Introduction
Traumatic brain injury (TBI) is a primary health
concern and leading cause of mortality worldwide. Neurological
deficits resulting from TBI present a severe burden to patient
families and society. There is no current effective treatment
method to promote functional recovery except for routine
rehabilitation, hyperbaric oxygen and basic care. It has previously
been demonstrated that neural stem cells (NSCs) may promote
neurological recovery following brain injury (1). Stem cell therapy is a cellular
approach that has the potential to induce a variety of beneficial
neurorestorative processes that may facilitate the recovery of
neurological function (2). Over
the last decade, bone marrow stromal cells (BMSCs) have been used
as therapeutic vectors or tools for the treatment of a variety of
diseases. Previous studies have demonstrated that the
transplantation of BMSCs in animal models of injury exhibits
protective effects following acute spinal cord (3), lung (4) and liver (5) injuries. BMSCs have been demonstrated
improve the neurological functional outcome of central nervous
system (CNS) disorders, including stroke and TBI (6,7).
Furthermore, BMSCs are capable of proliferating and differentiating
into neurons, astrocytes or oligodendrocytes in vitro
(8,9).
Previous studies have revealed a significant loss of
synapses in the days following brain injury, notably in the brain
regions connected to the site of initial injury, including the
hippocampus (10). Post-traumatic
neuronal plasticity involves aspects of neurogenesis, including
axonal sprouting, synaptic formation and remodeling. Previous
studies have reported that BMSCs increase synapse protein
expression in the ischemic brain and reconstructed neuronal
networks (11,12). A further study reported that statin
therapy had a synergetic effect with BMSCs, and mobilized engrafted
BMSCs to the lesion area, promoting repair following stroke
(13). The transplantation of
BMSCs is therefore considered a promising strategy for TBI
treatment and may promote morphological and functional recovery
post-TBI. However, the neuroprotective effect and underlying
mechanisms of BMSCs following TBI require further examination and
remain to be fully elucidated.
The present study hypothesized that the effect of
BMSCs on motor function may be associated with the expression of
synaptophysin (SYN). Therefore, a rat model of TBI was constructed
to investigate if BMSCs migrate to injured areas and promote
functional recovery via upregulation of neurotrophic factors and
synaptic proteins.
Materials and methods
Cell culture
A total of 15 Sprague-Dawley (SD) female rats (age,
1 month; weight, 20–24 g), were obtained from the Hebei Medical
University Experimental Animal Center (Shi Jiazhuang, China) and
were housed in a temperature-controlled (22–24°C) room with a 12-h
light/dark cycle and with water and food freely available. Rats
were anaesthetized with 10% chloral hydrate (3 ml/kg; Bio-Rad
Biotechnology, Inc., Shanghai, China) and BMSCs were isolated and
cultured. Briefly, fresh bone marrow cells were collected from the
femurs of SD rats, via suction from the medullary cavity using a
20-ml sterile syringe. All rats were sacrificed following cell
harvesting. For anticoagulation, 5 ml heparin (100 IU/ml) was used.
Following filtration, cells were centrifuged at 1,000 × g for 5 min
at 4°C. The purified cells were cultured in a 25 cm2
flask with Dulbecco's modified Eagle's medium/nutrient mixture F12
(DMEM/F12; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare,
Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), and incubated
at 37°C in 5% CO2. After 48 h, non-adherent cells were
removed and fresh media was added. When adherent cells reached ~80%
confluency, the cells were dissociated with 0.25% trypsin solution
and re-seeded again. Following three passages of culture, passage 3
BMSCs were used for subsequent experiments.
Animals and TBI model
Adult male SD rats (weight, 250–300 g; age, 12–16
weeks), were obtained from Hebei Medical University Experimental
Animal Center and were housed in a temperature-controlled (22–24°C)
room with a 12-h light/dark cycle and with water and food freely
available. A total of 105 rats were utilized in this study. All
experimental procedures were performed in accordance with the
guidelines of the Chinese council on animal protection, and were
approved by the Hebei Medical University Committee (Shijiazhuang,
China) for the use of animals in research (Permit Number: 2015046).
The TBI model was developed as described using a weight-drop device
(14). Briefly, the rats were
anaesthetized with 10% chloral hydrate (3 ml/kg) and fixed onto the
stereotactic device. Aseptic techniques were used throughout
surgery. A midline incision was made to expose the skull and right
parietal craniotomy (5-mm in diameter, 1.5 mm posterior, and 2.5 mm
lateral to the bregma) using a high-speed microdrill. The dura was
exposed and kept intact. A 40-g steel weight fell freely through a
vertical tube from 2.5 m onto the motor cortex to induce TBI.
Following injury, the bone flap was placed in situ and the
scalp was sutured. Rats were placed on heat pads (37°C) for 4 h to
maintain normal body temperature during the recovery period.
Sham-operated animals underwent procedures identical to those of
the TBI animals, including anesthesia and surgery; however, did not
receive TBI.
Groups and drug administration
The 105 adult rats were randomly divided into 3
groups (n=35/group): Sham, TBI and TBI + BMSC-treated. Prior to
transplantation, BMSCs were digested with trypsin, washed twice
with DMEM and centrifuged at 1,000 × g for 5 min at 4°C. In
BMSC-transplanted animals, the BMSCs (3×106 cells/ml) in
1 ml of phosphate-buffered saline (PBS) were transplanted via a
tail vein puncture into rats 30 min following the induction of TBI.
The sham and TBI groups received equal volumes of saline injection.
Each subgroup was composed of five rats, and rats were anesthetized
with 10% chloral hydrate and decapitated 1, 3, 5, 7 and 14 days
following TBI. The remainder of the rats (n=10 per treatment group)
underwent neurobehavioral examinations. All investigations were
blind and the groups were revealed at the end of the behavioral and
histological analyses.
Rotarod task
A rotarod was used to assess motor function as
previously described (15).
Briefly, a 7-cm diameter cylinder was positioned 1.2 m above a foam
pad while speed and acceleration were controlled by computer
interface (San Diego Instruments, San Diego, CA, USA). For each
trial, the rat was placed on the rotating barrel, the speed was
accelerated from 4 to 40 rpm over a period of 5 min, and the
latency to fall was recorded. Pre-training occurred once a day for
the 3 days preceding injury. Following injury, rats were re-tested
on the rotarod task on days 1, 3, 5, 7 and 14 post-TBI. Each rat
underwent four test trials per day. The average latency of the
total of the four trials was calculated.
Modified neurological severity score
(mNSS)
Neurological deficits were evaluated using the mNSS
on an 18-point scale by a researcher blinded to treatment, which
tested reflexes, alertness, coordination and motor abilities. One
point was awarded for failure to perform a particular task; thus,
the higher the score, the more severe the injury, whereas a healthy
rat scored zero. Post-injury, mNSS was evaluated at days 1–14
post-TBI.
Histological analysis
Brain tissues were fixed in 4% paraformaldehyde
solution for 24 h, washed with running water for 4 h, and embedded
in paraffin and dehydrated with gradient alcohol and xylene. The
samples were serially sectioned at a thickness of 5 µm. All
sections were mounted on glass slides and subsequently stained with
hematoxylin and eosin (H&E). Sections were observed and
analyzed using an optical microscope.
Western blot analysis
Rats were decapitated under deep anesthesia and the
brains were rapidly isolated. The hippocampal tissues were
dissected on ice, the proteins were extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) from the cortex surrounding the
injured area and the protein concentration was determined using a
bicinchoninic acid kit (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China). Samples (50 µg) were separated by 10%
SDS-PAGE and subsequently transferred onto polyvinylidene membranes
(Roche Diagnostics GmbH, Mannheim, Germany). The blots were blocked
with 5% fat-free dry milk for 2 h at room temperature, followed by
incubation with the following rabbit primary antibodies:
Anti-vascular endothelial growth factor (VEGF) polyclonal (1:1,000;
Abcam, ab53465, Cambridge, UK), anti-brain derived neurotrophic
factor (BDNF) monoclonal (1:1,000; Abcam, ab216443) and
anti-β-actin monoclonal (1:1,000; Affinity Biologicals Inc.,
AF7018, Ancaster, ON, Canada) at 4°C overnight. Following this, the
membranes were incubated with horse-radish peroxidase
(HRP)-conjugated donkey anti-mouse immunoglobulin (Ig)-G or donkey
anti-rabbit IgG (sc-2314 and sc-2313; 1:5,000; both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) secondary antibodies at 37°C
for 1 h. Signals were detected by Enhanced Chemiluminescence using
a Western Lightning® Plus-ECL kit (Perkin-Elmer, Inc.,
Waltham, MA, USA). (Densitometric analysis for the blots was
performed using National Institutes of Health Image software
version 1.41 (Bethesda, MD, USA).
Immunohistochemical and
immunofluorescence analyses
Rats were perfused transcardially with saline under
deep anesthesia, followed by 4% paraformaldehyde for 24 h, and
placed into 30% sucrose solution (0.1 M PBS, pH 7.4) until they
sank to the bottom. The brain tissues were embedded in optimum
cutting temperature compound and cut into 15 µm-thick sections
coronally from the anterior to posterior hippocampus (bregma −2.0
to −3.0 mm) using a cryostat. Frozen sections were sliced with a
microtome, treated with 0.4% Triton X-100 for 20 min and blocked at
room temperature in normal donkey serum (017–000-121; Shanghai
Solarbio Bioscience & Technology Co., Ltd., Shanghai, China)
for 2 h. For immunohistochemical analyses, sections were incubated
overnight at 4°C with rabbit anti-VEGF (1:100) and rabbit anti-BDNF
polyclonal antibodies (1:100), and subsequently with HRP-conjugated
anti-rabbit IgG antibodies at 37°C in the dark for 30 min.
3,3′-Diaminobenzidine was used to reveal the immunohistochemical
reaction. For double labeling, the frozen sections were incubated
with rabbit anti-sex determining region Y (SRY) polyclonal (1:100;
Abcam, ab209858), mouse anti-neuronal nuclear antigen (NeuN)
monoclonal (1:100; EMD Millipore, Billerica, MA, USA, MAB324-K) or
anti-glial fibrillary acidic protein (GFAP) monoclonal (1:100; EMD
Millipore, IF03L) antibodies overnight at 4°C. The following day,
the sections were incubated with fluorescein
isothiocyanate-conjugated anti-rabbit IgG or anti-mouse IgG
secondary antibodies (sc-2090 and sc-2099; 1:1,000; Santa Cruz
Biotechnology, Inc.) for 2 h at 37°C in the dark. All cell nuclei
were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). PBS
was substituted for the primary antibody as the negative control.
Sections were imaged under a laser scanning confocal microscope
(Olympus Fluoview™ FV1000; Olympus Corporation, Tokyo, Japan).
Statistical analysis
All experiments were repeated three times and
similar results were obtained. Statistical analysis was performed
using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation and the
significance of the experimental results was determined using
one-way analysis of variance followed by the Student-Newman-Keuls
post hoc multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
BMSC treatment improves motor
deficits
To determine the neuroprotective effects of BMSCs
against TBI-induced brain damage, the present study examined the
effects of BMSCs on motor deficits via a rotarod task and mNSS
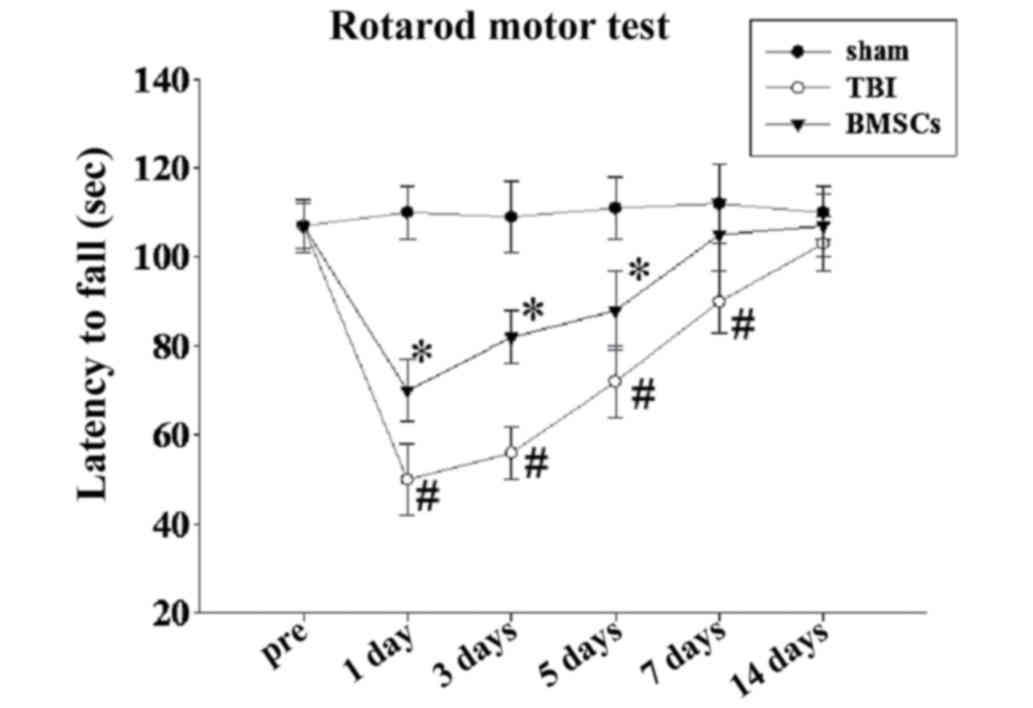
score following TBI at 1, 3, 5, 7 and 14 days. As presented in
Fig. 1, TBI resulted in a
significant motor deficit at 1, 3, 5 and 7 days compared with the
sham group, and BMSC treatment significantly improved motor
function recovery and latency(s) at 1, 3 and 5 days compared with
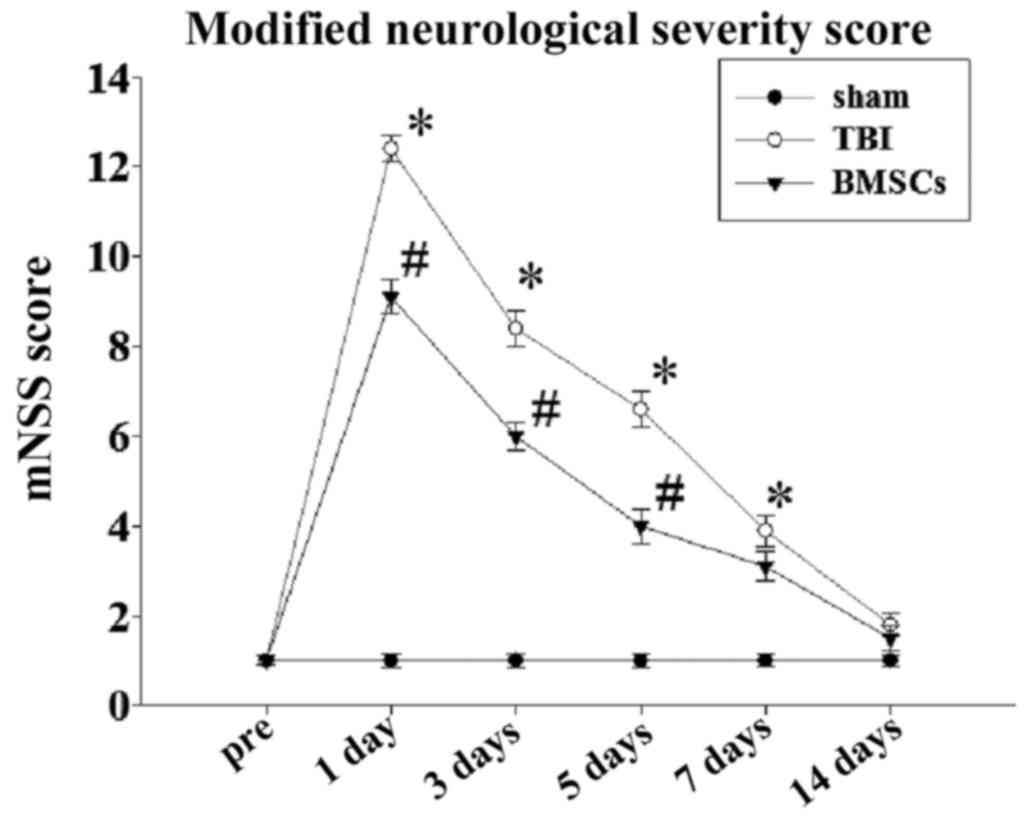
the TBI group. In addition, it was demonstrated that the mNSS of
the rats in the TBI group was significantly increased in comparison
with the sham group at 1–7 days, and BMSC treatment significantly
reduced the mNSS score compared with the TBI group at 1–5 days
(Fig. 2), and additionally
improved neuromotor function.
BMSC treatment reduces cortex neuronal
death
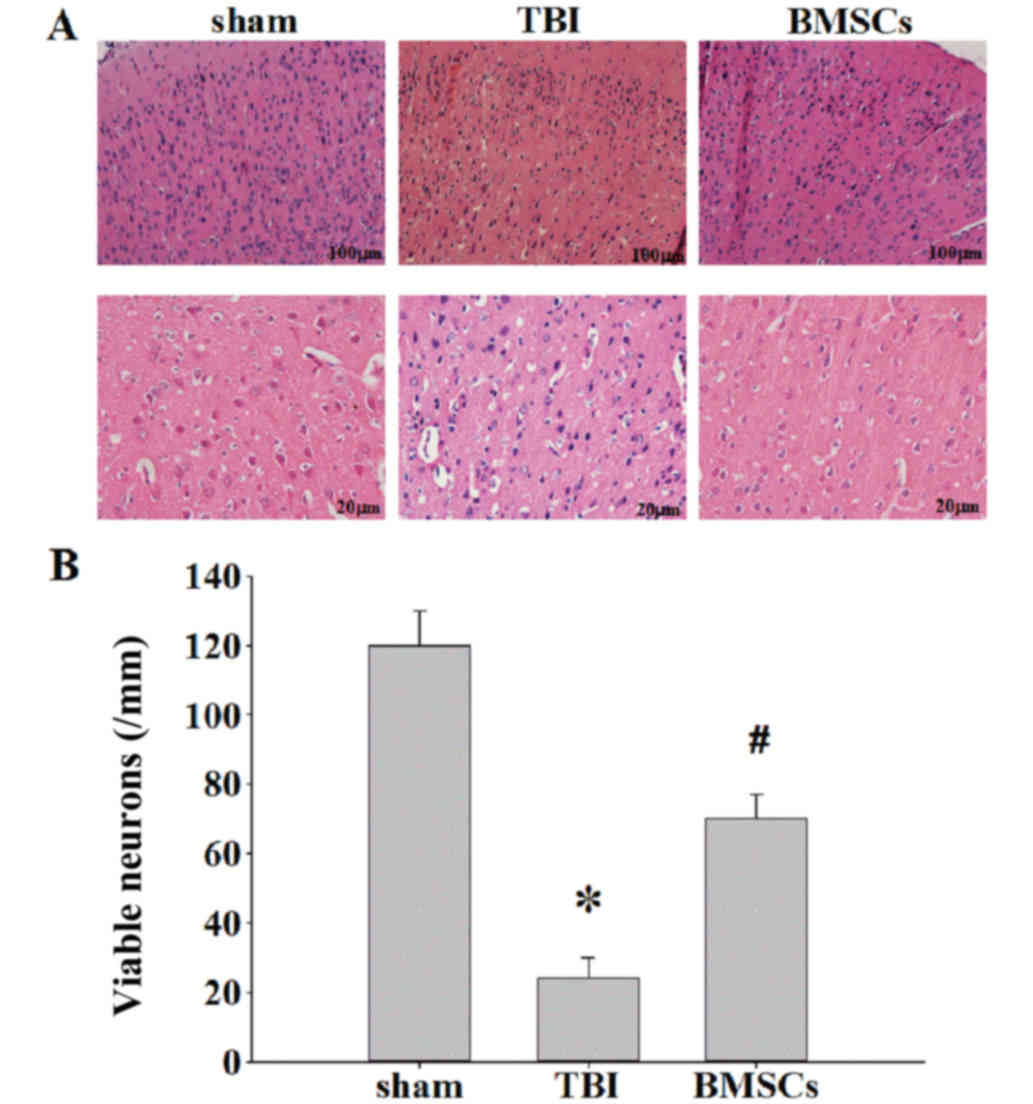
H&E staining was performed to examine the effect
of BMSCs on ipsilateral cerebral cortex neuronal damage 7 days
following TBI. In the TBI rats, there were marked morphological
alterations in the cortex compared with the sham group. Neuronal
cell body swelling and disorder was observed, in addition to
intercellular broadening, cell loss and nuclear pyknosis and
karyolysis in the TBI group (Fig.
3A). Treatment with BMSCs significantly moderated morphological
alterations and reduced neuronal loss induced by injury (Fig. 3B, *P<0.01 vs. sham group;
#P<0.05 vs. TBI group).
BMSC treatment increases expression of
VEGF and BDNF
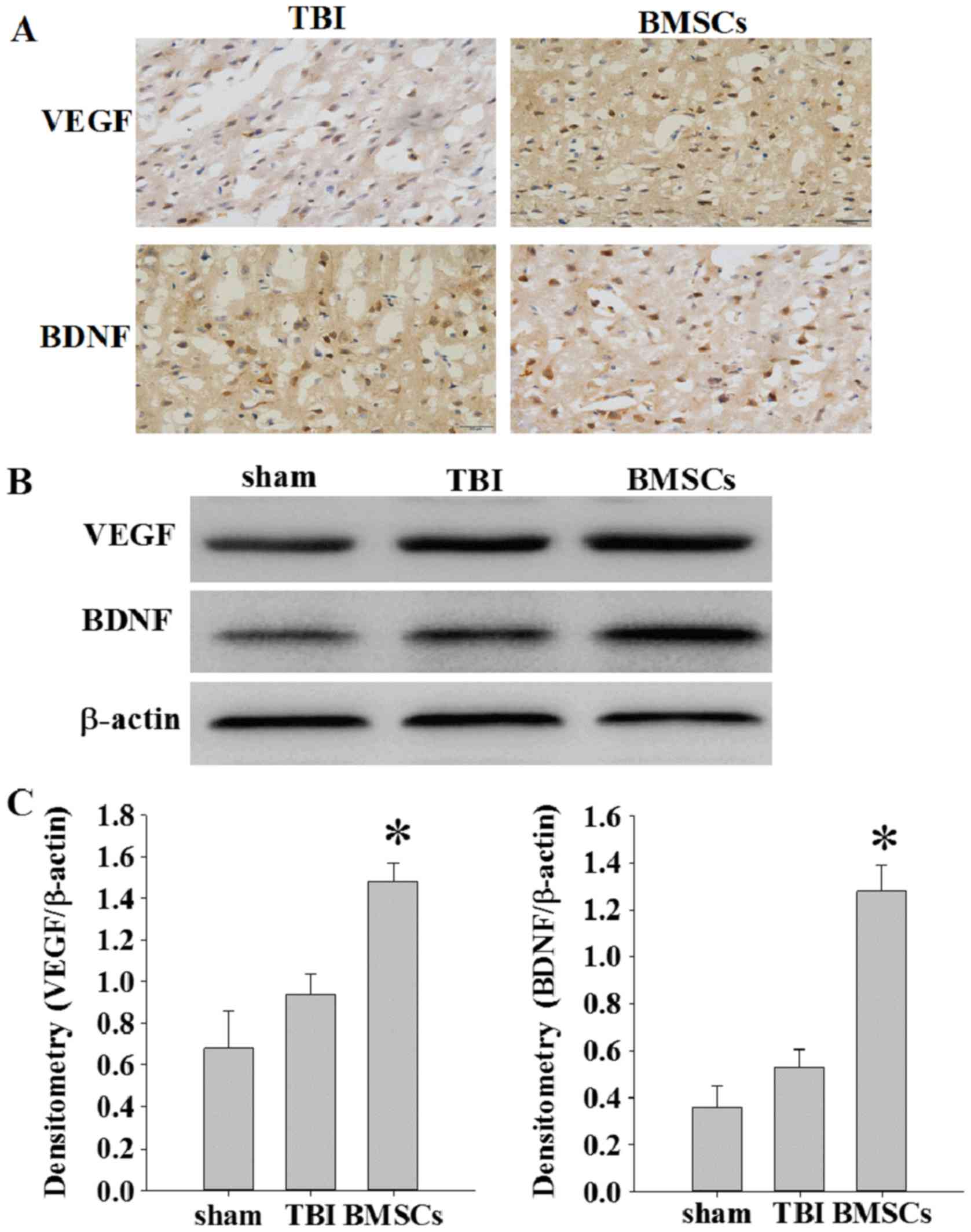
The expression of VEGF and BDNF was detected at 14
days following TBI via immunochemistry and western blot assay. As
presented in Fig. 4A, weak
immunopositive staining was observed in the sham group; however,
strong immunohistochemical staining for VEGF or BDNF was observed
in the cytoplasm of cells around the injury site in the BMSC group.
The protein expression levels of VEGF and BDNF were significantly
increased in the BMSC group compared with the sham and TBI groups
(Fig. 4B and C; *P<0.05).
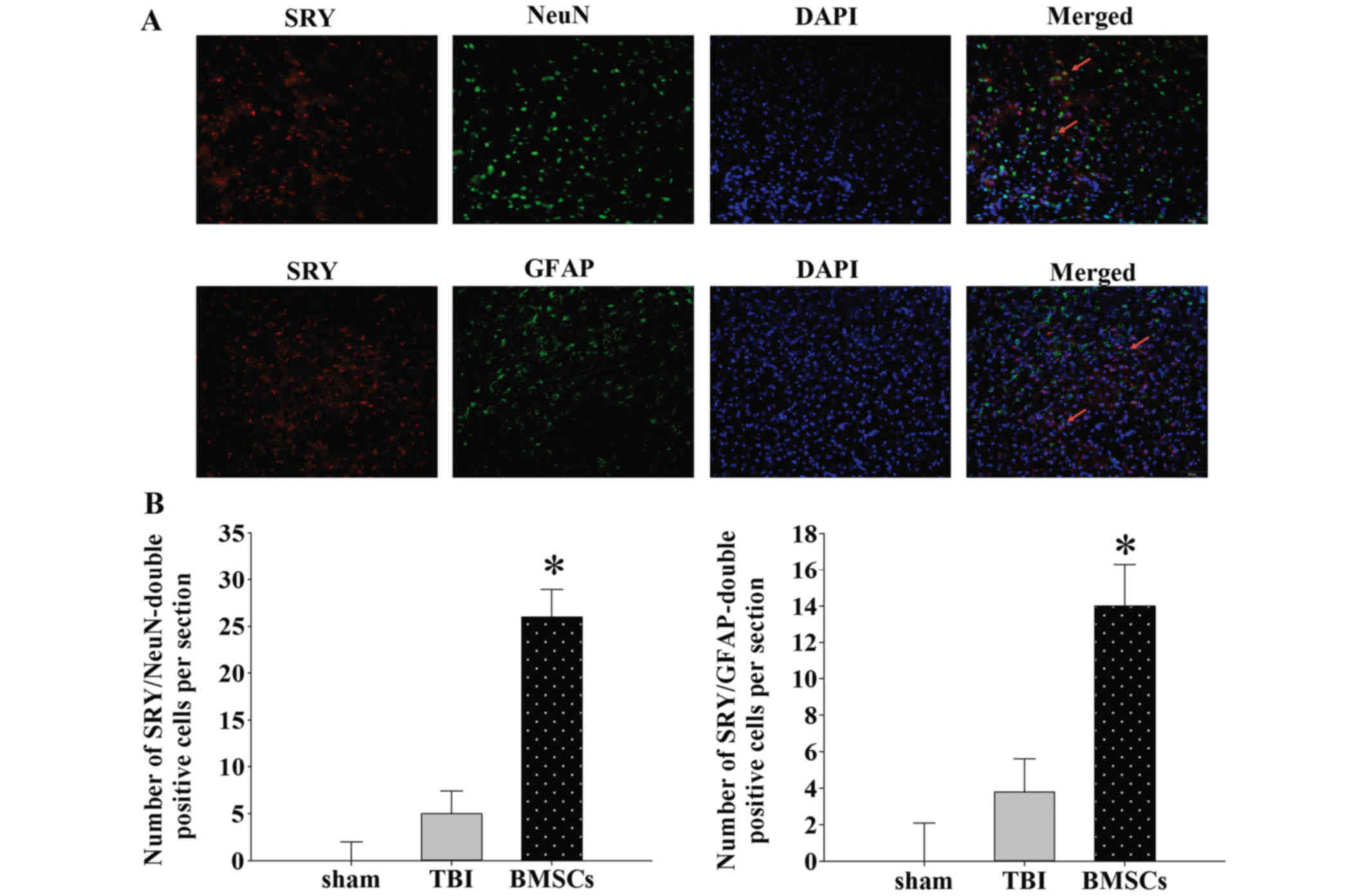
BMSCs may migrate to injured areas and
differentiate into neurons or astrocytes
BMSCs were tracked to evaluate their migration and
distribution patterns in rats. BMSCs isolated from female rats were
injected into male rats in vivo. The expression of SRY was
detected via laser scanning confocal microscopy to trace
transplanted BMSCs in the injured site. Double immunofluorescence
staining was performed to investigate the co-localization of SRY
and NeuN or GFAP expression. As presented in Fig. 5, SRY was stained with rabbit
anti-SRY antibody and secondary antibodies labeled with red
fluorescence. In addition, neurons or astrocytes were stained with
mouse anti-NeuN antibody or mouse anti-GFAP antibody and secondary
antibody labeled with green fluorescence. Sections were stained
with DAPI (blue) to reveal all nuclei. The images were merged, and
various SRY-positive cells in the lesion epicenter were observed to
be positive for NeuN or GFAP at 14 days following TBI (Fig. 5A). The number of cells
double-positive for SRY/NeuN or SRY/GFAP was greater in the BMSC
group compared with the TBI group (Fig. 5B; *P<0.01). These results
suggested that BMSCs migrate to injured areas and differentiate
into neurons and astrocytes following induction of TBI in the
rat.
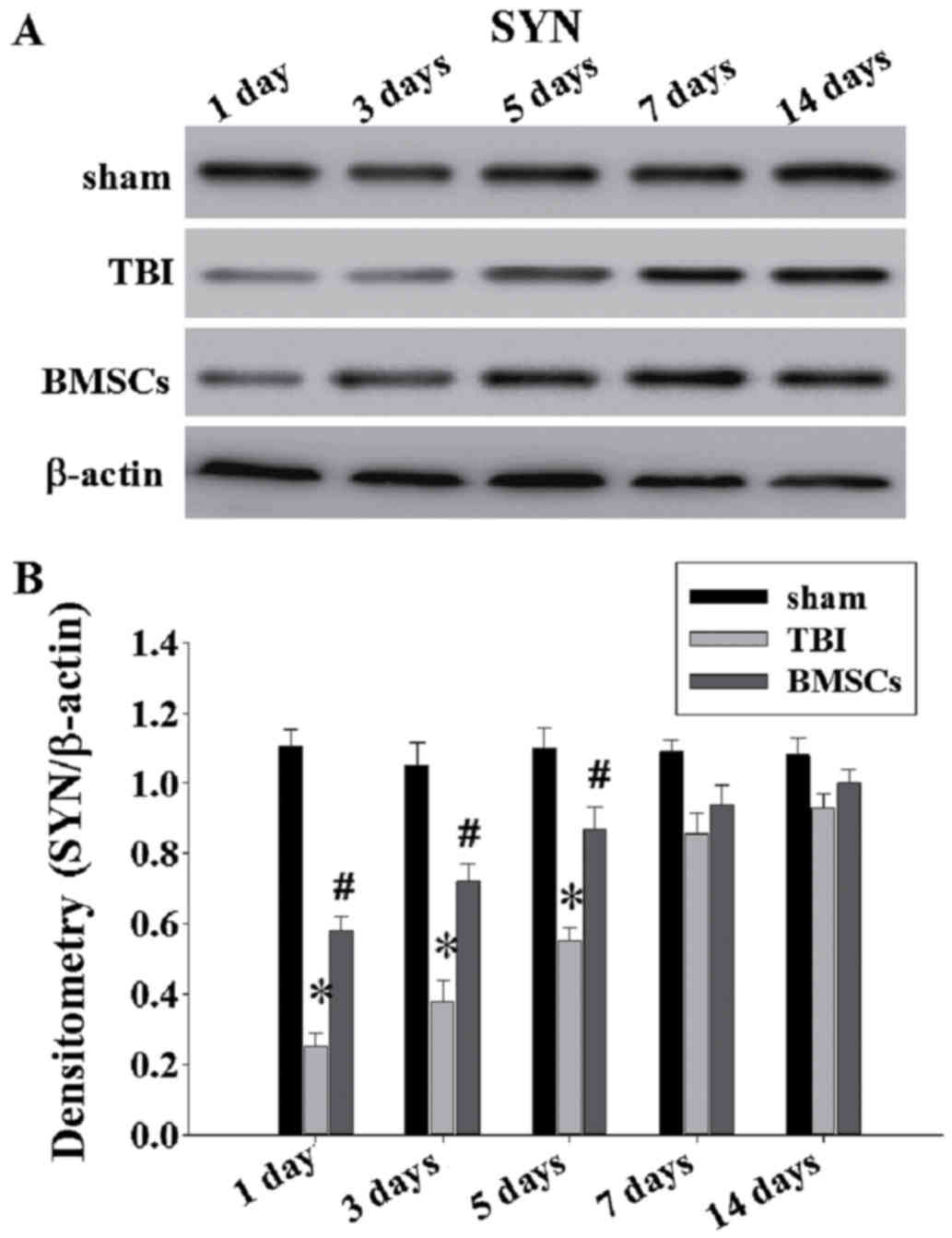
BMSC treatment attenuates synapse
protein loss
To further investigate the BMSC underlying
mechanisms of action, western blotting was performed to examine the
expression of SYN at 1, 3, 5, 7 and 14 days post-TBI (Fig. 6A). As presented in Fig. 6 and B, there was a significant
downregulation of SYN expression in the TBI group compared with the
sham group at 1, 3 and 5 days (*P<0.05). Reduced levels of SYN
in TBI animals indicates loss of synapses. Treatment with BMSCs
resulted in significantly greater levels of SYN at 1–5 days
compared with the TBI group (Fig.
6; #P<0.05).
Discussion
The results from the present study are similar to
the findings of previous investigations, indicating that in animal
models of TBI or stroke, BMSCs may effectively reduce brain damage
and improve functional recovery (6,13,16).
The present study verified and expanded previous results by
demonstrating that BMSCs migrate to injured brain tissue, reduce
motor deficits and neuronal injuries, increase the expression of
VEGF and BDNF and induce a greater expression of synaptophysin
following TBI. These results thus demonstrated that BMSCs exhibit
potential as an effective treatment to promote recovery following
TBI.
TBI is a highly complex disorder, resulting from
injury to primary and secondary brain signaling pathways.
Currently, there is no effective treatment for brain injury to
promote functional recovery, except for routine rehabilitation and
basic care. Notably, BMSCs have previously been demonstrated to
improve neurological functional recovery in experimental TBI
models. There are various explanations regarding the broad
underlying mechanisms by which BMSCs exert their beneficial
effects. Previous findings indicated that injected BMSCs cross the
blood brain barrier and actively migrate to sites of tissue damage
(17,18). Additionally, BMSCs expressing C-X-C
chemokine receptor type 4 have an active tropism toward zones of
tissue damage where the expression of stromal cell derived factor
(SDF)-1 is increased (19). The
tracking of BMSCs is essential for evaluation of their migration
and distribution patterns in rats following TBI. The present study
harvested BMSCs from female rats, cultured them and injected them
into male rats in vivo. Following this, the expression of
SRY was detected via immunofluorescence microscopy to track BMSC
survival and further co-localization with NeuN and GFAP. It was
demonstrated that the number of cells double-positive for SRY/NeuN
or SRY/GFAP was increased in the BMSC group compared with the TBI
group. This was consistent with the hypothesis that BMSCs migrate
to injured areas and differentiate into neurons and astrocytes
following induction of TBI in the rat.
An increase in the expression level of neurotrophic
factors is considered as one of the primary underlying mechanisms
to promote neuroprotection and neurorepair following damage
(20). Numerous growth factors are
important in brain development, including basic fibroblast growth
factor, insulin-like growth factor-1, VEGF and BDNF; thus, their
increased expression following brain injury may recapitulate the
processes involved in brain growth and acceleration of neuronal
repair. In particular, increased production of VEGF and BDNF in the
injured brain has been reported to lead to functional recovery
(21,22). Previous studies revealed that
simvastatin may significantly promote the migration of BMSCs to the
injured spinal cord, increase the expression of BDNF and VEGF,
reduce the lesion cavity and accelerate the recovery of hind limb
function in rats (23). Song et
al (24) revealed that BMSC
treatment increases the expression of SDF-1, VEGF and BDNF in the
peri-infarct region following focal ischemic stroke. The present
study demonstrated that BMSCs may significantly increase the
expression of VEGF and BDNF around the site of injury at 14 days
following TBI. Therefore, it may be hypothesized that the
protective effect of BMSCs on TBI may be associated with increased
expression of neurotrophic factors.
In addition, the present study demonstrated that
BMSC treatment significantly increased the expression of
synaptophysin at 1, 3 and 5 days compared with the TBI group.
Synaptophysin has been extensively used as a marker protein to
quantify the number of synapses during neuroanatomical remodeling,
or following injury (25).
Enhanced synaptic plasticity may be beneficial as synaptogenesis
promotes neurorestorative effects and enhances functional recovery,
post TBI (26). Our results
demonstrated that treatment with BMSCs significantly reduced
TBI-induced neuromotor impairment, as assessed by rotarod testing
and mNSS. Furthermore, BMSCs increased expression of VEGF and BDNF.
Protein expression levels of synaptophysin were downregulated
following TBI, and this was reversed in part by treatment with
BMSCs. Therefore, it may be hypothesized that BMSC treatment may
contribute to the improvement of synaptic plasticity in the injured
brain, enhancing motor functional outcome following TBI. In
conclusion, the results of the present study revealed that
treatment for TBI with BMSCs, administered by tail vein puncture,
may significantly promote the migration of BMSCs to the injured
brain area, reduce neuronal damage, increase the expression of
BDNF, VEGF and synaptophysin, and accelerate the recovery of motor
function in rats. Furthermore, these findings implicate the
therapeutic potential of BMSCs as an effective treatment following
TBI; however, further investigation is necessary in order to
develop a novel, suitable treatment for general clinical
application.
Glossary
Abbreviations
Abbreviations:
|
BMSCs
|
bone marrow stromal cells
|
|
mNSS
|
modified neurologic severity score
|
|
VEGF
|
vascular endothelial growth factor
|
|
BDNF
|
brain derived neurotrophic factor
|
|
SRY
|
sex determining region Y
|
|
SYN
|
synaptophysin
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Logan TT, Villapol S and Symes AJ: TGF-β
superfamily gene expression and induction of the Runx1
transcription factor in adult neurogenic regions after brain
injury. PLoS One. 8:e592502013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dharmasaroja P: Bone marrow-derived
mesenchymal stem cells for the treatment of ischemic stroke. J Clin
Neurosci. 16:12–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida H, Nakayama M, Tanaka H, Kitamura
M, Hatoya S, Sugiura K, Harada Y, Suzuki Y, Ide C and Inaba T:
Safety of autologous bone marrow stromal cell transplantation in
dogs with acute spinal cord injury. Vet Surg. 41:437–442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Islam MN, Das SR, Emin MT, Wei M, Sun L,
Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S and
Bhattacharya J: Mitochondrial transfer from bone-marrow-derived
stromal cells to pulmonary alveoli protects against acute lung
injury. Nat Med. 18:759–765. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Feng Z, Hu B, Chi X and Jiao S: Ex
vivo-expanded bone marrow mesenchymal stem cells facilitate
recovery from chemically induced acute liver damage.
Hepatogastroenterology. 59:2389–2394. 2012.PubMed/NCBI
|
|
6
|
Li Y, Chen J, Chen XG, Wang L, Gautam SC,
Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N and Chopp M:
Human marrow stromal cell therapy for stroke in rat: Neurotrophins
and functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osanai T, Kuroda S, Sugiyama T, Kawabori
M, Ito M, Shichinohe H, Kuge Y, Houkin K, Tamaki N and Iwasaki Y:
Therapeutic effects of intra-arterial delivery of bone marrow
stromal cells in traumatic brain injury of rats-in vivo cell
tracking study by near-infrared fluorescence imaging. Neurosurgery.
70:435–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bae KS, Park JB, Kim HS, Kim DS, Park DJ
and Kang SJ: Neuron-like differentiation of bone marrow-derived
mesenchymal stem cells. Yonsei Med J. 52:401–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tohill M, Mantovani C, Wiberg M and
Terenghi G: Rat bone marrow mesenchymal stem cells express glial
markers and stimulate nerve regeneration. Neurosci Lett.
362:200–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao X, Deng P, Xu ZC and Chen J: Moderate
traumatic brain injury causes acute dendritic and synaptic
degeneration in the hippocampa dentate gyrus. PLoS One.
6:e245662011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye X, Yan T, Chopp M, Zacharek A, Ning R,
Venkat P, Roberts C and Chen J: Combination BMSC and Niaspan
treatment of stroke enhances white matter remodeling and synaptic
protein expression in diabetic rats. Int J Mol Sci. 14:22221–22232.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aizawa-Kohama M, Endo T, Kitada M, Wakao
S, Sumiyoshi A, Matsuse D, Kuroda Y, Morita T, Riera JJ, Kawashima
R, et al: Transplantation of bone marrow stromal cell-derived
neural precursor cells ameliorates deficits in a rat model of
complete spinal cord transaction. Cell Transplant. 22:1613–1625.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui X, Chopp M, Zacharek A, Roberts C, Lu
M, Savant-Bhonsale S and Chen J: Chemokine, vascular and
therapeutic effects of combination Simvastatin and BMSCs treatment
of stroke. Neurobiol Dis. 36:35–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marmarou A, Foda MA, van den Brink W,
Campbell J, Kita H and Demetriadou K: A new model of diffuse brain
injury in rats. Part I: Pathophysiology and biomechanics. J
Neurosurg. 80:291–300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vonder Haar C, Emery MA and Hoane MR:
Chronic folic acid administration confers no treatment effects in
either a high or low dose following unilateral controlled cortical
impact injury in the rat. Restor Neurol Neurosci. 30:291–302.
2012.PubMed/NCBI
|
|
16
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S and
Díez-Tejedor E: Effects of intravenous administration of allogenic
bone marrow-and adipose tissue-derived mesenchymal stem cells on
functional recovery and brain repair markers in experimental
ischemic stroke. Stem Cell Res Ther. 4:112013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pavlichenko N, Sokolova I, Vijde S,
Shvedova E, Alexandrov G, Krouglyakov P, Fedotova O, Gilerovich EG,
Polyntsev DG and Otellin VA: Mesenchymal stem cells transplantation
could be beneficial for treatment of experimental ischemic stroke
in rats. Brain Res. 1233:203–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhakta S, Hong P and Koc O: The surface
adhesion molecule CXCR4 stimulates mesenchymal stem cell migration
to stromal cell-derived factor-1 in vitro but does not decrease
apoptosis under serum deprivation. Cardiovasc Revasc Med. 7:19–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan J, Bennet L, Gluckman PD and Gunn AJ:
Insulin-like growth factor-1 and post-ischemic brain injury. Progr
Neurobiol. 70:443–462. 2003. View Article : Google Scholar
|
|
21
|
Zhang HY, Jin XB and Lue TF: Three
important components in the regeneration of the cavernous nerve:
Brain-derived neurotrophic factor, vascular endothelial growth
factor and the JAK/STAT signaling pathway. Asian J Androl.
13:231–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Choi KH, Jang YJ, Kim HN, Bae SS,
Choi BT and Shin HK: Electroacupuncture preconditioning reduces
cerebral ischemic injury via BDNF and SDF-1α in mice. BMC
Complement Altern Med. 13:222013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han X, Yang N, Cui Y, Xu Y, Dang G and
Song C: Simvastatin mobilizes bone marrow stromal cells migrating
to injured areas and promotes functional recovery after spinal cord
injury in the rat. Neurosci Lett. 521:136–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song M, Mohamad O, Gu X, Wei L and Yu SP:
Restoration of intracortical and thalamocortical circuits after
transplantation of bone marrow mesenchymal stem cells into the
ischemic brain of mice. Cell Transplant. 22:2001–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brock TO and O'Callaghan JP: Quantitative
changes in the synaptic vesicle proteins synapsin I and p38 and the
astrocyte-specific protein glial fibrillary acidic protein are
associated with chemical-induced injury to the rat central nervous
system. J Neurosci. 7:931–942. 1987.PubMed/NCBI
|
|
26
|
Pati S, Muthuraju S, Hadi RA, Huat TJ,
Singh S, Maletic-Savatic M, Abdullah JM and Jaafar H: Neurogenic
plasticity of mesenchymal stem cell, an alluring cellular
replacement for traumatic brain injury. Curr Stem Cell Res Ther.
11:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|