Introduction
Gastric cancer is one of the most common types of
malignant cancer worldwide (1). In
addition tosurgery, chemotherapy and radio chemotherapy, there is a
demand for novel treatment approaches for gastric cancer due to its
poor prognosis (2).
Metformin is a widely used anti-diabetic drug, which
has been reported to exhibit potential anticancer activity
(3). Previous studies have shown
that metformin administration significantly reduces the incidence
and mortality rates of various types of cancer, including gastric
cancer (4–6). According to the findings reported by
a previous study, patients who were treated with metformin hada
lower incidence of gastric cancer, compared with those who did not
receive metformin treatment (7).
However, the mechanism underlying the anticancer effects of
metformin is complex and remains to be fully elucidated. Activation
of the AMP-activated protein kinase (AMPK) pathway has been
recognized as a key aspect of the anticancer effects of metformin
(8).
The Sonic hedgehog (Shh) signaling pathway is one of
the most common signal transduction pathways in human cells
(9). It is critical in normal cell
differentiation and embryonic development (10). However, abnormal activation of the
Shh pathway has been identified in several types of human cancer
(11). Substantial evidence has
shown that the Shh pathway is crucial to the development and
homeostasis of gastric glands. In addition, abnormal activation of
the Shh pathway has been found toresult in gastric cancer (12–14).
The aim of the present study was to investigate the
effects of metformin on the regulation of the Shh signaling pathway
in gastric cancer cells. The expression levels of Shh, Glioma
associated oncogene (Gli)-1, Gli-2 and Smoothened (SMO) were
examined using western blot and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses. The RNA interference
technique was also used to detect whether the effects of metformin
on the Shh signaling pathway are dependent on AMPK.
Materials and methods
Cell culture
The HGC-27 and MKN-45 human gastric cancer cell
lines were obtained from the Peking Union Medical College (Beijing,
China). The cell lines were cultured in 1640 medium containing 10%
fetal bovine serum and penicillin streptomycin (100 mg/l;
Invitrogen; Thermo Fisher Scientific, Inc.). The cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
RNA interference
Small interfering RNAs (siRNAs) specific for the
AMPKα1 and control siRNA were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The cells were seeded
into 12-well plates at a concentration of 3×105 cells/well.
Following incubation for 24 h, the cells were transfected with the
AMPKα1 siRNAs or control siRNA for 48 h using
Lipofectamine® 2000 transfection reagent at 37°C
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following transfection, the cells were
treated in serum-free medium for 24 h, and then used in the
following experiments. Cells were divided into five groups: HGC-27
cells treated with PBS alone for 12 h as a blank control at 37°C;
untransfected HGC-27 cells treated with rhShh (0.5 µg/ml) for 12
hat 37°C; untransfected HGC-27 cells treated with combination of
rhShh (0.5 µg/ml) and metformin (5 mM) for 12 h at 37°C; HCG-27
cells transfected with siControl and treated with the combination
of rhShh (0.5 µg/ml) and metformin (5 mM) for 12 h at 37°C; HCG-27
cells transfected with siAMPK and treated with the combination of
rhShh (0.5 µg/ml) and metformin (5 mM) for 12 h at 37°C.
Western blot analysis
RIPA buffer supplemented with protease inhibitor was
used to lyse the cells for western blot analysis. The protein
concentrations were determined using an enhanced BCA protein assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein
lysates (30 µg/sample) were separated using SDS-PAGE onan 8 or 15%
gel, and transferred onto a nitrocellulose membrane (GE Healthcare
Life Sciences, Piscataway, NJ, USA). The membrane was blocked with
5% non-fat dry milk in Tris-buffered saline (TBS) containing 0.1%
Tween-20 (TBST). The membranes were then incubated with primary
antibody at 4°C overnight, and subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies (dilution,
1:3,000; cat. no. BM2006; Boster Systems, Inc., Pleasanton, CA,
USA) for 1 h at room temperature. The primary antibodies are listed
as follows: Rabbit anti-human monoclonal Shh (dilution, 1:1,000;
cat. no. EP1190Y; Abcam, Cambridge, MA, USA), rabbit anti-human
monoclonal Gli-1 (dilution, 1:1,000; cat. no. AB49314; Abcam),
rabbit anti-human monoclonal Gli-2 (dilution, 1:1,000; cat. no.
AB26056; Abcam), rabbit anti-human monoclonal Gli-3 (dilution,
1:1,000; cat. no. AB6050; Abcam), rabbit anti-human monoclonal AMPK
(dilution, 1:1,000; cat. no. AB32047; Abcam), and rabbit anti-human
monoclonal GAPDH (dilution, 1:1,000; cat. no. AB9485; Abcam). The
bands were visualized using chemiluminescence (Santa Cruz
Biotechnology, Inc.) and exposed on BioMax film (Kodak, Rochester,
NY, USA). Image J software (version 1.41; National Institutes of
Health, Bethesda, MD, USA) was used for quantitative densitometric
analysis.
RT-qPCR analysis
The total RNA was extracted from the cells using
anRNeasy mini kit (Qiagen, Inc., Valencia, CA, USA) according to
the manufacturer's protocol. cDNA was synthesized using 2 µg of RNA
with an RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) and the cDNA was then used as a template for RT-qPCR
analysis. RT-qPCR was performed using 2 µl cDNA in a 20 µl reaction
system with Maxima SYBR-Green qPCR Master Mix (Thermo Fisher
Scientific, Inc.) using the Bio-Rad CFX96 Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.). The specific primers (Table I) were designed using Primer
Premier software (version, 5.0; Premier Bio soft International,
Palo Alto, CA, USA). The thermal cycling parameters for RT-qPCR
reactions consisted of an initial denaturation step at 95°C for 10
min followed by 40 cycles of denaturation at 95°C for 15 sec,
annealing at 55°C for 15 sec and extension at 55°C for 15 sec.
GAPDH was used as the internal control. The qPCR amplification was
performed using an ABI Prism 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and SYBR-Green PCR master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative mRNA
expression was calculated using the delta-delta Cq method (15).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Primer | Forward | Reverse |
|---|
| Gli-1 |
5′-TCCTTTGGGGTCCAGCCTTG-3′ |
5′-ATGCCTGTGGAGTTGGGGCT-3′ |
| Gli-2 |
5′-ACGCTAAGTGGCAGTCCTGT-3′ |
5′-TGGGGCAGCGAGACTAAATA-3′ |
| Gli-3 |
5′-GGGGACAAAGATGAAAGCAA-3′ |
5′-GCTTTGAACGGTTTCTGCTC-3′ |
| Shh |
5′-CGCACGGGGACAGCTCGGAAGT-3′ |
5′-CTGCGCGGCCCTCGTAGTGC-3′ |
| SMO |
5′-TTACCTTCAGCTGCCACTTCTACG-3′ |
5′-GCCTTGGCAATCATCTTGCTCTTC-3′ |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGT-3′ |
5′-GAAGATGGTGATGGGATTTC-3′ |
Statistical analysis
All experiments were repeated at least three times,
and representative results are presented. All quantitative data are
presented as the mean + standard deviation. Differences between two
groups were compared using the two tailed Student's t-test with
SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
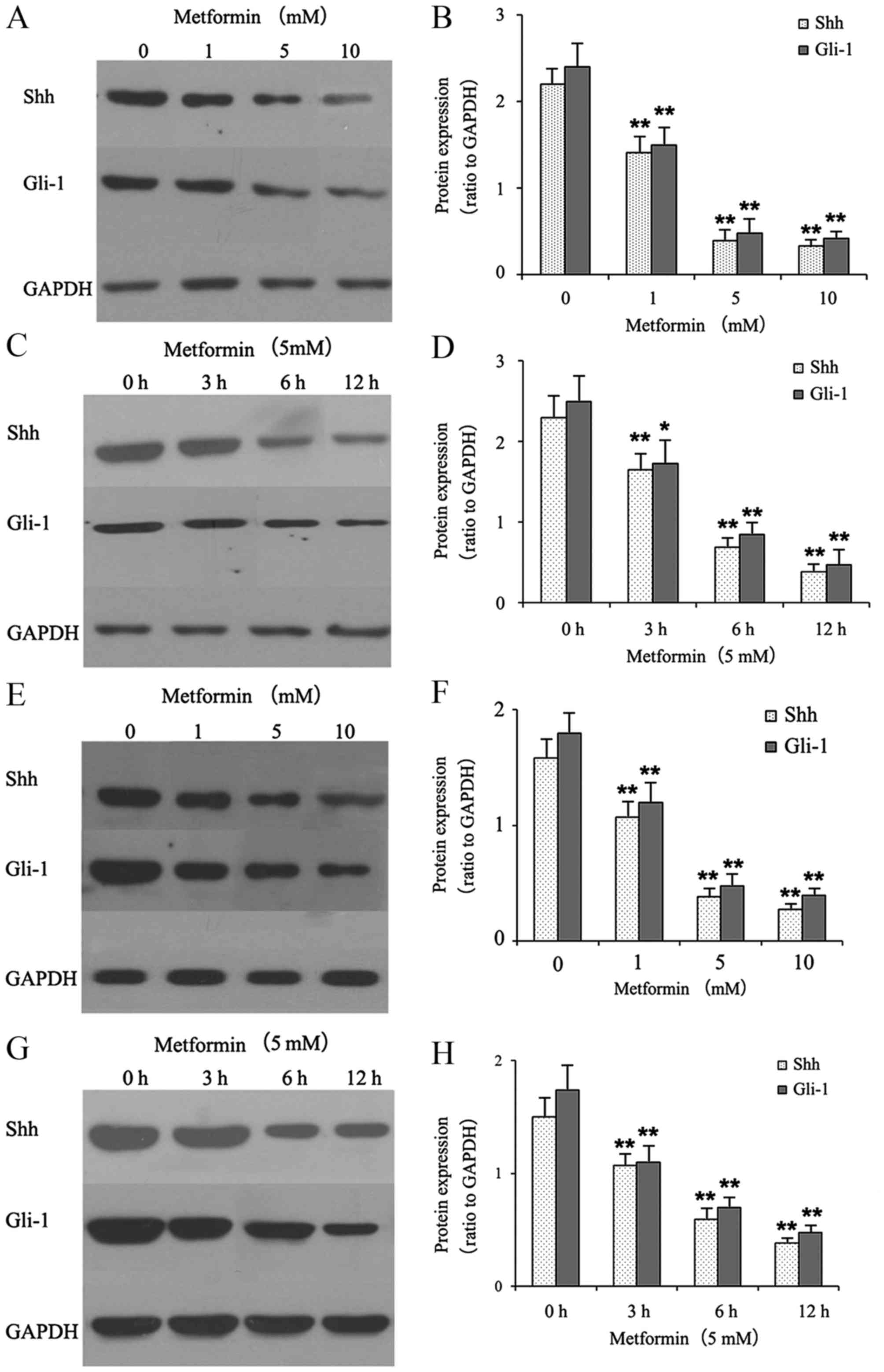
Metformin decreases the protein
expression levels of Shh and Gli-1 in gastric cancer cells
The effects of metformin on the protein expression
of Shh and Gli-1 in gastric cancer cells were examined using
western blot analysis. The gastric cancer cells were treated with
different concentrations of metformin (0, 1, 5 and 10 mmol/l) for
12 h or with 5 mM metformin for different durations (0, 3, 6 and 12
h). As shown in Fig. 1A-D,
metformin suppressed the protein expression levels of Shh and Gli-1
in a dose- and time-dependent manner in the HGC-27 cells. As shown
in Fig. 1E-H, treatment with
metformin also suppressed the protein expression levels of Shh and
Gli-1 in the MKN-45 cells.
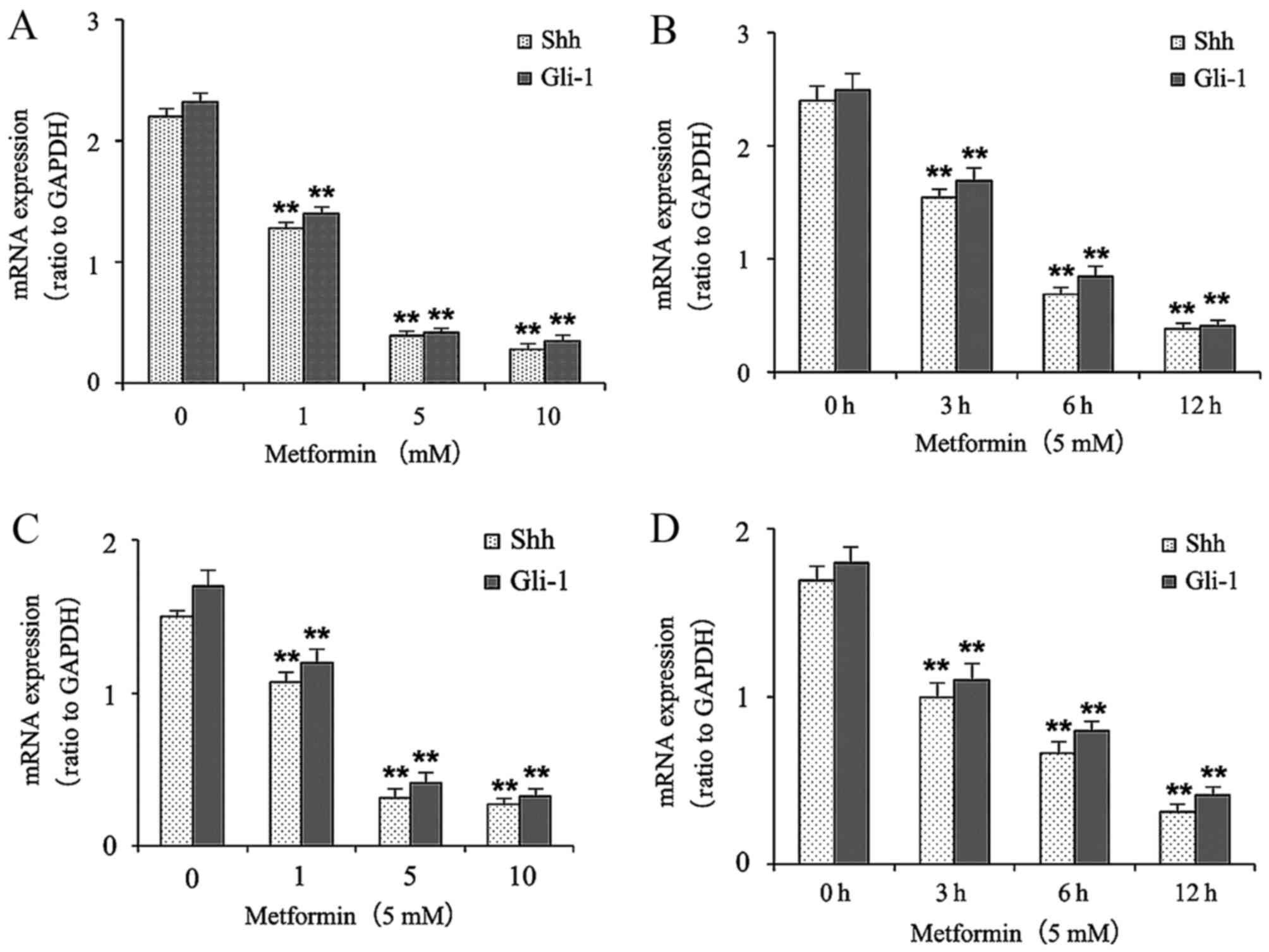
Metformin decreases the mRNA
expression levels of Shh and Gli-1 in gastric cancer cells
The effects of metformin on the mRNA levels of Shh
and Gli-1 in the gastric cancer cells were determined using RT-qPCR
analysis. The gastric cancer cells were treated with different
concentrations of metformin (0, 1, 5 and 10 mmol/l) for 12 h or
with 5 mM metformin for different durations (0, 3, 6 and 12 h). As
shown in Fig. 2A and B, treatment
with metformin decreased the mRNA expression levels of Shh and
Gli-1 in a dose- and time-dependent manner in the HGC-27 cells.
Treatment of metformin also decreased the mRNA expression levels of
Shh and Gli-1 in a dose- and time-dependent manner in the MKN-45
cells (Fig. 2C and D).
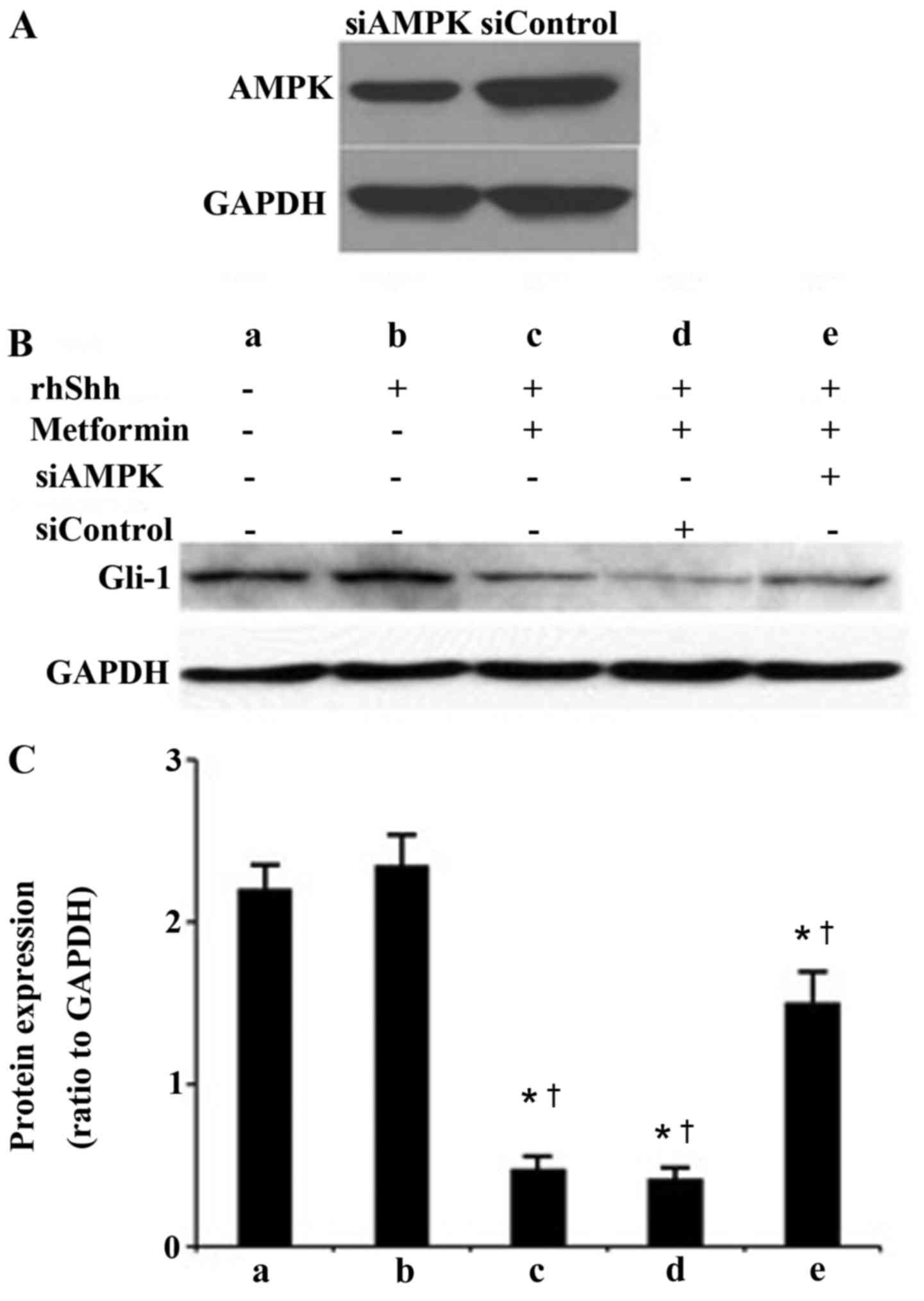
AMPK is involved in metformin-induced
suppression of the Shh signaling pathway in gastric cancer
cells
As shown in Fig.
3A, siRNA specific to AMPKα1 effectively depleted the
expression of AMPK at the protein level in the transfected HGC-27
gastric cells, compared with the cells transfected with control
siRNA. The protein levels of Gli-1 were also examined using western
blot analysis. As presented in Fig.
3B, the blank control group was treated with PBS only.
Untransfected cells were treated with recombinant human (rh) Shhor
the combination of rhShh and metformin. HCG-27 gastric cells
transfected with siControlor siAMPK were also treated with a
combination of rhShh and metformin. As shown in Fig. 3B and C, treatment with rhShh
significantly upregulated the expression of Gli-1, whereas
metformin significantly inhibited the upregulation of Gli-1 induced
by rhShh. The depletion of AMPK induced by siRNA reversed the
suppressive effect of metformin on the rhShh-induced expression of
Gli-1in the HGC-27 gastric cancer cells (Fig. 3B and C).
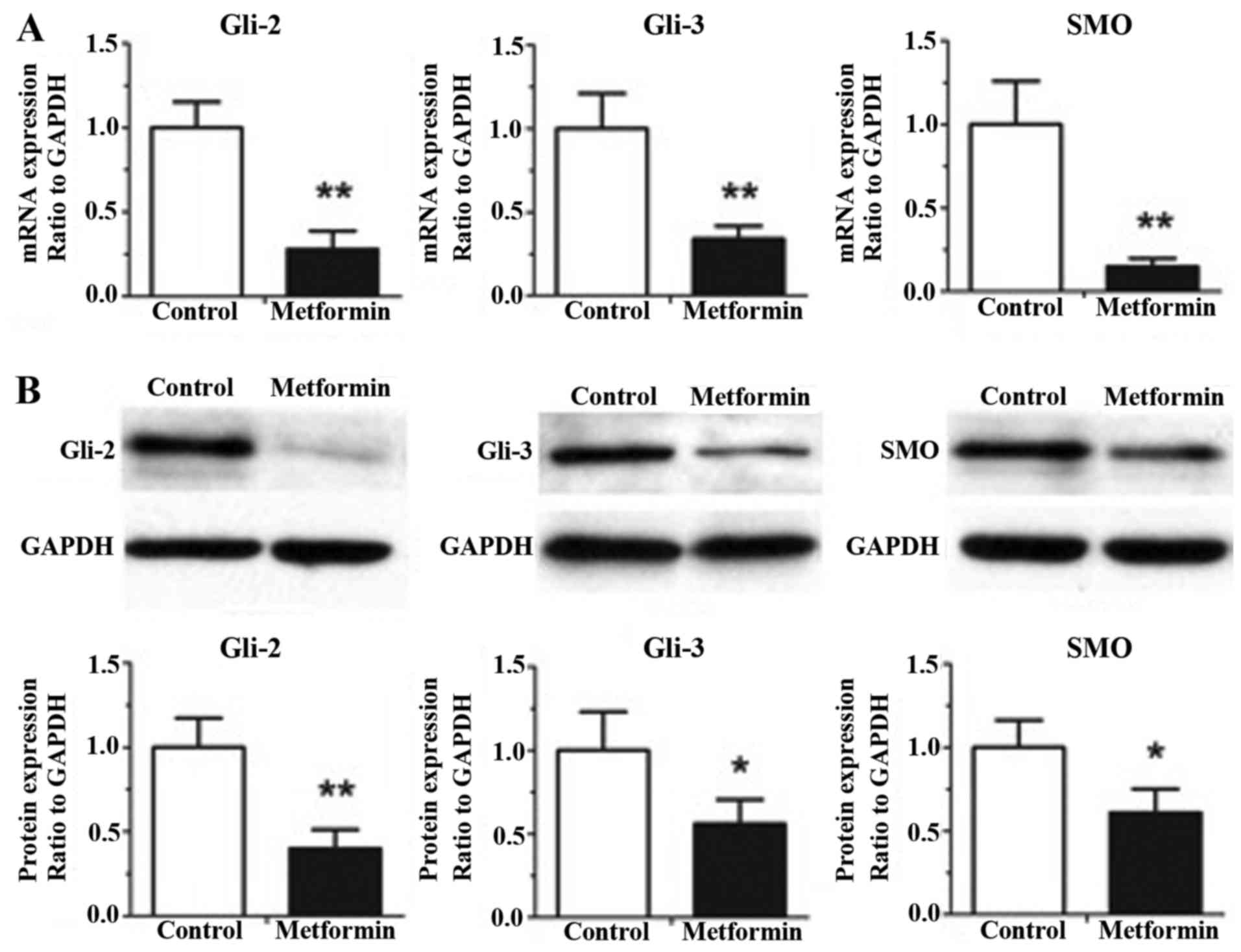
Metformin decreases the gene and
protein expression levels of Gli-2, Gli-3 and SMO in gastric cancer
cells
The effects of metformin on the gene and protein
expression levels of other hedgehog pathway components in the
gastric cancer HGC-27 cells, including Gli-2, Gli-3 and SMO, are
shown in Fig. 4. As shown in
Fig. 4A, treatment with metformin
at a concentration of 5 mM for 12 h exhibited significant decreases
in the mRNA expression levels of Gli-2, Gli-3 and SMO, compared
with the control group, determined using RT-qPCR analysis
(P<0.01). The results of the western blot analysis (Fig. 4B) demonstrated that 5 mM metformin
treatment for 12 h induced significant decreases in the protein
expression levels of Gli-2, Gli-3 and SMO, compared with the
control group (P<0.05).
Discussion
In the present study, it was demonstrated that
metformin significantly inhibited the Shh signaling pathway in
gastric cancer cells. It was also shown that AMPK was involved in
suppression of the Shh signaling pathway by metformin in gastric
cancer cells.
As the leading cause of cancer-associated mortality
worldwide, although there have been advances in the treatment of
gastric cancer, the prognosis of gastric cancer remains poor
(1,2). Thus, there is an urgent requirement
to examine novel strategies for the prevention and treatment of
gastric cancer. Metformin is generally accepted as an anti-diabetic
drug and is also considered a first-line treatment for type 2
diabetes (16). However, in
previous years, numerous studies have confirmed that patients
treated with metformin have lower incidence and mortality rates of
various types of cancer, including lung cancer, breast cancer,
pancreatic cancer and gastric cancer (5–8). A
study by Kim et al (7)
reported that diabetic patients with no administration of insulin
had a decreased incidence of gastric cancer when treated with
metformin, compared with those who were not treated with metformin.
Another previous study showed that the use of metformin as a single
agent induced the activation and phosphorylation of
mitogen-activated-protein-kinase through the increased
heterodimerization of C-RAF/B-RAF in non-small cell lung carcinoma
cells (17). In addition, it has
been demonstrated that the duration of metformin treatment is
associated with the decreased risk of gastric cancer, particularly
in patients who used metformin for >3 years (7).
However, the mechanism underlying the anticancer
effects of metformin remain to be fully elucidated Previous studies
have shown that, despite the complexity of the molecular signaling
mechanisms of metformin, several critical molecules and pathways
have been confirmed to be involved in this regulation, including
the activation of AMPK (8,18), inhibition of mammalian target of
rapamycin complex-1 (19),
interruption of the crosstalk between insulin and insulin-like
growth factor-1 receptor (20) and
reductions in the expression of vascular endothelial growth factor
and plasminogen activator inhibitor-1 (21).
The Shh signaling pathway is well accepted as being
key in numerous cellular processes controlling oncogenesis,
angiogenesis and cancer development (9,10).
Previous evidence has demonstrated that abnormal activation and/or
overexpression of Shh protein is involved in the genesis of gastric
cancer (11). Regulation of the
Shh signaling pathway may serve as a novel treatment approach for
gastric cancer.
A previous study showed that metformin reduced the
expression of Shh in pancreatic cancer cells (22). Although the use of metformin has
been associated with a reduction of gastric cancer, the effects of
metformin on the Shh signaling pathway have not been reported
previously. The present study showed that treatment with metformin
decreased the expression levels of Shh and SMO, and their
downstream molecules, Gli-1, Gli-2 and Gli-3, in gastric cancer
cells at the mRNA and protein levels. These results indicated that
inhibition of the Shh signaling pathway is one of the underlying
mechanisms of the anticancer effects of metformin.
As Shh binds to the transmembrane receptor, Patched,
another transmembrane protein, SMO, is depressed (23). Consequently, theGli transcription
factors, predominantly Gli-1, are activated (23). Gli-1 is a potent positive activator
of downstream target genes (24).
Thus, Gli-1 was considered a marker of abnormal activation of the
Shh signaling pathway in the present study.
As the activation of AMPK has been considered as one
important mechanism of the anticancer effects of metformin
(8,18), the present study investigated
whether the suppression of the Shh signaling pathway induced by
metformin was AMPK-dependent. Firstly, it was demonstrated that the
expression of AMPK was suppressed by siRNA specific to AMPK. The
cells were then treated with rhShh and metformin. Compared with
untransfected cells or cells transfected with control siRNA, the
expression of Gli-1 induced by rhShh was significantly increased in
the cells transfected with siRNA specific to AMPK. These results
indicated that the presence of AMPK was essential in
metformin-induced inhibition of the Shh signaling pathway.
In conclusion, the findings of the present study
demonstrated that metformin regulated the expression of Shh in
gastric cancer cells, and that inhibition of the Shh signaling
pathway by metformin was, at least in part, dependent on AMPK.
Further investigations are required to examine the mechanisms
underlying the association between the anticancer effects of
metformin and the Shh signaling pathway.
Acknowledgements
The authors would like to acknowledge the support
from the National Natural Science Foundation of China (grant no.
81172368) and WuJieping Medical Foundation of China (grant no.
32067001203).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: A representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Micic D, Cvijovic G, Trajkovic V, Duntas
LH and Polovina S: Metformin: Its emerging role in oncology.
Hormones (Athens). 10:5–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ,
Lee YJ, Lee EK, Kook MC, Kim CG, Ryu KW and Kim YW: Long-term
metformin use reduces gastric cancer risk in type 2 diabetics
without insulin treatment: A nationwide cohort study. Aliment
Pharmacol Ther. 39:854–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vallianou NG, Evangelopoulos A and Kazazis
C: Metformin and cancer. Rev Diabet Stud. 10:228–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varjosalo M and Taipale J: Hedgehog
signaling. J Cell Sci. 120:3–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ingham PW: Hedgehog signaling. Cold Spring
Harb Perspect Biol. 4(pii): a0112212012.PubMed/NCBI
|
|
11
|
di Magliano M Pasca and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki H, Minegishi Y, Nomoto Y, Ota T,
Masaoka T, van den Brink GR and Hibi T: Down-regulation of a
morphogen (sonic hedgehog) gradient in the gastric epithelium of
helicobacter pylori-infected mongolian gerbils. J Pathol.
206:186–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman DM, Karhadkar SS, Maitra A, De Oca
R Montes, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman
JR, et al: Widespread requirement for Hedgehog ligand stimulation
in growth of digestive tract tumours. Nature. 425:846–851. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Chen K, Huang S, Zhang X, Adegboyega
PA, Evers BM, Zhang H and Xie J: Frequent activation of the
hedgehog pathway in advanced gastric adenocarcinomas.
Carcinogenesis. 26:1698–1705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexander GC, Sehgal NL, Moloney RM and
Stafford RS: National trends in treatment of type 2 diabetes
mellitus, 1994–2007. Arch Intern Med. 168:2088–2094. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corte CM Della, Ciaramella V, Di Mauro C,
Castellone MD, Papaccio F, Fasano M, Sasso FC, Martinelli E,
Troiani T, De Vita F, et al: Metformin increases antitumor activity
of MEK inhibitors through GLI1 downregulation in LKB1 positive
human NSCLC cancer cells. Oncotarget. 7:4265–4278. 2016.PubMed/NCBI
|
|
18
|
Zheng L, Yang W, Wu F, Wang C, Yu L, Tang
L, Qiu B, Li Y, Guo L, Wu M, et al: Prognostic significance of AMPK
activation and therapeutic effects of metformin in hepatocellular
carcinoma. Clin Cancer Res. 19:5372–5380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinnett-Smith J, Kisfalvi K, Kui R and
Rozengurt E: Metformin inhibition of mTORC1 activation, DNA
synthesis and proliferation in pancreatic cancer cells: Dependence
on glucose concentration and role of AMPK. Biochem Biophys Res
Commun. 430:352–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rozengurt E, Sinnett-Smith J and Kisfalvi
K: Crosstalk between insulin/insulin-like growth factor-1 receptors
and G protein-coupled receptor signaling systems: A novel target
for the antidiabetic drug metformin in pancreatic cancer. Clin
Cancer Res. 16:2505–2511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ersoy C, Kiyici S, Budak F, Oral B, Guclu
M, Duran C, Selimoglu H, Erturk E, Tuncel E and Imamoglu S: The
effect of metformin treatment on VEGF and PAI-1 levels in obese
type 2 diabetic patients. Diabetes Res Clin Pract. 81:56–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura M, Ogo A, Yamura M, Yamaguchi Y
and Nakashima H: Metformin suppresses sonic hedgehog expression in
pancreatic cancer cells. Anticancer Res. 34:1765–1769.
2014.PubMed/NCBI
|
|
23
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, Platt KA, Censullo P and i Altaba A
Ruiz: Gli1 is a target of Sonic hedgehog that induces ventral
neural tube development. Development. 124:2537–2552.
1997.PubMed/NCBI
|