lancet. 375:2267–2277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gray NE, Lam LN, Yang K, Zhou AY, Koliwad
S and Wang JC: Angiopoietin-like 4 (Angptl4) protein is a
physiological mediator of intracellular lipolysis in murine
adipocytes. J Biol Chem. 287:8444–8456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ono H, Shimano H, Katagiri H, Yahagi N,
Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, et
al: Hepatic Akt activation induces marked hypoglycemia,
hepatomegaly, and hypertriglyceridemia with sterol regulatory
element binding protein involvement. Diabetes. 52:2905–2913. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stiles B, Wang Y, Stahl A, Bassilian S,
Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA and Wu
H: Liver-specific deletion of negative regulator Pten results in
fatty liver and insulin hypersensitivity. Proc Natl Acad Sci USA.
101:2082–2087. 2004. View Article : Google Scholar :
|
|
26
|
Mizutani N, Ozaki N, Seino Y, Fukami A,
Sakamoto E, Fukuyama T, Sugimura Y, Nagasaki H, Arima H and Oiso Y:
Reduction of insulin signaling upregulates angiopoietin-like
protein 4 through elevated free fatty acids in diabetic mice. Exp
Clin Endocrinol Diabetes. 120:139–144. 2012. View Article : Google Scholar
|
|
27
|
Yamada T, Ozaki N, Kato Y, Miura Y and
Oiso Y: Insulin downregulates angiopoietin-like protein 4 mRNA in
3T3-L1 adipocytes. Biochem Biophys Res Commun. 347:1138–1144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prudente S, Morini E and Trischitta V:
Insulin signaling regulating genes: Effect on T2DM and
cardiovascular risk. Nat Rev Endocrinol. 5:682–693. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karlsson HK and Zierath JR: Insulin
signaling and glucose transport in insulin resistant human skeletal
muscle. Cell Biochem Biophys. 48:103–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou M, Cui J, Liu J, Liu F, Jiang R, Liu
K, Wang Y, Yin L, Liu W and Yu B: Angiopoietin-like 4 confers
resistance to hypoxia/serum deprivation-induced apoptosis through
PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells.
PLoS One. 9:e858082014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chong HC, Chan JS, Goh CQ, Gounko NV, Luo
B, Wang X, Foo S, Wong MT, Choong C, Kersten S and Tan NS:
Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and
enhances angiogenesis to accelerate wound healing in diabetic mice.
Mol Ther. 22:1593–1604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan ZK, Yang ZJ and Chen F: Effect of
electroacupuncture stimulation of 'Housanli' (ST 36) and 'Zhongwan'
(CV 12) on serum leptin and hepatocellular JAK 2-STAT 3 signaling
in obese rats. Zhen Ci Yan Jiu. 40:1–5. 2015.In Chinese. PubMed/NCBI
|