Introduction
Mammalian stanniocalcin 2 (STC2) is a type of
glycoprotein hormone, which regulates calcium/phosphate levels
(1,2). STC2 has been primarily investigated
in cancer and has been identified to promote tumor growth and/or
invasion in gastric cancer, neuroblastoma and laryngeal squamous
cell cancer (3–9). Conversely, STC2 has been identified
to reduce the migration and invasion of breast tumor cells
(10). STC2 may also affect
postnatal growth and animal size (11–13).
Notably, STC2 may be stimulated by retinoic acid and vitamin D3
(14,15), two important triggers for
osteoblast differentiation, indicating a possible function of STC2
in osteogenesis.
Extracellular signal-regulated kinase 1/2 (ERK1/2)
is important for numerous cellular responses, including cell
proliferation, differentiation and survival. ERK may be activated
by growth factors to regulate osteoblast differentiation via
collagen and α2β1 integrin-mediated signaling (16,17).
A previous study determined that the introduction of a mutated ERK1
in human osteoblast cells decreased alkaline phosphatase (ALP)
activity and deposition of bone matrix proteins, resulting in
reduced osteoblast differentiation and matrix mineralization
(18). U0126, a specific inhibitor
of mitogen-activated protein kinase (MAPK)/ERK, has been determined
to block ascorbic acid (AA)-or bone morphogenetic protein
7/AA-dependent osteoblast-specific gene expression (19). Previous studies have demonstrated
that ERK may regulate osteoblast differentiation via various
molecules, including Schnurri-3, secreted phosphoprotein 24, ETS2
repressor factor, twist family bHLH transcription factor 1 and
vasopressin (20–24). As STC2 was reported to regulate the
expression of cyclin D1 and activate ERK1/2 in a dominant-positive
manner (25) the present study
investigated whether STC2 contributed to osteoblast differentiation
in association with the MAPK/ERK signaling pathway.
Materials and methods
Cell culture and transfection
C2C12 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
Millipore), 100 U/ml penicillin and 100 mg/ml streptomycin.
MC3T3-E1 cells were cultured in α-minimum essential medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin. C2C12 is a
mouse myoblast cell line capable of osteoblastic differentiation,
which has been identified as a type of mesenchymal stem cell
(26,27). MC3T3-E1 is an osteoblast precursor
cell line. In order to induce osteoblast differentiation, medium
was added with 50 mg/ml ascorbic acid, 10 mM sodium
β-glycerophosphate, 1 µM dexamethasone and 50 ng/ml bone
morphogenetic protein 2 (all obtained from Sigma-Aldrich; Merck
Millipore). To block the activation of the ERK1/2 signaling
pathway, 20 µM U0126 (Sigma-Aldrich; Merck Millipore) was added to
the medium.
STC2 cDNA was cloned into a plenti6 vector
(Invitrogen; Thermo Fisher Scientific, Inc.). STC2 short hairpin
RNA (shRNA) plasmid was purchased from GeneChem Co., Ltd.
(Shanghai, China). Cells were transfected using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol for 6 h at 37°C.
ALP activity and mineralization
analysis
Cells were plated in 96 or 24-well plates at a
density of 0.5×104 cells/well and cultured in
differentiation medium for 0, 3 or 6 days. The medium was changed
every 3 days. ALP activity and staining was completed with 1-Step
NBT/BCIP substrate solution (Thermo Fisher Scientific, Inc.) as
previously described (28). For
mineralization detection, cells were fixed in 70% ethanol for 10
min after 7–14 days induction to differentiation, then stained with
Alizarin red solution (2%, pH 4.2) for 15 min at room temperature,
then washed with deionized water for removal of nonspecific
staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). cDNA was prepared using
PrimeScript RT-PCR kit (Takara Bio, Inc., Otsu, Japan) and was
subsequently used for the RT-qPCR analysis performed using
FastStart Universal SYBRGreen Master (Roche Diagnostics GmbH,
Mannheim, Germany). Primers used were as described by Wang et
al (29). The thermocycling
conditions were as follows: First cycle for 10 min at 95°C,
followed by and 40 cycles for 15 sec at 95°C and 1 min at 60°C. The
primer sequences were as follows: Forward
5′-CAATAAGGTAGTGAACAGAC-3′, and reverse, 5′-CTTCAAGCCATACTGGTCT-3′
for osteocalcin (OCN); forward, 5′-CCTGGTAAAGATGGTGCC-3′ and
reverse, 5′-CACCAGGTTCACCTTTCGCACC-3′ for collagen type I α 1 chain
(Col1α1); forward, 5′-GAATGCACTACCCAGCCAC-3′ and reverse,
5′-TGGCAGGTACGTGTGGTAG-3′ for runt-related transcription factor 2
(Runx2); forward, 5′-GTCAAGAGTCTTAGCCAAACTC-3′ and reverse,
5′-AAATGATGTGAGGCCAGATGG-3′ for osterix (OSX); and forward,
5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse,
5′-CCTGCTTCACCACCTTCTTGAT-3′ for GAPDH. Results were quantified
using the 2−∆∆Cq method (30).
Western blotting
Cells were harvested and treated with lysis buffer
[50 mM Tris-HCl (pH 6.8), 100 mM dithiothreitol, 2% SDS, 10%
glycerol, and 1 mM phenylmethylsulfonyl fluoride]. Cell lysates
were centrifuged at 12,000 × g for 15 min at 4°C, and cell
debris was then discarded. Proteins were quantified by
bicinchoninic acid assay and 40 µg samples were electrophoresed on
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Following blocking with milk, membranes were incubated with primary
antibodies against STC2 (1:500; Santa Cruz Biotechnology, Dallas,
TX, USA; cat. no. sc-14350), phosphorylated (p)-ERK1/2 (1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA; cat. no. 9102)
and β-actin (1:3,000; Beyotime Institute of Biotechnology, Haimen,
China; cat. no. AF0003) overnight at 4°C, followed by incubation
with horseradish peroxidase (HRP)-labeled anti-mouse (cat. no.
7076) or anti-rabbit (cat. no. 7074) IgG secondary antibodies
(1:2,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The proteins were visualized using an enhanced
chemiluminescent substrate for detection of HRP (Beyotime Institute
of Biotechnology).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using unpaired Student's t test with Microsoft Excel
software (Microsoft Corporation, Redmond, WA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Knockdown of STC2 reduces osteoblast
differentiation and mineralization
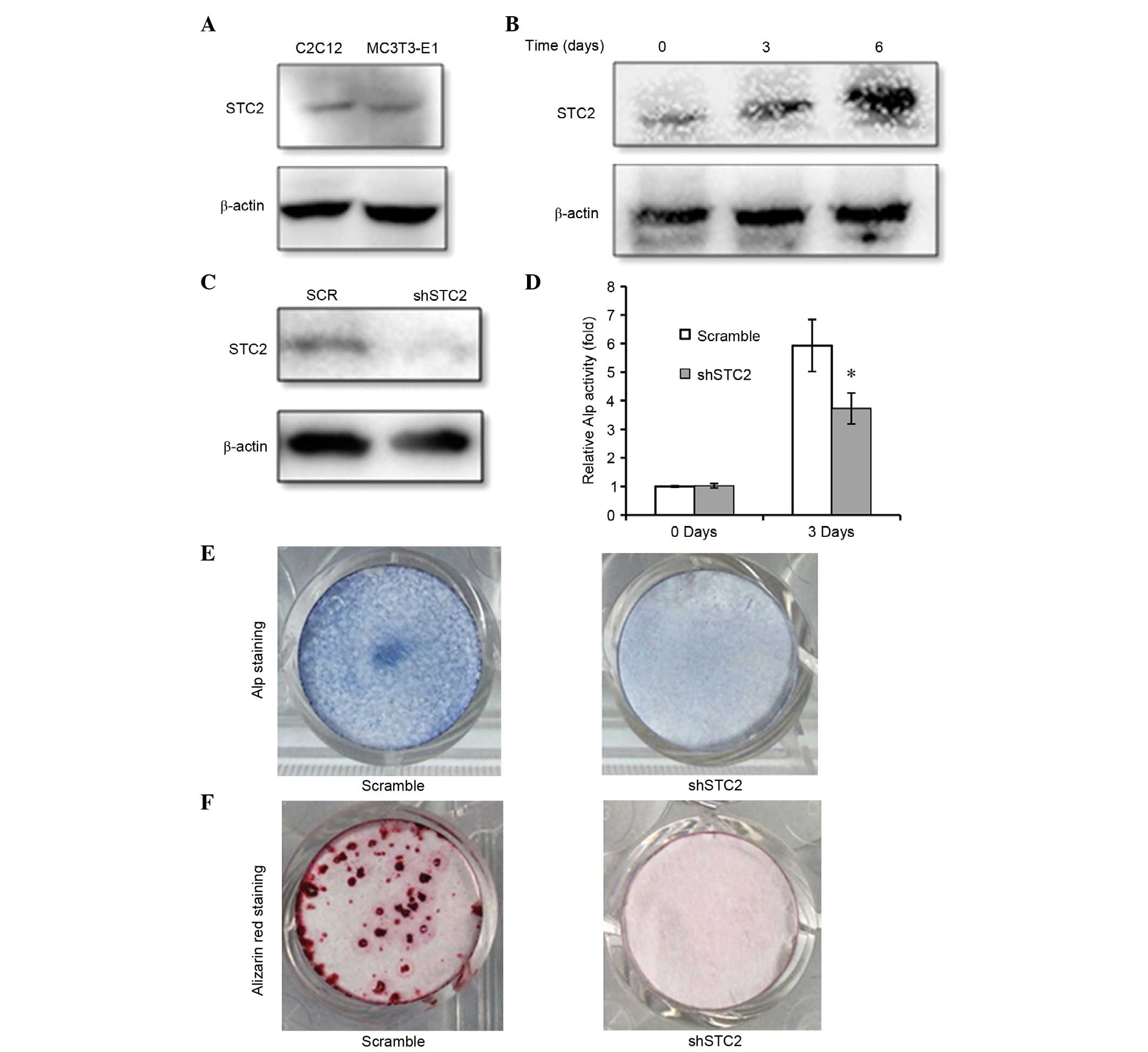
STC2 was expressed in the C2C12 and MC3T3-E1 cell
lines (Fig. 1A) and STC2 protein
expression levels were increased during differentiation of MC3T3-E1
cells to osteoblasts (Fig.
1B).
To investigate the function of STC2 in osteoblast
differentiation, STC2 expression was silenced with a specific shRNA
in MC3T3-E1 cells (Fig. 1C). ALP
activity is an early marker for osteoblast differentiation;
therefore, it was used in the present study to determine whether
cells were differentiating. It was revealed that the ALP activity
was significantly lower in the shSTC2 group of MC3T3-E1 cells
compared with the scramble group (P<0.05; Fig. 1D and E). Following the induction of
osteoblast differentiation, the mineralized nodules detected by
Alizarin red staining on day 14 were markedly reduced in the shSTC2
group of MC3T3-E1 cells when compared with the control cells
(Fig. 1F).
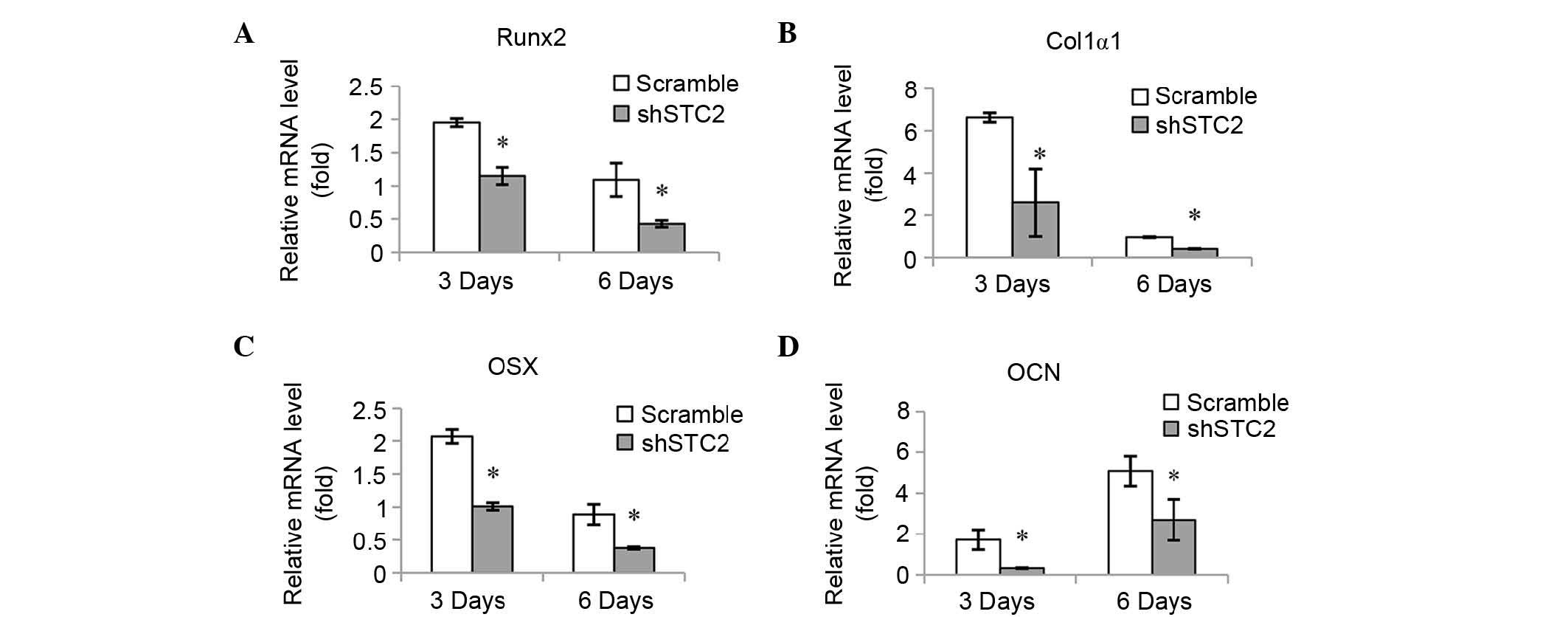
Knockdown of STC2 decreases expression
levels of osteoblast-specific genes
The mRNA expression levels of various
osteoblast-associated genes were quantified in order to determine
the molecular mechanisms underlying the effect STC2 may have on
osteoblast differentiation. It was determined that mRNA expression
levels of Runx2 (Fig. 2A), Col1α1
(Fig. 2B), OSX (Fig. 2C) and OCN (Fig. 2D) were significantly reduced in the
shSTC2 group of the MC3T3-E1 cells compared with the scramble group
at 3 and 6 days of induction (P<0.01).
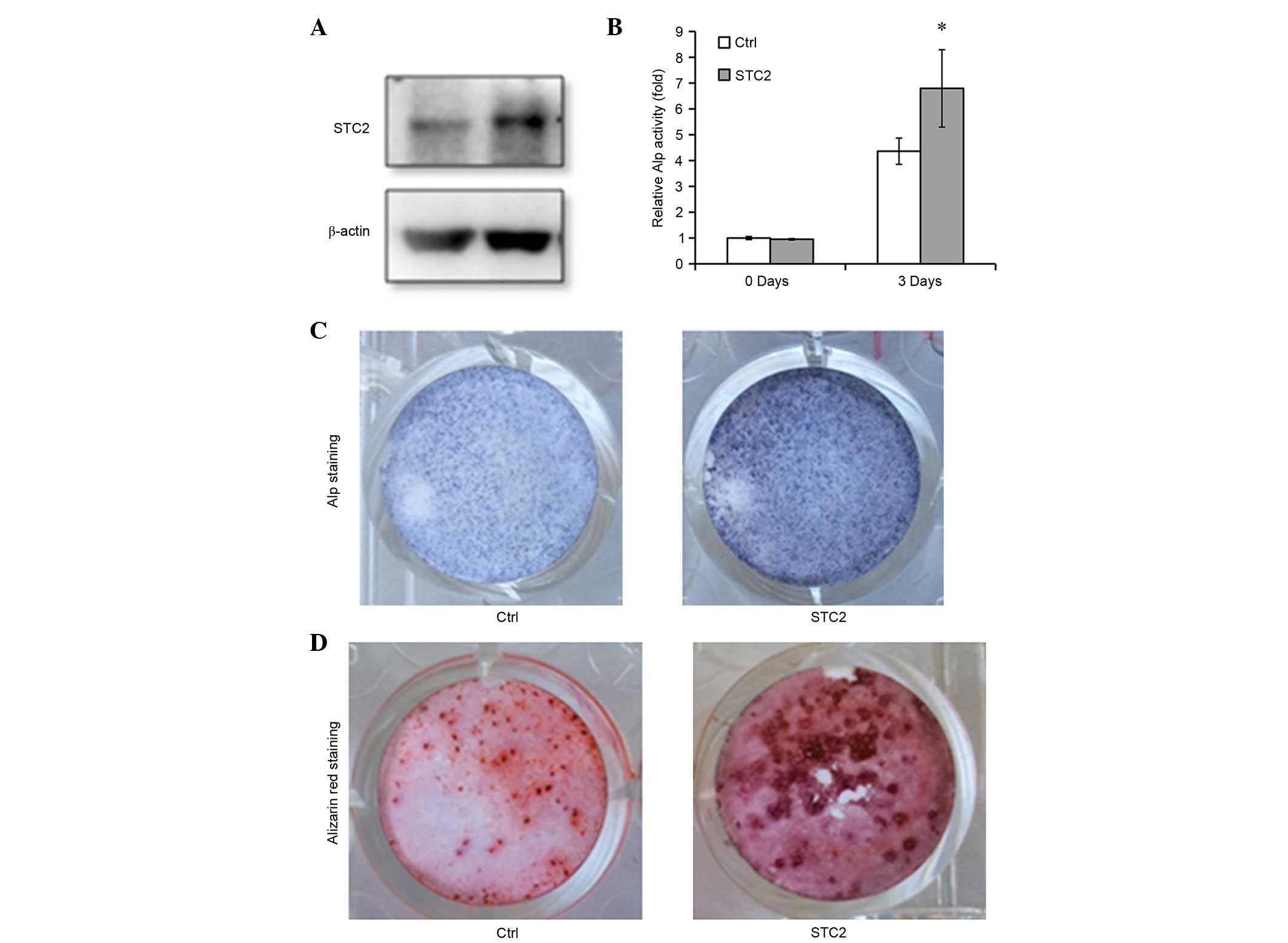
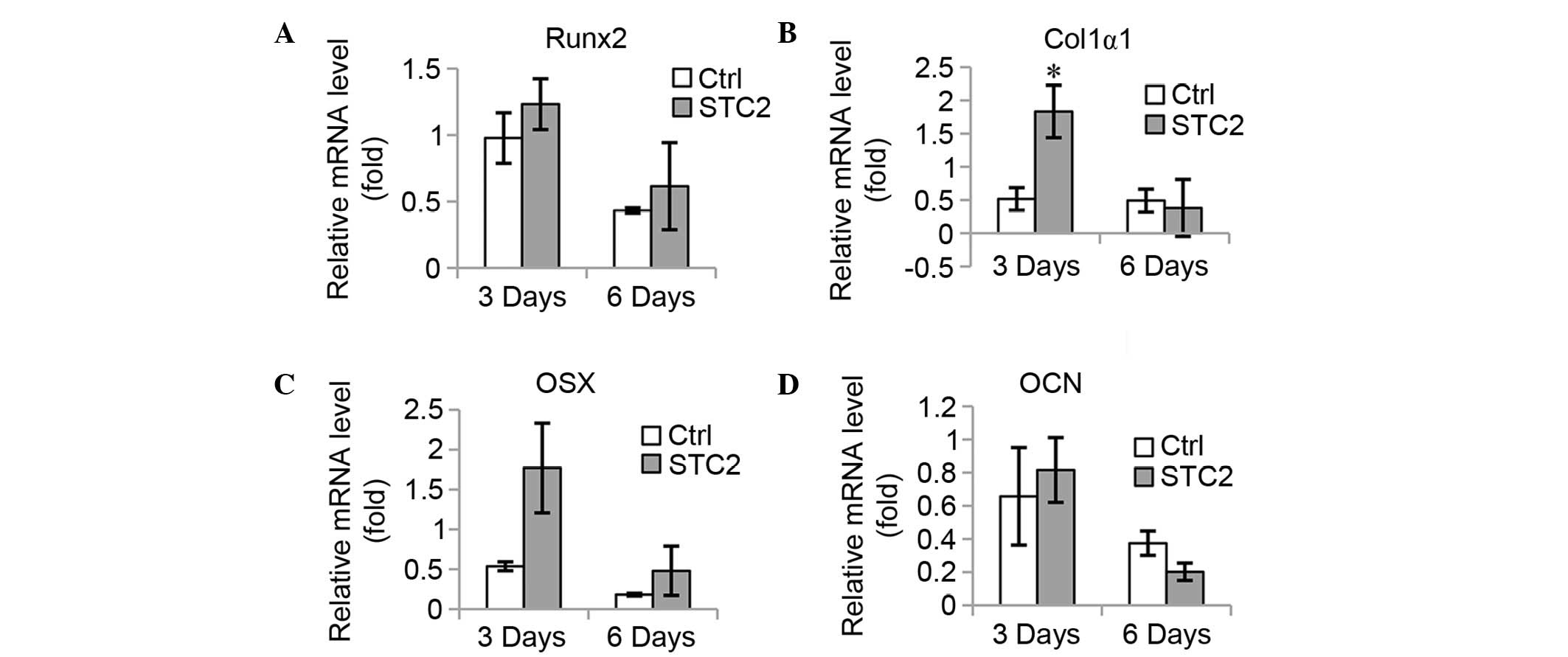
Overexpression of STC2 facilitates osteoblast
differentiation and mineralization. To confirm the function of STC2
in osteoblast differentiation, STC2 cDNA was transfected into
MC3T3-E1 cells (Fig. 3A) and the
cells were treated with differentiation medium. The ALP activity
was significantly higher in the STC2 overexpressing cells compared
with the control cells (P<0.05; Fig. 3B and C) and an increased number of
mineralized nodules were observed in the STC2 overexpressing cells
compared with the control cells on day 12 following the induction
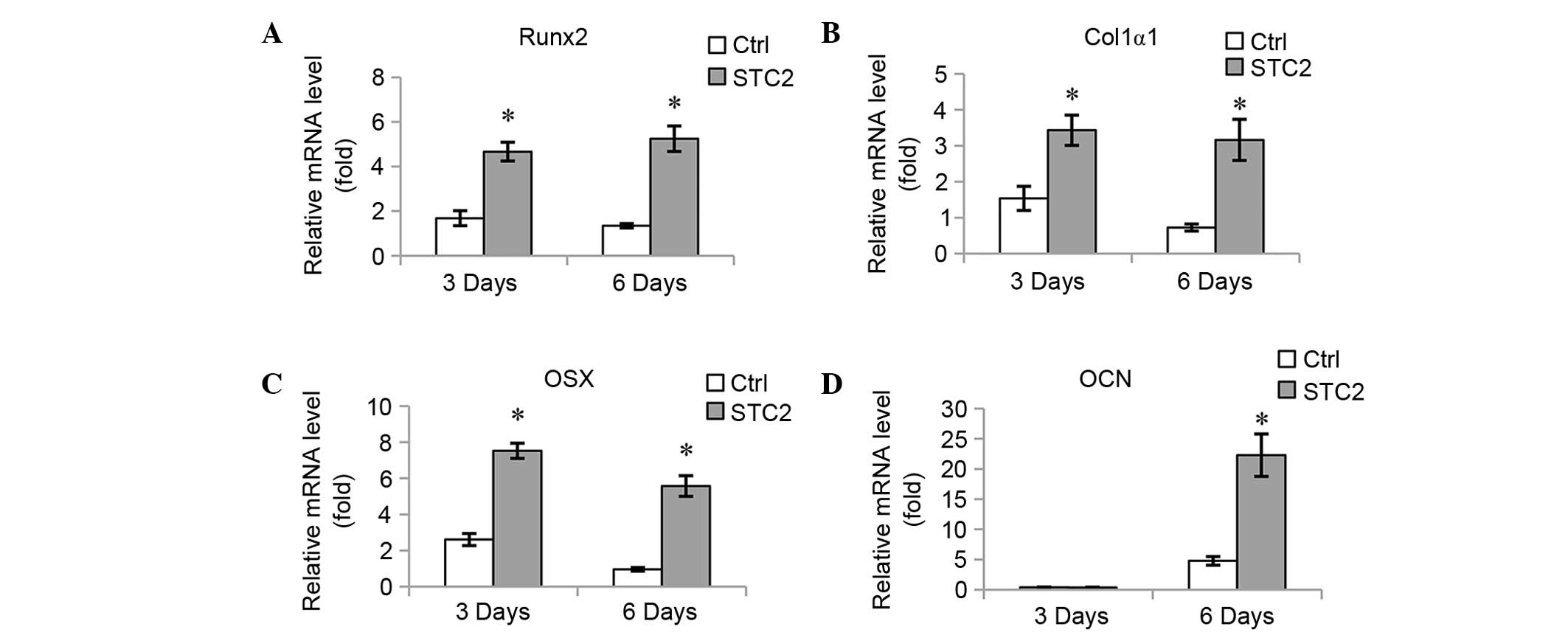
(Fig. 3D). The mRNA expression
levels of osteoblast-specific genes, including Runx2 (Fig. 4A), Col1α1 (Fig. 4B), OSX (Fig. 4C), and OCN (Fig. 4D) were significantly increased at 3
and 6 days of induction, with the exception of OCN at 3 days as no
significant difference between the expression levels of the
different groups was identified.
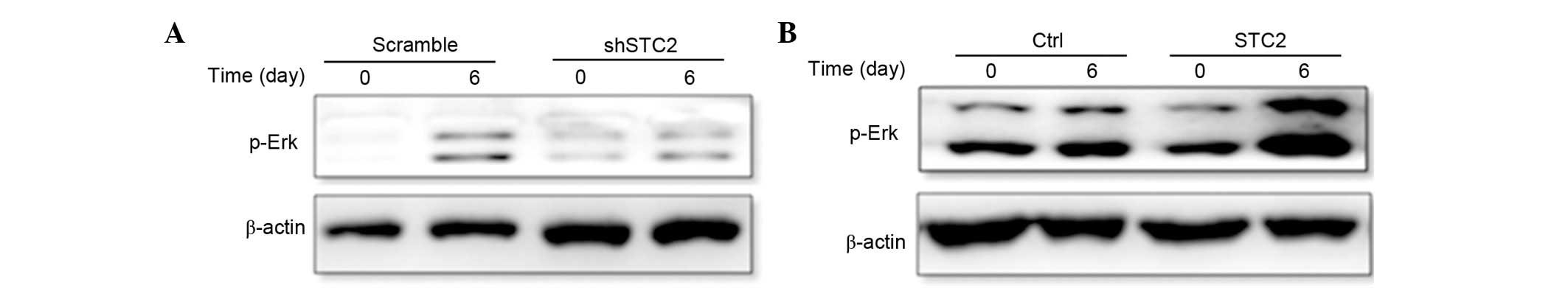
STC2 regulates ERK phosphorylation. To determine how
STC2 promoted the differentiation of MC3T3-E1 cells, the present
study investigated the MAPK/ERK signaling pathway using western
blotting. It was determined that the protein expression level of
p-ERK was reduced in the shSTC2 group cells (Fig. 5A). However, in the MC2T3-E1 cell
line transfected with a STC2 overexpressing plasmid, p-ERK
expression was increased compared with the control cells (Fig. 5B). These findings suggest that the
regulation of ERK phosphorylation by STC2 may be essential to
osteoblast differentiation.
Inhibition of ERK phosphorylation
reduces osteoblast-specific gene expression stimulated by STC2
overexpression
In order to validate the aforementioned findings,
the cells were treated with U0126, an effective and selective
inhibitor of MAPK kinase (a kinase upstream of ERK1/2) to block the
activation of the ERK1/2 signaling pathway and the expression
levels of osteoblast-specific genes were determined (Fig. 6). It was demonstrated that
treatment of MC3T3-E1 cells with 20 µM U0126 reduced the
upregulation of Runx2 (Fig. 6A),
OSX (Fig. 6C), and OCN (Fig. 6D) in the 3 and 6 day groups
compared with the controls. However, this was not observed for
Col1α1 expression in cells that were stimulated by overexpression
of STC2 at 6 days.
Discussion
STC2 has been identified to regulate
calcium/phosphate levels and was expressed in limb buds (31). The present study determined STC2
was expressed in osteoblast precursor cell lines. Thus, the
mechanism underlying the effect of STC2 on osteoblast
differentiation was investigated using overexpression or knockdown
methods. It was determined that overexpression of STC2 promoted ALP
activity, mineralization and increased the expression levels of
osteoblast-associated genes, including Runx2, Col1α1, OSX, and OCN.
Conversely, knockdown of STC2 reduced ALP activity, mineralization
and expression levels of osteoblast-associated gene expression.
These findings suggest that STC2 may regulate osteoblast
differentiation.
Activation of the ERK1/2 pathway is essential for
osteoblast differentiation and STC2 has been identified to
stimulate the activation of ERK1/2 in a dominant-positive manner
(25); therefore, STC2 may be
important for osteoblast differentiation via regulation of the ERK
signaling pathway. It was determined the expression level of p-ERK
was higher in MC3T3-E1 cells transfected with a plasmid
overexpressing STC2 cells, however, this was reduced in the cells
where STC2 was knocked-down compared with the control cells,
indicating a possible regulation of STC2 on ERK activation during
the differentiation of MC3T3-E1 cells.
In addition, it was revealed that treatment of
MC3T3-E1 cells with the ERK inhibitor, U0126, reduced the
upregulation of osteoblast-specific genes stimulated by the
overexpression of STC2, which was consistent with other results
indicating ERK activation was required for STC2 to be able to
regulate osteogenic differentiation. To the best of our knowledge,
this is the first study to determine that STC2 promoted osteoblast
differentiation via regulation of ERK phosphorylation.
In conclusion, the present study identified that
STC2 regulated osteoblast differentiation and subsequent matrix
mineralization via an ERK-mediated signaling pathway. This suggests
STC2 may be a promising target in bone development-associated
diseases.
Acknowledgements
The present study was supported by the Starting Fund
(grant no. 2014RCK02), the Postdoctoral Research Fund from the
Fifth People's Hospital of Shanghai, Fudan University for Dr Juan
Zhou (grant no. 2014WYYJ06), the National Natural Science
Foundation of China (grant nos. 81171911, 81372797 and 91129721 for
Professor Gong Yang) and by the Cooperative Projects in Colleges
and Universities (grant no. 20141001) for Dr Yang Hong.
References
|
1
|
Ishibashi K, Miyamoto K, Taketani Y,
Morita K, Takeda E, Sasaki S and Imai M: Molecular cloning of a
second human stanniocalcin homologue (STC2). Biochem Biophys Res
Commun. 250:252–258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeiger W, Ito D, Swetlik C, Oh-hora M,
Villereal ML and Thinakaran G: Stanniocalcin 2 is a negative
modulator of store-operated calcium entry. Mol Cell Biol.
31:3710–3722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang Z, Tian Z, Luo K, Song H and Yi J:
Clinical significance of stanniocalcin expression in tissue and
serum of gastric cancer patients. Chin J Cancer Res. 26:602–610.
2014.PubMed/NCBI
|
|
4
|
Arigami T, Uenosono Y, Ishigami S,
Yanagita S, Hagihara T, Haraguchi N, Matsushita D, Hirahara T,
Okumura H, Uchikado Y, et al: Clinical significance of
stanniocalcin 2 expression as a predictor of tumor progression in
gastric cancer. Oncol Rep. 30:2838–2844. 2013.PubMed/NCBI
|
|
5
|
Yokobori T, Mimori K, Ishii H, Iwatsuki M,
Tanaka F, Kamohara Y, Ieta K, Kita Y, Doki Y, Kuwano H and Mori M:
Clinical significance of stanniocalcin 2 as a prognostic marker in
gastric cancer. Ann Surg Oncol. 17:2601–2607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YY, Li L, Zhao ZS and Wang HJ:
Clinical utility of measuring expression levels of KAP1, TIMP1 and
STC2 in peripheral blood of patients with gastric cancer. World J
Surg Oncol. 11:812013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volland S, Kugler W, Schweigerer L,
Wilting J and Becker J: Stanniocalcin 2 promotes invasion and is
associated with metastatic stages in neuroblastoma. Int J Cancer.
125:2049–2057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Li YY, Zhang WQ, Lin D, Zhang WM
and Dong WD: Expression of stanniocalcin-1 and stanniocalcin-2 in
laryngeal squamous cell carcinoma and correlations with clinical
and pathological parameters. PLoS One. 9:e954662014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kita Y, Mimori K, Iwatsuki M, Yokobori T,
Ieta K, Tanaka F, Ishii H, Okumura H, Natsugoe S and Mori M: STC2:
A predictive marker for lymph node metastasis in esophageal
squamous-cell carcinoma. Ann Surg Oncol. 18:261–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou J, Wang Z, Xu H, Yang L, Yu X, Yang Z,
Deng Y, Meng J, Feng Y, Guo X and Yang G: Stanniocalicin 2
suppresses breast cancer cell migration and invasion via the
PKC/claudin-1-mediated signaling. PLoS One. 10:e01221792015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang AC, Hook J, Lemckert FA, McDonald
MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW and Reddel RR:
The murine stanniocalcin 2 gene is a negative regulator of
postnatal growth. Endocrinology. 149:2403–2410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gagliardi AD, Kuo EY, Raulic S, Wagner GF
and DiMattia GE: Human stanniocalcin-2 exhibits potent
growth-suppressive properties in transgenic mice independently of
growth hormone and IGFs. Am J Physiol Endocrinol Metab.
288:E92–E105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rimbault M, Beale HC, Schoenebeck JJ,
Hoopes BC, Allen JJ, Kilroy-Glynn P, Wayne RK, Sutter NB and
Ostrander EA: Derived variants at six genes explain nearly half of
size reduction in dog breeds. Genome Res. 23:1985–1995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raulic S, Ramos-Valdes Y and DiMattia GE:
Stanniocalcin 2 expression is regulated by hormone signalling and
negatively affects breast cancer cell viability in vitro. J
Endocrinol. 197:517–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takei Y, Yamamoto H, Masuda M, Sato T,
Taketani Y and Takeda E: Stanniocalcin 2 is positively and
negatively controlled by 1,25(OH)(2)D(3) and PTH in renal proximal
tubular cells. J Mol Endocrinol. 42:261–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W and Aletta JM: EGF-mediated
phosphorylation of extracellular signal-regulated kinases in
osteoblastic cells. J Cell Physiol. 162:348–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhary LR and Avioli LV: Activation of
extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) by
FGF-2 and PDGF-BB in normal human osteoblastic and bone marrow
stromal cells: Differences in mobility and in-gel renaturation of
ERK1 in human, rat, and mouse osteoblastic cells. Biochem Biophys
Res Commun. 238:134–139. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai CF, Chaudhary L, Fausto A, Halstead
LR, Ory DS, Avioli LV and Cheng SL: Erk is essential for growth,
differentiation, integrin expression and cell function in human
osteoblastic cells. J Biol Chem. 276:14443–14450. 2001.PubMed/NCBI
|
|
19
|
Xiao G, Gopalakrishnan R, Jiang D, Reith
E, Benson MD and Franceschi RT: Bone morphogenetic proteins,
extracellular matrix, and mitogen-activated protein kinase
signaling pathways are required for osteoblast-specific gene
expression and differentiation in MC3T3-E1 cells. J Bone Miner Res.
17:101–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shim JH, Greenblatt MB, Zou W, Huang Z,
Wein MN, Brady N, Hu D, Charron J, Brodkin HR, Petsko GA, et al:
Schnurri-3 regulates ERK downstream of WNT signaling in
osteoblasts. J Clin Invest. 123:4010–4022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao KW, Murray EJ and Murray SS: Spp24
derivatives stimulate a Gi-protein coupled receptor-Erk1/2
signaling pathway and modulate gene expressions in W-20-17 cells. J
Cell Biochem. 116:767–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Twigg SR, Vorgia E, McGowan SJ, Peraki I,
Fenwick AL, Sharma VP, Allegra M, Zaragkoulias A, Akha E Sadighi,
Knight SJ, et al: Reduced dosage of ERF causes complex
craniosynostosis in humans and mice and links ERK1/2 signaling to
regulation of osteogenesis. Nat Genet. 45:308–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quarto N, Senarath-Yapa K, Renda A and
Longaker MT: TWIST1 silencing enhances in vitro and in vivo
osteogenic differentiation of human adipose-derived stem cells by
triggering activation of BMP-ERK/FGF signaling and TAZ
upregulation. Stem Cells. 33:833–847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamma R, Sun L, Cuscito C, Lu P, Corcelli
M, Li J, Colaianni G, Moonga SS, Di Benedetto A, Grano M, et al:
Regulation of bone remodeling by vasopressin explains the bone loss
in hyponatremia. Proc Natl Acad Sci USA. 110:18644–18649. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Wu K, Sun Y, Li Y, Wu M, Qiao Q,
Wei Y, Han ZG and Cai B: STC2 is upregulated in hepatocellular
carcinoma and promotes cell proliferation and migration in vitro.
BMB Rep. 45:629–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yaffe D and Saxel O: Serial passaging and
differentiation of myogenic cells isolated from dystrophic mouse
muscle. Nature. 270:725–727. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang YF, Lin JJ, Lin CH, Su Y and Hung
SC: c-Jun N-terminal kinase 1 negatively regulates osteoblastic
differentiation induced by BMP2 via phosphorylation of Runx2 at
Ser104. J Bone Miner Res. 27:1093–1105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong Z, Zylstra-Diegel CR, Schumacher CA,
Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, et
al: Wntless functions in mature osteoblasts to regulate bone mass.
Proc Natl Acad Sci USA. 109:E2197–E2204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Harimoto K, Liu J, Guo J, Hinshaw
S, Chang Z and Wang Z: Spata4 promotes osteoblast differentiation
through Erk-activated Runx2 pathway. J Bone Miner Res.
26:1964–1973. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stasko SE and Wagner GF: Possible roles
for stanniocalcin during early skeletal patterning and joint
formation in the mouse. J Endocrinol:. 171:237–248. 2001.
View Article : Google Scholar : PubMed/NCBI
|