Introduction
Reactive oxygen species (ROS), including superoxide
anions, hydroxyl radicals and hydrogen peroxide
(H2O2), are generated in cells by
environmental elements, primarily via mitochondrial respiratory
cellular metabolism (1). Under
normal conditions, ROS are efficiently neutralized by cellular
antioxidant mechanisms. However, when the generation of ROS
increases, the resulting imbalance can cause various cellular
dysfunctions. This type of cellular damage, particularly in the
pancreas, may lead to deleterious effects, and could potentially
cause diabetes (2,3). Diabetes mellitus is a common
metabolic disease that is associated with chronic inflammation,
hyperglycemia, obesity, hyperlipidemia, hyperinsulinemia and
insulin resistance (4).
Individuals with diabetes have high blood sugar caused by β-cell
dysfunction (5).
H2O2 can inflict damage on vulnerable cell
types, including RINm5F pancreatic β-cells, which may lead to
apoptosis due to intracellular ROS generation (6,7).
Mushrooms have been used as an effective medicinal
food and traditional therapy for centuries; they contain several
compounds, including polyphenols and polysaccharides (particularly
beta-glucan), which provide health benefits due to their
antioxidative effects (8–12). Among them, the mushroom Inonotus
obliquus has been used as a traditional natural medicine with
notable efficacy (13–15). Several studies have reported that
I. obliquus does not induce any adverse side effects when
used in drugs and food for the prevention and treatment of
diabetes. In a previous study, a culture broth of I.
obliquus had significant effects on alloxan-induced diabetic
mice (16,17). However, it has been previously
noted that while its effects on diabetes have been studied
extensively in vivo, the number of in vitro studies
is insufficient. Therefore, the present study aimed to confirm that
the antioxidant potential of I. obliquus protects against
β-cell death and may therefore prevent diabetes. The present study
examined the preventive effects of polysaccharides isolated from
I. obliquus on H2O2-induced oxidative
damage in RINm5F pancreatic β-cells. In addition, polysaccharides
from the fruiting body of I. obliquus (PFIO) and
polysaccharides from a liquid culture broth of I. obliquus
(PLIO) were compared, and the results of the study confirmed that
they inhibit the destruction of pancreatic β-cells in
H2O2-induced oxidative stress via the
modulation of cellular signaling pathways.
Materials and methods
Materials
RPMI-1640 media, fetal bovine serum (FBS),
penicillin/streptomycin and trypsin-ethylenediaminetetraacetic acid
(EDTA) were obtained from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Dichlorodihydrofluorescein diacetate
(H2DCF-DA) and an apoptotic assay kit were obtained from
Molecular Probes (Thermo Fisher Scientific, Inc.).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
isopropyl alcohol, H2O2, Hoechst 33342,
mitochondria isolation kit and the rat/mouse insulin enzyme-linked
immunosorbent assay (ELISA) kit (cat. no. EZRMI-13K) were purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Antibodies against c-Jun N-terminal kinase (JNK; dilution, 1:1,000;
cat. no. 9252), phosphorylated (p)-JNK (dilution, 1:1,000; cat. no.
9255S), extracellular signal-regulated kinase (ERK; dilution,
1:1,000; cat. no. 4695), p-ERK (dilution, 1:1,000; cat. no. 9101S),
p38 (dilution, 1:1,000; cat. no. 9212), p-p38 (dilution, 1:1,000;
cat. no. 4631S), cleaved caspase-3 (dilution, 1:1,000; cat. no.
9664S), nuclear factor (NF)-κB p65 (dilution, 1:1,000; cat. no.
3034), and horseradish peroxidase (HRP)-conjugated anti-rabbit
immunoglobulin (Ig)G (dilution, 1:2,000; cat. no. 7074) were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Antibodies against β-actin (dilution, 1:1,000; cat. no. sc-47778),
B-cell lymphoma 2 (Bcl-2; dilution, 1:1,000; cat. no. sc-7382),
Bcl-2-associated X protein (Bax; dilution, 1:1,000; cat. no.
sc-493), caspase-3 (dilution, 1:1,000; cat. no. sc-7272),
apoptosis-inducing factor (dilution, 1:200; AIF; cat. no.
sc-13116), cytochrome c (dilution, 1:200; cat. no. sc-7159), and
HRP-conjugated goat anti-mouse IgG (dilution, 1:2,000; cat. no.
sc-2005) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The RINm5F (CRL-11605; American Type Culture
Collection, Manassas, VA, USA) cell line was a clone derived from
the RIN-m rat islet cell line. The cells were kindly provided by
Professor S. Y. Choi (Hallym University, Chuncheon, South Korea).
All other chemicals were analytical grade.
Preparation of samples
Dried fruiting bodies of I. obliquus (IO)
were purchased from ChagaIn (Seoul, South Korea) and were
pulverized in a blender. Ground mushroom (20 g) was subsequently
extracted with distilled water (60 ml) at 121°C for 2 h. Extracts
were centrifuged at 600 × g for 25 min at 4°C and were filtered
through 0.45 µm Whatman filter paper (Whatman 4) to remove
insoluble matter prior to freeze-drying. The entire procedure was
repeated three times. Polysaccharides were precipitated from the
resuspended extracts using 95% ethanol, and were collected by
filtration through 0.45 µm Whatman filter paper. The supernatant
precipitant was dialyzed using a dialysis tube (molecular weight
cut-off, 12,400; Sigma-Aldrich; Merck Millipore) for 5 days to
remove low-molecular-weight compounds. The extracted PFIO was then
used for further experiments. The liquid culture broth of I.
obliquus was filtered and centrifuged (600 × g, 25 min, 4°C) to
remove fragments of debris. The supernatant was extracted in the
same manner as PFIO. The extracted PLIO were then used for further
experiments.
Cell culture
RINm5F cells were maintained in RPMI-1640 medium
supplemented with 10% inactivated FBS, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. The cells were cultured to ~80% confluence and were
harvested with 0.25% trypsin-EDTA. The resulting cells were diluted
appropriately for reseeding in culture petri dishes or in test
plates.
Cell viability assay
To determine the effects of PFIO and PLIO on cell
viability the cells were treated with H2O2.
Briefly, RINm5F cells were seeded in 12-well plates
(2.5×104 cells/well in 1 ml medium) and were incubated
for 72 h. Subsequently, 300 µM H2O2 was added
to the cells (for 2 h) that had been pretreated with or without
PFIO or PLIO (1–100 µg/ml for 24 h). Cell viability was evaluated
using the MTT assay. MTT solution (0.5 ml) was added to each well,
which was then incubated for 2 h at 37°C. The formazan crystals in
each well were then dissolved in isopropyl alcohol, and the
absorbance was measured at 595 nm using an ELISA microplate reader
(model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Intracellular ROS scavenging activity
and image analysis
To determine the effects of PFIO and PLIO on
oxidative stress-induced ROS generation, the cells were treated
with or without PFIO or PLIO (1–100 µg/ml) for 20 h, and were then
treated with 0.3 mM H2O2 for 2 h. After 2 h,
5 µM H2DCF-DA solution in phosphate-buffered saline
(PBS) was added to each well of the plate, which was incubated for
2 h at 37°C and the fluorescence was measured at excitation and
emission wavelengths of 485 and 535 nm, respectively, using a
microplate spectrofluorometer. Image analysis of intracellular ROS
production was performed by seeding RINm5F β-cells in
coverslip-loaded 12-well plates and treating in the aforementioned
manner. After washing twice with PBS, the cells were mounted under
glass coverslips using Vectashield (Brunschwig Chemie, Amsterdam,
Netherlands) and the cells were observed. Images of the stained
cells were captured using a fluorescence microscope (Nikon
Corporation, Tokyo, Japan).
Annexin V/propidium iodide (PI)
staining
Cells undergoing apoptosis were identified using a
fluorescein isothiocyanate (FITC)-labeled Annexin V/PI apoptosis
detection kit (Molecular Probes; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. PI can be used to
differentiate necrotic, apoptotic and normal cells, since this
agent cannot penetrate the membrane and is generally excluded from
viable cells. Cells were pretreated with or without various
concentrations of PFIO or PLIO (50 or 100 µg/ml) for 20 h, and/or
were then treated with 0.3 mM H2O2 for 2 h. Briefly, the cells were
harvested with trypsin-EDTA, washed with PBS, and were centrifuged
at 600 × g for 5 min to collect the cell pellet. The number of
cells was adjusted to 1×106 cells/ml. The cells were
then resuspended in binding buffer [10 mM HEPES, 140 mM NaCl, and
2.5 mM CaCl2 (pH 7.4)] and were stained with
FITC-labeled Annexin V/PI at room temperature for 15 min in the
dark. Flow cytometric analysis was performed using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). The percentage
of apoptotic cells was calculated using Cell Quest software
(version 4.0.4; BD Biosciences). Cells in the early phase of
apoptosis were Annexin V-positive and PI-negative; however, cells
in the late stages of apoptosis were Annexin V-positive and
PI-positive. The apoptotic index (%) was calculated as the sum of
cells in the early and late phases of apoptosis divided by the
total number of events.
Hoechst 33342 staining
In order to examine the degree of nuclear
condensation, the nuclear morphology of cells was evaluated using
the cell-permeable, DNA-specific fluorescent dye Hoechst 33342.
RINm5F cells were seeded in 24-well plates and incubated for 24 h.
Cells were pretreated with or without various concentrations of
PFIO or PLIO (50 or 100 µg/ml) for 20 h, and/or were then treated
with 0.3 mM H2O2 for 2 h. Cells were incubated for 30 min with 5 µg
Hoechst 33342 (stock solution, 10 mg/ml), and were fixed for 20 min
at room temperature in 4% formaldehyde. Images of the stained cells
were collected using a Nikon fluorescence microscope in order to
examine the degree of nuclear condensation. Cells with
homogeneously stained nuclei were considered viable, whereas the
presence of chromatin condensation and/or fragmentation was
indicative of apoptosis.
Measurement of caspase-3
activities
Caspase activity was determined with a fluorimetric
assay using the enzyme substrate Z-DEVDAMC for caspase-3 (Molecular
Probes; Thermo Fisher Scientific, Inc.), which is specifically
cleaved by the enzyme at the Asp residue to release the fluorescent
group, 7-amino-4-methyl coumarin. Cells were pretreated with or
without various concentrations of PFIO or PLIO (50 or 100 µg/ml)
for 20 h, and/or were then treated with 0.3 mM H2O2 for 2 h. Cells
were harvested and processed according to the manufacturer's
protocol. Fluorescence was measured continuously for a period of 60
min at multiple time points at 350 and 450 nm excitation and
emission, respectively.
Measurement of insulin secretion
To determine the amount of insulin secreted, cells
were pretreated with or without various concentrations of PFIO or
PLIO (50 or 100 µg/ml) for 20 h, and/or were then treated with 0.3
mM H2O2 for 2 h. After incubation, 1 ml of Krebs-Ringer's
bicarbonate buffer [115 mM NaCl, 4.7 mM KCl, 2.5 mM
CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 20 mM NaHCO3, 10 mM
HEPES (pH 7.4), and 0.2% bovine serum albumin] was added for 30 min
at 37°C, after which the cells were incubated in Krebs-Ringer's
bicarbonate buffer containing 5 or 20 mM glucose for 2 h at 37°C.
The cell culture medium was collected from the treated cells, and
the level of insulin released into the medium was measured using a
rat/mouse insulin ELISA kit according to the manufacturer's
protocol.
Preparation of subcellular
fractions
After various treatments, the mitochondrial fraction
was prepared using a mitochondria isolation kit (Sigma-Aldrich;
Merck Millipore) according to the manufacturer's protocol. Briefly,
after various treatments, cells were harvested and resuspended in
0.65–2 ml lysis buffer. The homogenate was incubated on ice for 5
min, two volumes of 1X extraction buffer were added, and the
solution was centrifuged at 600 × g for 10 min at 4°C. Following
centrifugation, the supernatant was transferred to fresh 1.5 ml
tubes and centrifuged at 11,000 × g for 10 min at 4°C. The
supernatant was removed, and the pellet was suspended in a CelLytic
M cell lysis reagent with protease inhibitor cocktail (1:100; v/v).
Nuclear extracts were prepared by lysing nuclei in a high salt
buffer supplemented with protease and phosphatase inhibitors using
a nuclear extraction kit (Affymetrix, Inc., Santa Clara, CA, USA)
according to the manufacturer's protocol.
Western blot analysis
The treated cells were washed in 1X PBS and were
lysed in lysis buffer (10 mM Tris-HCl, pH 7.5; 10 mM
NaH2PO4/NaHPO4, pH 7.5; 130 mM NaCl; 1% Triton X-100, 10 mM NaPPi;
1 mM phenylmethylsulphonyl fluoride; 2 µg/ml pepstatin A) for 30
min on ice. The lysates were centrifuged at 12,000 × g for 30 min
at 4°C. The supernatant was collected, and protein content in the
supernatant was measured using a Bio-Rad Protein Assay kit (Bio-Rad
Laboratories, Inc.) prior to western blot analysis. The total or
fractionated protein samples (50 µg per lane) were loaded and
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes. Membranes were blocked with 1.5% skim milk in 1X
Tris-buffered saline containing 0.1% Tween 20 for 30 min, prior to
incubation with the appropriate primary antibodies at 4°C
overnight. Subsequently, the samples were incubated with
HRP-conjugated secondary antibodies for 1 h at room temperature. An
enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA)
was used to develop the luminescent signal.
Statistical analysis
Experimental results are presented as the mean ±
standard error of the mean, and were from at least three
independent experiments. Statistical analysis was performed to
evaluate significant differences using Student's t-test, or one-way
analysis of variance and Duncan's multiple range tests (SAS version
9.1; SAS Institute, Inc., Cary, NC, USA) for comparing multiple
groups. P<0.05 was considered to indicate a statistically
significant different.
Results
Protective effects of PFIO and PLIO on
H2O2-treated RINm5F cells
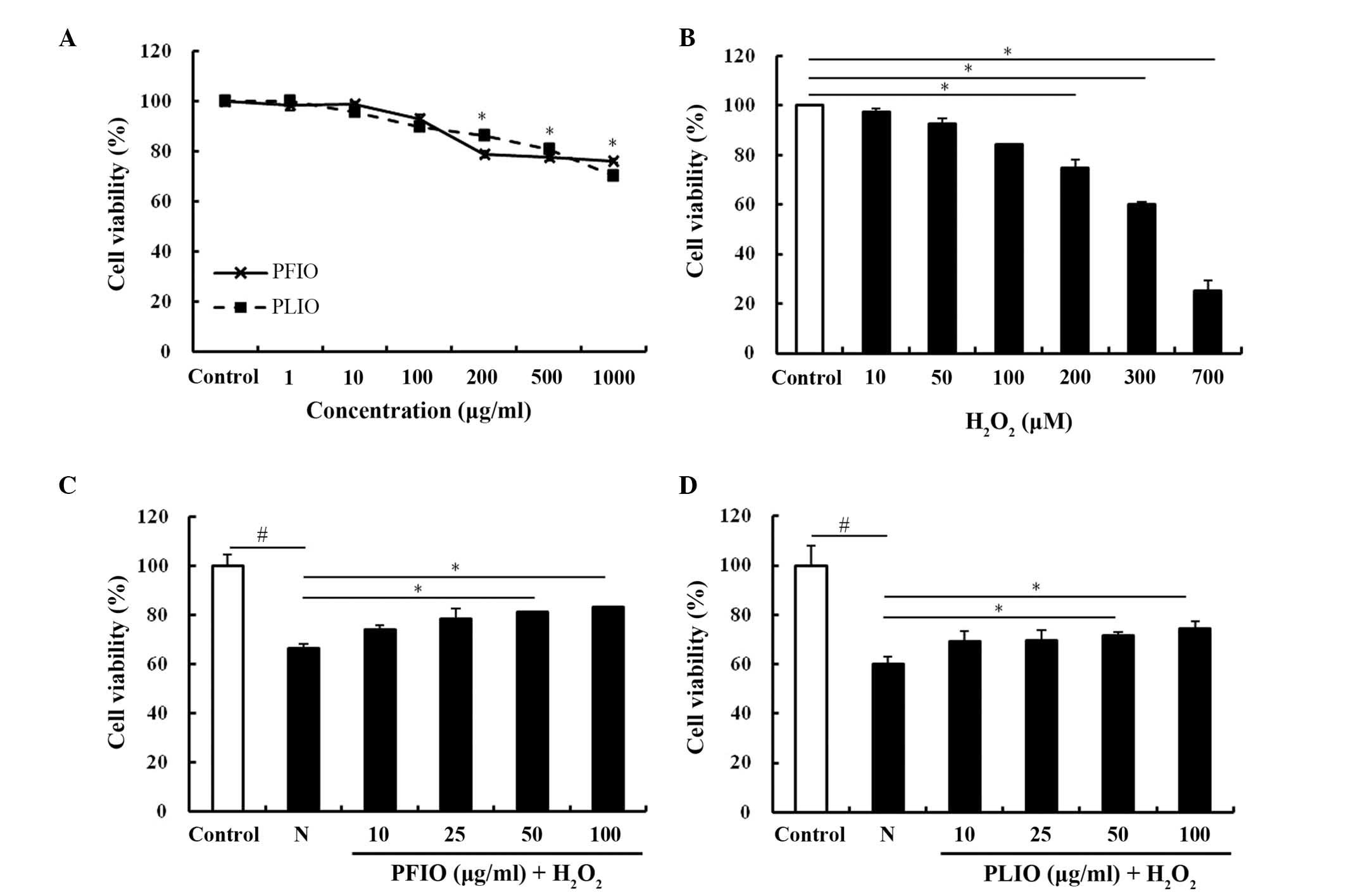
To determine the cytotoxic effects of PFIO and PLIO,
cell viability was determined using the MTT assay. PFIO and PLIO
did not cause any cytotoxicity at a 100 µg/ml concentration
(Fig. 1A). Therefore, the maximum
concentration of PFIO and PLIO used for follow-up studies was 100
µg/ml. In the present study, H2O2 was used to
induce oxidative stress in RINm5F cells. To confirm the
cytotoxicity of H2O2, it was added in various
concentrations (10–700 µg/ml). H2O2 was able
to induce oxidative stress in RINm5F cells, and decreased viability
in a dose-dependent manner. Compared with the control group, the
viability of RINm5F cells treated with 300 µg/ml
H2O2 for 2 h was reduced by ~60% (Fig. 1B). Therefore, 2 h duration was
selected for the exposure to 300 µg/ml H2O2.
To evaluate whether PFIO and PLIO exerted protective effects on
H2O2-treated cells, cells were pretreated
with PFIO or PLIO. The viability of PFIO- and PLIO-treated cells
increased; however, the protective effect of PFIO was greater than
the effect of PLIO (Fig. 1C and
D).
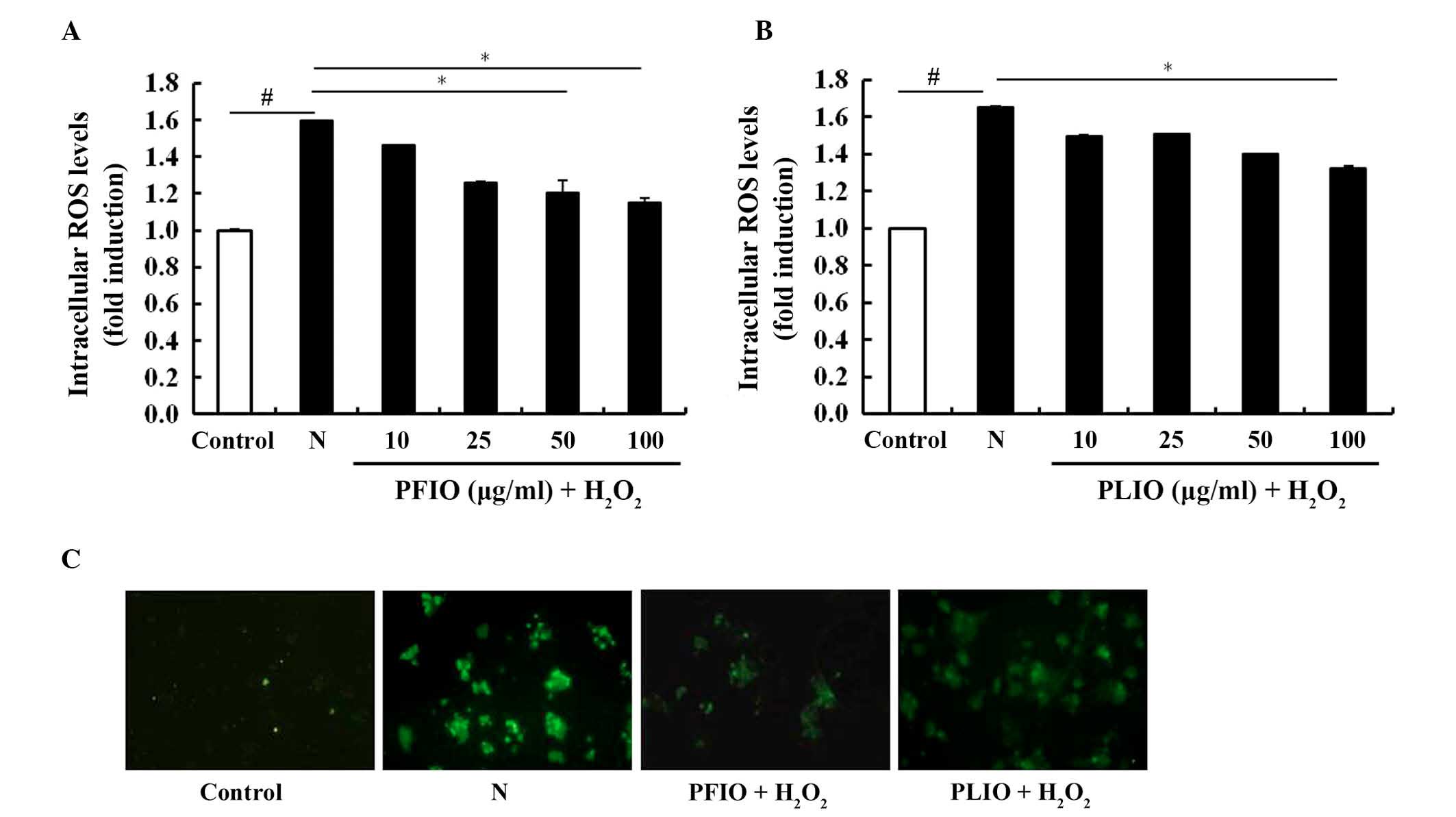
Inhibitory effects of PFIO and PLIO on
ROS generation in H2O2-treated RINm5F cells
The inhibitory effects of PFIO and PLIO on
H2O2-induced ROS generation in RINm5F β-cells
was determined using the ROS-sensitive fluorescent probe,
H2DCF-DA. H2DCF-DA is a cell-permeable dye
that is diverted by intracellular esterase into its non-fluorescent
form, DCFH. DCFH is not cell permeable and is oxidized by
H2O2 to DCF. PFIO (100 µg/ml) exerted an
inhibitory effect on H2O2-treated cells, as
demonstrated by a decrease in intracellular ROS levels, which was
similar to untreated controls (Fig.
2A). The inhibitory effects of PLIO on
H2O2-treated cells were weaker compared with
PFIO (Fig. 2B). Furthermore, the
fluorescence intensity of H2DCF-DA was enhanced in the
microscopic images of H2O2-treated RINm5F
cells; however, the fluorescence intensity of cells pretreated with
PFIO and PLIO was decreased (Fig.
2C). These data suggest that PFIO and PLIO may prevent
H2O2-induced oxidative stress through the
scavenging of intracellular ROS.
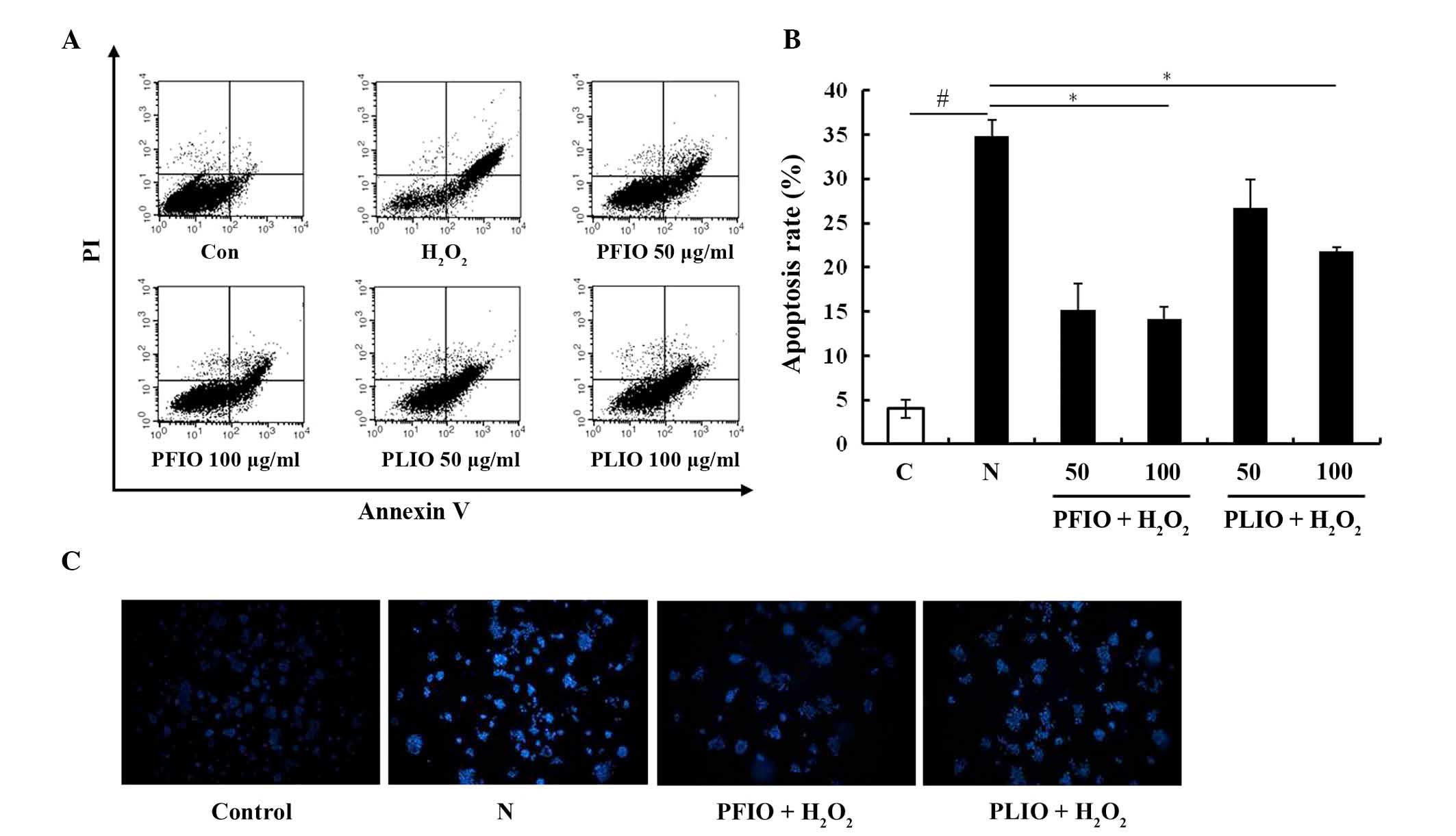
Effects of PFIO and PLIO on
H2O2-induced apoptosis of RINm5F cells
To evaluate whether the inhibitory effects of
H2O2 on RINm5F β-cells were associated with
apoptosis, double staining using FITC-labeled Annexin V and PI was
performed by flow cytometry. The apoptotic rate was significantly
increased to 35.6% in RINm5F β-cells following treatment with
H2O2 for 2 h; however, pretreatment with 100
µg/ml PFIO markedly inhibited H2O2-induced
apoptosis in RINm5F β-cells, and the inhibitory effects of PLIO
were also confirmed (Fig. 3A and
B). To investigate DNA condensation and/or fragmentation in
H2O2-induced apoptosis, chromatin in RINm5F
β-cells was stained using Hoechst 33342. Only in RINm5F β-cells
treated with H2O2 was microscopic DNA
fragmentation detected. Pretreatment with 100 µg/ml PFIO or PLIO
decreased H2O2-induced chromatin condensation, suggesting that PFIO
and PLIO may exert protective effects on oxidative stress-induced
apoptotic cell death in RINm5F β-cells by inhibiting DNA
fragmentation (Fig. 3C).
Effects of PFIO and PLIO treatment on
the expression of mitogen-activated protein kinases (MAPKs) and
apoptosis-associated proteins
A previous study demonstrated that the
phosphorylation of MAPK proteins is associated with the regulation
of mitochon-drial permeability-mediated activation of apoptotic
proteins, including the Bcl-2 protein family and cytochrome c
(18). To further confirm the
effects of PFIO and PLIO on the H2O2-induced
apoptosis of RINm5F β-cells, the present study detected the
expression of phosphorylated proteins from the MAPK signaling
pathway (ERK, JNK and p38) in RINm5F cells using western blot
analysis. In cultured cells exposed to H2O2, the N group exhibited
increased levels of p-MAPKs compared with the control. Conversely,
pretreatment with 100 µg/ml PFIO or PLIO inhibited the
H2O2-dependent phosphorylation of ERK.
However, PFIO and PLIO did not alter the phosphorylation of JNK
(Fig. 4A). Apoptosis is well known
to occur via two pathways, the intrinsic and extrinsic pathways
(18). The intrinsic pathway is
associated with activation of the Bcl-2 family of proteins and the
release of cytochrome c, whereas the extrinsic pathway is
characterized by the activation of AIF, caspase-8 and caspase-10.
The present study confirmed that pretreatment with 100 µg/ml PFIO
or PLIO did not alter the activation of AIF compared with in the
H2O2-only RINm5F cells (data not shown);
however, treatment with H2O2 alone decreased the expression of
Bcl-2 compared with the control, whereas treatment with H2O2 alone
increased the expression of Bax compared to the control. Treatment
with H2O2 alone increased mitochondrial release of cytochrome c
into the cytosol compared with the control. However, treatment with
100 µg/ml PFIO or PLIO inhibited mitochondrial release of
cytochrome c into the cytosol compared with the N group.
Furthermore, pretreatment with PFIO increased the expression of
Bcl-2 compared with the N group, whereas PFIO treatment decreased
the expression of Bax compared to the N group (Fig. 4B).
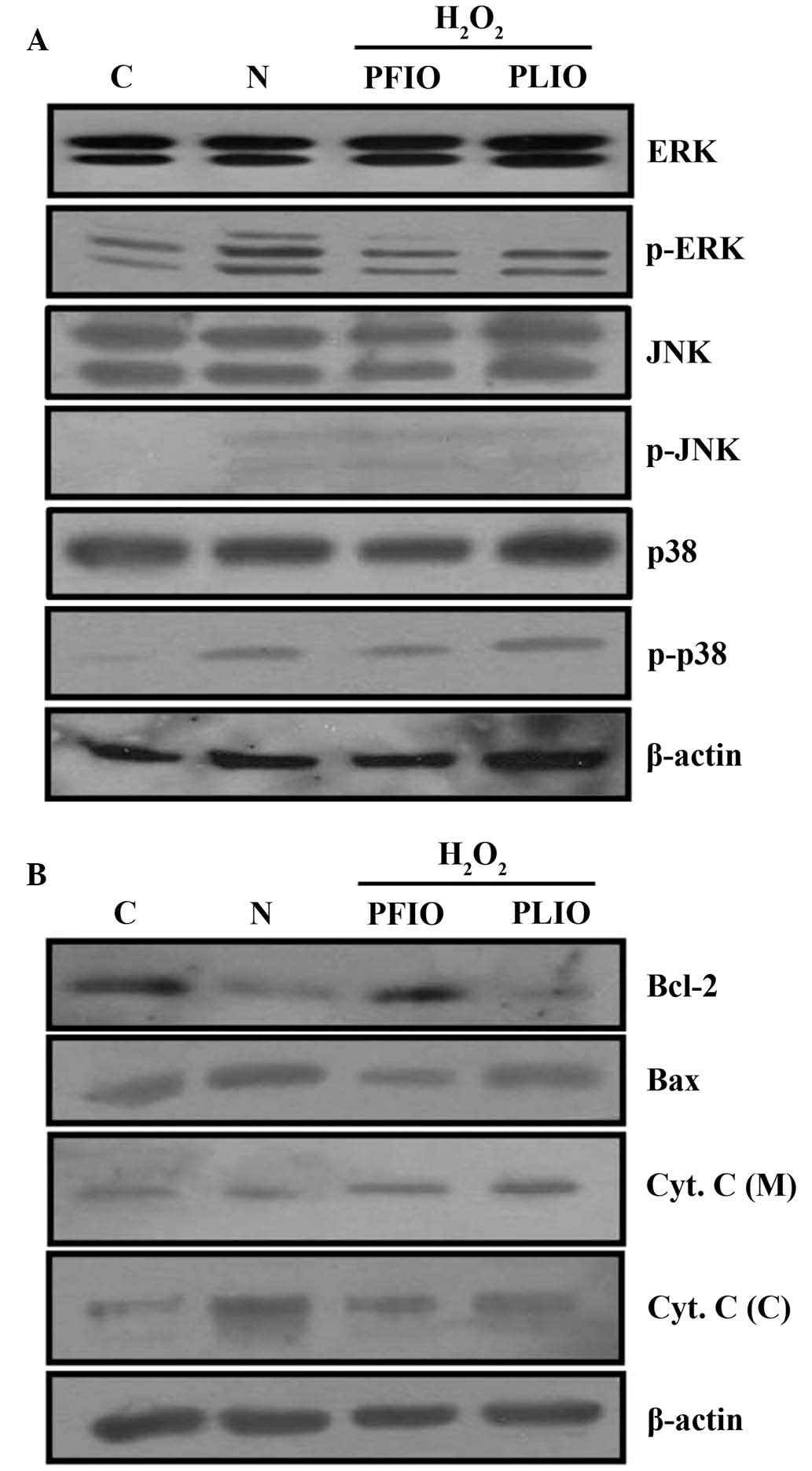 | Figure 4.Effects of PFIO and PLIO on
H2O2-induced apoptosis-associated protein
expression in RINm5F pancreatic cells. Treated cells were harvested
and lysates were prepared. (A) Expression levels of ERK, p-ERK,
JNK, p-JNK, p38 and p-p38 were assessed by western blot analysis.
(B) Expression levels of the Bcl-2 family of proteins and
fractional Cyt. C were determined by western blot analysis. Equal
loading of total proteins in each sample was confirmed by β-actin
expression. C, control; N, H2O2 treatment alone; H2O2, hydrogen
peroxide; PFIO, polysaccharides derived from Inonotus obliquus
fruiting body; PLIO, polysaccharides derived from I. obliquus
liquid culture broth; ERK, extracellular signal-regulated kinase;
JNK, c-Jun N-terminal kinase; p-, phosphorylated; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; Cyt. C, cytochrome c;
(C), cytosolic fraction; (M), mitochondrial fraction. |
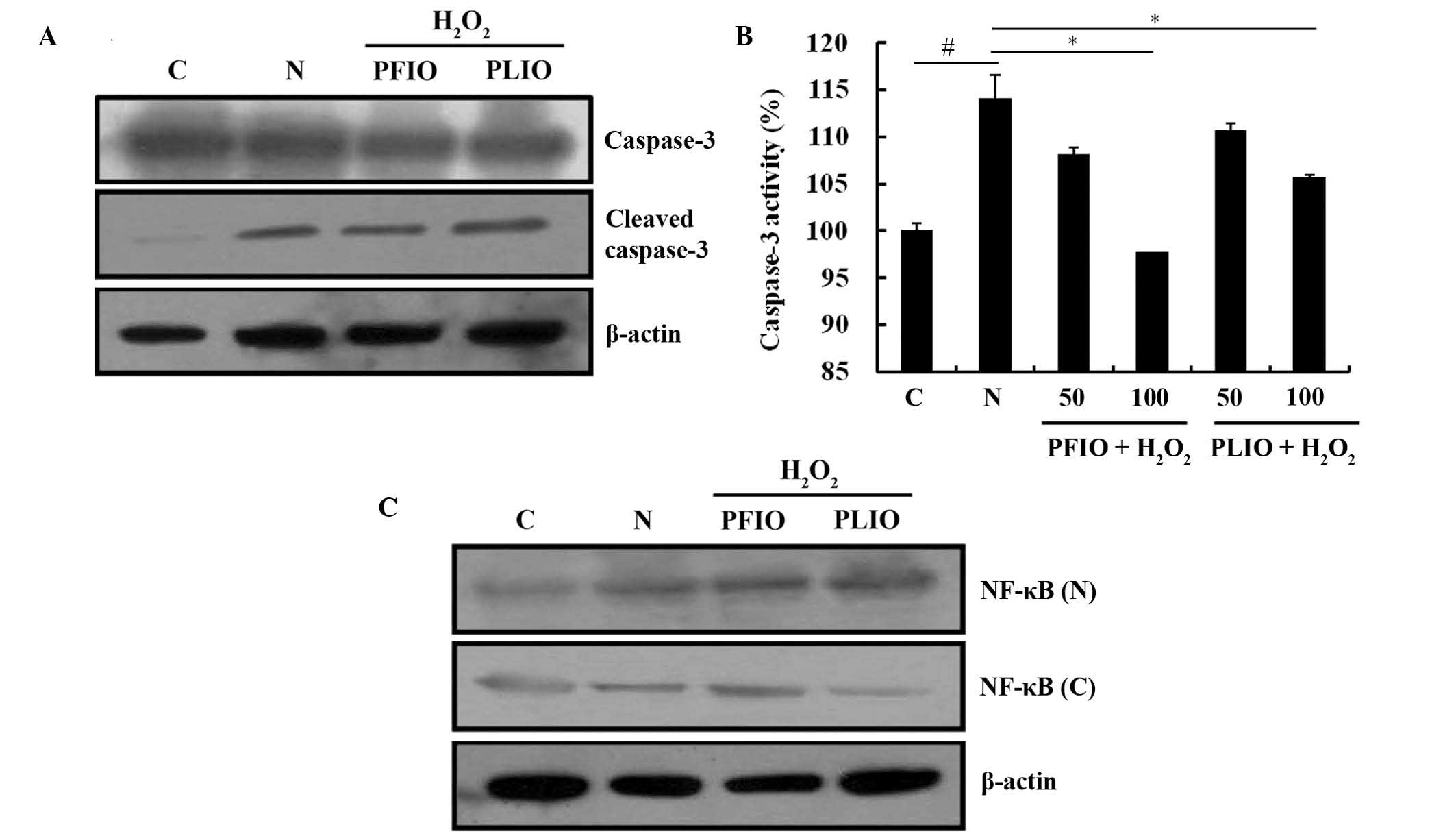
Effects of PFIO and PLIO treatment on
NF-κB translocation and caspase-3 activation
Caspase-3 is an important protein in the procession
of apoptosis; therefore, the present study examined the activation
of caspase-3 and its expression using a caspase-3 assay kit and
western blot analysis. Initially, the effects of PFIO or PLIO on
cleaved caspase-3 expression in RINm5F cells were determined. The
expression level of cleaved caspase-3 in H2O2-treated cells was
increased compared with the control. However, there were no marked
differences in cleaved caspases-3 expression between the PFIO or
PLIO groups and the N group (Fig.
5A). Caspase activity was also measured; treatment with 100
µg/ml PFIO or PLIO significantly inhibited H2O2-induced caspase-3
activity compared with the N group (Fig. 5B). NF-κB is involved in oxidative
stress-induced cell death in several cell types (19). The present study examined the
translocation of NF-κB from the cytosol to the nucleus, and
demonstrated that H2O2 treatment of RINm5F cells increased the
NF-κB p65 translocation from the cytosol into the nucleus. However,
pretreatment with PFIO or PLIO in RINm5F cells prior to H2O2
treatment did not induce any change in the translocation of NF-κB
p65 compared with the N group (Fig.
5C).
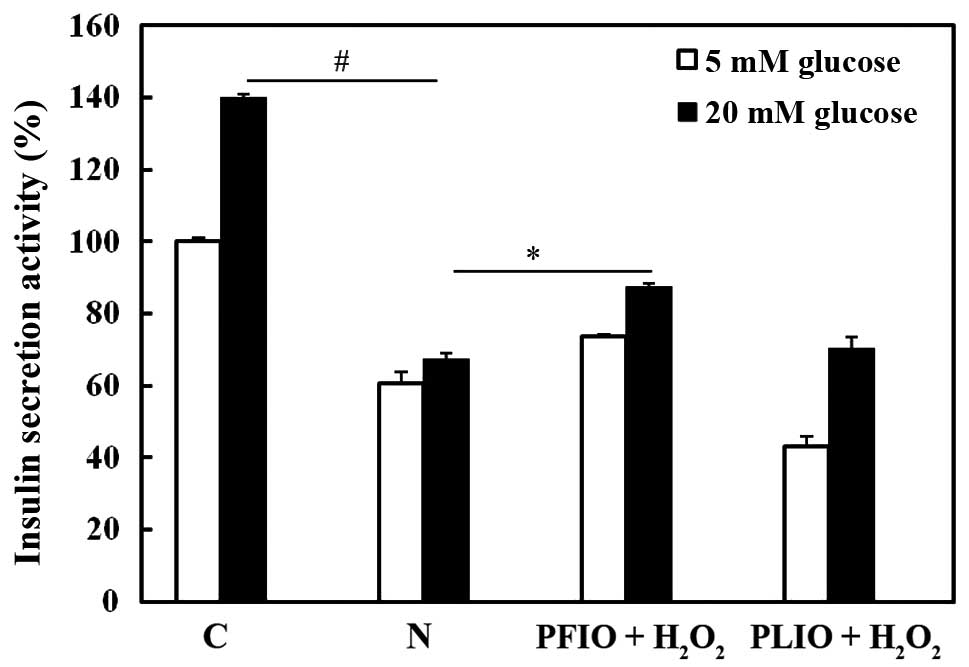
Effects of PFIO and PLIO on insulin
secretion of H2O2-treated RINm5F cells
To investigate whether PFIO and PLIO have potential
for the prevention of H2O2-induced β-cell dysfunction in RINm5F rat
insulinoma cells, the efficacy of insulin release was examined
following pretreatment with PFIO or PLIO. Insulin secretion was
measured using a rat/mouse insulin ELISA kit. Compared with
H2O2-treated RINm5F cells, pretreatment with
PFIO increased insulin secretion; however, pretreatment with PLIO
did not increase insulin secretion (Fig. 6).
Discussion
Increased exposure to H2O2
generates ROS, which induces exogenous stress in RINm5F cells.
Excess ROS generation can be inhibited by natural antioxidants, as
well as synthetic antioxidants, including butylated hydroxyanisole
and butylated hydroxytoluene (20,21).
However, synthetic antioxidants possess adverse side effects and
toxicity compared with natural antioxidants (22). Therefore, there has been a gradual
increase in demand for a safe substitute, such as antioxidants
extracted from natural foods (23,24).
Among natural foods, polysaccharides isolated from mushrooms have
previously been reported to be bioavailable and non-toxic compounds
that possess antioxidant activity (25–28).
Therefore, the present study evaluated the anti-diabetic efficacy
of a natural antioxidant isolated from I. obliquus on
H2O2-induced generation of ROS in pancreatic
β-cells.
Treatment with H2O2
significantly decreased cell viability, which was restored by PFIO
and PLIO pretreatment (Fig. 1C and
D). Excessive ROS generation is associated with apoptosis,
resulting in mitochondrial translocation of Bax, and the release of
cytochrome c from the mitochondrial fraction to the cytosol.
Subsequently, cytochrome c in the cytosol activates
caspase-3, which has a crucial role in the apoptotic pathway. In
the present study, H2O2 treatment of RINm5F cells increased the
protein expression levels of the pro-apoptotic protein Bax and the
release of cytochrome c from the mitochondria to the cytosol
compared with the control, whereas H2O2 treatment in RINm5F cells
decreased the expression levels of the anti-apoptotic protein Bcl-2
compared with the control, suggesting H2O2 treatment induced
apoptosis of RIN5mF pancreatic cells (Fig. 4B). Among the various signaling
pathways that respond to stress, MAPK family members are crucial
for the maintenance of cells. It has been previously demonstrated
that ERK is important for cell survival, whereas JNK and p38 are
considered to be stress responsive and, thus, involved in apoptosis
(29). The present study
demonstrated that H2O2-induced apoptosis is
prevented by pretreatment with PFIO and PLIO. According to the
results of the present study, the H2O2-only
group exhibited upregulation of MAPK phosphorylation and other
distinct characteristics of apoptosis. However, pretreatment of
cells with PFIO and PLIO reduced phosphorylation of MAPKs (Fig. 4A). Although the effects of the
PLIO-treated group may seem insignificant, it did have efficacy.
The translocation of NF-κB from the cytosol to the nucleus is
associated with the phosphorylation of MAPK proteins. However, in
the present study, H2O2-treated cells with or without PFIO and PLIO
treatment did not induce marked changes in the levels of NF-κB
nuclear translocation. The treatment of RINm5F cells with PFIO or
PLIO would explain the effects on oxidative stress-induced cell
damages independent of NF-κB activation (Fig. 5C). Finally, insulin secretion was
significantly inhibited in RINm5F cells exposed to H2O
(30,31). PFIO-treated cells exhibited a
marked increase in insulin secretion compared with cells treated
with PLIO (Fig. 6).
In conclusion, the results of the present study
demonstrated that PFIO and PLIO not only scavenged intracellular
ROS but also downregulated the phosphorylation of ERK, which may
lead to inhibition of cleaved caspase-3 in RINm5F pancreatic
β-cells after H2O2-treatment. These effects
may result in a decreased apoptotic cell rate. Therefore, these
results indicated that since ROS scavenging in cells is important
for cellular therapy, I. obliquus may be considered a
potential therapeutic agent for the prevention of diabetes.
Acknowledgements
The present study was supported by a grant from the
National Institute of Biological Resources (NIBR), funded by the
Ministry of Environment (MOE) of the Republic of Korea (grant no.
NIBR201528101).
References
|
1
|
Casteilla L, Rigoulet M and Pénicaud L:
Mitochondrial ROS metabolism: Modulation by uncoupling proteins.
IUBMB Life. 52:181–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimoto K, Suzuki K, Kizaki T, Hitomi Y,
Ishida H, Katsuta H, Itoh E, Ookawara T, Suzuki K, Honke K and Ohno
H: Gliclazide protects pancreatic beta-cells from damage by
hydrogen peroxide. Biochem Biophys Res Commun. 303:112–119. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redondo PC, Jardin I, Hernández-Cruz JM,
Pariente JA, Salido GM and Rosado JA: Hydrogen peroxide and
peroxynitrite enhance Ca2+ mobilization and aggregation in
platelets from type 2 diabetic patients. Biochem Biophys Res
Commun. 333:794–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weir GC and Bonner-Weir S: Five stages of
evolving beta-cell dysfunction during progression to diabetes.
Diabetes. 53:(Suppl 3). S16–S21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans JL, Maddux BA and Goldfine ID: The
molecular basis for oxidative stress-induced insulin resistance.
Antioxid Redox Signal. 7:1040–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang JS, Lee JS, Lee JH, Kwon DS, Lee KE,
Lee SY and Hong EK: Hispidin produced from Phellinus linteus
protects pancreatic beta-cells from damage by hydrogen peroxide.
Arch Pharm Res. 33:853–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima Y, Sato Y and Konishi T:
Antioxidant small phenolic ingredients in Inonotus obliquus
(persoon) Pilat (Chaga). Chem Pharm Bull (Tokyo). 55:1222–1226.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao XQ, Yu F, Wang N, Wu Y, Zou F, Wu K,
Liu M and Ouyang JP: Hypoglycemic effect of polysaccharide enriched
extract of Astragalus membranaceus in diet induced insulin
resistant C57BL/6J mice and its potential mechanism. Phytomedicine.
16:416–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perera PK and Li Y: Mushrooms as a
functional food mediator in preventing and ameliorating diabetes.
Functional Foods in Health and Disease. 4:161–171. 2011.
|
|
11
|
Kim YJ, Park J, Min BS and Shim SH:
Chemical constituents from the sclerotia of Inonotus obliquus. J
Korean Soc Appl Biol Chem. 54:287–294. 2011. View Article : Google Scholar
|
|
12
|
De Silva DD, Rapior S, Hyde KD and Bahkali
AH: Medicinal mushrooms in prevention and control of diabetes
mellitus. Fungal Divers. 56:1–29. 2012. View Article : Google Scholar
|
|
13
|
Cha JY, Jun BS, Kim JW, Park SH, Lee CH
and Cho YS: Hypoglycemic effects of fermented Chaga mushroom
(Inonotus obliquus) in the diabetic Otsuka Long-Evans Tokushima
Fatty (OLETF) rat. Food Sci Biotechnol. 15:739–745. 2006.
|
|
14
|
Zheng W, Zhang M, Zhao Y, Wang Y, Miao K
and Wei Z: Accumulation of antioxidant phenolic constituents in
submerged cultures of Inonotus obliquus. Bioresour Technol.
100:1327–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Lu X, Qu Z, Wang Z and Zhang L:
Glycosidase inhibitory activity and antioxidant properties of a
polysaccharide from the mushroom Inonotus obliquus. J Food Biochem.
34:178–191. 2010. View Article : Google Scholar
|
|
16
|
Shashkina MY, Shashkin PN and Sergeev AV:
Chemical and medicobiological properties of chaga (review).
Pharmaceutical Chemistry Journal. 40:560–568. 2006. View Article : Google Scholar
|
|
17
|
Sun JE, Ao ZH, Lu ZM, Xu HY, Zhang XM, Dou
WF and Xu ZH: Antihyperglycemic and antilipidperoxidative effects
of dry matter of culture broth of Inonotus obliquus in submerged
culture on normal and alloxan-diabetes mice. J Ethnopharmacol.
118:7–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dumont A, Hehner SP, Hofmann TG, Ueffing
M, Dröge W and Schmitz ML: Hydrogen peroxide-induced apoptosis is
CD95-independent, requires the release of mitochondria-derived
reactive oxygen species and the activation of NF-kappaB. Oncogene.
18:747–757. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito M, Sakagami H and Fujisawa S:
Cytotoxicity and apoptosis induction by butylated hydroxyanisole
(BHA) and butylated hydroxytoluene (BHT). Anticancer Res.
23:4693–4701. 2003.PubMed/NCBI
|
|
21
|
Kahl R and Kappus H: Toxicology of the
synthetic antioxidants BHA and BHT in comparison with the natural
antioxidant vitamin E. Z Lebensm Unters Forsch. 196:329–338.
1993.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C, Pearson AM and Gray JI: Effects of
synthetic antioxidants (BHA, BHT and PG) on the mutagenicity of
IQ-like compounds. Food Chemistry. 3:177–183. 1992. View Article : Google Scholar
|
|
23
|
Cordero-Herrera I, Martín MA, Goya L and
Ramos S: Cocoa flavonoids protect hepatic cells function against
high glucose-induced oxidative stress: Relevance of MAPKs. Mol Nutr
Food Res. 59:597–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuda H, Watanabe M, Hui SP, Joko S, Okabe
H, Jin S, Takeda S, Miki E, Watanabe T and Chiba H: Anti-apoptotic
effects of novel phenolic antioxidant isolated from the Pacific
oyster (Crassostrea gigas) on cultured human hepatocytes under
oxidative stress. Food Chem. 176:226–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee IK and Yun BS: Highly oxygenated and
unsaturated metabolites providing a diversity of hispidin class
antioxidants in the medicinal mushrooms Inonotus and Phellinus.
Bioorg Med Chem. 15:3309–3314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai MC, Song TY, Shih PH and Yen GC:
Antioxidant properties of water-soluble polysaccharides from
Antrodia cinnamomea in submerged culture. Food Chemistry.
104:1115–1122. 2007. View Article : Google Scholar
|
|
27
|
Lee IK, Kim YS, Jang YW, Jung JY and Yun
BS: New antioxidant polyphenols from the medicinal mushroom
Inonotus obliquus. Bioorg Med Chem Lett. 17:6678–6681. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui Y, Kim DS and Park KC: Antioxidant
effect of Inonotus obliquus. J Ethnopharmacol. 96:79–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaneto H, Nakatani Y, Kawamori D,
Miyatsuka T and Matsuoka TA: Involvement of oxidative stress and
the JNK pathway in glucose toxicity. Rev Diabet Stud. 1:165–174.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leibiger IB, Leibiger B and Berggren PO:
Insulin signaling in the pancreatic beta-cell. Annu Rev Nutr.
28:233–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim S, Rashid MA, Jang M, Kim Y, Won H,
Lee J, Woo JT, Kim YS, Murphy MP, Ali L, et al:
Mitochondria-targeted antioxidants protect pancreatic β-cells
against oxidative stress and improve insulin secretion in
glucotoxicity and glucolipotoxicity. Cell Physiol Biochem.
28:873–886. 2011. View Article : Google Scholar : PubMed/NCBI
|