Introduction
Oxidative stress is involved in the progress of
neuronal degenerative disorders, such as multiple sclerosis
(1). Glutamate is a crucial
neurotransmitter for neural activation. However, when released as a
result of neural injury, high concentrations of extracellular
glutamate are toxic to neurons. Glutamate cytotoxicity is a result
of non-receptor-mediated oxidative stress and receptor-initiated
excitotoxicity (2). Mouse
hippocampal neuronal HT22 cells have previously been used to
examine the mechanism of glutamate-induced oxidative stress
(3); because HT22 cells express
low levels of glutamate receptors, glutamate-mediated cell damage
in these cells is primarily due to non-receptor-mediated oxidative
stress (4,5).
The rate limiting enzyme in heme catabolism is heme
oxygenase-1 (HO-1; official gene symbol HMOX1), which is thus a
crucial component in the cellular antioxidant system. HO-1 gene
expression is inducible (6) and
HO-1 has been demonstrated to be involved in cytoprotection against
glutamate-induced oxidative damage in HT22 cells (7). Induction of HO-1 expression is
performed at the transcriptional level, and its expression is
regulated by nuclear factor erythroid 2 like 2 (Nrf2; official gene
symbol Nfe2l2) (8). Nrf2 has been
reported to induce expression of various antioxidant stress-related
proteins, including glutathione (GSH) and HO-1 (9).
Taraxacum coreanum Nakai (Asteraceae family)
is a dandelion native to Korea, and it is extensively consumed as a
vegetable and used as a traditional therapeutic agent for
inflammatory diseases in Korea. Previous studies of this species
have revealed the presence of taraxinic
acid-1′-O-β-D-glucopyranoside, of which the hydrolysate,
taraxinic acid, possesses an anti-leukemic effect (10). The anti-inflammatory effect of a
methanolic extract of the aerial part of T. coreanum has
also been reported (11). In the
present study, which aimed to investigate the neuroprotective
potential of natural Korean medicinal sources, the cytoprotective
effect of T. coreanum against oxidative stress was evaluated
in vitro in HT22 cells.
Materials and methods
Preparation of plant extract
The whole plant of Taraxacum coreanum Nakai was
collected from the Botanical Garden of Wonkwang University (Iksan,
Korea) in May 2014. This species was identified by Dr Kyu-Kwan
Chang, and the voucher specimen (WK-2014-028) was deposited in
Wonkwang University. Each fresh aerial and root part (50 g) was
soaked in 500 ml ethanol and left for 7 days at room temperature.
Following filtration with filter paper, the solvent was dried using
rotary evaporator to yield 7.5 g of aerial ethanolic extract (TCAE)
and 12.8 g of root ethanolic extract (TCRE).
High performance liquid chromatography
(HPLC)
Chromatography was performed using a HPLC instrument
of the YL-9100 series (YoungLin Instrument Co., Ltd., Anyang,
Korea). In all experiments, a Capcell Pak C18 column (4.6×250 mm, 5
µm; Shiseido Co., Ltd, Tokyo, Japan) was used as the stationary
phase, and the injection volume was 20 µl. Samples containing 2
mg/ml of TCAE or TCRE were prepared. The mobile phase consisted of
water containing 0.1% formic acid (A) and acetonitrile (B) in a
gradient system: 0–50 min linearly changed 10 to 50% B, 50–55 min
linearly changed 50 to 100% B, 55–60 min remained at 100% B. The
detection wavelength was adjusted to 254 nm, and the flow rate was
0.7 ml/min.
Chemicals and reagents
All cell culture reagents were obtained from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Tin
protoporphyrin (SnPP) and cobalt protoporphyrin IX (CoPP) were
obtained from Frontier Scientific, Inc. (Logan, UT, USA). Caffeic
acid, chlorogenic acid, ferulic acid, and all other chemicals,
unless indicated otherwise, were obtained from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany).
Cell culture and viability assay
Mouse hippocampal HT22 cells were obtained from
Professor Hyun Park (Wonkwang University). Cell culture and MTT
assay were conducted as described previously (12). Briefly, a total of 2×104 cells/well
were seeded in 96-well plates. They were pre-treated with the
indicated concentration of TCAE or TCRE for 3 h, and this was
followed by treatment with 5 mM glutamate. For measurement of cell
viability, cells were maintained with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
at a final concentration of 0.5 mg/ml for 4 h, and the formazan
formed was dissolved in acidic 2-propanol.
Western blot analysis
HT22 cells were harvested and pelleted by
centrifugation at 200 × g for 3 min. Subsequently, the cells were
rinsed with PBS and lysed using radioimmunoprecipitation assay
(RIPA) lysis buffer containing 25 mmol/l Tris-HCl buffer (pH 7.6),
150 mmol/l NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS for
15 min at 4°C, and then underwent centrifugation at 15,000 × g for
10 min at 4°C. The protein concentration was determined using
Bradford Assay Reagent (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). A total of 30 µg protein samples were resolved using
SDS-polyacrylamide gel electrophoresis and electrophoretically
transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% skimmed milk, washed with TBST buffer, and then
incubated with the following primary antibodies: Anti-HO-1 (catalog
no. sc-10789; 1:1,000), anti-Nrf2, (catalog no. sc-722; 1:1,000)
anti-Lamin B, (catalog no. sc-6216; 1:1,000), anti-Actin (catalog
no. sc-1616; 1:1,000) all from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). They were then incubated with horseradish
peroxidase-conjugated goat (catalog no. ap106p; 1:1,000) and rabbit
(catalog no. ap132p; 1:1,000) secondary antibodies, obtained from
EMD Millipore (Billerica, MA, USA), followed by ECL detection.
Primary and secondary antibodies were diluted with 3% skimmed milk
in TBST buffer. The bands were visualized with enhanced
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK) and
quantified by densitometry (Image J, National Institutes of Health,
USA). Nuclear and cytoplasmic extracts of cells were prepared using
NE-PER reagents, as per the manufacturer's instructions (Thermo
Fisher Scientific, Inc.).
HO-1 activity
Determination of HO activity occurred as previously
described by Motterlini et al (13). Briefly, the HT22 cells were scraped
off the dish, and centrifuged (1,000 × g for 10 min at 4°C). The
pellet of HT22 cell was suspended in MgCl2 phosphate
buffer (20 mM, pH 7.4), frozen at −70°C, and finally sonicated on
ice prior to centrifugation at 18,000 × g for 10 min at 4°C. The
supernatant was added to a nicotinamide adenine dinucleotide
phosphate (NADPH)-generating system containing 0.8 mM NADPH, 2 mM
glucose-6-phosphate, 0.2 U
glucose-6-phosphate-L-dehydrogenase, and 2 mg protein of
rat liver cytosol prepared from the 15,000 × g supernatant fraction
as a source of biliverdin reductase, potassium phosphate buffer
(100 mM, pH 7.4), and hemin (10 µM) in a final volume of 200 µl.
The reaction was conducted for 1 h at 37°C in the dark and
terminated by addition of 1 ml chloroform. The extracted bilirubin
was calculated by the difference in absorption between wavelengths
of 464 and 530 nm using a quartz cuvette (extinction coefficient,
40 mM-1 cm-1 for bilirubin).
Preparation of cytosolic and nuclear
fractions
HT22 Cells were homogenized (1:20, w:v) in
PER-Mammalian Protein Extraction buffer (Pierce; Thermo Fisher
Scientific, Inc.) including freshly added 1 mM phenylmethylsulfonyl
fluoride and protease inhibitor cocktail I (EMD Millipore). The
cytosolic fraction of the cell was prepared by centrifugation at
15,000 × g for 10 min at 4°C. Cytoplasmic and nuclear extracts of
the cells were prepared using NE-PER cytoplasmic and nuclear
extraction reagents (Pierce; Thermo Fisher Scientific Inc.),
respectively. These methods were conducted as previously described
(12).
Statistical analysis
Data were expressed as the mean ± standard deviation
of at least 3 independent experiments. To compare 3 or more groups,
one-way analysis of variance followed by the Newman-Keuls post hoc
test was used. Statistical analysis was performed using GraphPad
Prism software version 3.03 (GraphPad Software Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
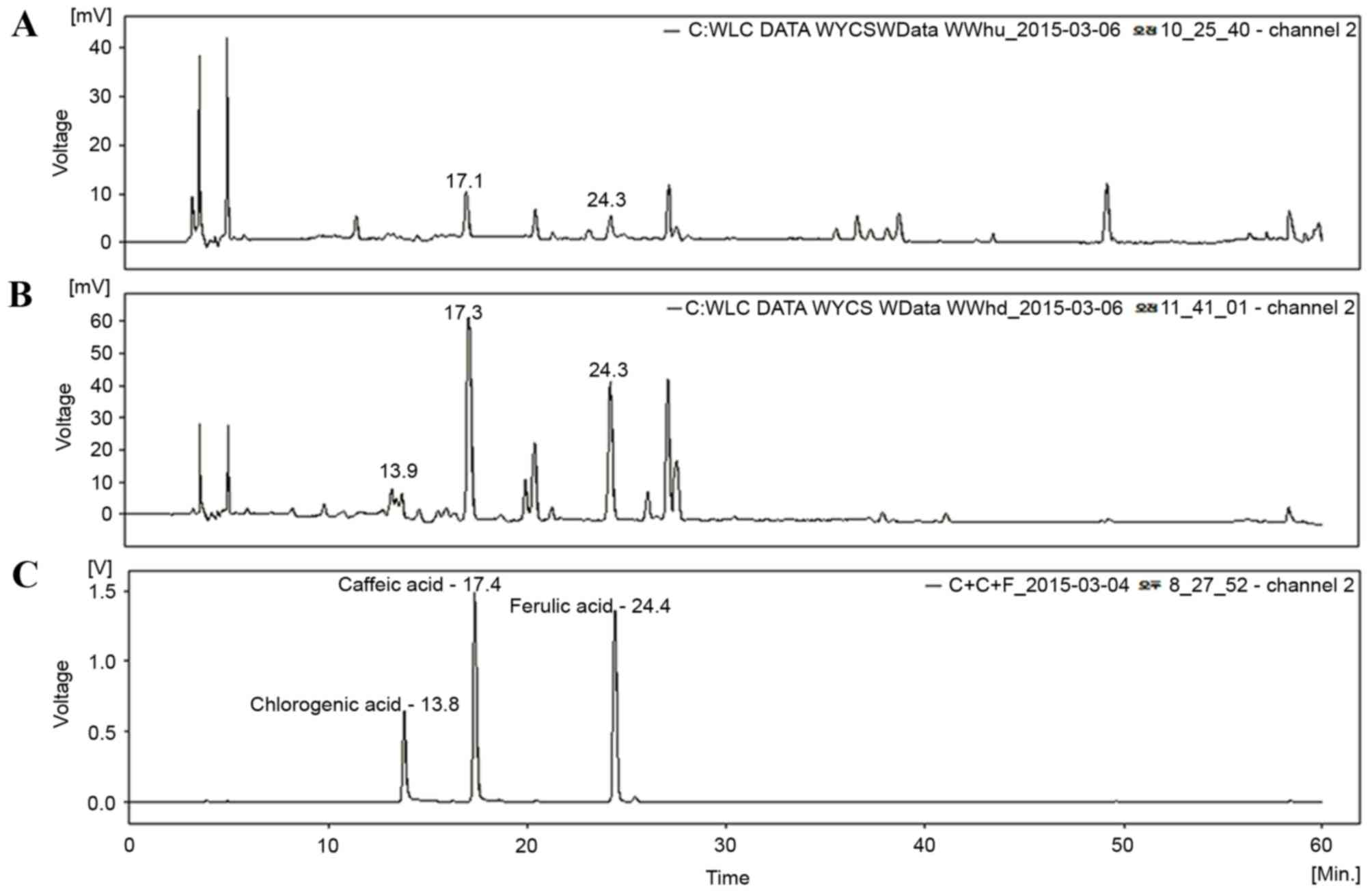
HPLC analysis of TCAE and TCRE
Three phenolic compounds, ferulic acid, caffeic acid
and chlorogenic acid, have been isolated from plants of the
Taraxacum genus (14,15). Therefore, TCAE and TCRE were
analyzed by HPLC in order to assess the presence of these
compounds. As demonstrated in Fig.
1, the peaks of ferulic acid and caffeic acid appeared clearly
in the HPLC chromatograms of both TCAE (Fig. 1A) and TCRE (Fig. 1B), however, chlorogenic acid was
only detected in TCRE (Fig.
1B).
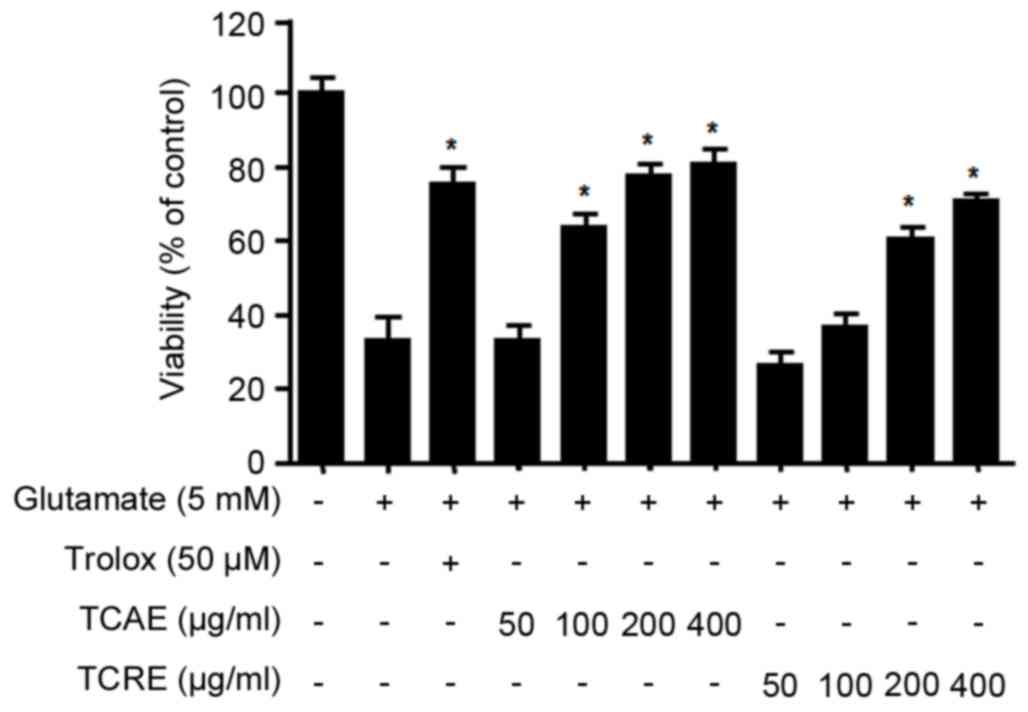
Effects of TCAE and TCRE on oxidative
toxicity
To investigate the protective effects of TCAE and
TCRE against glutamate-induced cytotoxicity, HT22 cell viability
was measured by MTT assay. Non-cytotoxic effects were obtained with
concentrations of up to 400 µg/ml for both TCAE and TCRE (data not
shown). The viability of glutamate-treated HT22 cells (5 mM for 24
h) was tested following treatment with 0, 50, 100, 200 and 400
µg/ml of each extract (Fig. 2).
The results demonstrated that both TCAE and TCRE significantly
restored the cell viability following glutamate-mediated damage in
a concentration-dependent manner compared with cells treated with
glutamate only (Fig. 2). Trolox, a
well-known antioxidant, was used as a positive control in this
assay and was confirmed to exhibit a significant protective effect
(Fig. 2). The present results
suggested that both aerial and root ethanolic extracts of T.
coreanum could protect HT22 cells against oxidative stress.
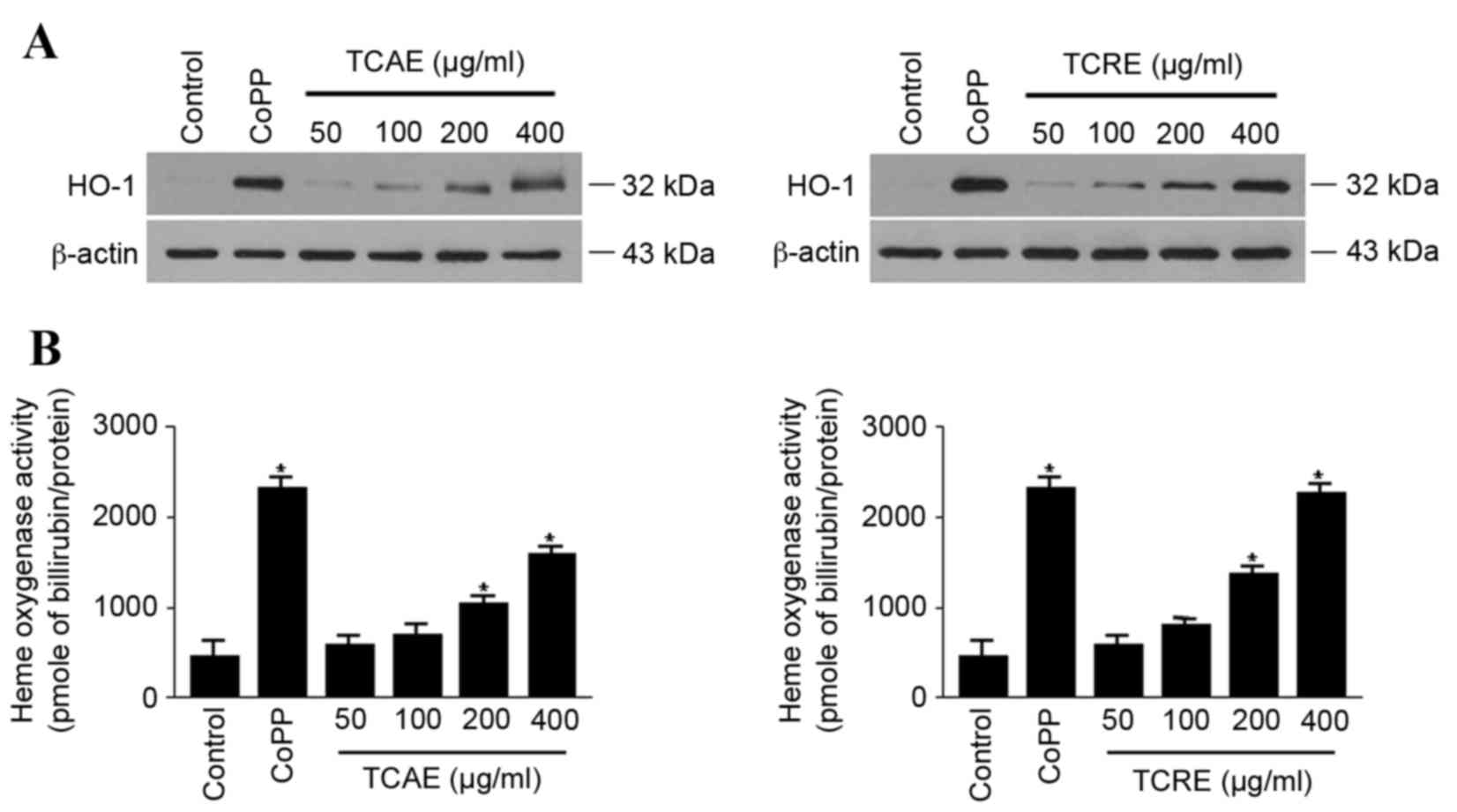
Effects of TCAE and TCRE on HO-1
expression and activity
The effect of TCAE and TCRE on HO-1 expression was
examined in HT22 cells. When cells were treated with 50, 100, 200
or 400 µg/ml TCAE or TCRE for 12 h, both extracts visibly increased
the expression of HO-1 protein in a concentration-dependent manner,
compared with untreated cells (Fig.
3A). In agreement with the concentration-dependent expression
of HO-1, both TCAE and TCRE treatments also significantly increased
HO-1 activity in HT22 cells compared with untreated cells (Fig. 3B). CoPP, used as a positive
control, exhibited a prominent induction of both HO-1 protein
expression and activity (Fig.
3).
Effects of HO-1 inhibitor on the
cytoprotection activity of TCAE and TCRE
The hypothesis that the protective effect of TCAE
and TCRE in HT22 cells arose from HO-1 expression was further
tested. Pretreatment of cells with SnPP, a competitive inhibitor of
HO-1, significantly reduced the cytoprotective effects of TCAE and
TCRE on glutamate-induced cell damage (P<0.05; Fig. 4). The present data, therefore,
suggested that the neuroprotective effects of TCAE and TCRE against
oxidative stress are a result of their ability to induce HO-1
expression.
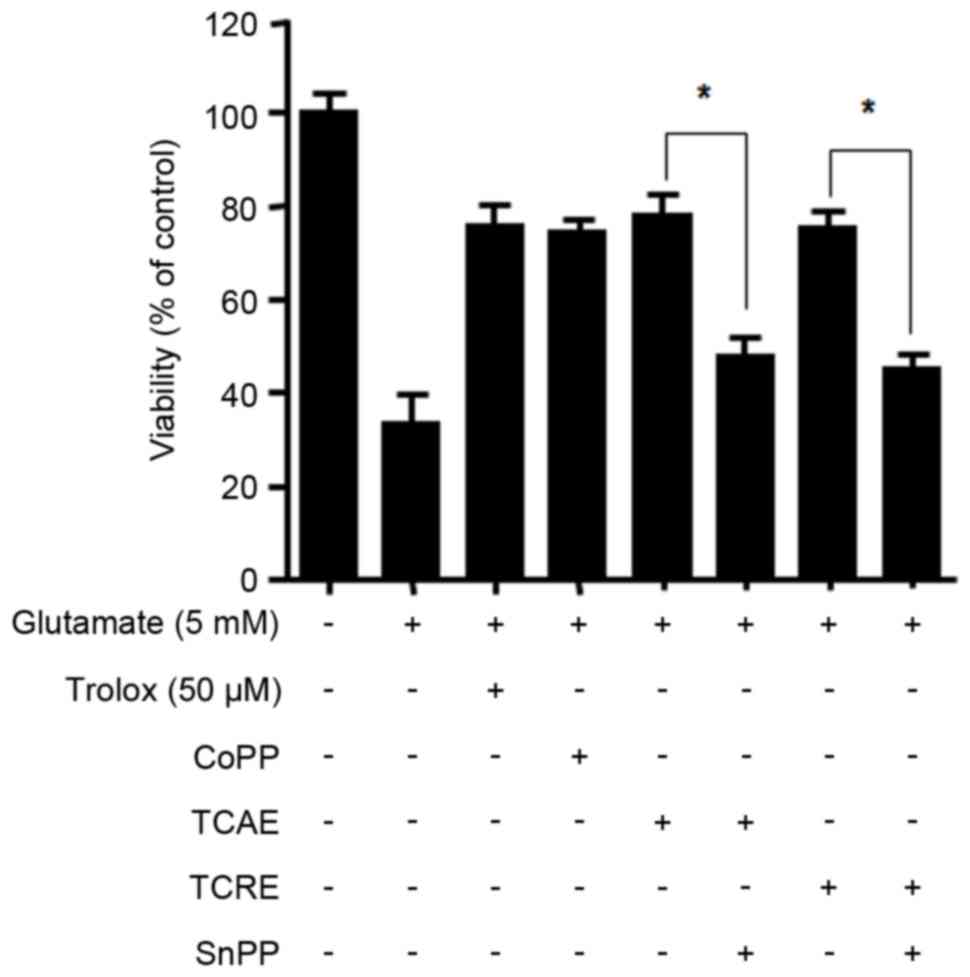 | Figure 4.Effects of HO-1 inhibitor SnPP on
TCAE- and TCRE-mediated cytoprotection. To test the effect of HO-1
inhibition, cells were pretreated with SnPP (50 µM) prior to
TCAE/TCRE-treatment. Cells were then incubated with either trolox
(50 µM, positive control), CoPP (20 µM, positive control), TCAE
(400 µg/ml) or TCRE (400 µg/ml). Cell damage was induced by
treatment with 5 mM glutamate in HT22 cells for 12 h. Viability was
measured by MTT assay. *P<0.05, with comparisons indicated by
brackets. HO-1, heme oxygenase-1; SnPP, tin protoporphyrin; TCAE,
Taraxacum coreanum aerial extract; TCRE, T. coreanum root extract;
CoPP, cobalt protoporphyrin IX. |
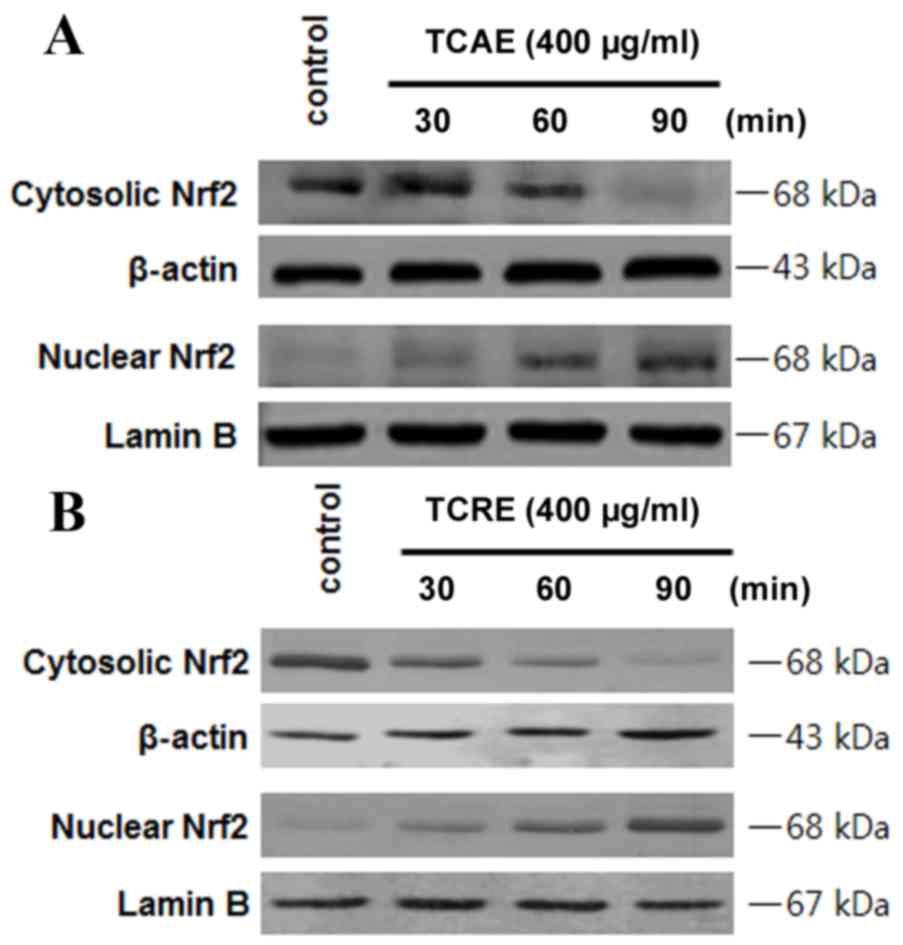
Effects of TCAE and TCRE on Nrf2
nuclear translocation
In order to examine whether Nrf2 is involved in the
extract-mediated HO-1 induction, the effect on Nrf2 nuclear
translocation in HT22 cells treated with TCAE and TCRE was tested
(Fig. 5). HT22 cells were
incubated with 400 µg/ml TCAE (Fig.
5A) or TCRE (Fig. 5B) for 30,
60 and 90 min, then Nrf2 protein levels in the nuclear and
cytosolic fractions were examined by western blot analysis. The
nuclear extracts of both TCAE and TCRE-treated cells exhibited a
time-dependent increase in Nrf2 levels compared with untreated
control, while Nrf2 levels decreased in the cytoplasmic fraction of
HT22 cells (Fig. 5).
Discussion
Taraxacum is a taxonomically complex genus of
the family Asteraceae. Some botanists have classified the genus
into ~34 macrospecies, and ~2000 microspecies, while other
botanists only accept a total of ~60 species (16). Taraxacum coreanum Nakai is a
dandelion native to Korea and is widely consumed as a vegetable and
as a traditional medicine for inflammatory disorders in Korea.
Although the whole plant of T. coreanum has been used in
Korean traditional medicine, the present study aimed to evaluate
the neuroprotective effects of its aerial and root parts
separately. TCAE and TCRE ethanolic extracts were subjected to HPLC
for evaluation of their chemical profile. As demonstrated in
Fig. 1, the peaks of caffeic acid
and ferulic acid were apparent in both extracts from their HPLC
chromatograms. These results, therefore, provide insights into the
chemical composition in the aerial and the root parts of T.
coreanum.
Glutamate is one of the most important transmitters
for brain function and is an important neural activator, however,
excess glutamate can trigger neurodegenerative diseases (2). It is generally acknowledged that
glutamate neurotoxicity is mediated by non-receptor-mediated
oxidative stress and receptor-initiated excitotoxicity (2). To examine the neuroprotective effects
of TCAE and TCRE, these extracts were added at non-toxic
concentrations (50–400 µg/ml) in mouse hippocampal HT22 cells
treated with glutamate. Both extracts significantly increased the
survival of glutamate-treated HT22 cells (Fig. 2). HT22 cells lack glutamate
receptors (5), suggesting that the
protective effects of TCAE and TCRE are likely derived from their
anti-oxidative properties rather than receptor-mediated signaling.
In addition, caffeic acid and ferulic acid, which were used as
internal standards in this study, did not exhibit any
cytoprotective effects at their non-toxic concentrations against
glutamate-induced HT22 cells (data not shown), suggesting that
other components exist in TCAE and TCRE that are responsible for
their neuroprotective activity.
HO-1 is induced as a protective enzyme in response
to diverse stimuli, therefore it may be suitable for the therapy of
oxidative tissue damage (17).
Therefore, the hypothesis that TCAE and TCRE could affect HO-1
expression was tested. The present study demonstrated that both
extracts induced expression and activity of HO-1 in a
concentration-dependent manner (Fig.
3). Furthermore, the neuroprotective activity of TCAE and TCRE
were reversed by the HO-1 inhibitor SnPP (Fig. 4), suggesting that the protective
properties of these extracts are mediated by HO-1.
HO-1 expression is associated with Nrf2
translocation (18). Under normal
conditions, Nrf2 interacts with Kelch-like ECH-associated protein 1
(Keap1) in the cytoplasm, but signals from electrophilic stimuli or
increased reactive oxygen species production result in separation
of the Nrf2-Keap1 complex, and Nrf2 translocation to the nucleus.
Following nuclear translocation, Nrf2 interacts with antioxidant
response element sites in the promoter regions of specific target
genes, initiating transcription of antioxidative genes, including
GSH and HO-1 (19). The hypothesis
that TCAE and TCRE may cause Nrf2 to translocate into the nucleus
was, therefore, examined in HT22 cells. The results confirmed that
Nrf2 levels were increased in the nuclear fractions of HT22 cells
following TCAE and TCRE treatments, while Nrf2 levels decreased in
the cytoplasm (Fig. 5), indicating
increased translocation as a result of TCAE and TCRE treatment.
The present study revealed that T. coreanum,
a native plant of Korea, confers a protective effect against
glutamate-induced oxidative stress in HT22 cells. This
cytoprotective effect of T. coreanum is mediated by
induction of HO-1 expression, via Nrf2 nuclear translocation.
Therefore, T. coreanum might serve as a potential
therapeutic agent for preventing neurodegeneration and further
studies are warranted for the isolation and characterization of its
active substances.
Acknowledgements
The authors acknowledge the support from the
Wonkwang University (Iksan, Korea) in 2015.
References
|
1
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schubert D and Piasecki D: Oxidative
glutamate toxicity can be a component of the excitotoxicity
cascade. J Neurosci. 21:7455–7462. 2001.PubMed/NCBI
|
|
3
|
Davis JB and Maher P: Protein kinase C
activation inhibits glutamate-induced cytotoxicity in a neuronal
cell lines. Brain Res. 652:169–173. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy TH, Miyamoto M, Sastre A, Schnaar
RL and Coyle JT: Glutamate toxicity in a neuronal cell line
involves inhibition of cystine transport leading to oxidative
stress. Neuron. 2:1547–1558. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rössler OG, Bauer I, Chung HY and Thiel G:
Glutamate-induced cell death of immortalized murine hippocampal
neurons: Neuroprotective activity of heme oxygenase-1, heat shock
protein 70 and sodium selenite. Neurosci Lett. 362:253–257. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabell L, Ferguson C, Luginbill D, Kern M,
Weingart A and Audesirk G: Differential induction of heme oxygenase
and other stress proteins in cultured hippocampal astrocytes and
neurons by inorganic lead. Toxicol Appl Pharmacol. 198:49–60. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satoh T, Baba M, Nakatsuka D, Ishikawa Y,
Aburatani H, Furuta K, Ishikawa T, Hatanaka H, Suzuki M and
Watanabe Y: Role of heme oxygenase-1 protein in the neuroprotective
effects of cyclopentenone prostaglandin derivatives under oxidative
stress. Eur J Neurosci. 17:2249–2255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi JH, Shin KM, Kim NY, Hong JP, Lee YS,
Kim HJ, Park HJ and Lee KT: Taraxinic acid, a hydrolysate of
sesquiterpene lactone glycoside from the Taraxacum coreanum NAKAI,
induces the differentiation of human acute promyelocytic leukemia
HL-60 cells. Biol Pharm Bull. 25:1446–1450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MH, Kang H, Lee K, Yang G, Ham I, Bu
Y, Kim H and Choi HY: The aerial part of Taraxacum coreanum extract
has an anti-inflammatory effect on peritoneal macrophages in vitro
and increases survival in a mouse model of septic shock. J
Ethnopharmacol. 146:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DS, Ko W, Kim DC, Kim YC and Jeong GS:
Cudarflavone B provides neuroprotection against glutamate-induced
mouse hippocampal HT22 cell damage through the Nrf2 and PI3K/Akt
signaling pathways. Molecules. 19:10818–10831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motterlini R, Foresti R, Intaglietta M and
Winslow RM: NO-mediated activation of heme oxygenase: Endogenous
cytoprotection against oxidative stress to endothelium. Am J
Physiol. 270:H107–H114. 1996.PubMed/NCBI
|
|
14
|
Li XP, Yu J, Luo JY, Li HS, Han FJ, Chen
XG and Hu ZD: Simultaneous determination of chlorogenic acid,
caffeic acid, ferulic acid, protocatechuic acid and protocatechuic
aldehyde in Chinese herbal preparation by RP-HPLC. Chem Pharm Bull.
52:1251–1254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling Y, Zhang Y, Cai S, Xiao Y, Cai S and
Zheng J: Chemical constituents of Taraxacum sinicum Kitag. Zhongguo
Zhing Yao Za Zhi. 23:232–234. 1998.(In Chinese).
|
|
16
|
Richards AJ: Eutriploid facultative
agamospermy in Taraxacum. New Phytologist. 69:761–774. 1970.
View Article : Google Scholar
|
|
17
|
Farombi EO and Surh YJ: Heme oxygenase-1
as a potential therapeutic target for hepatoprotection. J Biochem
Mol Biol. 39:479–491. 2006.PubMed/NCBI
|
|
18
|
Moi P, Chan K, Asunis I, Cao A and Kan YW:
Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic
leucine zipper transcriptional activator that binds to the tandem
NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl
Acad Sci USA. 91:9926–9930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiang W, Cahill JM, Liu J, Kuang X, Liu N,
Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS and Wong PK:
Activation of transcription factor Nrf-2 and its downstream targets
in response to moloney murine leukemia virus ts1-induced thiol
depletion and oxidative stress in astrocytes. J Virol.
78:11926–11938. 2004. View Article : Google Scholar : PubMed/NCBI
|