Introduction
Atherosclerosis (AS) is the primary factor
underlying cardiovascular and cerebrovascular diseases with high
morbidity and mortality rates (1).
Endothelial dysfunction, which is closely associated with vascular
endothelial cell injury, is implicated in a number of
pathophysiologic processes, including atheromatous plaque
formation, coronary artery diseases, diabetes and hypertension
(2–4). Of note, vascular endothelial cell
injury as a result of apoptosis is essential in the initiation of
AS (5).
The excessive generation of reactive oxygen species
(ROS) from oxidative stress is also considered to be crucial in the
development of AS, which results in apoptosis or necrosis through
the induction of mitochondrial membrane depolarization and caspase
signaling cascades in endothelial cells (6,7).
Oxidized low-density lipoprotein (ox-LDL) is the primary stimuli of
oxidative stress, which accelerates smooth muscle cell
proliferation, foam cell formation and endothelial cell apoptosis
(8). These pathological
alterations contribute to disruption of the endothelium barrier and
subsequently facilitate the formation of atherosclerotic plaques
(9). The increased apoptosis of
endothelial cells induced by ox-LDL has been suggested to be
associated with the binding of lectin-like ox-LDL receptor 1
(LOX-1) (10), activation of the
caspase signaling pathway, the imbalance of B cell lymphoma-2
(Bcl-2) and Bcl-2-associated X protein (Bax) (11) and the translocation of nuclear
factor-κB (12).
Dietary polyphenols in human food are the most
abundant natural antioxidants, and have been shown to be efficient
in treating various diseases, including cancer and cardiovascular
diseases (13). Resveratrol
(trans-3,4,5-trihydroxystilbene) is a natural phytochemical found
in grapes, red wine and certain Chinese medicinal herbs (14). It initially generated considerable
attention due to its anticancer properties. In addition to cancer
therapy, resveratrol possesses critical cardioprotective effects
(15). These biological activities
may be associated with its anti-inflammatory, anti-oxidative and
immune-modulatory properties (16). Although increasing evidence
supports that resveratrol exerts a protective effect against
endothelial cell apoptosis and endothelial dysfunction through
biological processes, including the suppression of superoxide
production and regulation of the Lox-1-dependent signaling pathway
(17–19), the importance of resveratrol in
endothelial cell apoptosis has received less attention. Therefore,
the aim of the present study was to examine the role of resveratrol
in HUVEC apoptosis induced by ox-LDL and further examine its
possible mechanisms.
Materials and methods
Materials
Resveratrol was purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). The powder was dissolved in
dimethyl sulfoxide (DMSO; <0.1% v/v) as a stock solution with a
concentration of 200 mg/ml. HUVECs were obtained from KeyGen
Biotech Co., Ltd. (Nanjing, China). M199 cell culture medium, fetal
bovine serum (FBS), endothelial growth factors (EGFs), penicillin
and streptomycin were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Ox-LDL was obtained from
Yiyuan Biotechnologies (Guangzhou, China).
5,5′,6,6′-tetrachloro-1,1′,
3,3′-tetraethyl-benza-midazolocarbocyanin iodide (JC-1) dye, the
Cell Counting Kit-8 (CCK-8) assay and
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained
from Beyotime Institute of Biotechnology (Jiangsu, China).
Antibodies targeting 5-bromo-2′-deoxyuridine (BrdU; cat. no.
sc-70443; 1:2,000), Bcl-2 (cat no. sc-509; 1:2,000), Bax (cat. no.
sc-70407; 1:2,000), cytochrome c (cat. no. sc-514435; 1:2,500),
caspase-3 (cat. no. sc-271759; 1:2,500), caspase-9 (cat. no.
sc-81589; 1:2,500), caspase-8 (cat. no. sc-6136; 1:2,500), poly
(ADP-ribose) polymerase 1 (PARP; cat. no. sc-136208; 1:2,500),
cyclooxygenase IV (COX IV) (cat. no. sc-69359; 1:2,500) and β-actin
(cat. no. sc-130301; 1:4,000) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). All other reagents were
purchased from Sigma-Aldrich; Merck Millipore, unless otherwise
specified.
Cell culture and treatment
The HUVECs were cultured in M199 medium supplemented
with 10% FBS, 100 µg/ml EGF, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere of 95% air; 5%
CO2. In selected experiments, the HUVECs were incubated
with various concentrations of ox-LDL (30, 60, 90, 120, 150 and 180
µg/ml), with or without resveratrol (5, 10, 20, 40, 80 and 120
µg/ml), for 24 h at 37°C.
Cell viability
Cell viability was measured using CCK-8 and BrdU
assays, according to an established method (20). The HUVECs were seeded in 96-well
plates at a density of 2×105 cells/well and then exposed
to different treatments, as indicated. Finally, CCK-8 (10 µl/well)
was added and incubated at 37°C for 2 h. The absorbance was read at
450 nm using a plate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The BrdU assay was measured according to BrdU uptake. The
cells were incubated with 50 mM BrdU for 4 h at 37°C and then fixed
with 4% paraformaldehyde. Following being permeabilized with 0.1%
Triton X-100 for 5 min, the samples were incubated with anti-BrdU
monoclonal antibody at 4°C overnight, followed by treatment with
biotinylated goat anti-mouse IgG antibody (Santa Cruz
Biotechnology, Inc.; cat. no. sc-130301; 1:4,000) for 1 h at room
temperature. The percentage of BrdU+ cells was
determined by counting the numbers of stained cells and total cells
using a light microscope (Olympus Corporation, Tokyo, Japan).
Analysis of apoptosis
HUVEC apoptosis was measured using an Annexin V-FITC
Apoptosis Detection kit (KeyGEN Biotech Co., Ltd.) using flow
cytometry according to the manufacturer's protocol. Briefly, the
HUVECs were trypsinized and then harvested by centrifugation at
12,000 × g at 4°C for 15 min. The cells were suspended in a binding
buffer of Annexin V-FITC and propidium iodide (PI) at room
temperature in the dark for 15 min. The stained samples were then
analyzed using flow cytometry (Beckman Coulter, Inc., Fullerton,
CA, USA) and the percentage of apoptotic cells was reflected by the
Annexin V/PI ratio. Annexin V was set as the horizontal axis and PI
as the vertical axis.
Western blot analysis
The HUVECs were collected and lysed in RIPA lysis
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
containing protease and phosphatase inhibitor cocktail. The protein
concentration was determined using a BCA protein content kit
(Beyotime Institute of Biotechnology). Proteins (40 µg) were
separated using 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and electrotransferred onto PVDF
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat milk for 1 h at room temperature and then
incubated with the following appropriate primary antibodies
overnight at 4°C: Bcl-2, Bax; cytochrome c, COX IV, cleaved
caspase-9, cleaved caspase-3, cleaved caspase-8, cleaved-PARP and
β-actin. Following incubation with the secondary antibodies
including horseradish peroxidase-conjugated rabbit anti-mouse (cat.
no. 58802; 1:1,000; Cell Signaling Technology, Inc.) or horseradish
peroxidase-conjugated donkey anti-goat (cat. no. sc-2056; 1:1,000;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature, the
signals were determined using an ECL kit and the intensity of the
protein bands was analyzed using ImageJ software (version 1.41;
National Institutes of Health, Bethesda, MA, USA).
Isolation of mitochondria
Intact mitochondria were isolated using a
Mitochondria Isolation kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The cytosolic and
mitochondrial fractions were used for western blot analysis. Cox IV
was used as the loading control for the mitochondrial fraction.
Mitochondria membrane potential (MMP)
analysis
MMP was measured using JC-1, which is an indicator
of the loss of MMP. The aggregate red fluorescent form indicates
healthy MMP, whereas the green fluorescent monomeric form indicates
loss of MMP. The fluorescence was observed using a confocal laser
scanning microscope (Zeiss 710; Zeiss GmbH, Munich, Germany) and
the fluorescence intensity was calculated using ImageJ software
(NIH).
ROS detection
ROS in the HUVECs was visualized using
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as previously
described (21). Briefly, the
HUVECs were washed with PBS three times and incubated with DCFH-DA
(5 µM) in serum-free M199 medium for 30 min at 37°C in the dark.
The cells were then washed in PBS and images were captured using a
fluorescence microscope (BX61; Olympus Corporation, Tokyo, Japan)
with an excitation wavelength of 488 nm and emission wavelength of
525 nm. The fluorescence intensity was calculated using ImageJ
software (NIH).
Superoxide dismutase (SOD) and lipid
peroxidation assay
The activity of SOD was measured using a commercial
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Additionally, the level of lipid
peroxidation was detected using a malondialdehyde (MDA) assay kit
(Beyotime Institute of Biotechnology). The activity of SOD and
levels of MDA are presented as U/mg protein and nmol/mg protein,
respectively.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean from at least 4 independent experiments. Data analysis
was performed using one-way or two-way analysis of variance using
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Resveratrol reverses ox-LDL-induced
apoptosis in HUVECs
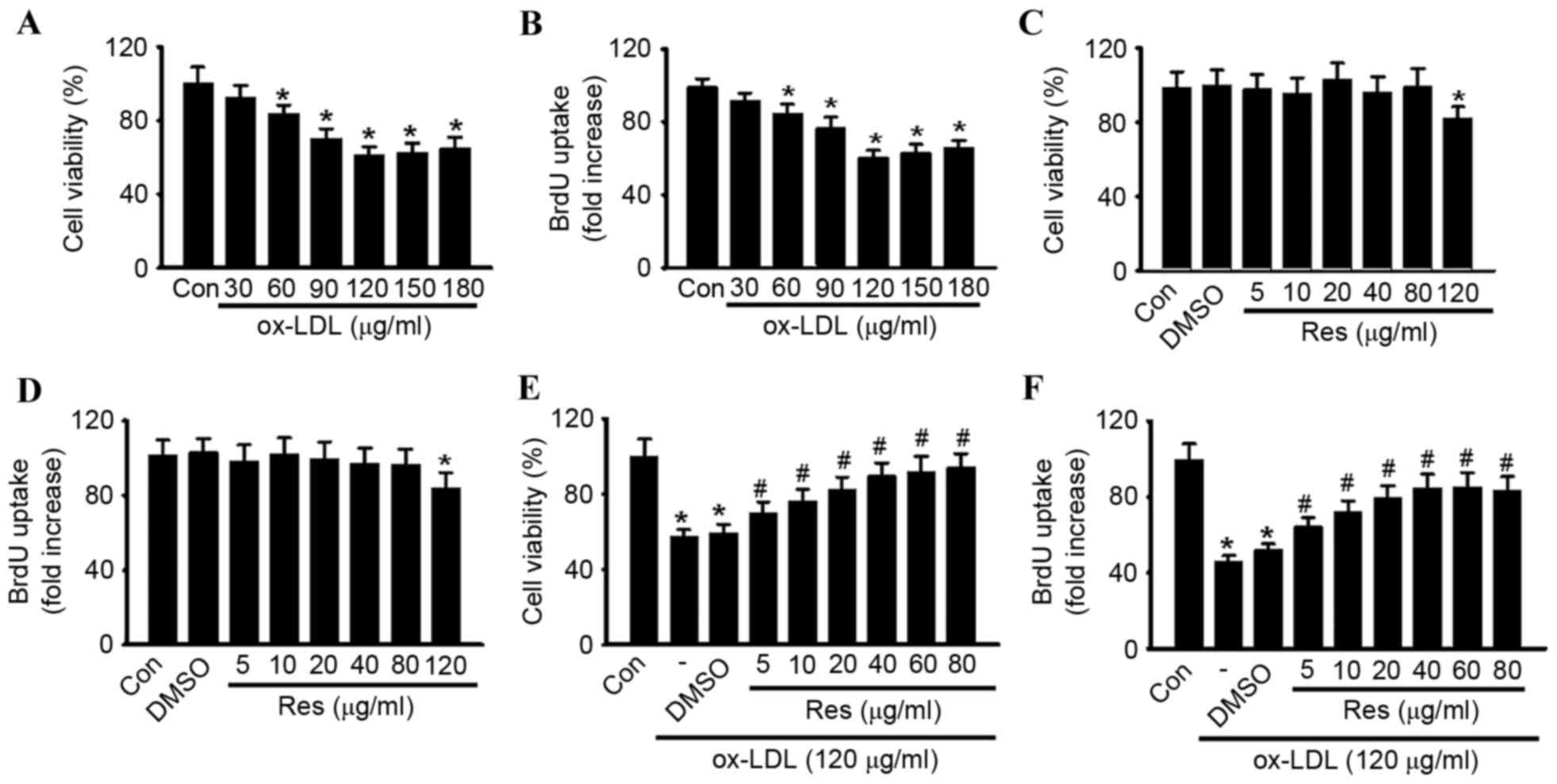
To confirm the pro-apoptotic effect of ox-LDL in
HUVECs, the cells were treated with various concentrations of
ox-LDL (30, 60, 90, 120, 150 and 180 µg/ml) for 24 h, and cell
viability was examined using CCK-8 and BrdU assays, respectively.
Compared with the control group, the cell viability of the HUVECs
was significantly decreased, in a dose-dependent manner, in the
cells treated with ox-LDL (Fig.
1A). At concentrations of 30, 60, 90, 120, 150 and 180 µg/ml,
cell viability decreased from 100.5±8.61% to 92.6±6.7, 83.9±4.8,
70.3±5.2, 61.5±4.1, 62.8±4.9 and 64.8±5.8%, respectively. The
maximal inhibitory effect of ox-LDL on cell viability was observed
at a concentration of 120 µg/ml. Similar results were obtained
using a BrdU uptake assay (Fig.
1B). A concentration of 120 µg/ml ox-LDL was selected to
perform the experiments. The CCK-8 and BrdU assays showed that
resveratrol alone had no cytotoxic effect towards the HUVECs at
concentrations <120 µg/ml (Fig. 1C
and D). To investigate whether resveratrol protected against
ox-LDL-induced cell injury, the HUVECs were treated with ox-LDL
(120 µg/ml) for 24 h in the presence or absence of resveratrol, and
cell viability was examined. As shown in Fig. 1E, resveratrol attenuated ox-LDL
induced cell injury dose-dependently. In accordance with the
results of the CCK-8 assay, a similar trend was observed in the
BrdU uptake assay (Fig. 1F).
Notably, no significant difference was found between 40 and 80
µg/ml. Thus, 40 µg/ml resveratrol was selected for use in the
following experiments. Using Annexin V-FITC/PI staining, the
apoptotic rates of the cells treated with ox-LDL in the presence or
absence of resveratrol were quantified by flow cytometry (Fig. 2A and B). Compared with the control,
the percentage of apoptotic cells was significantly increased in
the HUVECs treated with ox-LDL. However, the increased apoptotic
rate was significantly inhibited following resveratrol
exposure.
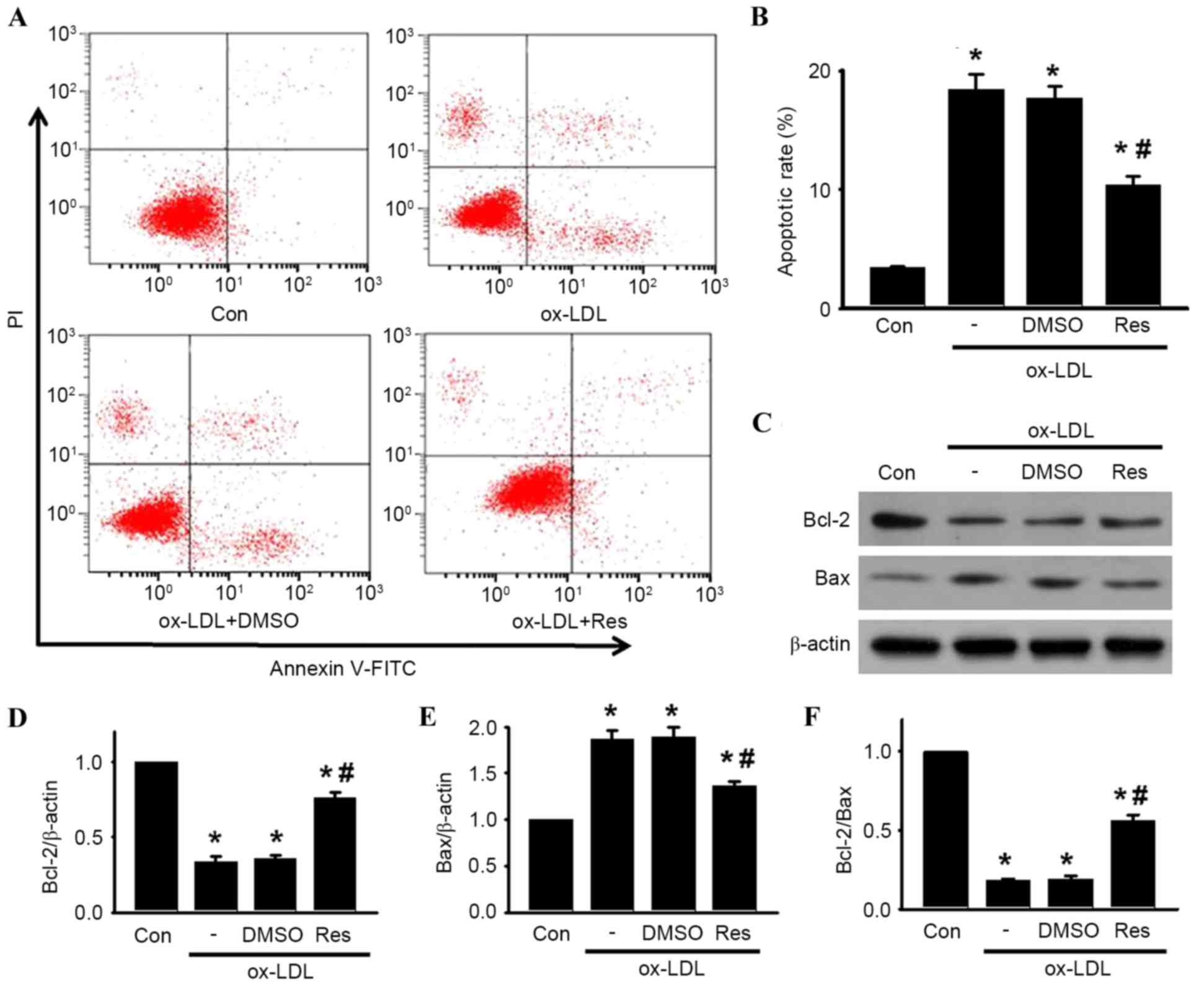 | Figure 2.Res attenuates ox-LDL-induced
apoptosis of HUVECs. Cells were incubated with ox-LDL (120 µg/ml)
in the presence or absence of Res (40 µg/ml) for 24 h. (A) Cell
apoptosis was examined using Annexin V/PI staining followed by flow
cytometry. (B) Percentages of apoptotic cells were calculated. (C)
Protein expression levels of Bcl-2 and Bax were measured using
western blot analysis. Representative western blot images are
shown. (D) Densitometric analysis of Bcl-2, (E) Bax and (F)
Bcl-2/Bax ratio. All data are presented as the mean + standard
error of the mean. *P<0.05, vs. control; #P<0.05,
vs. ox-LDL alone (n=4). HUVECs, human umbilical vein endothelial
cells; ox-LDL, oxidized-low density lipoprotein; Res, resveratrol;
Con, control; PI, propidium iodide; Bcl-2, B cell lymphoma-2; Bax,
Bcl-2-associated X protein; DMSO, dimethyl sulfoxide. |
Effect of resveratrol on
ox-LDL-induced expression of Bcl-2 and Bax in HUVECs
To investigate the mechanism by which resveratrol
attenuated ox-LDL-induced apoptosis in HUVECs, the protein
expression levels of Bcl-2 and Bax were determined. Bcl-2 is an
anti-apoptotic protein, which inhibits programmed cell death; Bax
is a pro-apoptotic protein, which drives the cell towards apoptosis
(22). The ratio of Bcl-2/Bax
appears to be a determinant of the survival or death of cells. As
shown in Fig. 2C-F, ox-LDL
treatment decreased the protein expression of Bcl-2 and increased
the protein expression of Bax, resulting in a decrease in the
Bcl-2/Bax ratio. By contrast, resveratrol attenuated the
ox-LDL-induced decrease of the Bcl-2/Bax ratio, which was due to
the increased level of Bcl-2 and decreased level of Bax.
Effects of resveratrol on the
ox-LDL-induced alteration of MMP
The loss of MMP is a distinguishing feature during
the process of cell apoptosis (23). To investigate the association
between resveratrol and mitochondrial membrane depolarization in
HUVECs, the present study examined the MMP using JC-1 staining.
Following ox-LDL treatment, the red fluorescence of JC-1 was
significantly decreased and the green fluorescence was markedly
increased, resulting in an augmented ratio of green/red
fluorescence intensity (Fig. 3A and
B). As expected, the increase in the green/red fluorescence
ratio induced by ox-LDL was counteracted by resveratrol treatment.
These results suggested that resveratrol inhibited ox-LDL-induced
apoptosis via the stabilization of MMP.
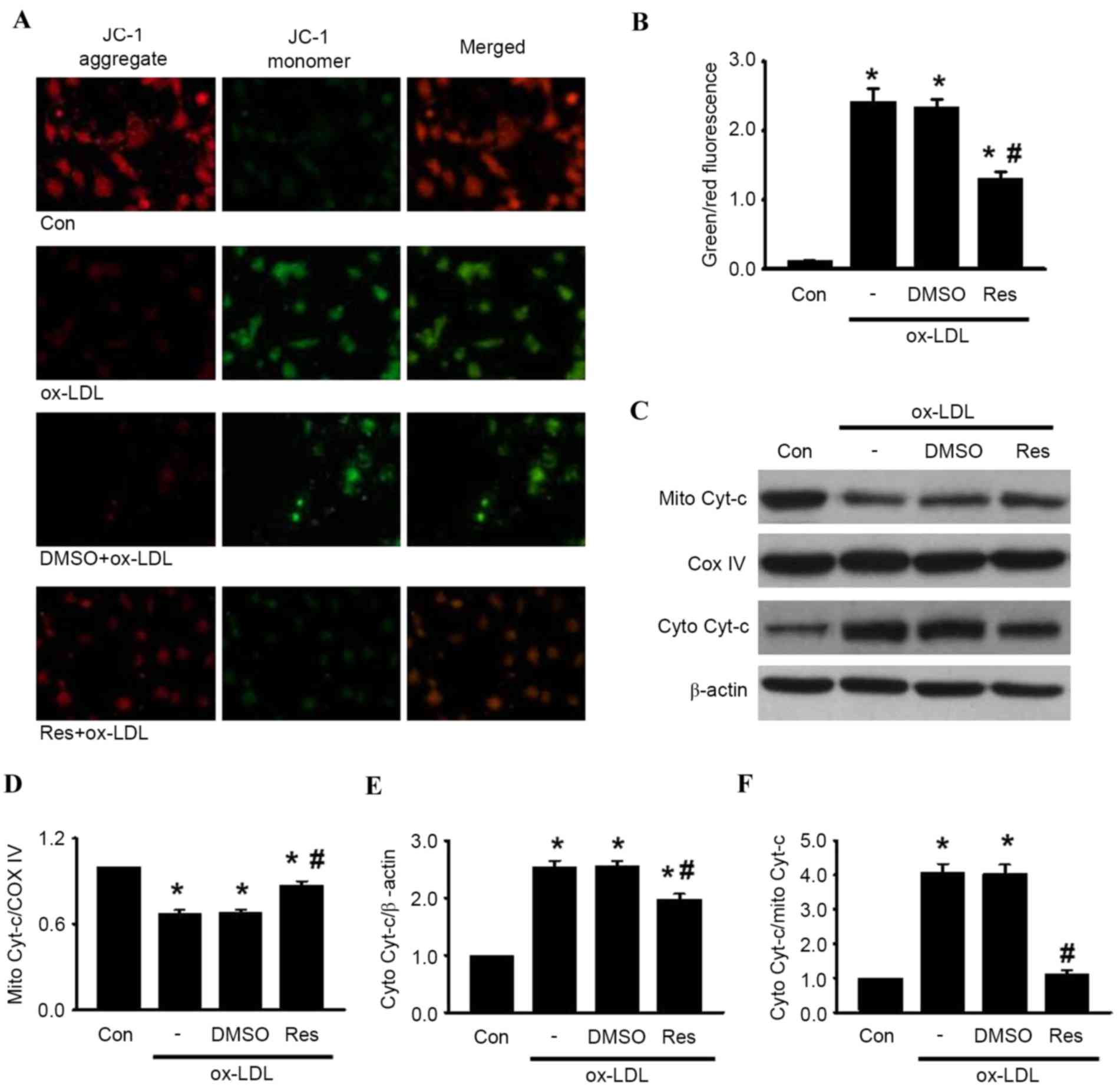 | Figure 3.Res inhibits ox-LDL-induced apoptosis
in HUVECs through the mitochondria-dependent pathway. HUVECs were
treated with ox-LDL (120 µg/ml) in the presence or absence of Res
(40 µg/ml) for 24 h. (A) Mitochondrial membrane potential was
measured using JC-1 dye staining under confocal microscopy. Green
fluorescence represents JC-1 monomer and red fluorescence
represents JC-1 aggregate; merged images show combined green and
red images (x400). (B) Quantitative analysis of the ratio of
green/red fluorescence (C) Protein expression of cytochrome
c in mitochondria and cytosol were measured using western
blot analysis. Densitometric analyses of the expression of
cytochrome c in the (D) mitochondria and (E) cytosol. (F)
Densitometric analysis of the release of cytochrome c from
the mitochondria to the cytoplasm. All data are presented as the
mean + standard error of the mean. *P<0.05, vs. control;
#P<0.05 m vs. ox-LDL group (n=4–6). HUVECs, human
umbilical vein endothelial cells; ox-LDL, oxidized-low density
lipoprotein; Res, resveratrol; Con, control; DMSO, dimethyl
sulfoxide; JC-1, 5,5′, 6,6′-tetrachloro-1,1′,
3,3′-tetraethyl-benza-midazolocarbocyanin iodide; Mito,
mitochondrial; Cyto, cytosolic; Cyt-c, cytochrome c; COX IV,
cyclooxygenase IV. |
Resveratrol inhibits HUVEC apoptosis
induced by ox-LDL through the mitochondria-dependent pathway
It has been documented that MMP destabilization
leads to the release of cytochrome c, sequentially initiating
activation of the caspase cascade, including caspase-3 and
caspase-9 (24). The results of
the western blot analysis showed that treatment with ox-LDL
enhanced the release of cytochrome c from the mitochondria to the
cytosol. The increased release of cytochrome c induced by ox-LDL
was alleviated by resveratrol exposure (Fig. 3C-F). Subsequently, the levesl of
caspase-9, caspase-3, PARP and caspase-8 cleavage was determined in
the HUVECs. The western blot analysis revealed that the cleavage of
caspase-9, caspase-3 and PARP were significantly increased
following ox-LDL treatment. However, these elevations were
inhibited by resveratrol treatment (Fig. 4A-C). Of note, the level of
caspase-8 cleavage remained unaltered in the presence or absence of
resveratrol (Fig. 4D) Taken
together, these results indicated that resveratrol inhibited
ox-LDL-induced apoptosis through the mitochondria-dependent pathway
instead of the extrinsic death receptor-mediated pathway.
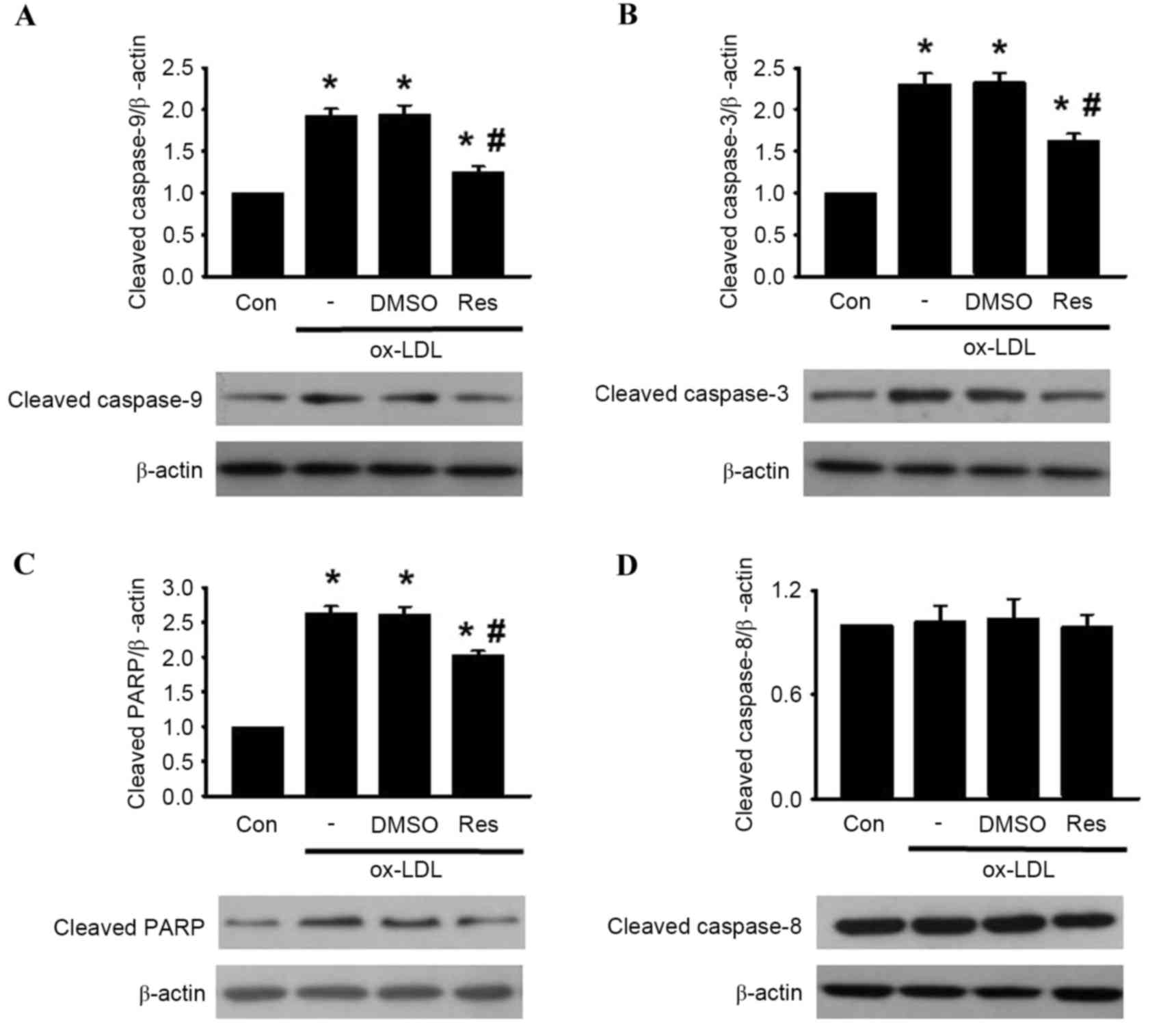 | Figure 4.Res reverses ox-LDL-induced caspase
activation. HUVECs were co-incubated with ox-LDL (120 µg/ml) and
Res (40 µg/ml) for 24 h. Expression levels of (A) cleaved
caspase-9, (B) cleaved caspase-3, (C) cleaved PARP and (D) cleaved
caspase-8 were determined using western blot analysis.
Densitometric analysis showed that Res inhibited the increased
expression of cleaved caspase-9, cleaved caspase-3 and cleaved
PARP, but had no effect on the expression of cleaved caspase-8.
Representative western blots are shown. All data are presented as
the mean + standard error of the mean. *P<0.05, vs. control;
#P<0.05, vs. ox-LDL group (n=3). HUVECs, human
umbilical vein endothelial cells; ox-LDL, oxidized-low density
lipoprotein; Res, resveratrol; Con, control; DMSO, dimethyl
sulfoxide; PARP, poly (ADP-ribose) polymerase 1. |
Resveratrol protects HUVECs from
oxidative damage induced by ox-LDL
Oxidative damage can be caused by excessive
intracellular ROS generation, a critical contributor to the
pathological process of endothelial cell apoptosis and
atherosclerosis (7,10). To elucidate the effects of
resveratrol on the oxidative stress induced by ox-LDL in HUVECs,
the present study examined ROS generation using DCFH-DA. As shown
in Fig. 5A and B, DCF fluorescence
intensity was significantly increased in the presence of ox-LDL,
which was alleviated by resveratrol. Similarly, resveratrol
decreased ox-LDL-induced lipid peroxidation, indicated by MDA
generation (Fig. 5C). By contrast,
the activity of SOD, an antioxidant enzyme, was markedly decreased
by ox-LDL. As expected, resveratrol markedly attenuated the
decreased activity of SOD (Fig.
5D). Together, these data suggested that resveratrol inhibited
ox-LDL-induced oxidative damage, which may be associated with its
protective effect on endothelial cell apoptosis.
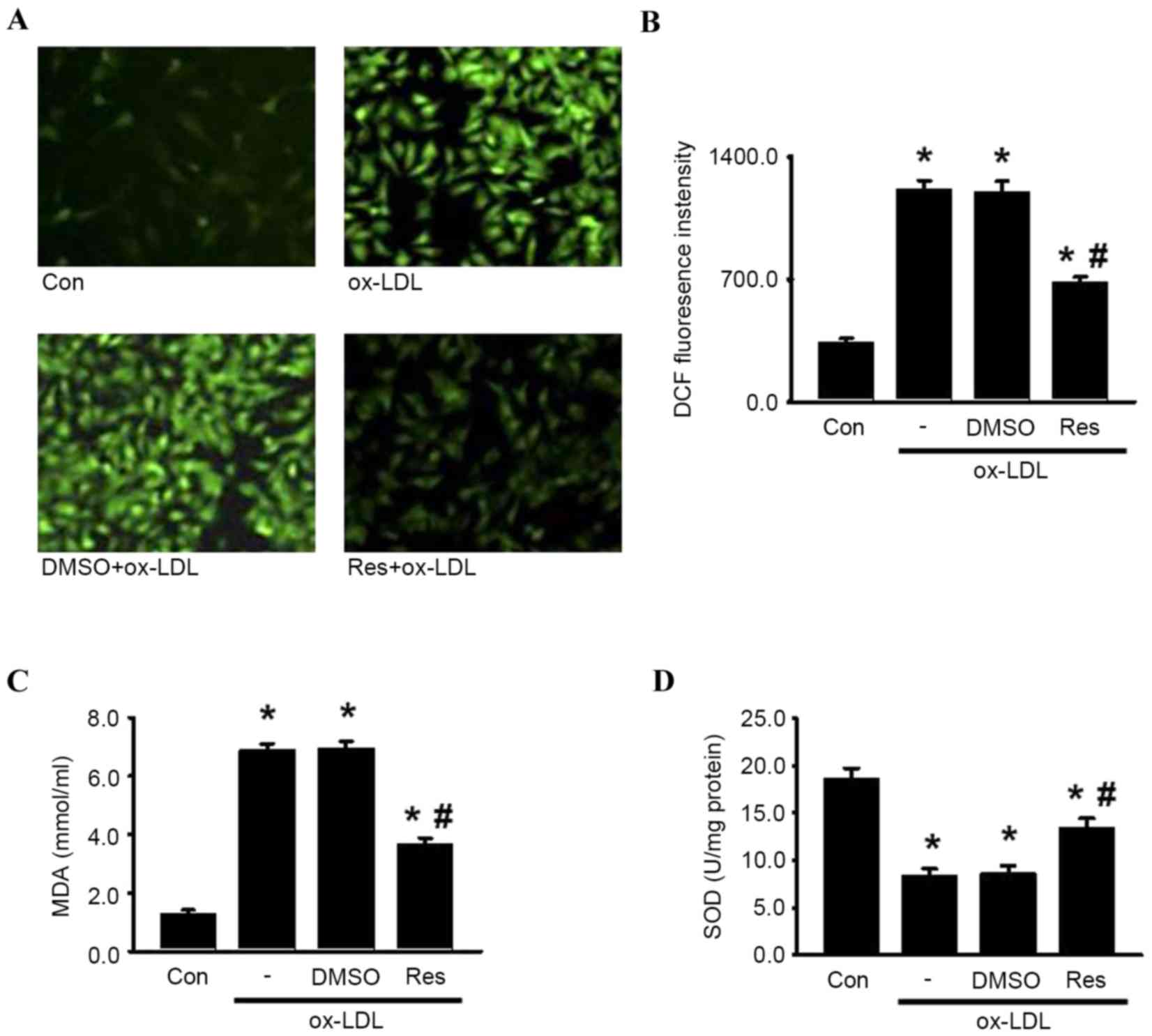 | Figure 5.Res attenuates oxidase damage induced
by ox-LDL in HUVECs. HUVECs were treated with ox-LDL (120 µg/ml) in
the presence or absence of Res (40 µg/ml) for 24 h. (A) ROS
generation was measured. Representative images of HUVECs loaded
with DCFH-DA (5 µM) were captured using a fluorescence microscope
(x200). (B) Quantitative analysis of DCF fluorescence intensity.
(C) Levels of lipid peroxidation were detected using an MDA assay
kit. (D) Intracellular activity of SOD was decreased by treatment
of ox-LDL, which were inhibited by Res. Data are presented as the
mean ± standard error of the mean. *P<0.05, vs. control;
#P<0.05, vs. ox-LDL group (n=4). HUVECs, human
umbilical vein endothelial cells; ox-LDL, oxidized-low density
lipoprotein; Res, resveratrol; Con, control; DMSO, dimethyl
sulfoxide; ROS, reactive oxygen species; DCF,
2′,7′-dichlorodihydrofluorescein; MDA, malondialdehyde; SOD,
superoxide dismutase. |
Discussion
Epidemiological studies have revealed a negative
association between cardiovascular disease-associated mortality and
the consumption of red wine (25,26).
This has been documented as the ‘French paradox’, which partly
indicates that the resveratrol present in red wine may assist in
preventing cardiovascular diseases (27). A number of molecular targets have
been found to be modulated by resveratrol in several cardiovascular
diseases, including atherosclerosis, hypertension and inflammation
(28,29). Although resveratrol has been shown
to contribute to improvements in endothelial dysfunction, its
effect on endothelial apoptosis, the initial event of
atherosclerosis, remains to be poorly elucidated. In the present
study, it was demonstrated that resveratrol restored ox-LDL-induced
endothelial cell apoptosis. The underlying mechanisms included
alteration in the activation of Bcl-2/Bax proteins and associated
proteins of the intrinsic mitochondrial pathway in HUVECs following
ox-LDL treatment. In addition, the present study found that
resveratrol attenuated ox-LDL-induced oxidative damage, as
evidenced by the attenuation of ROS and MDA generation, and the
restoration of SOD activity.
Endothelial cell apoptosis has been implicated in
the development of a variety of vascular diseases, including
atherosclerosis (5). Of note, a
previous study showed that increased apoptotic endothelial cells
were observed in atheromatous plaques, resulting in monocyte
adhesion and foam cell formation (7). Thus, the identification of effective
regimens with low toxicity for the protection against endothelial
cell apoptosis is urgently required. In the present study, it was
found that resveratrol dose-dependently reversed ox-LDL-induced
HUVEC injury and apoptosis. Of note, resveratrol had no cytotoxic
effect on HUVECs at concentrations <120 µg/ml, indicating the
safety of resveratrol for clinical use.
Abnormal expression of the Bcl-2 family is usually
associated with the apoptotic process, consisting of alterations in
the protein levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax.
Upon apoptotic stimulation, the expression of Bax is increased,
leading to a lower level of Bcl-2 (30). Bax forms oligomers and transfers
from the cytoplasm to the mitochondrial membrane, resulting in
mitochondrial membrane depolarization (31). Subsequently, cytochrome c is
released from the mitochondria to the cytosol, and triggers caspase
pathway activation (23). In the
present study, it was demonstrated that ox-LDL resulted in an
increase in the expression of Bax, and a decrease in the expression
of Bcl-2 and the Bcl-2/Bax ratio, all of which were attenuated
following exposure to resveratrol. Furthermore, resveratrol
attenuated ox-LDL-induced mitochondrial membrane depolarization and
cytochrome c release. Consequently, resveratrol inhibited
the elevation of caspase-9, caspase-3 and PARP cleavage induced by
ox-LDL, suggesting that resveratrol prevented ox-LDL-induced
apoptosis in the HUVECs predominantly through the
mitochondria-dependent pathway. Of note, neither ox-LDL nor
resveratrol affected caspase-8 activation, indicating that the
extrinsic death receptor-mediated pathway was not involved in this
process.
Oxidative damage induced by excessive ROS generation
is known to be a potential inducer of apoptosis, and mitochondrial
pathway dysfunction is the major cause of the overproduction of ROS
(31,32). Additionally, accumulating evidence
has indicated that resveratrol improves cardiovascular disorders
via reducing oxidative stress and damage (33,34).
Therefore, the present study aimed to examine whether resveratrol
inhibited oxidative damage and subsequently protected HUVECs from
apoptosis. The results showed that resveratrol inhibited the
ox-LDL-induced increased ROS generation and lipid peroxidation, and
decreased SOD activity.
In conclusion, the data obtained in the present
study demonstrated the protective role of resveratrol on
endothelial cell apoptosis induced by ox-LDL. Resveratrol
effectively inhibited ox-LDL-induced apoptosis through inhibition
of the mitochondrial pathway and oxidative damage. This novel
mechanism may assist in elucidating the functional role of
resveratrol for the treatment of endothelial cell apoptosis and
atherosclerosis.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities in China (grant no. 12ykpy26),
the Guangdong Natural Science Foundation (grant nos. S2012040008068
and S2013010014514) and the Guangdong Province-Ministry of
Education Joint Research Program (grant no. 2012B091100454).
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Executive summary: Heart disease and stroke statistics-2014 update:
A report from the American Heart Association. Circulation.
129:399–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajendran P, Rengarajan T, Thangavel J,
Nishigaki Y, Sakthisekaran D, Sethi G and Nishigaki I: The vascular
endothelium and human diseases. Int J Biol Sci. 9:1057–1069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Griendling KK and FitzGerald GA: Oxidative
stress and cardiovascular injury: Part I: Basic mechanisms and in
vivo monitoring of ROS. Circulation. 108:1912–1916. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park YS, Kim J, Misonou Y, Takamiya R,
Takahashi M, Freeman MR and Taniguchi N: Acrolein induces
cyclooxygenase-2 and prostaglandin production in human umbilical
vein endothelial cells: Roles of p38 MAP kinase. Arterioscler
Thromb Vasc Biol. 27:1319–1325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiro A, Wilkinson FL, Weston R, Smyth
JV, Serracino-Inglott F and Alexander MY: Endothelial
microparticles as conveyors of information in atherosclerotic
disease. Atherosclerosis. 234:295–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: Apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra S, Deshmukh A, Sachdeva R, Lu J and
Mehta JL: Oxidized low-density lipoprotein and atherosclerosis
implications in antioxidant therapy. Am J Med Sci. 342:135–142.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tricot O, Mallat Z, Heymes C, Belmin J,
Leseche G and Tedgui A: Relation between endothelial cell apoptosis
and blood flow direction in human atherosclerotic plaques.
Circulation. 101:2450–2453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Mitra S, Wang X, Khaidakov M and
Mehta JL: Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in
atherogenesis and tumorigenesis. Antioxid Redox Signal.
15:2301–2333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen TG, Chen TL, Chang HC, Tai YT, Cherng
YG, Chang YT and Chen RM: Oxidized low-density lipoprotein induces
apoptotic insults to mouse cerebral endothelial cells via a
Bax-mitochondria-caspase protease pathway. Toxicol Appl Pharmacol.
219:42–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D and Mehta JL: Intracellular signaling
of LOX-1 in endothelial cell apoptosis. Circ Res. 104:566–568.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: Potential as anticancer
agent. J Diet Suppl. 9:45–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cavallaro A, Ainis T, Bottari C and
Fimiani V: Effect of resveratrol on some activities of isolated and
in whole blood human neutrophils. Physiol Res. 52:555–562.
2003.PubMed/NCBI
|
|
15
|
Wang H, Yang YJ, Qian HY, Zhang Q, Xu H
and Li JJ: Resveratrol in cardiovascular disease: What is known
from current research? Heart Fail Rev. 17:437–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakata R, Takahashi S and Inoue H: Recent
advances in the study on resveratrol. Biol Pharm Bull. 35:273–279.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H and Förstermann U: Resveratrol: A
multifunctional compound improving endothelial function. Editorial
to: ‘Resveratrol supplementation gender independently improves
endothelial reactivity and suppresses superoxide production in
healthy rats’ by S. Soylemez et al. Cardiovasc Drugs Ther.
23:425–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmitt CA, Heiss EH and Dirsch VM: Effect
of resveratrol on endothelial cell function: Molecular mechanisms.
Biofactors. 36:342–349. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin YL, Chang HC, Chen TL, Chang JH, Chiu
WT, Lin JW and Chen RM: Resveratrol protects against oxidized
LDL-induced breakage of the blood-brain barrier by lessening
disruption of tight junctions and apoptotic insults to mouse
cerebrovascular endothelial cells. J Nutr. 140:2187–2192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang EW, Xue SJ, Li XY, Xu SW, Cheng JD,
Zheng JX, Shi H, Lv GL, Li ZG, Li Y, et al: EEN regulates the
proliferation and survival of multiple myeloma cells by
potentiating IGF-1 secretion. Biochem Biophys Res Commun.
447:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng H, Zhong W, Zhao H, Chen L, Zhou X,
Li F, Zhu W and Li G: Lack of PGC-1α exacerbates high
glucose-induced apoptosis in human umbilical vein endothelial cells
through activation of VADC1. Int J Clin Exp Pathol. 8:4639–4650.
2015.PubMed/NCBI
|
|
22
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deshmukh M and Johnson EM Jr: Evidence of
a novel event during neuronal death: Development of
competence-to-die in response to cytoplasmic cytochrome c. Neuron.
21:695–705. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renaud SC, Gueguen R, Schenker J and
d'Houtaud A: Alcohol and mortality in middle-aged men from eastern
France. Epidemiology. 9:184–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukao H, Ijiri Y, Miura M, Hashimoto M,
Yamashita T, Fukunaga C, Oiwa K, Kawai Y, Suwa M and Yamamoto J:
Effect of trans-resveratrol on the thrombogenicity and
atherogenicity in apolipoprotein E-deficient and low-density
lipoprotein receptor-deficient mice. Blood Coagul Fibrinolysis.
15:441–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Li X and Ren J: From French
Paradox to cancer treatment: Anti-cancer activities and mechanisms
of resveratrol. Anticancer Agents Med Chem. 14:806–825. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perez-Vizcaino F, Duarte J and
Andriantsitohaina R: Endothelial function and cardiovascular
disease: Effects of quercetin and wine polyphenols. Free Radic Res.
40:1054–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossi L, Mazzitelli S, Arciello M, Capo CR
and Rotilio G: Benefits from dietary polyphenols for brain aging
and Alzheimer's disease. Neurochem Res. 33:2390–2400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Besbes S, Mirshahi M, Pocard M and Billard
C: New dimension in therapeutic targeting of BCL-2 family proteins.
Oncotarget. 6:12862–12871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson TM, Yu ZX, Ferrans VJ, Lowenstein
RA and Finkel T: Reactive oxygen species are downstream mediators
of p53-dependent apoptosis. Proc Natl Acad Sci USA. 93:11848–11852.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ladurner A, Schachner D, Schueller K,
Pignitter M, Heiss EH, Somoza V and Dirsch VM: Impact of
trans-resveratrol-sulfates and -glucuronides on endothelial nitric
oxide synthase activity, nitric oxide release and intracellular
reactive oxygen species. Molecules. 19:16724–16736. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Xia N and Förstermann U:
Cardiovascular effects and molecular targets of resveratrol. Nitric
Oxide. 26:102–110. 2012. View Article : Google Scholar : PubMed/NCBI
|