Introduction
Raynaud's phenomenon (RP) is characterized by
transient vasospasms within the fingers and/or toes, under cold or
emotional stress conditions (1).
RP is clinically classified into primary and secondary subtypes
(2,3), however the pathogenesis is not fully
understood. Whereas primary RP does not reflect any other
disorders, secondary RP is closely associated with life-threatening
morbidities, including autoimmune disease or scleroderma (1). The cold-induced molecular mechanisms
in vascular cells remain to be fully elucidated, however, previous
research has indicated that cold-mediated vasoconstriction is
regulated by ras homolog gene family member A (RhoA) (4–6). In
endothelial cells (ECs), cold-induced RhoA activation increases the
production of endothlein-1 (ET-1), a key vasoconstrictor in RP
(7–9). Paracrine release of ET-1 from ECs
activates RhoA in vascular smooth muscle cells (VSMCs) and
pericytes (9–11). This RhoA activation induces the
vasoconstriction of VSMCs (4,6).
Therefore, targeting RhoA may be an effective strategy for
treatment of RP.
Previous studies have investigated the biological
and chemical efficacy of herbal medicines for the treatment of RP
(4,12–16).
Angelica gigas (AG) is currently used for the management of
vascular diseases, including menopausal symptoms (17,18),
atherosclerosis (19) and brain
ischemia (20). The
anti-vasoconstrictive effect of AG has been demonstrated in
vitro and in vivo (21,22),
suggesting that AG may be useful in managing the vascular
dysfunction observed in RP. However, the effect of AG on
cold-induced VSMC responses has not been studied.
The present study evaluated the inhibitory effect of
AG on cold-induced vascular cell contraction. AG treatment
inhibited cold- and ET-1-mediated RhoA activation in both pericytes
and ECs. However, AG treatment had no effect on cold-induced ET-1
production in ECs. These results suggest that AG may be beneficial
for relieving cold-induced vasoconstriction in RP, via the
inhibition of RhoA.
Materials and methods
AG preparation and cell culture
AG was purchased from Hanpoong Pharm and Foods
Company (Jeonju, Korea). AG roots were ground and subsequently
extracted with 30% ethanol. The freeze-dried mixture was stored at
−80°C. Human umbilical vein endothelial cells (HUVECs) were gifted
by Kwang Seok Kim at Korea Institute of Radiological and Medical
Science (Seoul, Korea). Human brain microvascular pericytes were
purchased from ScienCell Research Laboratories, Inc. (1200;
Carlsbad, CA, USA). HUVECs were cultured in endothelial medium
supplemented with 5% fetal bovine serum (FBS) and 1% endothelial
cell growth supplement, microvascular pericytes were cultured in
pericyte medium supplemented with 5% FBS, 1% pericyte growth
supplement, and 1% penicillin/streptomycin solution. FBS, growth
supplements and media were purchased from ScienCell Research
Laboratories, Inc. (Carlsbad, CA, USA). Cells were cultured at
humidified incubator (5% CO2; 95% relative humidity) at
37°C.
Western blot analysis
To investigate ET-1-mediated RhoA activation,
pericytes were incubated with 100 nM/l ET-1 (Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany). To measure the inhibitory effect of AG
on cold-induced RhoA activation, HUVECs and pericytes were
pretreated with AG (100 or 200 µg/ml) for 30 min, followed by a
warm (37°C) or cold (25°C) incubation for 30 min. Cells were lysed
for protein extraction using ice-cold radioimmunoprecipitation
assay buffer containing 50 mM Tris-HCl, (pH 7.5), 150 mM NaCl, 1%
triton X-100, 2 mM EDTA, 0.1% SDS and 1% sodium deoxycholate
(R2002; Biosesang Co., Ltd., Seoul, Korea). The extracted proteins
were mixed with sample buffer (EBA-1052; Daejeon, Korea) and boiled
at 100°C for 10 min. Protein amount was analyzed by using Bio-Rad
protein assay kit (500–0006; Bio-Rad GmbH, Munchen, Germany). Equal
amount of proteins (10 µg) were separated by 8–12% SDS-PAGE and
transferred onto a nitrocellulose blotting membrane. The blots were
probed with the following primary antibodies: Mouse-anti-active
RhoA monoclonal (26904; 1:500; NewEast Biosciences, Malvern, PA,
USA), rabbit-anti-phospho-focal adhesion kinase (FAK) monoclonal
(8556; 1:500) and rabbit-anti-proto-oncogene tyrosine-protein
kinase Src (SRC) polyclonal (2101; 1:1,000; both from Cell
Signaling Technology, Inc., Danvers, MA, USA),
mouse-anti-phospho-extracellular signal-related kinase (ERK)
monoclonal (sc7383; 1:1,000) and mouse-anti-β-actin monoclonal
(sc73615; 1:1,000; both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). After blocking the membrane with 2% skim milk for
non-phosphoform of proteins or 2% Bovine serum albumin (BSA) in
TBST for phosphoform of proteins, primary antibodies were incubated
with the membrane overnight at 4°C on a shaker. Secondary
antibodies for mouse (7076; 1:1,000–3,000) and rabbit (074;
1:1,000–3,000) were purchased from Cell Signaling Technology
(Danvers, MA, USA) and incubated with the membrane for 1 h at room
temperature on shaker. Antibody-conjugated membranes were incubated
with ECL reagent (DG-WP250; DoGen, Seoul, Korea).
ELISA
ET-1 production in HUVEC-conditioned medium was
evaluated using an endothelin-1 ELISA kit, according to the
manufacturer's protocol (ADI-900-020A; Enzo Life Sciences, Inc.,
Farmingdale, NY, USA).
Phalloidin staining
The cold-induced formation of stress fibers and
focal adhesion complexes was assessed in HUVECs. Cells were treated
as aforementioned, and fixed with 4% paraformaldehyde (Junsei
Chemical Co., Ltd., Tokyo, Japan) for 30 min and permeabilized with
0.1% Triton X-100 (T8787; Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) for 15 min. Cells were stained with rhodamine-phalloidin
(R415; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells
were visualized with Olympus FV10i self-contained confocal laser
system (Olympus America Inc., PA, USA).
Statistical analysis
The differences of means between the groups were
analyzed one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
AG reduces cold- and ET-1-induced RhoA
activation in pericytes
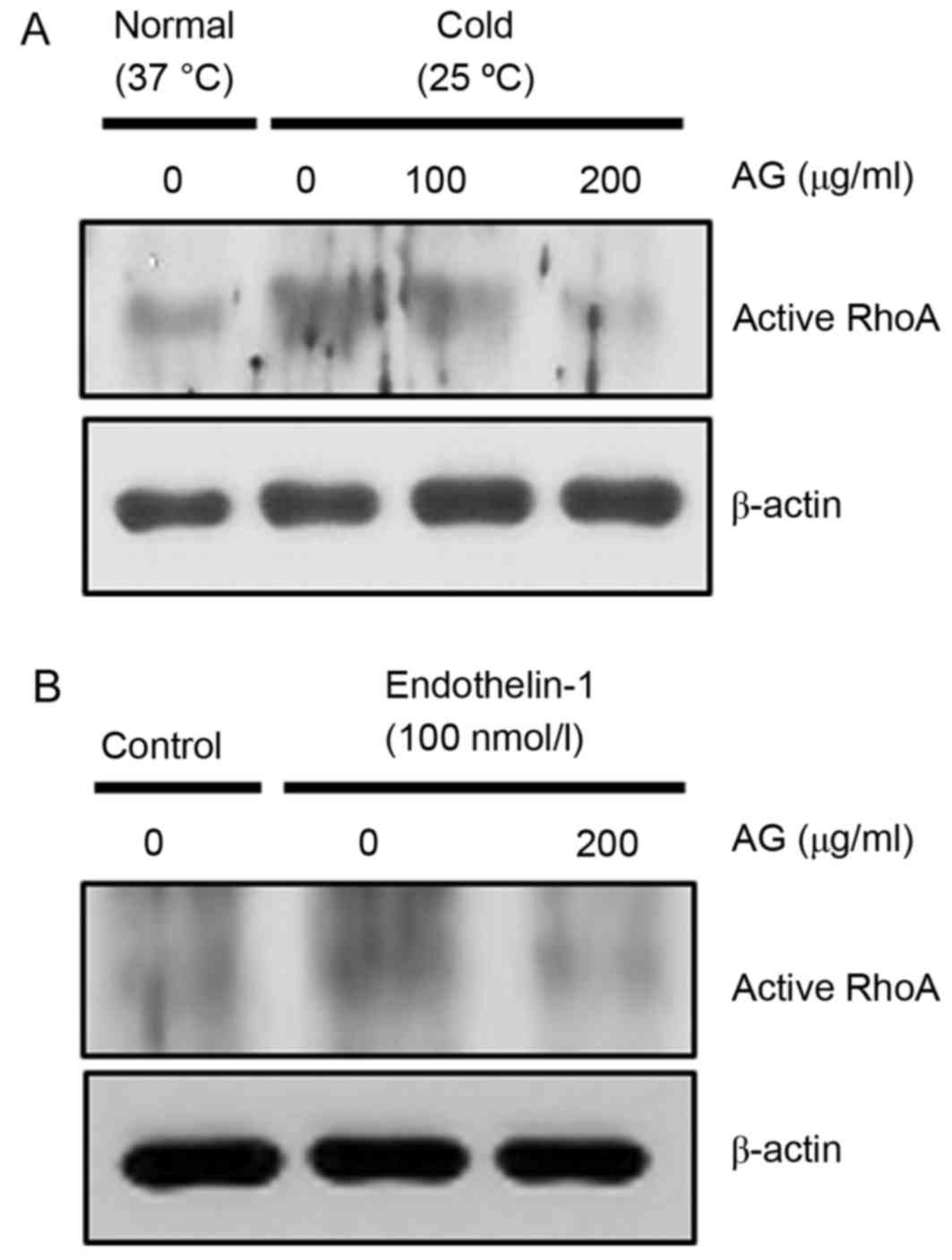
Pericytes contribute to microvascular contraction;
therefore, the inhibitory effect of AG on the activity of RhoA in
cold- or ET-1-exposed pericytes was examined by western blotting
with active RhoA-GTP monoclonal antibody. AG treatment inhibited
cold-induced RhoA activation (Fig.
1A), and ET-1-induced RhoA activation (Fig. 1B).
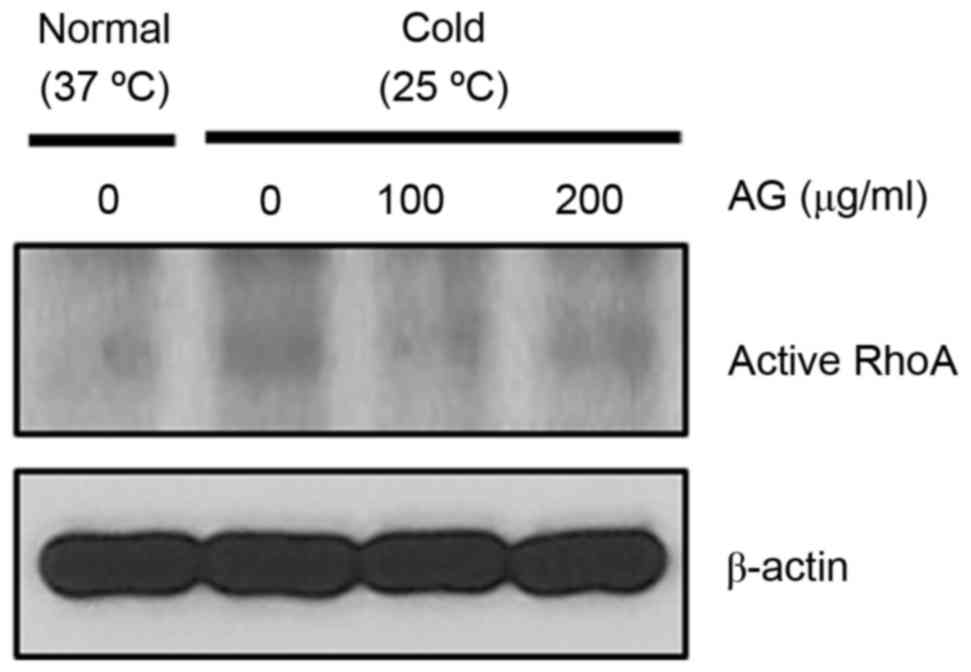
AG reduces cold-induced RhoA
activation in HUVECs
The impact of AG treatment on cold-induced RhoA
activation was investigated in HUVECs. AG treatment decreased
cold-induced RhoA activation (Fig.
2). Therefore, AG treatment demonstrated an ability to inhibit
cold-induced RhoA activation in both pericytes and HUVECs.
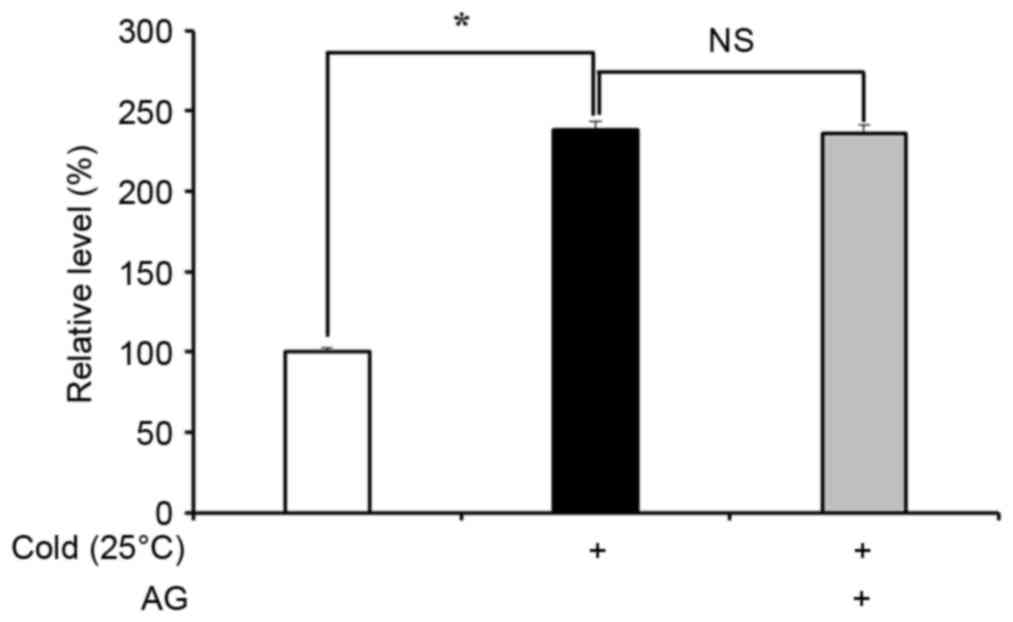
AG reduces cold-induced ET-1
production in HUVECs
ET-1 is produced by ECs and serves a key role in
vasoconstriction under cold conditions (7–9).
Therefore, the inhibitory effect of AG treatment on ET-1 production
in cold-exposed ECs was examined. HUVECs exposed to cold for 30 min
increased ET-1 production by approximately 2-fold compared with the
cells under normal conditions (P<0.05), however, AG pretreatment
did not impact on cold-induced ET-1 production (Fig. 3).
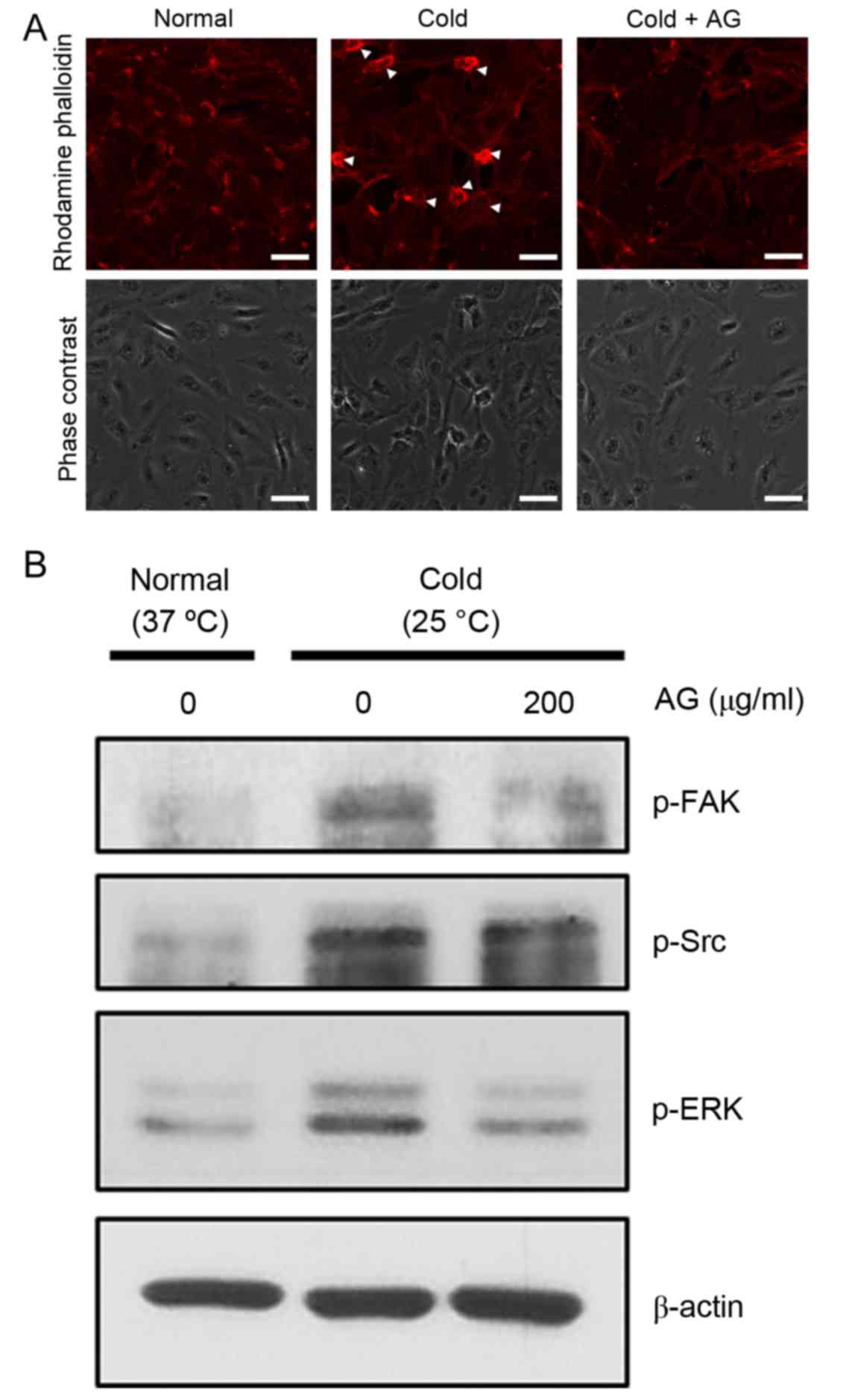
AG reduces the cold-mediated formation
of stress fibers and focal adhesion complexes in HUVECs
Cold-mediated RhoA activation induces the
phosphorylation of FAK, which stimulates the formation of stress
fibers and focal adhesion complexes (4). Cold exposure induced the formation of
stress fibers and focal adhesion complexes, whereas AG treatment
limited these cold-mediated responses (Fig. 4A). Furthermore, FAK phosphorylation
stimulates the phosphorylation of SRC and ERK, and western blot
analysis demonstrated that the cold-induced phosphorylation of FAK,
SRC and ERK was inhibited by AG treatment (Fig. 4B). Expression of unphosphorylated
FAK, SRC and ERK was not changed (data not shown).
Discussion
AG has been used in the treatment vascular
disorders, however its molecular mechanism remains unclear
(18–21). The activation of RhoA may serve as
an indicator for cold responses in vascular cells, however the
cold-mediated mechanisms of RP remain to be elucidated (4–6). Our
previous study demonstrated that cold-mediated contraction is
tightly regulated by RhoA activity (4). In the present study, cold- and
ET-1-induced RhoA activation in vascular cells was reduced by AG
treatment. Furthermore, the data suggested that AG treatment may
inhibit vascular cellular contraction via RhoA suppression,
in HUVECs and pericytes. Although AG treatment did not affect
cold-mediated ET-1 production in HUVECs, ET-1-mediated RhoA
activation in pericytes was reduced, indicating that AG may
demonstrate efficacy these cells. In addition, it may be
hypothesized that cold-induced ET-1 production is likely to be
regulated by a mechanism other than the RhoA-mediated pathway,
suggesting that studies should be performed to elucidate the
precise mechanism of cold-induced ET-1 production. However, further
in vitro and in vivo experiments are necessary to
elucidate which active compounds in AG are responsible for the
observed RhoA-inhibitory effects. In addition, the results
indicated that cold exposure may induce the formation of stress
fibers and focal adhesion complexes, and these responses were
reduced by AG treatment. Previous research has demonstrated that
cell contraction and the formation of focal adhesions depend on
RhoA signaling pathways (23,24).
The present study indicated that AG treatment was able to inhibit
cold-mediated RhoA activation, thus resulting in a blockade of
RhoA-mediated cell contraction and formation of focal adhesion
complexes. Therefore, AG inhibition of RhoA activation may suppress
vascular cellular contraction in cold conditions.
In conclusion, the present study demonstrated the
inhibitory effect of AG on cold-induced contractile responses in
vascular cells. Targeting RhoA by AG may be a useful therapeutic
strategy for the treatment of vascular diseases, including RP.
Further in vitro and in vivo studies are required,
and these should investigate the vasodilatory impact of AG, and
elucidate the active compound responsible for these effects.
Acknowledgements
Human umbilical vein endothelial cells (HUVECs) were
gifted by Kwang Seok Kim at Korea Institute of Radiological and
Medical Science (Seoul, Korea). This study was supported by the
Korean Medicine R&D Project of the Ministry of Health and
Welfare (grant no. HI13C0530).
References
|
1
|
Cooke JP and Marshall JM: Mechanisms of
Raynaud's disease. Vasc Med. 10:293–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herrick AL: Pathogenesis of Raynaud's
phenomenon. Rheumatology (Oxford). 44:587–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeRoy EC and Medsger TA Jr: Raynaud's
phenomenon: A proposal for classification. Clin Exp Rheumatol.
10:485–488. 1992.PubMed/NCBI
|
|
4
|
Cho SG, Go HY, Park JS, Jung KY, Sun SH,
Choi YK, Song YK, Park JH, Jun CY and Ko SG: Herbal prescription,
DSGOST, prevents cold-induced RhoA activation and endothelin-1
production in endothelial Cells. Evid Based Complement Alternat
Med. 2014:5493072014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson-Torgerson CS, Holowatz LA,
Flavahan NA and Kenney WL: Cold-induced cutaneous vasoconstriction
is mediated by rho kinase in vivo in human skin. Am J Physiol Heart
Circ Physiol. 292:H1700–H1705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bailey SR, Eid AH, Mitra S, Flavahan S and
Flavahan NA: Rho kinase mediates cold-induced constriction of
cutaneous arteries: Role of alpha2C-adrenoceptor translocation.
Circ Res. 94:1367–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rychlik-Golema W, Mastej K and Adamiec R:
The role of endothelin-1 and selected cytokines in the pathogenesis
of Raynaud's phenomenon associated with systemic connective tissue
diseases. Int Angiol. 25:221–227. 2006.PubMed/NCBI
|
|
8
|
Zamora MR, O'Brien RF, Rutherford RB and
Weil JV: Serum endothelin-1 concentrations and cold provocation in
primary Raynaud's phenomenon. Lancet. 336:1144–1147. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barman SA: Vasoconstrictor effect of
endothelin-1 on hypertensive pulmonary arterial smooth muscle
involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell
Mol Physiol. 293:L472–L479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakurada S, Okamoto H, Takuwa N, Sugimoto
N and Takuwa Y: Rho activation in excitatory agonist-stimulated
vascular smooth muscle. Am J Physiol Cell Physiol. 281:C571–C578.
2001.PubMed/NCBI
|
|
11
|
Dehouck MP, Vigne P, Torpier G,
Breittmayer JP, Cecchelli R and Frelin C: Endothelin-1 as a
mediator of endothelial cell-pericyte interactions in bovine brain
capillaries. J Cereb Blood Flow Metab. 17:464–469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YJ, Luo SF, Yang SH, Chen JY, Yu KH and
See LC: Vascular response of Raynaud's phenomenon to nifedipine or
herbal medication (duhuo-tisheng tang with danggui-sini tang): A
preliminary study. Chang Gung Med J. 31:492–502. 2008.PubMed/NCBI
|
|
13
|
Malenfant D, Catton M and Pope JE: The
efficacy of complementary and alternative medicine in the treatment
of Raynaud's phenomenon: A literature review and meta-analysis.
Rheumatology (Oxford). 48:791–795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ninomiya F: Clinical evaluation of
perspiration reducing effects of a kampo formula, shigyaku-san, on
palmoplantar hidrosis. Evid Based Complement Alternat Med.
5:199–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanai S, Okano H and Abe H: Efficacy of
toki-shigyakuka-gosyuyu-syokyoto
(danggui-sini-jia-wuzhuyu-shengjiang-tang) on peripheral
circulation in autonomic disorders. Am J Chinese Med. 25:69–78.
1997. View Article : Google Scholar
|
|
16
|
Park KS, Park KI, Kim JW, Yun YJ, Kim SH,
Lee CH, Park JW and Lee JM: Efficacy and safety of Korean red
ginseng for cold hypersensitivity in the hands and feet: A
randomized, double-blind, placebo-controlled trial. J
Ethnopharmacol. 158:Pt A. 25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi KO, Lee I, Paik SY, Kim DE, Lim JD,
Kang WS and Ko S: Ultrafine Angelica gigas powder normalizes
ovarian hormone levels and has antiosteoporosis properties in
ovariectomized rats: Particle size effect. J Med Food. 15:863–872.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KM, Kim MJ and Kang JS: Absorption,
distribution, metabolism, and excretion of decursin and decursinol
angelate from Angelica gigas Nakai. J Microbiol Biotechnol.
19:1569–1572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang JY, Kim J, Cai J, Kim Y, Shin K, Kim
TS, Lee SP, Park SK, Choi EK and Kim YB: An ethanolic extract of
Angelica gigas improves atherosclerosis by inhibiting
vascular smooth muscle cell proliferation. Lab Anim Res. 30:84–89.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh TW, Park KH, Jung HW and Park YK:
Neuroprotective effect of the hairy root extract of Angelica
gigas NAKAI on transient focal cerebral ischemia in rats
through the regulation of angiogenesis. BMC Complement Altern Med.
15:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhyu MR, Kim EY, Yoon BK, Lee YJ and Chen
SN: Aqueous extract of Schizandra chinensis fruit causes
endothelium-dependent and -independent relaxation of isolated rat
thoracic aorta. Phytomedicine. 13:651–657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhyu MR, Kim JH and Kim EY: Radix Angelica
elicits both nitric oxide-dependent and calcium influx-mediated
relaxation in rat aorta. J Cardiovasc Pharmacol. 46:99–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao L, Romero MJ, Toque HA, Yang G,
Caldwell RB and Caldwell RW: The role of RhoA/Rho kinase pathway in
endothelial dysfunction. J Cardiovasc Dis Res. 1:165–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Nieuw Amerongen GP, Koolwijk P,
Versteilen A and van Hinsbergh VW: Involvement of RhoA/Rho kinase
signaling in VEGF-induced endothelial cell migration and
angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 23:211–217.
2003. View Article : Google Scholar : PubMed/NCBI
|