Introduction
In the US, thyroid cancer constitutes ~2% of
malignancies, with cancer-associated mortality rates of >1,600
per year (1). Thyroid cancer
accounts for 1% of cases of cancer in humans, and includes
well-differentiated thyroid carcinoma of papillary and follicular
types, which account for >95% of cases of thyroid cancer, and
anaplastic thyroid carcinoma (ATC), which accounts for 1–5% of
thyroid malignancies (2–4). ATC is a life threatening disease with
a median survival rate of 6 months subsequent to diagnosis
(2–5). Of the patients diagnosed with ATC,
90% have extraglandular spread at the time of diagnosis, with 75%
of the patients developing distant metastases (6,7).
Consequently, cases of ATC are staged as stage IV in the American
Joint Commission on Cancer system (8).
Patients with ATC, as a life threatening type of
tumor, have a median survival rate of ~6 months and a 1 year
survival rate of <20% (9,10).
It is more common in women, compared with men (11) and commonly affects the lungs
(12). ATC is reported to induce
significant site-specific morbidity and is associated with a poor
prognosis. Patients with ATC present with an enlarged neck mass,
and the symptoms of ATC include dysphagia, odynophagia, dyspnea,
anxiety and vocal cord paralysis (13). If patients do not undergo
treatment, including surgical resection and external beam radiation
therapy, they develop uncontrolled local progression causing
suffocation and mass bleeding, and eventually succumb to mortality
(13,14). Despite tracheostomy, patients can
suffer from obstruction of the wind pipe (15,16).
Fucus vesiculosus, commonly known as
bladderwrack, is a brown edible seaweed, which has traditionally
been used as an anti-obesity treatment, health supplement and for
goiter treatment (17). It was the
first source of iodine to be identified and has been used in the
treatment of thyroid disorders (17). The brown color of the herb is due
to the presence of fucoxanthin pigment (18). The compound. fucoidan, has been
isolated from the extract of bladderwrack (19). The chemical structure of fucoidan
is similar to that of heparin, which is a compound used as an
anti-coagulant (20). In addition
to fucoidan, bladderwrack also contains fucophlorethol and
fucotriphlorethol A (20). The
present study aimed to determine the anticancer effect of fucoidan
in ATC cells by investigating apoptosis and anti-angiogenesis. The
current study demonstrated that fucoidan inhibited the expression
of vascular endothelial growth factor (VEGF) via suppression of
HIF-1α.
Materials and methods
Extraction of fucoidan
F. vesiculosus was collected from the coast
of the Baltic Sea and the identity of the specimens was confirmed
as previously described (21,22).
The fucoidan used in the present study was isolated from the
extract of F. vesiculosus in 20:80 ethyl acetate:hexane
using column chromatography and was characterized using
1H nuclear magnetic resonance (NMR), 13C NMR
and mass spectrometry.
Cell lines and cell culture
Human FTC133 and TPC1 ATC cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. The cell cultures were maintained at 37°C
in a humidified atmosphere containing 5% CO2.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) cell proliferation kit
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
analyze the effect of fucoidan on cell proliferation. The cells
were seeded into 96-well plates at a density of 2.5×105
cells in each well. After 24 h, different concentrations of
fucoidan (0, 1, 2, 4, 8, 10, 15 µM) were added to each well
containing the cells. Following incubation for 48 h at 37°C, 20 µl
of CCK-8 solution (5 mg/ml) was added to each well and incubation
was continued for another 4 h at 37°C in an incubator with 5%
CO2. The proliferation of the cells was measured using a
Multiskan Go spectrophotometer (Thermo Fisher Scientific, Inc.) at
450 nm. The proliferation index was calculated from the resulting
optical density values.
Western blot analysis
Following treatment of the cells with the
aforementioned concentrations of fucoidan for 72 h, the cells were
washed three times in ice-cold phosphate-buffered saline (PBS) and
then lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 137
mM NaCl, 10% glycerol, 100 mM sodium vanadate, 1 mM PMSF, 10 mg/ml
aprotinin, 10 mg/ml leupeptin, 1% NP-40 and 5 mM cocktail. The
concentration of proteins in the lysate was determined using a
bicinchoninic acid assay. The proteins (2 µg) were resolved on a
10% polyacrylamide gel by electrophoresis. For the transfer of
proteins onto a polyvinylidene difluoride membrane, a semi-dry
method was used. The membrane was blocked in 5% non-fat dry milk
overnight and then washed in Tris-buffered saline with Tween-20
(TBST). The membrane was then incubated overnight at 4°C with
anti-hypoxia inducible factor (HIF)-1α (1:50; cat. no. 100–449),
anti-B cell lymphoma-2 (Bcl-2)-associated X protein (Bax; 1:50;
cat. no. 100–56097), anti-Bcl-2 (1:50; cat. no. 100–2087),
anti-cleaved caspase-3 (1:50; cat. no. 100–56113).) and
anti-cleaved poly ADP-ribose polymerase (PARP; 1:50; cat. no.
100-56599; Cell Signaling Technology, Inc., Danvers, MA, USA). The
incubation with antibodies was followed by washing with TBST.
Subsequently, the membrane was incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
610-103-121; GE Healthcare Life Sciences, Chalfont, UK) for 1 h at
room temperature. Enhanced chemiluminescence (Pierce Biotechnology;
Thermo Fisher Scientific, Inc.) was used for the antigen detection.
β-actin was used as the control.
Assessment of apoptosis using
4,6-diamidino-2-phenylindole (DAPI) staining and terminal
deoxynucleotidyl transferasemediated dUTP nick-end labeling (TUNEL)
assays
Onto 18 mm cover slips, the cells in minimal
essential medium (Gibco; Thermo Fisher Scientific, Inc.) were
plated at a density of 3.0 106 and ~80% confluence for
24 h at 4°C. For angiogenesis analysis, cells were treated with 100
µM CoCl2 for 18 h to induce hypoxic conditions Treatment
of the cells with 100 µg/ml fucoidan for 24 h was followed by
fixing in 10% paraformaldehyde. The cells were then washed twice
with PBS and stained with 2 µg/ml DAPI for 20 min at 37°C. A
fluorescent microscope was then used to analyze the nuclear
fragmentation in the stained cells. A TUNEL kit (Chemicon,
Temecula, CA, USA) was used for the TUNEL assay, which was
performed according to the manufacturer's protocol.
Cell wounding assay
At 90% confluence, HUVECs were plated onto the 60 mm
diameter culture dishes and then scratched with pipette tip. The
cells were then subjected to PBS washing followed by incubation in
endothelial cell growth medium MV2 (ECGM2) supplemented with 2% FBS
along with recombinant human VEGF (50 ng/ml), thymidine (1 mM) and
fucoidin (10 µM) obtained from PromoCell GmbH (Heidelberg,
Germany). The cell cultures used as negative control were incubated
with ECGM2 medium containing 2% FBS alone. After 16 h, HUVECs were
rinsed in PBS followed by methanol fixation. The experiments were
performed in triplicates and the data are presented as the mean of
three experiments.
Tube formation assay
To each Matrigel-coated well (BD Biosciences,
Franklin Lakes, NJ, USA), 0.2 ml of cell suspension comprising
cells suspended in growth medium at a density of 2.5×105
cells/ml, was added. The cells were incubated at 37°C in an
incubator with 5% CO2 in the presence or absence of
fucoidan for 12 h. The cells were also incubated at 4°C in media
containing 50 ng/ml VEGF and 1 mM thymidine along with fucoidan (10
µM) for 16 h. Subsequently, a phase contrast microscope was used to
examine changes in the morphology of the cells.
Migration assay
To determine the migration potential of the
fucoidan-treated cells, Transwell cell culture inserts were used.
The cells (3×105) in 200 ml of growth medium were seeded
into the upper chambers of the Transwell inserts. To the lower
chamber, 750 ml of medium was added, which contained 20% FBS as a
chemoattractant. In the control wells, medium was added to the
upper and the lower chambers. The non-migrated cells in the upper
chamber were removed by swabbing following 24 h of incubation at
4°C. The inserts were observed under an inverted fluorescent
microscope subsequent to fixing and staining. The number of cells
in five randomly selected fields of view were counted in
triplicate.
Statistical analysis
One-way analysis of variance and Student's t-test
were used for statistical analysis. The data are presented as the
mean ± standard deviation. P≤0.05 was considered to indicate a
statistically significant difference. SPSS software (version 10.0;
SPSS, Inc., Chicago, IL, USA) was used for statistical
calculations.
Results
Inhibition of ATC cell growth by
fucoidan
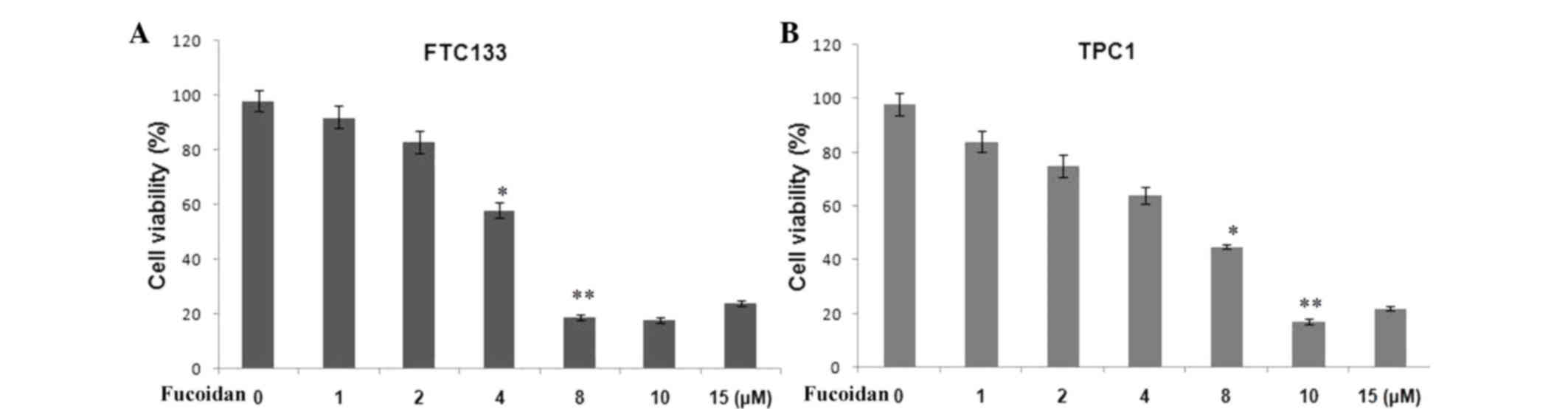
Treatment of the FTC133 and TPC1 ATC cell lines with
different doses of fucoidan (1–15 µM) led to inhibition of cell
growth 36 h following treatment. The inhibition of cell growth by
fucoidan was observed to be concentration-dependent (Fig. 1). For the FTC133 and TPC1 cell
lines, the half maximal inhibitory concentrations for growth
inhibition were 8 and 10 µM, respectively.
Effects of fucoidan on apoptotic cell
death in FTC133 ATC cells
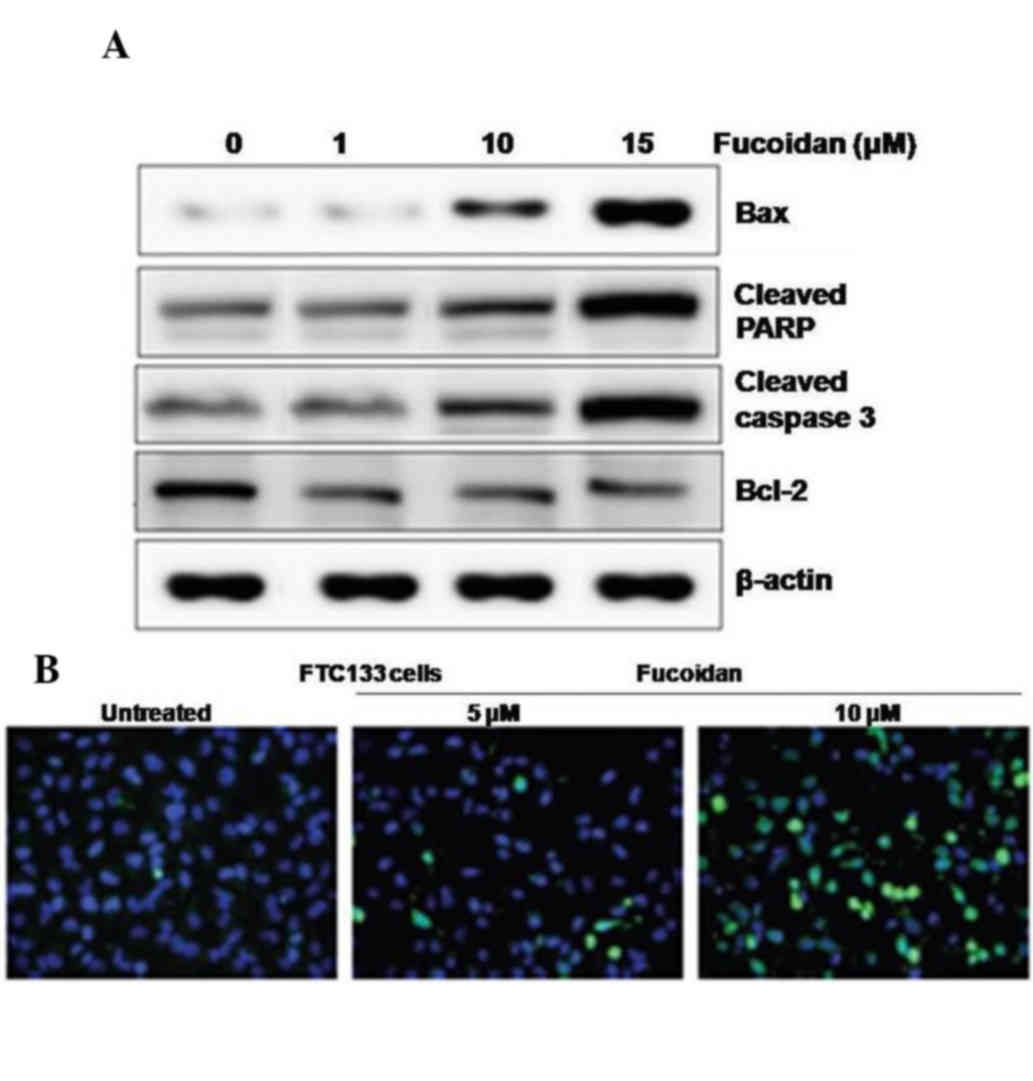
The results of the western blot analysis revealed
that the expression levels of Bax, cleaved PARP and caspase-3 were
increased, and the expression of Bcl-2 was decreased in the FTC133
cells treated with fucoidan for 36 h, compared with the control
cells (Fig. 2A). DAPI staining
showed the appearance of DNA fragmentation and perinuclear
apoptotic bodies in the FTC133 cells treated with 8 µM of fucoidan
(Fig. 2B). Thus, fucoidan led to
apoptosis of the FTC133 cells. The fucoidan-induced apoptosis was
indicated by DNA strand breakage, which were also observed in the
results of the TUNEL analysis.
Effects of fucoidan on
angiogenesis
The cells were treated with CoCl2 (100
µM) for 18 h to induce hypoxia-like conditions, and were then
treated with different doses of fucoidan. Under the hypoxic
conditions, the expression of HIF-1α was promoted, however,
fucoidan treatment (10 µM) reduced the hypoxia-induced expression
of HIF-1α. The expression of VEGF was also enhanced under the
hypoxic conditions and this hypoxia-induced expression of VEGF was
decreased by treatment with fucoidan. The results from the tube
formation assay clearly revealed that the formation of vessel-like
structures was suppressed following treatment with fucoidan
(Fig. 3A). Incubation of the
wounded cells in media containing 50 ng/ml VEGF and 1 mM thymidine
in the presence of fucoidan (10 µM) for 16 h showed failure of
wound healing capacity (Fig. 3B).
Investigation of the effect of fucoidan on the expression of VEGF
revealed a reduction in the expression of VEGF by fucoidan in
hypoxia (Fig. 3C). Thus, tube
formation and the migration of cells were inhibited by fucoidan,
suggesting that fucoidan has a potent anti-angiogenic effect.
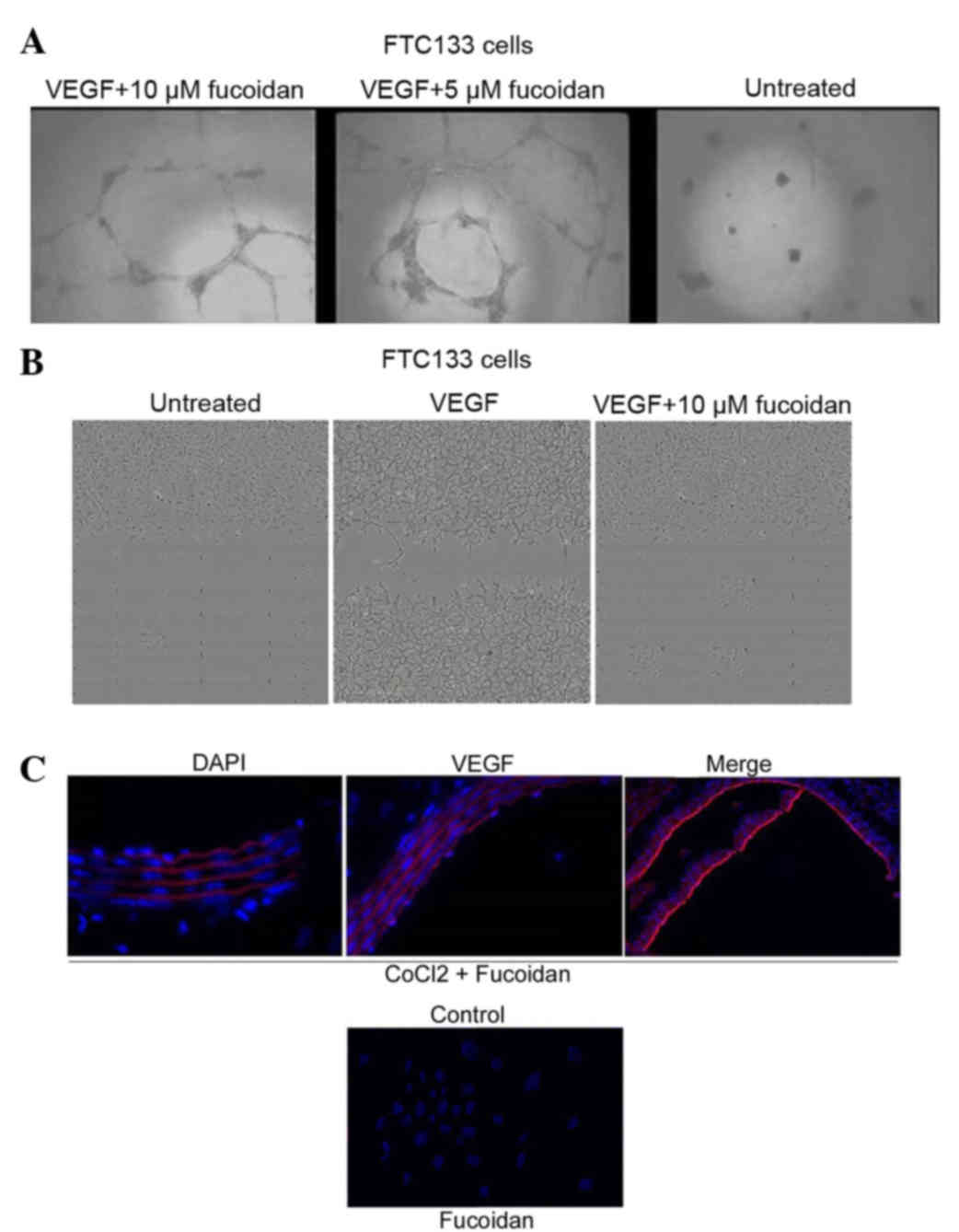 | Figure 3.Effects of fucoidan on angiogenesis in
human thyroid cells. (A) Cells were plated on Matrigel (200
µl/well) and treated with various concentrations of fucoidan. After
12 h, capillary tube formation was assessed under a phase-contrast
microscope and images were captured (magnification, ×400). (B)
Effects of fucoidan on migration. Following wounding, the cells
were washed with phosphate-buffered saline and incubated in ECGM2
containing 2% FBS and 50 ng/ml recombinant human VEGF, 1 mM
thymidine and/or fucoidan. ECGM2 medium with 2% FBS was used as a
negative control. Results are representative of at least three
independent experiments performed in triplicate. Magnification,
×350. (C) Immunocytochemistry of VEGF in hypoxia-induced cells (100
µM CoCl2). Magnification, vx350. VEGF, vascular
endothelial factor; FBS, fetal bovine serum; ECGM2, endothelial
cell growth medium 2; DAPI, 4,6-diamidino-2-phenylindole. |
Discussion
Fucus vseiculosus has been used in the
treatment of thyroid disorders (17) and fucoidan has been isolated from
its extract (19). Fucoidan is
structurally similar to heparin, which is used as an anti-coagulant
agent. In the present study, the effect of fucoidan on the growth
of ATC cells was investigated. A previous study investigated the
anticancer effects of plants or their components (23). Of the drugs used in cancer therapy,
~70% are natural products or their derivatives (24). The results of the present study
revealed that fucoidan inhibited the growth of tumor cells and
induced apoptosis of the cells. Fucoidan also suppressed
angiogenesis by decreasing the expression levels of HIF-1α and
VEGF. Apoptosis is vital in cancer treatment by removing infected
cells through programmed cell death (25). Caspase-3, a member of the caspase
family, is important for inducing apoptosis. It is involved in the
proteolytic cleavage of various key proteins, including PARP, a
protein repairing DNA and maintaining genomic DNA integrity
(26,27). The results from the present study
demonstrated that fucoidan promoted the expression levels of
cleaved caspase-3 and PARP, and induced apoptosis of the thyroid
cells. TUNEL and DAPI staining were used to investigate the
apoptotic effects of fucoidan. Anti-apoptotic regulators, including
Bcl-2, and pro-apoptotic regulators, including Bax, are responsible
for the maintenance of cell homeostasis. The results of the present
study showed that fucoidan enhanced the expression of Bax and
decreased the expression of Bcl-2 in the FTC133 human thyroid
cells. Angiogenic factors, including VEGF, are responsible for
inducing angiogenesis (28,29).
In the present study, the expression levels of HIF-1α and VEGF were
inhibited under CoCl2-induced hypoxic conditions in the
FTC133 cells. This anti-angiogenic effect of fucoidan was supported
by the inhibition of cell migration and tube formation observed,
indicating that fucoidan inhibited angiogenesis through VEGF, in
addition to targeting the endothelial cells directly. Thus fucoidan
offers significant potential as an anticancer agent. In conclusion,
the present study demonstrated the anticancer effects of fucoidan
in ATC cells, which involved the induction of apoptosis and
anti-angiogenesis by inhibiting the expression of VEGF via the
suppression of HIF-1α.
These findings suggested that fucoidan may be a
potential candidate for cancer therapy against ATC.
References
|
1
|
American Cancer Society: Cancer Facts and
Figures. American Cancer Society; Atlanta, GA: pp. 30303–1002.
2009
|
|
2
|
Catalano MG, Poli R, Pugliese M, Fortunati
N and Boccuzzi G: Emerging molecular therapies of advanced thyroid
cancer. Mol Aspects Med. 31:215–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fassnacht M, Kreissl MC, Weismann D and
Allolio B: New targets and therapeutic approaches for endocrine
malignancies. Pharmacol Ther. 123:117–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel KN and Shaha AR: Poorly
differentiated and anaplastic thyroid cancer. Cancer Control.
13:119–128. 2006.PubMed/NCBI
|
|
5
|
Sakorafas GH, Sampanis D and Safioleas M:
Cervical lymph node dissection in papillary thyroid cancer: Current
trends, persisting controversies, and unclarified uncertainties.
Surg Oncol. 19:e57–e70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimaoka K, Schoenfeld DA, DeWys WD,
Creech RH and DeConti R: A randomized trial of doxorubicin versus
doxorubicin plus cisplatin in patients with advanced thyroid
carcinoma. Cancer. 56:2155–2160. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahuja S and Ernst H: Chemotherapy of
thyroid carcinoma. J Endocrinol Invest. 10:303–310. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fleming ID, Phillips JL, Menck HR, Murphy
GP and Winchester DP: The national cancer data base report on
recent hospital cancer program progress toward complete American
joint committee on cancer/TNM staging. Cancer. 80:2305–2310. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilliland FD, Hunt WC, Morris DM and Key
CR: Prognostic factors for thyroid carcinoma. A population-based
study of 15, 698 cases from the surveillance, epidemiology and end
results (SEER) program 1973–1991. Cancer. 79:564–573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53, 856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see comments].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ain KB: Anaplastic thyroid carcinoma:
Behavior, biology, and therapeutic approaches. Thyroid. 8:715–726.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Are C and Shaha AR: Anaplastic thyroid
carcinoma: Biology, pathogenesis, prognostic factors, and treatment
approaches. Ann Surg Oncol. 13:453–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jereb B, Stjernswärd J and Löwhagen T:
Anaplastic giant cell carcinoma of the thyroid. A study of
treatment and prognosis. Cancer. 35:1293–1295. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Junor EJ, Paul J and Reed NS: Anaplastic
thyroid carcinoma: 91 patients treated by surgery and radiotherapy.
Eur J Surg Oncol. 18:83–88. 1992.PubMed/NCBI
|
|
15
|
Tan RK, Finley RK III, Driscoll D,
Bakamjian V, Hicks WL Jr and Shedd DP: Anaplastic carcinoma of the
thyroid: A 24-year experience. Head Neck. 17:41–47; discussion
47–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tallroth E, Wallin G, Lundell G, Löwhagen
T and Einhorn J: Multimodality treatment in anaplastic giant cell
thyroid carcinoma. Cancer. 60:1428–1431. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moro CO and Basile G: Obesity and
medicinal plants. Fitoterapia. 71:(Suppl 1). S73–S82. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saha M, Rempt M, Grosser K, Pohnert G and
Weinberger F: Surface-associated fucoxanthin mediates settlement of
bacterial epiphytes on the rockweed Fucus vesiculosus.
Biofouling. 27:423–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwak KW, Cho KS, Hahn OJ, Lee KH, Lee BY,
Ko JJ and Chung KH: Biological effects of fucoidan isolated from
Fucus vesiculosus on thrombosis and vascular cells. Korean J
Hematol. 45:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parys S, Kehraus S, Krick A, Glombitza KW,
Carmeli S, Klimo K, Gerhäuser C and König GM: In vitro
chemopreventive potential of fucophlorethols from the brown alga
Fucus vesiculosus L. by anti-oxidant activity and inhibition
of selected cytochrome P450 enzymes. Phytochemistry. 71:221–229.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Řezanka T, Vyhnálek O and Podojil M:
Separation and identification of lipids and fatty acids of the
marine alga Fucus vesiculosus by TLC and GC-MS. Folia
Microbiologica. 33:309–313. 1988. View Article : Google Scholar
|
|
22
|
Morris CA, Nicolaus B, Sampson V, Harwood
JL and Kille P: Identification and characterization of a
recombinant metallothionein protein from a marine alga Fucus
vesiculosus. Biochem J. 338:553–560. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin F, Giuliano AE and Van Herle AJ:
Growth inhibitory effects of flavonoids in human thyroid cancer
cell lines. Thyroid. 9:369–376. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cragg GM, Newman DJ and Yang SS: Natural
product extracts of plant and marine origin having antileukemia
potential. The NCI experience. J Nat Prod. 69:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connor L, Huang DC, O'Reilly LA and
Strasser A: Apoptosis and cell division. Curr Opin Cell Biol.
12:257–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: Molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|