Introduction
There is growing public concern regarding the
adverse effects of environmental chemicals with an estrogenic
influence on reproductive health. Phytoestrogens, including
daidzein, genistein and coumestrol, are defined as estrogenic
compounds. Daidzein and genistein, among all types of isoflavones,
are widely distributed in the daily human diet (1). In a typical Western diet, an average
of 0.2 mg/kg isoflavones are consumed daily, whereas a typical
Asian diet contains >1.5 mg/kg isoflavones per day (2), which can raise individual human
isoflavone serum levels to 500 nM (3). For infants fed soy-based formulas,
isoflavone intake can reach 9.3 mg/kg·bw/day (4).
Various studies have demonstrated that isoflavones
provide a protective barrier against cancers, osteoporosis,
menopausal syndromes and cardiovascular diseases (5–9).
These findings have evoked strong public and academic interest in
isoflavones. However, the negative effects of isoflavones,
particularly on the male reproductive system, have also been
reported (10). Previous studies
demonstrated that isoflavones may produce male reproductive
toxicity; their main adverse effects on the male reproductive
system include the disturbance of sex hormone release (11,12),
interference with the onset of puberty (13), altering penile corpus cavernosum
structure, weakening erectile function (14,15),
suppressing the activity of some steroidogenesis-associated enzymes
(16), and decreasing the weight
and epithelial height of accessory sex organs (12). Furthermore, a high intake of
soy-based food and soy isoflavones is associated with reduced sperm
concentration, as demonstrated in animal experiments and human
epidemiological studies (17,18).
As aforementioned, infants fed soy-based formulas may be exposed to
isoflavones, which may exert potential adverse effects. Although
previous in vivo experiments have been conducted to
determine the effects of isoflavone exposure on the testes
(10), data regarding exposure to
isoflavones during the early neonatal period is limited.
The mechanism underlying the effects of isoflavone
exposure on male reproductive function is not fully understood.
Several studies have reported that the effects of isoflavone
exposure differ to those of estradiol (19), which implies that they may have
different mechanisms of action. Isoflavones can suppress
testosterone production in Leydig cells by direct inhibition of
3β-hydroxysteroid dehydrogenase (3β-HSD) activity, and induce
adiponectin secretion, which can further suppress steroidogenic
acute regulatory protein (StAR) expression and decrease
testosterone (20). However,
existing studies cannot fully explain the observed toxic phenomena
associated with isoflavone exposure; for example, the associated
decreased testosterone levels and sperm count.
The present study aimed to explore the effects of
daidzein, a major type of isoflavone, on testes in the early
neonatal period. A testis culture system was used, in which the
testicular architecture was conserved and its development remains
similar to that in vivo (21,22),
in order to explore the effects of daidzein exposure on
steroidogenesis and Sertoli cell function. A cell culture
experiment was also performed. The results of the present study may
partially explain the adverse effects of daidzein on testes.
Materials and methods
Reagents
Daidzein (CAS# 486-66-8; purity ≥98%) was purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Daidzein
stock solution was generated using dimethyl sulfoxide (DMSO;
Amresco, LLC, Cleveland, OH, USA) and was diluted in culture
medium.
Experimental animals
Male neonatal mice (postnatal day 4) Kunming mice
were obtained from the Experimental Animal Center of Sichuan
University (SYXK 2009-045; Chengdu, China) and 10–15 mice were
used. The animals were maintained at 25°C, with access to food and
water ad libitum and a 12-h light/dark cycle. All animal
studies were conducted in accordance with the principles and
procedures for the care and use of laboratory animals. The present
study was approved by the ethics committee of West China School of
Public Health, Sichuan University (Chengdu, China).
Organ culture and treatment
Organ culture was performed as described in our
previous studies (23,24). The mice were anesthetized and
sacrificed by decapitation. Testes obtained from neonatal mice
[postnatal day 4 (PND4), 10–15 mice] were isolated and cut into six
to eight pieces. The pieces were pooled and transferred into 50 ml
bottles containing 6 ml culture medium; six or more pieces were
randomly put into each bottle. The bottles were attached to a
rotator at 30 rpm and were incubated at 34°C for 72 h. Organ
culture of the testes was performed in Dulbecco's modified Eagle's
medium (DMEM)/F-12 (Hali Biotech, Chengdu, China) supplemented with
10% calf serum (Hali Biotech), 15 mmol/l HEPES (pH 7.4), 5 µg/ml
transferrin, 10 µg/ml insulin, 2 mmol/l glutamine, 100 U/ml
penicillin G and 100 µg/ml streptomycin. The culture medium was
collected and changed daily. Mixed gas comprised of 50% O2, 45% N2
and 5% CO2 was injected into the bottles to maintain a fresh
atmosphere, as previously stated (24,25).
Testes were assigned to five groups: Control group and
daidzein-treated groups (0.03, 0.3, 3 and 30 µmol/l). At the end of
the 72 h culture, the testes were fixed for 12 h in Bouin's fluid
(trinitrophenol:methanal:ethanoic acid =15:5:1) at 4°C, embedded in
paraffin and cut into 5 µm sections. The media were collected and
stored at −80°C for the subsequent testosterone radioimmunoassay.
For mRNA analysis, testes were collected and immediately frozen in
liquid nitrogen. Furthermore, 30 µg/ml 5′-bromo-2′-deoxyuridine
(BrdU) was mixed into the culture system 3 h prior to testis
harvesting to determine proliferation of Sertoli cells. The data
were obtained from at least three independently repeated culture
bottles.
Leydig cell culture and treatment
Leydig cells were obtained from the testes of male
preadolescent mice (10–15 mice; age, 18–21 days) following
euthanasia by decapitation under anesthesia. Subsequently, Leydig
cells were isolated by a combination of collagenase digestion and
Percoll density centrifugation. After digestion with 8 ml
collagenase II with 1.5% bovine serum albumin (BSA; Sigma-Aldrich;
Merck Millipore) at 35°C for 25 min, and prior to Percoll density
centrifugation, seminiferous tubules were removed by passage of
testicular fractions through a 200-mesh filter. The dispersed cells
were washed with DMEM/F12 and layered over a Percoll gradient (70,
58, 30 and 5%; Pharmacia Biotech; GE Healthcare, Uppsala, Sweden).
The gradient was centrifuged for 30 min at 1,500 × g, and
cells localized between Percoll gradient 70 and 58% were isolated.
This step ensured the removal of heavier red blood cells and
lighter germ cells.
To determine the purity of the target cells, enzyme
histochemical and immunocytochemical methods were used to detect
3β-HSD. After a 24 h culture period, purified Leydig cells were
incubated for 60 min at 34°C in a phosphate buffer solution
containing 10% nitroblue tetrazolinum (Amresco, LLC), 10%
nicotanamide adenosine dinucleotide (Amresco, LLC), 6% DHEA (Merck
Millipore) and 6% DMSO (26).
Positive cells (containing blue granules) were identified as Leydig
cells. In addition, Leydig cell slides were fixed with 4%
paraformaldehyde, and were then immunostained according to standard
protocol. Briefly, fixed cell slides were treated with 5% Triton
X-100 and sealed with 5% BSA. Subsequently, the slides were
incubated with 3β-HSD antibodies (1:800; BIOSS, Beijing, China;
cat. no. bs-3906R) overnight at 4°C, then incubated with a
secondary antibody working solution from an immunohistochemistry
kit (SP-9000; ZSGB-BIO) for a further 15 min at room temperature,
followed by incubation with avidin-biotin peroxidase complex for 15
min. DAB was used as the chromogen and slides were observed under a
light microscope. Leydig cells were typically 90% pure as assessed
by these two staining methods.
Leydig cells were cultured in the same DMEM/F12
medium as the organ culture. Leydig cells (3×105/1 ml
medium) were plated into six-well plates and were cultured at 34°C
in a humidified atmosphere containing 95% air and 5%
CO2. A total of 1 day after plating, fresh medium was
added, and treatments were initiated. Doses of daidzein were added
to wells in triplicate, and cells were cultured at 34°C in a
humidified atmosphere containing 5% CO2 for 24 h.
Subsequently, the medium was collected and stored at −20°C until
further analysis.
Assessment of cellular viability
Cellular viability was evaluated using the MTT
proliferation assay. Briefly, cells were plated in a 96-well plate
at a density of 10,000 cells/well. Following 24 or 48 h incubation
at 37°C with various concentrations of daidzein, 20 µl MTT was
added to each well and the cells were incubated for 4 h at 37°C.
Subsequently, the medium was replaced with 150 µl DMSO and the
cells were oscillated for 15 min. Finally, absorbance was measured
at 490 nm. Results were presented as a percentage of the control
values from untreated cells.
Measurement of testosterone
production
Testosterone secreted into the culture medium was
determined in duplicate by Iodine [125I] Testosterone
Radioimmunoassay kit (Beijing North Institute of Biological
Technology, Beijing, China), according to the manufacturer's
protocol.
Histopathology and
immunohistochemistry
Histopathological evaluation was conducted using
hematoxylin and eosin (H&E) staining. Following fixation in
Bouin's fixative and dehydration, the testes were embedded in
paraffin and cut into 5 µm sections. Sections from the testes were
stained with H&E for histopathological evaluation, hematoxylin
was applied for 5 min and eosin for 2 min (both at room
temperature). Protein expression in tissue was detected by
immunohistochemical staining. Serial sections (5 µm) were then
mounted on slides, deparaffinized with xylene twice for 15 min and
rehydrated in an alcohol gradient. The sections were then
immunostained with antibodies according to manufacturer's protocol.
For antigen retrieval, sections were microwaved at 450 W in 10
mmol/l citrate buffer solution. For all immunohistological
procedures, slides were treated with Triton X-100 for 1 min, and
were then incubated in 0.3% H2O2 for 10 min and in 5% normal goat
serum albumin (ZSGB-BIO, Beijing, China) in PBS for 1 h, in order
to block nonspecific antigen-binding. Subsequently, the slides were
incubated with the following primary antibodies: Anti-3β-HSD
(1:800), anti-cholesterol side chain cleavage enzyme (P450scc;
1:200; Wuhan Boster Biological Technology Co., Ltd., Wuhan, China;
cat. no. BA3699), anti-17α-hydroxylase/20-lyase (P450C17α; 1:200;
BIOSS; cat. no. bs-6695R), anti-vimentin (1:400; BIOSS; cat. no.
bs-8533R) and anti-BrdU (1:100; Sigma-Aldrich; Merck Millipore;
cat. no. B2531) overnight at 4°C. The primary antibodies were
detected by incubation with a secondary antibody working solution
from a immunohistochemistry kit (SP-9000; ZSGB-BIO) for a further
15 min at room temperature, followed by incubation with
avidin-biotin peroxidase complex (Vector Laboratories, Inc.,
Burlingame, USA) for 15 min. 3′,3′-Diaminobenzidine (ZSGB-BIO) was
used as the chromogen and hematoxylin as the nuclear counterstain.
Negative control refers to samples in which the primary antibody
was omitted. Staining was observed under a light microscope.
RNA extraction, reverse transcription
and quantitative polymerase chain reaction (qPCR)
Testes were collected and homogenized, followed by
total RNA extraction from the testes and cells using the MicroElute
Total RNA kit (Omega Bio-tek, Norcross, GA, USA), and 0.8 µg total
RNA was reverse transcribed using Oligo (dT) 18 primers and
RevertAid M-MuLV reverse transcriptase (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in a 20 µl reaction mixture, according to
the manufacturer's protocol. To determine the expression levels of
mRNAs that code for proteins implicated in the steroidogenic
pathway (StAR, P450scc, P450C17α and 3β-HSD), qPCR amplification
was performed using a Bio-Rad CFX96 Detector system and SsoFast
EvaGreen Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
A reaction volume of 10 µl was used containing 2 µl cDNA, 5 µl
supermix, 0.2 µl each primer and 2.6 µl RNase/DNase-free water. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec; followed by 35 cycles of 95°C for 5 sec and Tm for
5 sec. Tm was 60°C for StAR and p450c17α, 55°C for P450scc and
3β-HSD, 56.3°C for inhibin B, 57.4 for vimentin and 57.4 for
β-actin. In testes, the expression levels of inhibin B and vimentin
were also detected. The primers used were as follows: StAR, forward
(f) 5′-cgg gtg gat ggg tca agt tc-3′, reverse (r) 5′-cca agc gaa
aca cct tgcc-3′; p450scc, f 5′-aca tgg cca aga tgg tac agt tg-3′, r
5′-acg aag cac cag gtc att cac-3′; 3β-HSD, f 5′-tgg aca aag tat tcc
gac caga-3′, r 5′-ggc aca ctt gct tga aca cag-3′; p450c17α, f
5′-tga cca gta tgt agg ctt cag tcg-3′, r 5′-tcc ttc ggg atg gca aac
tctc-3′; vimentin f 5′-cgt cca cac gca cct acag-3′, r 5′-ggg gga
tga gga ata gag gct-3′; and inhibin B, f 5′-ctt cgt ctc taa tga agg
caa cc-3′, and r 5′-ctc cac cac att cca cct gtc-3′. Gene expression
was normalized to the housekeeping gene β-actin: F 5′-ggc tgt att
ccc ctc cat cg-3′ and R 5′-cca gtt ggt aac aat gcc atgt-3′, and
expression levels are presented relative to vehicle control (DMSO)
at the same time point. All samples were run together in
triplicate. Quantification cycle (Cq) values obtained for
triplicates were averaged and normalized to β-actin for each RNA
sample. Analyses were performed using the 2−ΔΔCq method
(27).
Western blot analysis
Leydig cells were washed with PBS and lysed in cell
lysis/extraction reagent (Nanjing KeyGen Biotech, Co., Ltd.,
Nanjing, China), including phenylmethanesulfonyl fluoride. Protein
concentration was quantified using the Bradford method. Proteins
(40 µg) were separated by 10% SDS-PAGE, and were then
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membranes. The blots were incubated at 4°C overnight with specific
primary antibodies against StAR (1:100; cat. no. sc-25806), P450scc
(1:200; cat. no. 18043), P450C17α (1:200; cat. no. 66850) and
3β-HSD (1:200; cat. no. sc-28206), all obtained from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and β-actin (1:1,000;
ZSGB-BIO; cat. no. TA-09). Subsequently, membranes were incubated
with secondary antibodies (1:5,000; Santa Cruz Biotechnology, Inc.;
cat. nos. sc-2004 and sc-2020) for 1 h at room temperature,
according to the manufacturer's protocol. The membranes were then
washed with TBS-Tween 20, and immunoreactivity was visualized using
an enhanced chemiluminescence reagent and analyzed with ChemiDoc MP
system and ImageLab 4.0 (Bio-Rad Laboratories, Inc.).
Measurement of inhibin B
production
Inhibin B secreted into the medium by testes was
detected by ELISA [Human/Mouse/Rat Inhibin B (β B subunit);
RayBiotech, Norcross, GA, USA] according to the manufacturer's
protocol. Absorbance was measured using an ELISA plate reader at a
wavelength of 450 nm.
Statistical analysis
All values are expressed as the mean ± standard
error. Statistical analysis was performed using one-way analysis of
variance followed by Dunnett's t-test with SPSS 20.0 (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
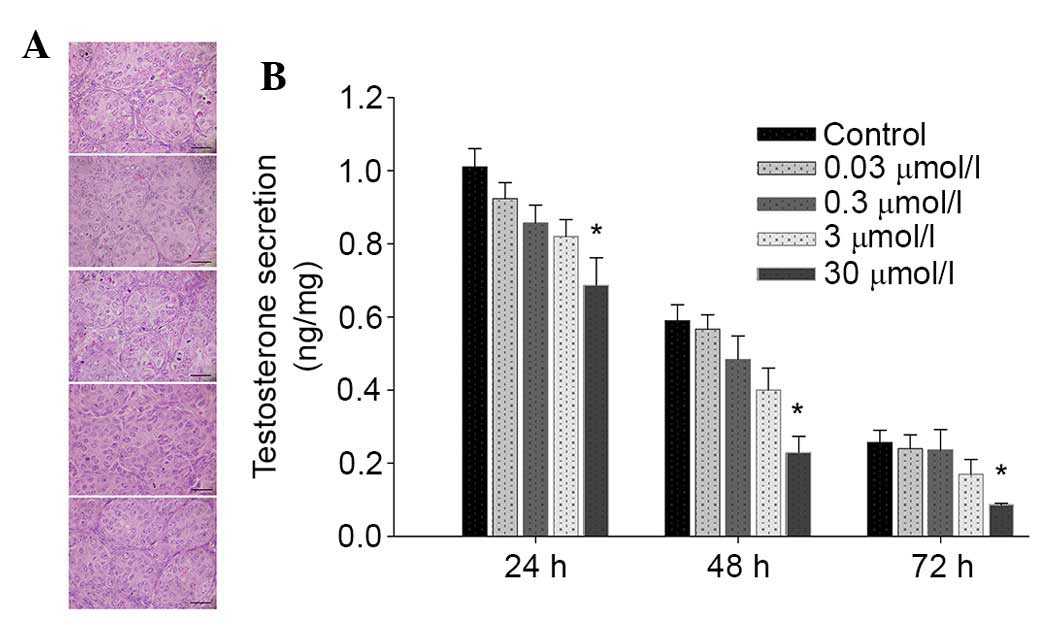
Exposure to daidzein suppresses
testosterone secretion in testes without inducing histopathological
changes
Testicular histopathology was observed 72 h
following daidzein administration, and testosterone secreted by
neonatal testes was collected every 24 h and analyzed. No obvious
histopathological alterations were observed in the daidzein-treated
testes (Fig. 1A). However,
testosterone secretion by PND4 testes during 3 days of culture was
reduced following incubation with 30 µmol/l daidzein (Fig. 1B).
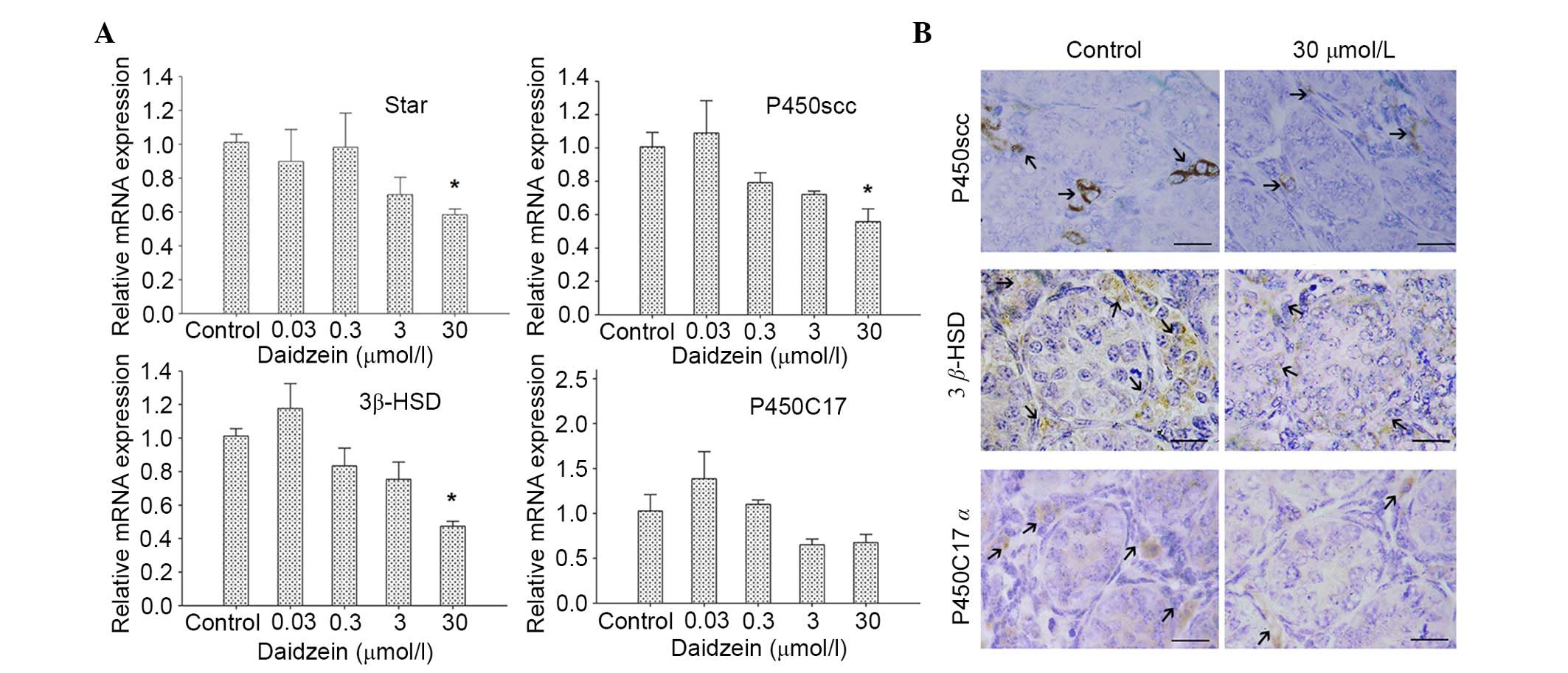
Daidzein alleviates StAR and
steroidogenic enzyme expression in testes
To determine the effects of daidzein on
steroidogenic-related protein and enzyme expression in neonatal
testes, qPCR and immunohistochemical analysis were performed. After
72 h culture with or without daidzein, the mRNA expression levels
of StAR, P450scc and 3β-HSD, which are involved in the
steroidogenic process, were downregulated following treatment with
30 µmol/l daidzein compared with the control (P<0.05; Fig. 2A). No obvious changes in the mRNA
expression levels of P450C17α were detected.
Corresponding with the findings of qPCR, the protein
expression levels of P450scc and 3β-HSD in the testes were reduced
following treatment with 30 µmol/l daidzein (Fig. 2B). No marked change in P450C17α
staining intensity was detected.
Exposure to daidzein reduces
testosterone production, and alters the expression of StAR and
steroidogenic enzymes in Leydig cells
To further verify the effects of daidzein on
testosterone production in testes, the present study investigated
testosterone secretion, and StAR, P450scc, 3β-HSD and P450C17α
expression in Leydig cells in vitro. Cell viability was
analyzed by MTT assay, and no changes were observed (Fig. 3A). Consistent with organ culture,
incubation with 30 µmol/l daidzein induced a marked suppression of
testosterone production by Leydig cells compared with in the
control cells (P<0.05; Fig.
3B). The qPCR results indicated that the mRNA expression levels
of StAR, P450scc, 3β-HSD and P450C17α were lower following
treatment with 30 µmol/l daidzein compared with the control
(P<0.05; Fig. 3C).
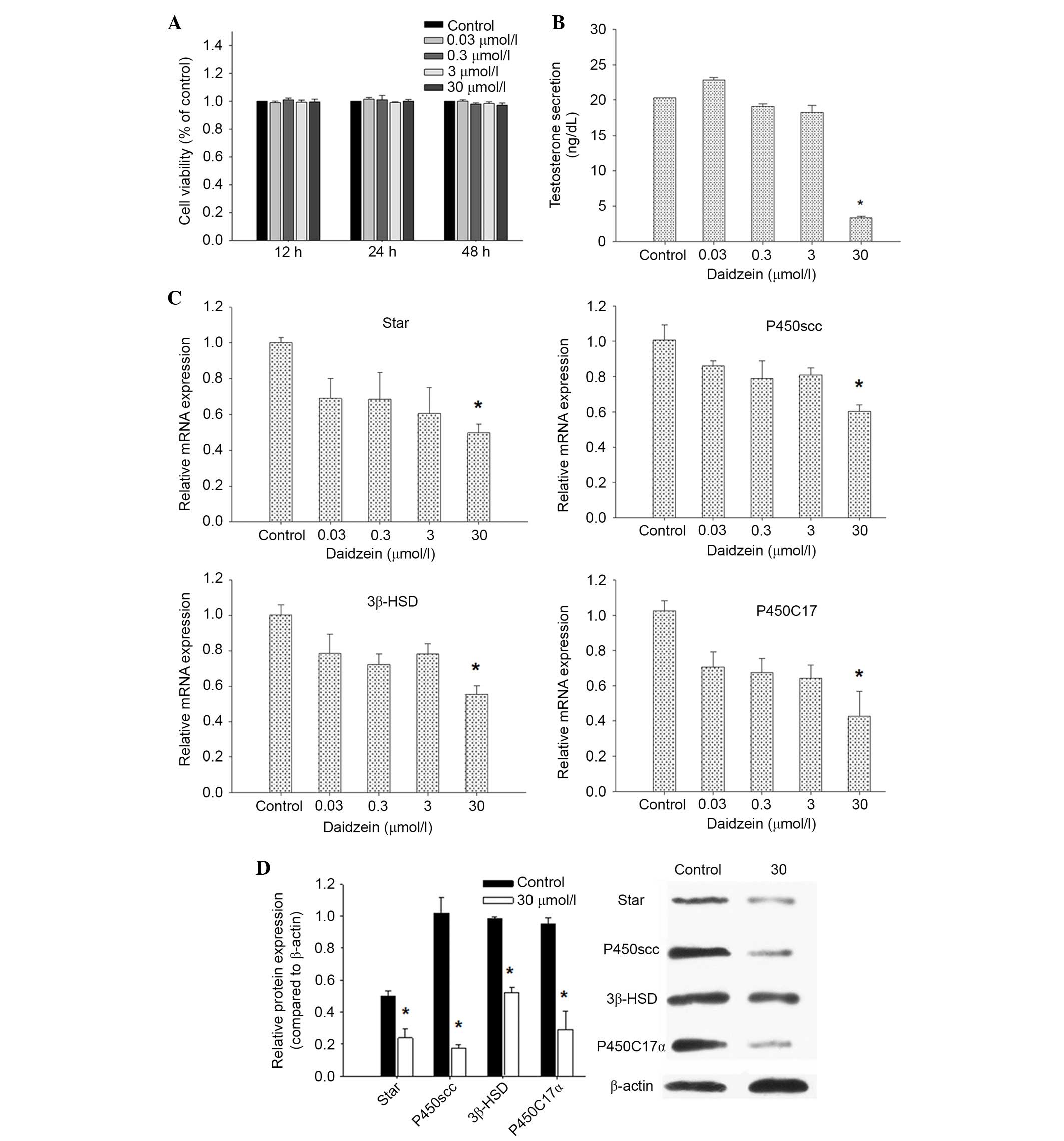 | Figure 3.Effects of daidzein on testosterone
production, and the expression of StAR and steroidogenic enzymes in
Leydig cells. (A) Cell viability assessment in Leydig cells. (B)
Testosterone secretion in Leydig cells, in the absence or presence
of daidzein. (C) Quantitative polymerase chain reaction analyses of
mRNA expression in primary cultured Leydig cells. (D) Western blot
analysis of StAR, P450scc, 3β-HSD, P450C17α and β-actin expression
in Leydig cells. A representative gel is shown. Densitometric
analysis of StAR, P450scc, 3β-HSD and P450C17α expression to
β-actin expression was conducted. Data are presented as the mean ±
standard error. *P<0.05 vs. control. StAR, steroidogenic acute
regulatory protein; P450scc, anti-cholesterol side chain cleavage
enzyme; 3β-HSD, 3β-hydroxysteroid dehydrogenase; P450C17α,
17α-hydroxylase/20-lyase. |
The present study also examined the protein
expression levels of StAR, P450scc, 3β-HSD and P450C17α in Leydig
cells. StAR, P450scc, 3β-HSD and P450C17α protein expression levels
were decreased in Leydig cells exposed to daidzein (Fig. 3D). Collectively, these effects on
mRNA and protein expression indicate that a general downregulation
of the steroid synthesis pathway leads to the observed low
testosterone levels.
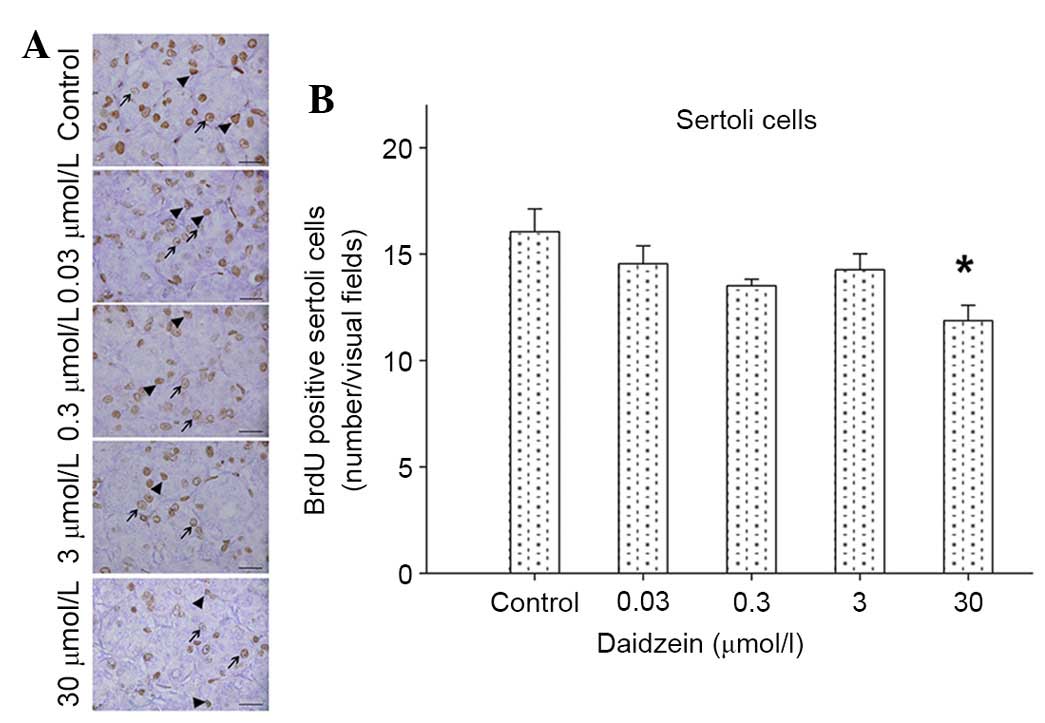
Daidzein inhibits Sertoli cell
proliferation in cultured neonatal mouse testes
To investigate the effects of daidzein exposure on
Sertoli cells in mouse testes, cell proliferation was analyzed with
BrdU staining. A total of 3 hours after BrdU was mixed into the
culture system, some labeled cells were observed in the
seminiferous tubules and in the interstitium of the testes. The
number of labeled Sertoli cells was reduced in the testes following
treatment with 30 µmol/l daidzein (Fig. 4A and B).
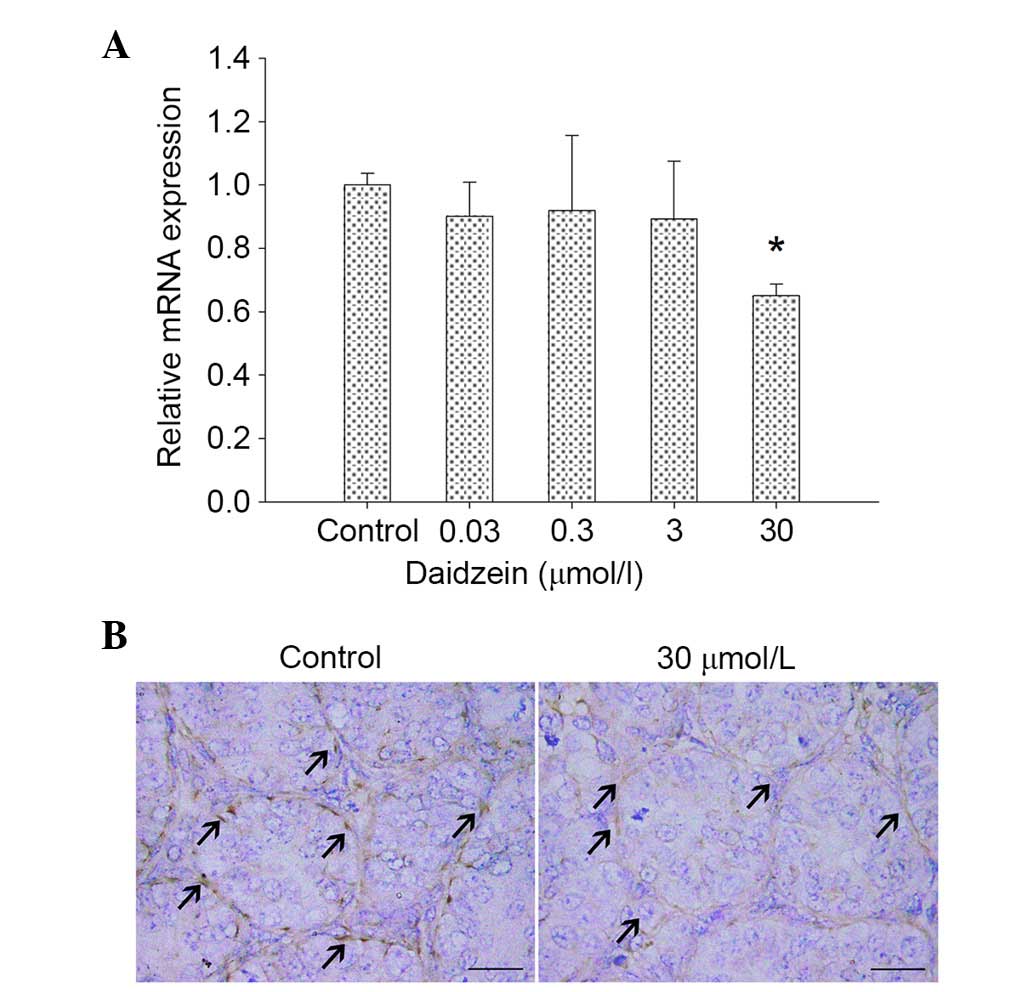
Daidzein inhibits vimentin expression
in cultured neonatal mouse testes
To further determine the effects of daidzein on
Sertoli cells in neonatal testes, the expression levels of vimentin
were analyzed. Vimentin mRNA expression levels were markedly lower
following treatment with 30 µmol/l daidzein compared with the
control (P<0.05; Fig. 5A). In
addition, immunostaining of Sertoli cells in daidzein-treated (30
µmol/l) testes was weaker compared with the control (Fig. 5B).
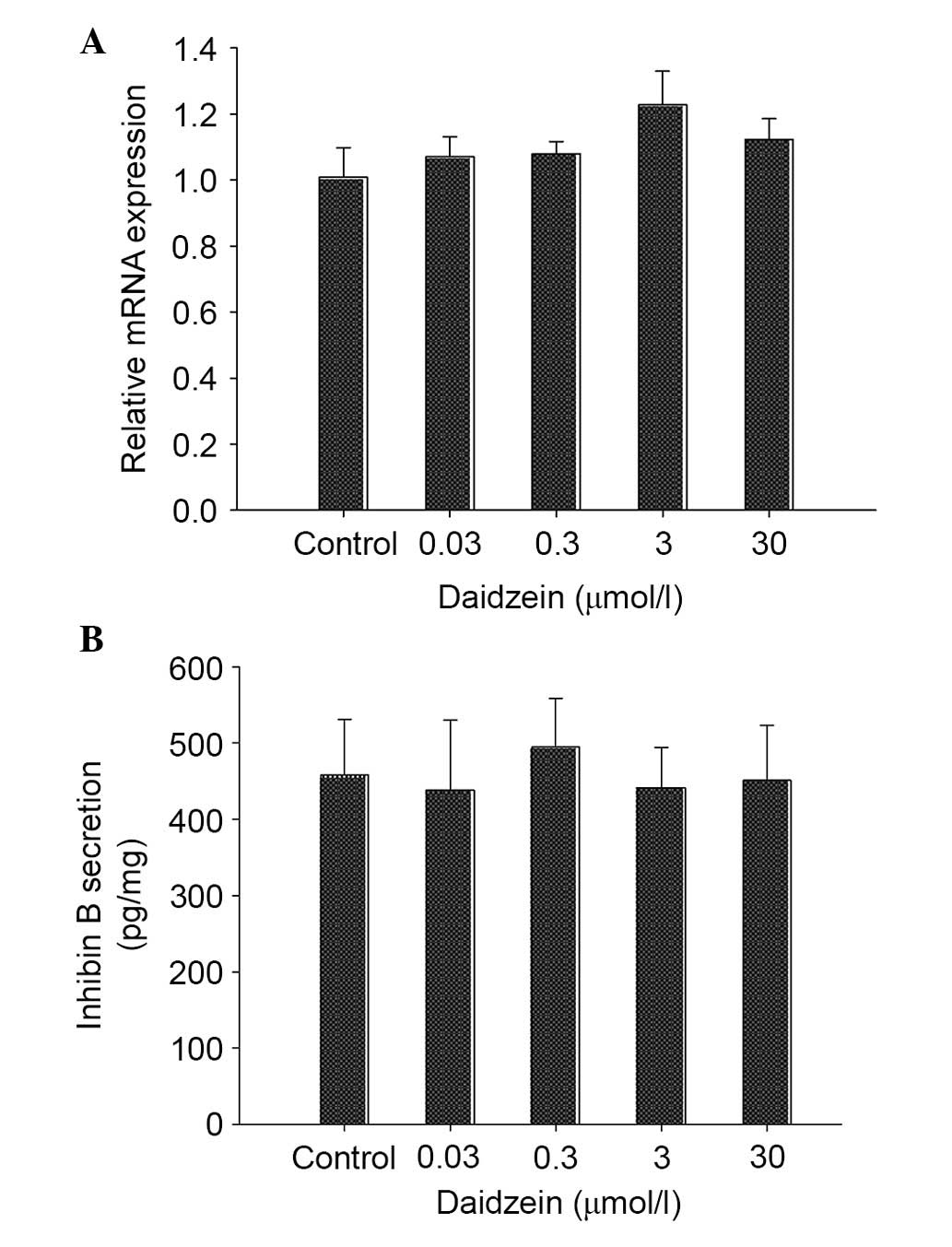
Exposure to daidzein has no effect on
inhibin B expression in neonatal mouse testes
The effects of daidzein on the expression levels of
inhibin B, which is a regulator involved in steroidogenesis, were
also analyzed. As shown in Fig.
6A, the mRNA expression levels of inhibin B exhibited no
alteration following treatment with daidzein. Consistent with this
result, the levels of inhibin B secreted by testes exhibited no
difference between daidzein-treated testes and controls (Fig. 6B).
Discussion
Daidzein is an active isoflavone, which is widely
consumed in the East. The present study focused on the effects of
daidzein on testosterone biosynthesis and Sertoli cell function in
neonatal mouse testes. The results demonstrated that daidzein was
able to decrease testosterone synthesis in vitro, and the
decrease was preceded by alterations in related protein expression,
e.g. StAR, P450scc and 3β-HSD. In addition, the present data
illustrated that high doses of daidzein may exert adverse effects
on Sertoli cells in neonatal mouse testes.
The present study investigated the effects of
daidzein on testes during the early neonatal period in vitro
using a testis culture system, since the dose of delivered
isoflavone to pups via milk is difficult to control. The dose has
been shown to be thousands of times lower compared with the dose
delivered by maternal diet (28).
Furthermore, the period of infancy is much longer in humans than it
is in mice. Therefore, the testis culture method was chosen to
determine the direct effects of daidzein on early neonatal mouse
testes (PDN4).
Testis culture was initially used in reproductive
physiology studies, including those focused on spermatogenesis,
meiosis and development (29–32).
In addition, the organ culture method has been used to develop
novel applications in toxicological studies, allowing in
vitro functional screening tools to select exogenous compounds
for further in vivo evaluation (33,34).
Cell lines or primary cultures are limited, since most only poorly
mimic the physiological situation; however, organ culture can
preserve intercellular relationships in tissue (35).
The present study demonstrated that short exposure
to high concentrations of daidzein may lead to reduced testosterone
levels, without histopathological changes, in neonatal mouse
testes, which consisted with the results of a previous study
(10). Pan et al reported
that genistein exposure at 20 and 100 mg/kg/day was able to
adversely affect testosterone production (15). Furthermore, Lehraiki et al
reported that genistein impairs early testosterone production in
fetal mouse testes in vitro (22). Genistein at a dose of 213
mg/kg.bw/day from gestational day 7 to PND13 also reduced plasma
testosterone levels in rat offspring (36). Similarly, a clear downtrend
tendency in plasma testosterone levels was detected in primary
Leydig cells treated with 30 µmol/l daidzein, which is similar to
the results of Opalka et al (37,38).
Nevertheless, no adverse effect on proliferation was detected in
Leydig cells treated with 30 µmol/l daidzein. The present findings
identified a potential harmful effect of daidzein exposure on
testis steroidogenesis function during the early neonatal
period.
Although the association between isoflavone exposure
and reduced testosterone production has been defined, the toxicant
mechanism remains unclear. Suppression of StAR and steroidogenic
enzymes (P450scc, 3β-HSD and P450C17α) has been suggested as
toxicant mechanisms underlying reduced testosterone levels
(39,40). Testosterone production can be
inhibited by exogenous compounds via the suppression of StAR,
P450scc and 3β-HSD (41,42). The present results detected reduced
expression levels of StAR, P450scc and 3β-HSD in the neonatal
testes when treated with a high concentration of daidzein.
Consistent with this result, it has previously been reported that
genistein, another type of isoflavone, suppresses StAR, P450scc,
3β-HSD and P450C17 expression, and decreases testosterone
production in fetal testes (22).
These findings may explain why testosterone levels in the testis or
plasma are decreased following treatment with isoflavones.
Concurrently, the expression levels of StAR and
steroidogenic enzymes were assessed in daidzein-treated Leydig
cells, in order to further verify the mechanistic activities of
daidzein on factors associated with steroid synthesis. The results
demonstrated an apparent decline in mRNA and protein expression
levels of StAR, P450scc, 3β-HSD and P450C17, which are consistent
with the organ culture results. With regards to P450C17α, the
discrepancy in expression levels between the testes and Leydig
cells may be due to different levels of sensitivity to isoflavones.
Leydig cells are known to be more sensitive to exogenous agents
compared with organ culture (43).
Prior to the onset of puberty, immature Sertoli
cells proliferate in parallel to spermatogonia until the
seminiferous epithelium reaches its final size (44). Our previous study indicated that
isoflavone exposure can downregulate follicle-stimulating hormone
receptor, transferrin and vimentin mRNA expression in Sertoli cells
in vitro (45). The present
results demonstrated that 30 µmol/l daidzein was able to interfere
with Sertoli cell proliferative activity in neonatal mouse testes
in vitro, thus implying a potential adverse effect on
spermatogenesis.
The present study also detected reduced expression
levels of vimentin, which is an essential Sertoli cell cytoskeletal
protein, following treatment with daidzein. Previous studies have
demonstrated that endocrine disruptors can alter the expression of
vimentin in vitro and in vivo (46,47).
In the present study, the mRNA and protein expression levels of
vimentin were reduced in daidzein-exposed testes; these results are
similar to our previous study (45). These findings demonstrated that
vimentin may be sensitive to daidzein treatment. Reduced vimentin
expression may help to explain the increased germ cell apoptosis,
which was observed in China mini-pig boars following exposure to
250 ppm soy isoflavone for 60 days (12). The association between altered
vimentin expression and reproductive toxicity due to isoflavone
exposure remains unclear. Therefore, further research is required
to clarify these associations and the mechanisms by which
isoflavone exerts effects on vimentin expression.
Inhibin B, which is secreted by Sertoli cells,
reflects spermatogenesis function in the testes, and, is an
essential biomarker of reproductive toxicity. Concentration of
inhibin B is correlated with testicular histology structure
(48), sperm concentration and
sperm count (49). Decreased
inhibin B levels may predict impaired secretory function of Sertoli
cells and damaged testicular spermatogenesis (50). However, in the present study, no
clear effects on inhibin B mRNA expression were detected in the
neonatal testes. Similarly, concentrations of inhibin B in the
supernatant were not altered in testes exposed to daidzein in
vitro. Therefore, inhibin B may not be affected following
daidzein exposure.
In conclusion, early neonatal exposure to daidzein
elicits adverse effects on testosterone biosynthesis and Sertoli
cell function. Daidzein exposure may inhibit the expression of StAR
and steroidogenic enzymes (P450scc and 3β-HSD). In addition, the
results of the present study revealed that exposure to daidzein
reduces the expression of vimentin in Sertoli cells, predicting a
potential adverse effect on sperm development. Therefore, these
results indicate that isoflavones exert potential harmful effects
on immature testes; however, the detailed mechanism of action of
this phytochemical remains to be elucidated. Further studies
investigating the effects of isoflavones on Sertoli cells are
required to adequately understand the role of isoflavones in sperm
development.
Acknowledgements
Financial support for this study was provided by the
National Natural Science Foundation of China (grant no.
81072309).
References
|
1
|
Kurzer MS and Xu X: Dietary
phytoestrogens. Annu Rev Nutr. 17:353–381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coward L, Barnes NC, Setchell KDR and
Barnes S: Genistein, daidzein, and their β-glycoside conjugates:
Antitumor isoflavones in soybean food from American and Asian
diets. J Agri Food Chem. 41:1961–1967. 1993. View Article : Google Scholar
|
|
3
|
Morton MS, Arisaka O, Miyake N, Morgan LD
and Evans BA: Phytoestrogen concentrations in serum from Japanese
men and women over forty years of age. J Nutr. 132:3168–3171.
2002.PubMed/NCBI
|
|
4
|
McCarver G, Bhatia J, Chambers C, Clarke
R, Etzel R, Foster W, Hoyer P, Leeder JS, Peters JM, Rissman E, et
al: NTP-CERHR expert panel report on the developmental toxicity of
soy infant formula. Birth Defects Res B Dev Reprod Toxicol.
92:421–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lagari VS and Levis S: Phytoestrogens for
menopausal bone loss and climacteric symptoms. J Steroid Biochem
Mol Biol. 139:294–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magee PJ and Rowland I: Soy products in
the management of breast cancer. Curr Opin Clin Nutr Metab Care.
15:586–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adjakly M, Ngollo M, Boiteux JP, Bignon
YJ, Guy L and Bernard-Gallon D: Genistein and daidzein: Different
molecular effects on prostate cancer. Anticancer Res. 33:39–44.
2013.PubMed/NCBI
|
|
8
|
Liu ZM, Ho SC, Chen YM and Ho YP: The
effects of isoflavones combined with soy protein on lipid profiles,
C-reactive protein and cardiovascular risk among postmenopausal
Chinese women. Nutr Metab Cardiovasc Dis. 22:712–719. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jungbauer A and Medjakovic S:
Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol
Biol. 139:277–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rozman KK, Bhatia J, Calafat AM, Chambers
C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, et
al: NTP-CERHR expert panel report on the reproductive and
developmental toxicity of genistein. Birth Defects Res B Dev Reprod
Toxicol. 77:485–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akingbemi BT, Braden TD, Kemppainen BW,
Hancock KD, Sherrill JD, Cook SJ, He X and Supko JG: Exposure to
phytoestrogens in the perinatal period affects androgen secretion
by testicular Leydig cells in the adult rat. Endocrinology.
148:4475–4488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan XX, Zhang B, Li LL, Xiao CW, Fan JX,
Geng MM and Yin YL: Effects of soybean isoflavones on reproductive
parameters in Chinese mini-pig boars. J Anim Sci Biotechnol.
3:312012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caceres S, Pena L, Moyano G,
Martinez-Fernandez L, Monsalve B, Illera MJ, Millan P, Illera JC
and Silvan G: Isoflavones and their effects on the onset of puberty
in male Wistar rats. Andrologia. 47:1139–1146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Pan L, Xia X, Feng Y, Jiang C and
Cui Y: Long-term effects of phytoestrogen daidzein on penile
cavernosal structures in adult rats. Urology. 72:220–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan L, Xia X, Feng Y, Jiang C and Huang Y:
Exposure to the phytoestrogen daidzein attenuates
apomorphine-induced penile erection concomitant with plasma
testosterone level reduction in dose- and time-related manner in
adult rats. Urology. 70:613–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu GX, Zhao BH, Chu YH, Zhou HY, Akingbemi
BT, Zheng ZQ and Ge RS: Effects of genistein and equol on human and
rat testicular 3beta-hydroxysteroid dehydrogenase and
17beta-hydroxysteroid dehydrogenase 3 activities. Asian J Androl.
12:519–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chavarro JE, Toth TL, Sadio SM and Hauser
R: Soy food and isoflavone intake in relation to semen quality
parameters among men from an infertility clinic. Hum Reprod.
23:2584–2590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cederroth CR, Zimmermann C, Beny JL,
Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli
JD and Nef S: Potential detrimental effects of a phytoestrogen-rich
diet on male fertility in mice. Mol Cell Endocrinol. 321:152–160.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adachi T, Okuno Y, Takenaka S, Matsuda K,
Ohta N, Takashima K, Yamazaki K, Nishimura D, Miyatake K, Mori C
and Tsujimoto G: Comprehensive analysis of the effect of
phytoestrogen, daidzein, on a testicular cell line, using mRNA and
protein expression profile. Food Chem Toxicol. 43:529–535. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfaehler A, Nanjappa MK, Coleman ES,
Mansour M, Wanders D, Plaisance EP, Judd RL and Akingbemi BT:
Regulation of adiponectin secretion by soy isoflavones has
implication for endocrine function of the testis. Toxicol Lett.
209:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livera G, Delbes G, Pairault C,
Rouiller-Fabre V and Habert R: Organotypic culture, a powerful
model for studying rat and mouse fetal testis development. Cell
Tissue Res. 324:507–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lehraiki A, Chamaillard C, Krust A, Habert
R and Levacher C: Genistein impairs early testosterone production
in fetal mouse testis via estrogen receptor alpha. Toxicol In
Vitro. 25:1542–1547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Huang J, Li M, Gao ZB, Zhu YF and Li
Y: The effects of di- (2-ethylhexyl) phthalate (DEHP) in
testosterone synthesis and its molecular mechanisms in the fetal
testis of male mouse by organ culture in vitro. Sichuan Da Xue Xue
Bao Yi Xue Ban. 44:511–516. 2013.(In Chinese). PubMed/NCBI
|
|
24
|
Liu Y, Zhu YF, Gao ZB, Li M, Zhong LY, Yin
DJ and Li Y: Establishment of a rotary aerobic culture system for
in vitro culture of mouse testis. Nan Fang Yi Ke Da Xue Xue Bao.
35:66–71. 2015.(In Chinese). PubMed/NCBI
|
|
25
|
Li Y and Yu ZL: Effect of zinc on bone
metabolism in fetal mouse limb culture. Biomed Environ Sci.
15:323–329. 2002.PubMed/NCBI
|
|
26
|
Liu JZ, Guo HB, Deng CH, Ou YH and Peng
AP: The culture and identification of rat testis Leydig cell.
Zhonghua Nan Ke Xue. 12:14–17. 2006.(In Chinese). PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doerge DR, Twaddle NC, Churchwell MI,
Newbold RR and Delclos KB: Lactational transfer of the soy
isoflavone, genistein, in Sprague-Dawley rats consuming dietary
genistein. Reprod Toxicol. 21:307–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steinberger A and Steinberger E:
Differentiation of rat seminiferous epithelium in organ culture. J
Reprod Fertil. 9:243–248. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokonishi T, Sato T, Katagiri K and Ogawa
T: In vitro spermatogenesis using an organ culture technique.
Methods Mol Biol. 927:479–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato T, Katagiri K, Gohbara A, Inoue K,
Ogonuki N, Ogura A, Kubota Y and Ogawa T: In vitro production of
functional sperm in cultured neonatal mouse testes. Nature.
471:504–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato T, Katagiri K, Kubota Y and Ogawa T:
In vitro sperm production from mouse spermatogonial stem cell lines
using an organ culture method. Nat Protoc. 8:2098–2104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lehraiki A, Racine C, Krust A, Habert R
and Levacher C: Phthalates impair germ cell number in the mouse
fetal testis by an androgen- and estrogen-independent mechanism.
Toxicol Sci. 111:372–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lambrot R, Livera G, Coffigny H, Pairault
C, Frydman R, Habert R and Rouiller-Fabre V: A new method for
toxicity assays on human and mouse fetal testis. Biochimie.
88:1831–1835. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Auger J, Eustache F, Rouiller-Fabre V,
Canivenc-Lavier MC and Livera G: Integrative rodent models for
assessing male reproductive toxicity of environmental endocrine
active substances. Asian J Androl. 16:60–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boberg J, Mandrup KR, Jacobsen PR, Isling
LK, Hadrup N, Berthelsen L, Elleby A, Kiersgaard M, Vinggaard AM,
Hass U and Nellemann C: Endocrine disrupting effects in rats
perinatally exposed to a dietary relevant mixture of
phytoestrogens. Reprod Toxicol. 40:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Opalka DM, Kaminska B, Piskula MK,
Puchajda-Skowronska H and Dusza L: Effects of phytoestrogens on
testosterone secretion by Leydig cells from Bilgoraj ganders (Anser
anser). Br Poult Sci. 47:237–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Opalka M, Kaminska B, Leska A and Dusza L:
Mechanism of phytoestrogen action in Leydig cells of ganders (Anser
anser domesticus): Interaction with estrogen receptors and
steroidogenic enzymes. J Environ Sci Health A Tox Hazard Subst
Environ Eng. 47:1335–1339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hannas BR, Lambright CS, Furr J, Evans N,
Foster PM, Gray EL and Wilson VS: Genomic biomarkers of
phthalate-induced male reproductive developmental toxicity: A
targeted RT-PCR array approach for defining relative potency.
Toxicol Sci. 125:544–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ye L, Su ZJ and Ge RS: Inhibitors of
testosterone biosynthetic and metabolic activation enzymes.
Molecules. 16:9983–10001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu S, Wang D, Zhang J, Zhang D, Gong M,
Wang C, Wei N, Liu W, Wang Y, Zhao C, et al: Citrinin reduces
testosterone secretion by inducing apoptosis in rat Leydig cells.
Toxicol In Vitro. 26:856–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
N'Tumba-Byn T, Moison D, Lacroix M,
Lecureuil C, Lesage L, Prud'homme SM, Pozzi-Gaudin S, Frydman R,
Benachi A, Livera G, et al: Differential effects of bisphenol A and
diethylstilbestrol on human, rat and mouse fetal Leydig cell
function. PLoS One. 7:e515792012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Delbès G, Duquenne C, Szenker J, Taccoen
J, Habert R and Levacher C: Developmental changes in testicular
sensitivity to estrogens throughout fetal and neonatal life.
Toxicol Sci. 99:234–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryser S, Glauser D, Vigier M, Zhang YQ,
Tachini P, Schlegel W, Durand P and Irminger-Finger I: Gene
expression profiling of rat spermatogonia and Sertoli cells reveals
signaling pathways from stem cells to niche and testicular cancer
cells to surrounding stroma. BMC Genomics. 12:292011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yin D, Zhu Y, Liu L, Xu H, Huang J and Li
Y: Potential detrimental effect of soy isoflavones on testis
sertoli cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 39:598–604.
2014.(In Chinese). PubMed/NCBI
|
|
46
|
Feng Y, Fang X, Shi Z, Xu M and Dai J:
Effects of PFNA exposure on expression of junction-associated
molecules and secretory function in rat Sertoli cells. Reprod
Toxicol. 30:429–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tay TW, Andriana BB, Ishii M, Tsunekawa N,
Kanai Y and Kurohmaru M: Disappearance of vimentin in Sertoli
cells: A mono(2-ethylhexyl) phthalate effect. Int J Toxicol.
26:289–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pierik FH, Vreeburg JT, Stijnen T, De Jong
FH and Weber RF: Serum inhibin B as a marker of spermatogenesis. J
Clin Endocrinol Metab. 83:3110–3114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Andersson AM, Petersen JH, Jørgensen N,
Jensen TK and Skakkebaek NE: Serum inhibin B and
follicle-stimulating hormone levels as tools in the evaluation of
infertile men: Significance of adequate reference values from
proven fertile men. J Clin Endocrinol Metab. 89:2873–2879. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Monsees TK, Franz M, Gebhardt S,
Winterstein U, Schill WB and Hayatpour J: Sertoli cells as a target
for reproductive hazards. Andrologia. 32:239–246. 2000. View Article : Google Scholar : PubMed/NCBI
|