Introduction
Curcumin is regarded as a direct and an indirect
antioxidant agent through its reduction of reactive oxygen species
(ROS) (1,2) and enhancement of the expression of
cytoprotective enzymes, including glutathione-S-transferase
(3,4). It has been reported that curcumin
induces antioxidant response mechanisms by regulating transcription
factors including the nuclear factor (NF)-κB pathway (5–7). The
nuclear factor (NF)-κB pathway modulates DNA repair enzymes and the
expression of anti-inflammatory response proteins. These proteins
increased the ability of the cell to repair oxidative damage.
Curcumin is therefore able to induce a protective effect and
activate the NF-κB pathway in cells following exposure to oxidative
and inflammatory influences.
There is a connection between the exposure to PM2.5
and cardiopulmonary diseases (8–10).
Between 2014 and 2016, the concentration of PM2.5 has risen to
>600 µg/m3 in Shanghai and Beijing, particularly in
winter (11–15). There is growing concern about
protecting people from PM2.5 injury. Although a number of findings
have produced experimental data confirming that PM2.5 injures the
cardiovascular system and other organs (16,17),
knowledge of the biological mechanisms remains limited.
The vascular endothelial cells (ECs) are the primary
organ system exposed to PM2.5, and have therefore been inferred to
be susceptible. In previous studies, HUVECs were treated with PM2.5
to detect the effects on ECs viability and apoptosis (18,19).
These studies demonstrated that PM2.5 induced HUVECs apoptosis via
ROS expression. ECs dysfunction is regarded as an important marker
in the development of atherosclerosis (AS) (20). A previous study also demonstrated
that air pollution enhanced the atherosclerosis pathogenesis via
abnormal oxidized lipid accumulation in vessels in vivo
(21). Oxidized low-density
lipoprotein (oxLDL), a key risk factor for atherosclerosis, has
several functions during AS development, including inducing EC
apoptosis (22,23). Ox-LDL induces EC apoptosis by
eliciting ROS expression. A previous study demonstrated that PM2.5
(200 µg/ml) induces apoptosis in HUVECs via the activation of ROS
and that a direct cytotoxic effect was observed in HUVECs (24). Therefore, new strategies are
required to counter PM2.5-induced injury. The strategies may
enhance the antioxidant potential of EC and in this context,
curcumin has a number of biological effects including direct and
indirect antioxidant response in animal and cell models.
A number of previous studies have demonstrated that
PM2.5 and oxLDL induce the inflammatory response (18,25).
The EC adhesion molecules vascular cell adhesion molecule-1
(VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are the
mark of the inflammatory response. A previous study demonstrated
that oxLDL increased the surface expression of ICAM-1 in HUVECs
(26). The present study
investigated whether curcumin could regulate PM2.5-treated HMEC-1
adhesion molecule expression.
The purpose of the present study was to investigate
the protective effects of curcumin on PM2.5-induced HMEC-1
cytotoxicity. It demonstrated, for the first time to the best of
the authors' knowledge, that curcumin reduced PM2.5-induced
oxLDL-mediated vascular inflammation in HMEC-1. In addition,
curcumin countered PM2.5-enhanced ROS, ICAM-1 and VCAM-1 expression
levels, which serve a key role in the inflammatory process via the
expression of NF-κB. An improved understanding of the multiple
functions of curcumin against PM2.5-induced vascular inflammation
may inform the prevention and treatment of PM2.5-induced
injury.
Materials and methods
Materials
Curcumin [1,7-bis
(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; high purity
≥98.5%] was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). PM2.5 (National Institute of Standards and Technology
Boulder Laboratories, Boulder, CO, USA) was suspended in PBS for 24
h and centrifuged at 17,000 × g and 4°C for 10 min.
HMEC-1 culture
Human microvascular endothelial cells (HMEC-1) were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI 1640 medium
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 100 U/ml penicillin with 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere under 5% CO2 at 37°C.
Cell viability assay
MTT assay was used to evaluate cell viability. In
the MTT assay, HMEC-1 (1×104 cells/ml) were plated in
96-well plates and treated with curcumin (0–50 µM) or PM2.5 (0–600
µg/ml) for 24 h. Then, 20 µl MTT was added to each well, and the
cells were incubated at 37°C for 4 h. The supernatant was removed
and 150 µl dimethyl sulfoxide was added to each well for 10 min in
darkness. The optical density was calculated at 490 nm with a
microplate reader.
Analysis of apoptotic cells
Apoptotic cells were determined by flow cytometry
using annexin V-fluorescein isothiocyanate/propidium iodide
staining following the manufacturer's protocols.
Measurement of intracellular oxidative
stress
To determine whether PM2.5 affected the ROS levels
of HMEC-1, a 2′,7′-dichlorofluorescin diacetate (DCFDA) kit
(Sigma-Aldrich; Merck KGaA) was used. Curcumin/PM2.5 treated HMEC-1
(2×105 cells/well) were added to DCFDA (20 µM) for 20
min, washed with PBS and then the ROS expression was analyzed by
flow cytometry All experiments were repeated three times.
The malondialdehyde (MDA) level and superoxide
dismutase (SOD) activity of HMEC-1 were detected by a Lipid
Peroxidation (MDA) assay kit (Sigma-Aldrich; Merck KGaA) and SOD
assay kit (Sigma-Aldrich; Merck KGaA), respectively.
Measurement of cytokine levels
The plasma oxLDL was measured using an ELISA kit
(cat. no. ABIN366743; Cusabio Biotech Co., Ltd., Wuhan, China) as a
marker of oxidative stress. Levels of tumor necrosis factor (TNF)-α
(cat. no. DTA00C; R&D Systems, Inc., Minneapolis, MN, USA),
interleukin (IL)-8 (cat. no. D8000C; R&D Systems, Inc.), ICAM-1
(cat. no. ELHS-ICAM1-1; RayBiotech, Inc., Norcross, GA, USA) and
VCAM-1 (cat. no. ab100661; Abcam, Cambridge, UK) were also measured
by ELISA kits, according to the manufacturer's protocols.
NF-κB p65 transcription factor
assay
To determine NF-κB activity, the nuclear fractions
of curcumin/PM2.5 treated HMEC-1 were isolated and measured by
NF-κB p65 Transcription Factor Assay kit (Abcam). The assay was
conducted according to the manufacturer's protocols.
Measurement of caspase 3 activity
The activity of caspase 3 was measured using a
Caspase Activity Detection kit (Sigma-Aldrich; Merck KGaA) was
detected according to the manufacturer's protocols. The cells were
lysed using the colorimetric buffers included in the Caspase
Activity Detection kit. Proteins were centrifuged at 12,000 × g for
10 min at 4°C and measured by the BCA Protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Subsequently, 100 µg protein was
incubated with 5 µl caspase 3 substrate (Ac-DEVD-pNA) in a 96-well
plate. The activity of caspase 3 was determined using a
spectrophotometer at 405 nm.
Statistical analysis
The experimental results are expressed as the mean ±
standard error and are accompanied by a number of observations. For
analysis of the results, one-way analysis of variance was used with
the post-hoc Bonferroni's test for multiple comparisons using
SigmaStat software, version 3.5 (Systat Software, Inc., San Jose,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Curcumin reduces PM2.5-induced HMEC-1
apoptosis
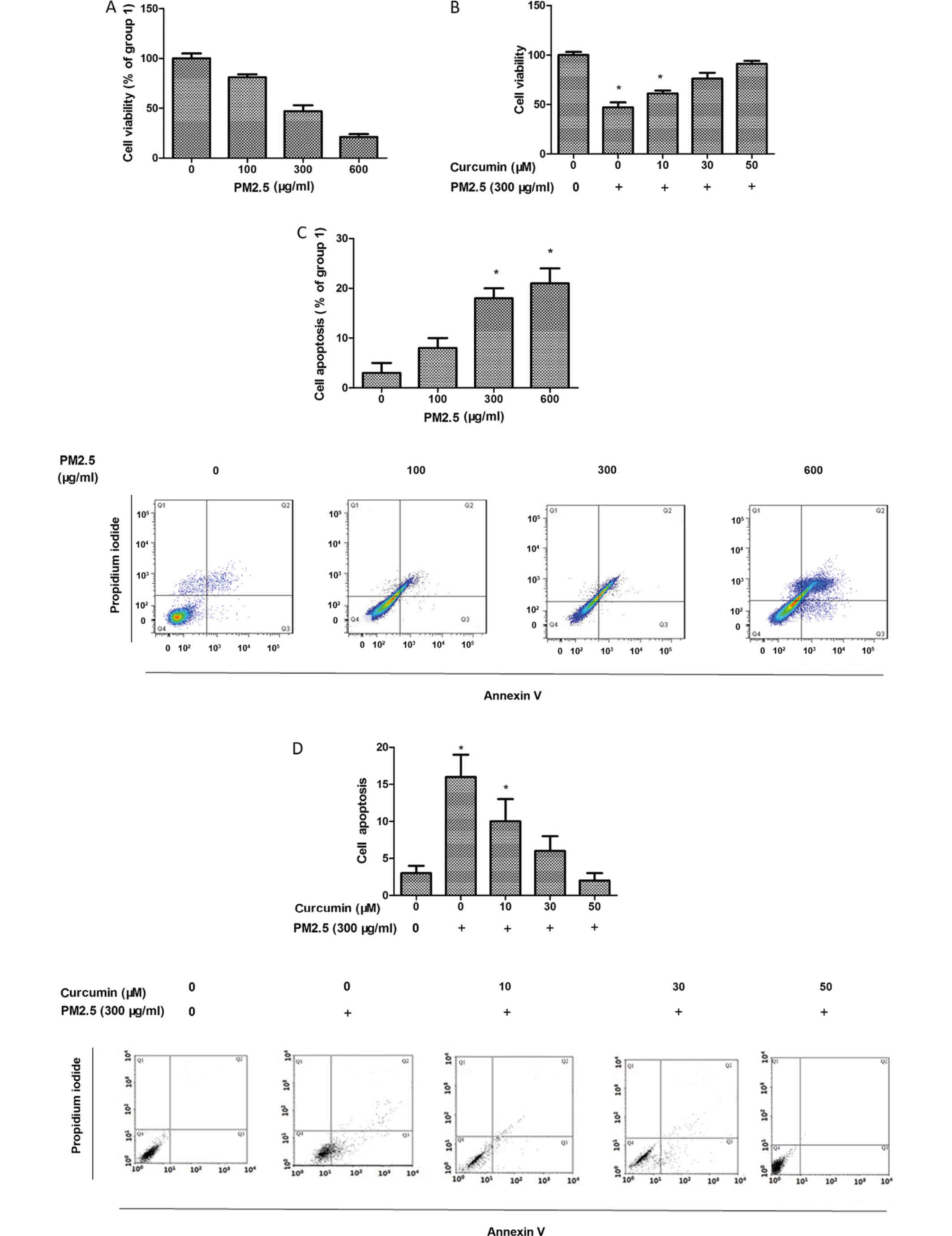
HMEC-1 viability was assessed using the MTT assay
and the data demonstrated that PM2.5 reduced HMEC-1 viability
(Fig. 1A). HMEC-1 viability
remained unchanged following 5–50 µM curcumin treatment for 24 h
(data not shown). Therefore, 50 µM curcumin was used for subsequent
experiments. In the cells exposed to PM2.5, curcumin pretreatment
increased HMEC-1 viability (Fig.
1B).
HMEC-1 apoptosis was detected using flow cytometry.
As presented in Fig. 1C, PM2.5
enhanced HMEC-1 apoptosis. In addition, the protective effect of
curcumin against PM2.5-induced HMEC-1 injury was also assessed.
Curcumin eliminated PM2.5-induced HMEC-1 apoptosis (Fig. 1D).
Curcumin reduces PM2.5-induced
oxidative stress in HMEC-1
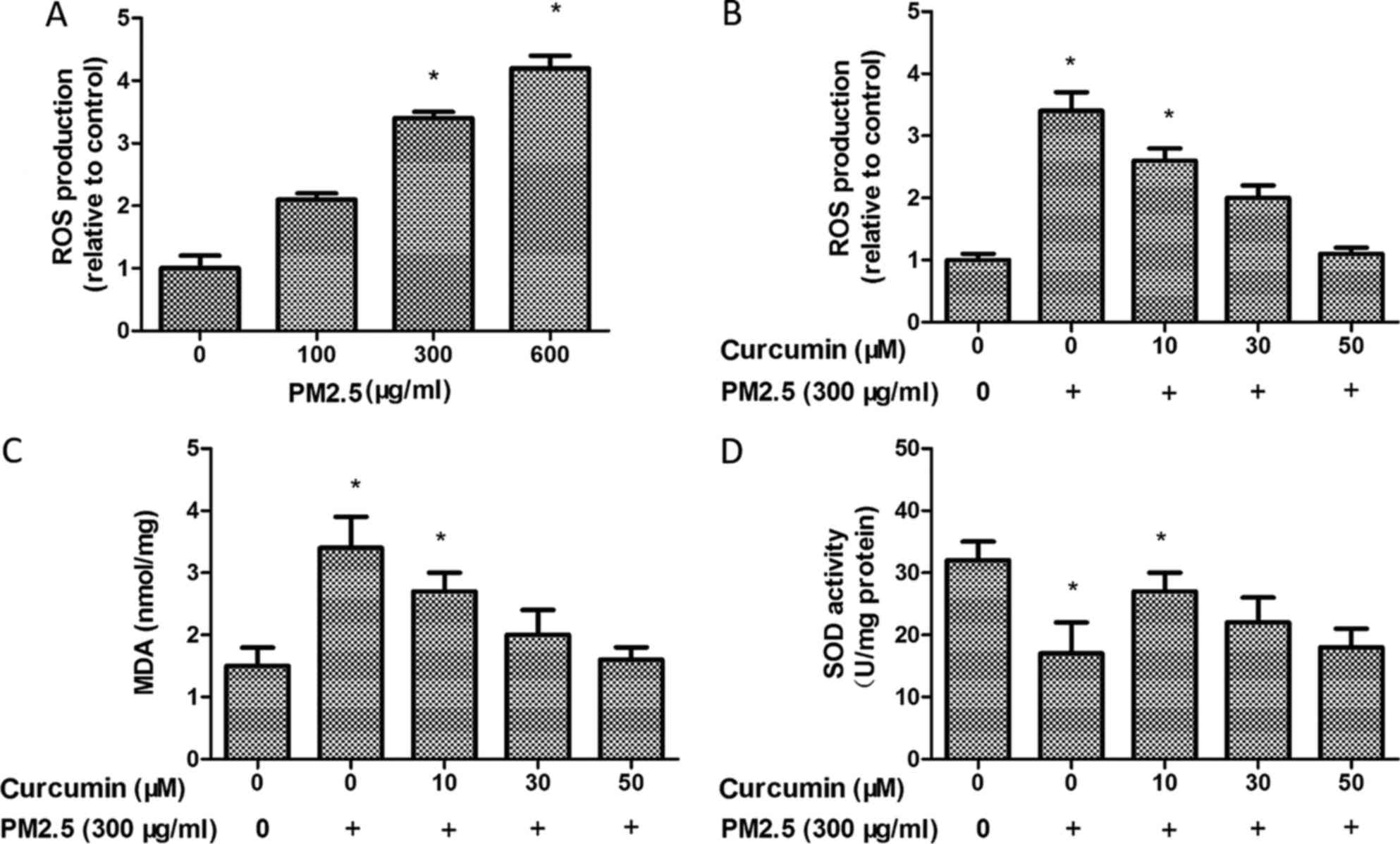
The key hypotheses concerning the effects of PM2.5
is the ability to enhance oxidative stress. The ROS production was
observed using H2DCF-DA by flow cytometry. It was observed that
PM2.5 treatment induced the production of ROS in HMEC-1 (Fig. 2A). However, ROS production was
decreased following curcumin (50 µM) pretreatment for 24 h prior to
PM2.5 exposure (Fig. 2B).
The MDA level was markedly increased in the PM2.5
group (Fig. 2C). By contrast, SOD
activity decreased in the PM2.5 group compared with the control
group (Fig. 2D). PM2.5
significantly decreased the MDA level (Fig. 2C) and enhanced SOD activity
(Fig. 2D) in the curcumin
pretreated HMEC-1 group compared with the PM2.5 group. Therefore,
curcumin may block PM2.5-induced oxidative stress in HMEC-1.
Curcumin reduces PM2.5-induced oxLDL
expression in HMEC-1
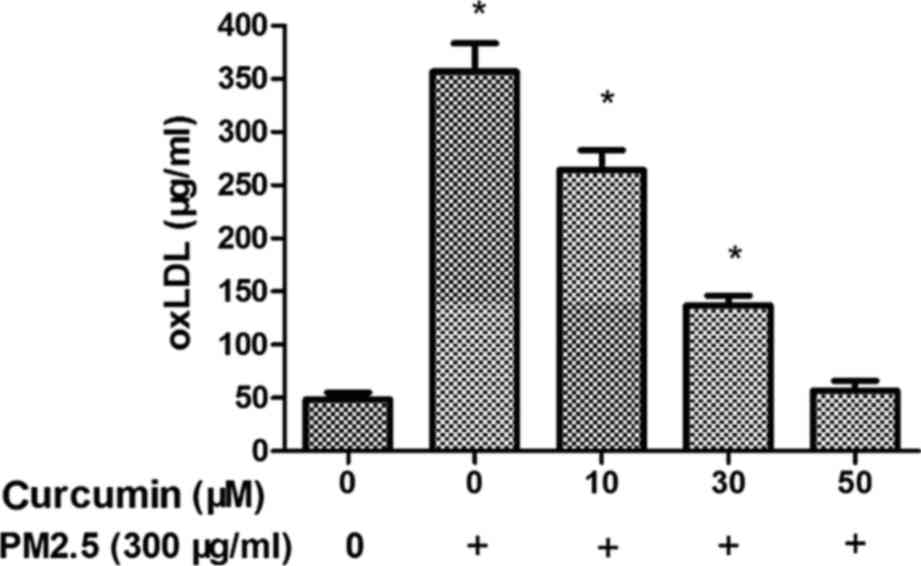
Fig. 3 demonstrates
that plasma oxLDL level was overexpressed in the PM2.5 group
compared with the control group. The data suggested that PM2.5
induces oxidative stress via the oxLDL level. Treatment with
curcumin (50 µM) decreased the expression of oxLDL in HMEC-1
exposed to PM2.5 (Fig. 3).
Curcumin reduces PM2.5-induced
inflammatory responses in HMEC-1
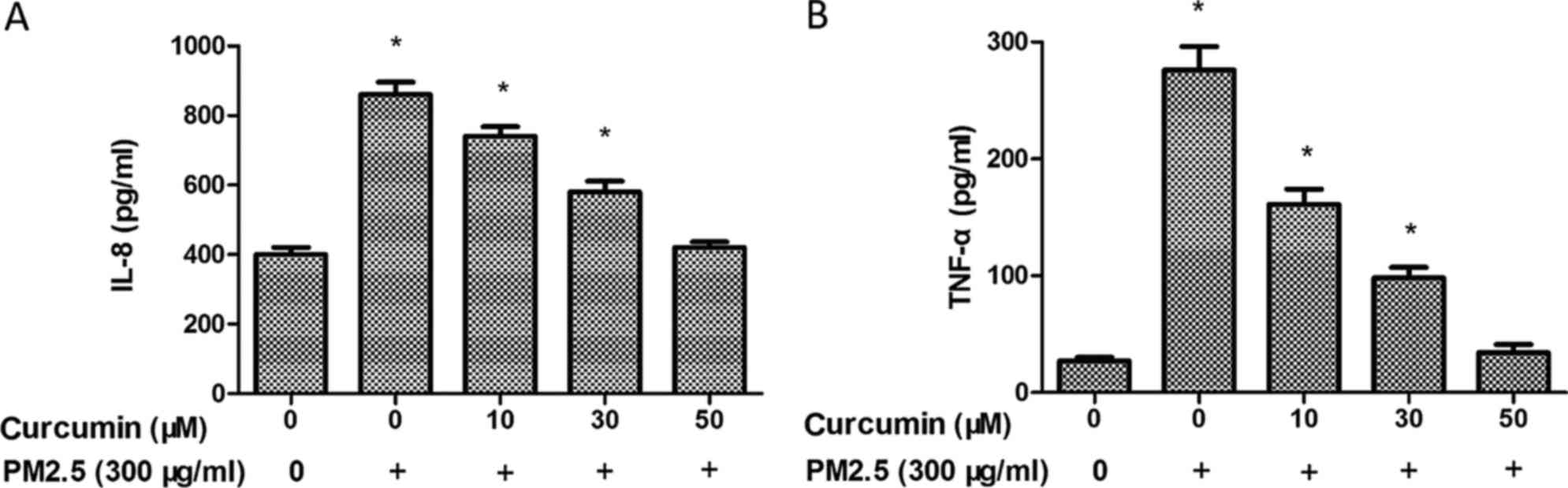
To further investigate the inflammatory effects of
PM2.5 in HMEC-1, the expression of inflammatory chemokines was
measured by ELISA. As demonstrated in Fig. 4, PM2.5 exposure induced IL-8
(Fig. 4A) and TNF-α (Fig. 4B) levels in the PM2.5 groups. A
significant decrease of the expression of IL-8 (Fig. 4A) and TNF-α was identified in
HMEC-1 pretreated with curcumin prior to PM2.5 exposure (Fig. 4B).
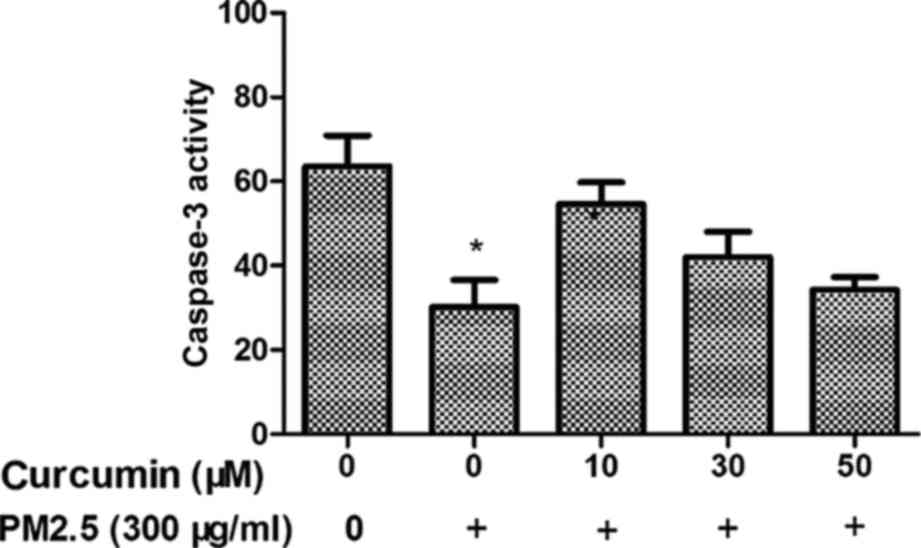
Curcumin reduces PM2.5-induced caspase
3 activity in HMEC-1
The caspase family of cysteine proteases have a key
role in cell death, and caspase 3 is an important member of the
caspase family. Therefore, the caspase 3 activity in PM2.5-treated
HMEC-1 was investigated. As demonstrated in Fig. 5, the activity of caspase 3 in the
PM2.5 group was increased. Furthermore, the activity of caspase 3
was suppressed in HMEC-1 pretreated with curcumin prior to PM2.5
exposure.
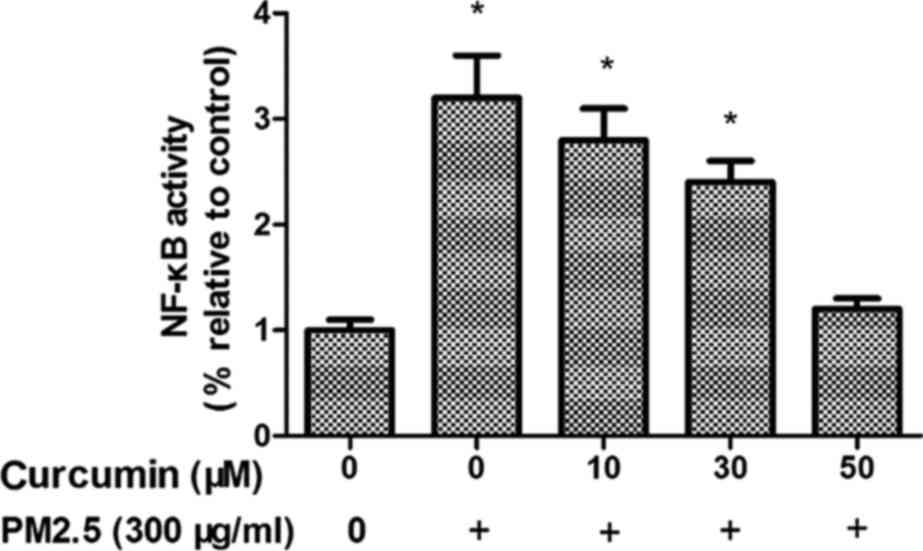
Curcumin reduces PM2.5-induced NF-κB
signaling in HMEC-1
To investigate whether NF-κB is regulated by PM2.5
treatment, phosphorylated (p)NF-κB expression was examined. As
presented in Fig. 6, PM2.5 induced
intracellular pNF-κB levels in HMEC-1 (P<0.05). Fig. 6 demonstrates the effects of
pretreatment with curcumin on PM2.5-induced pNF-κB upregulation in
HMEC-1.
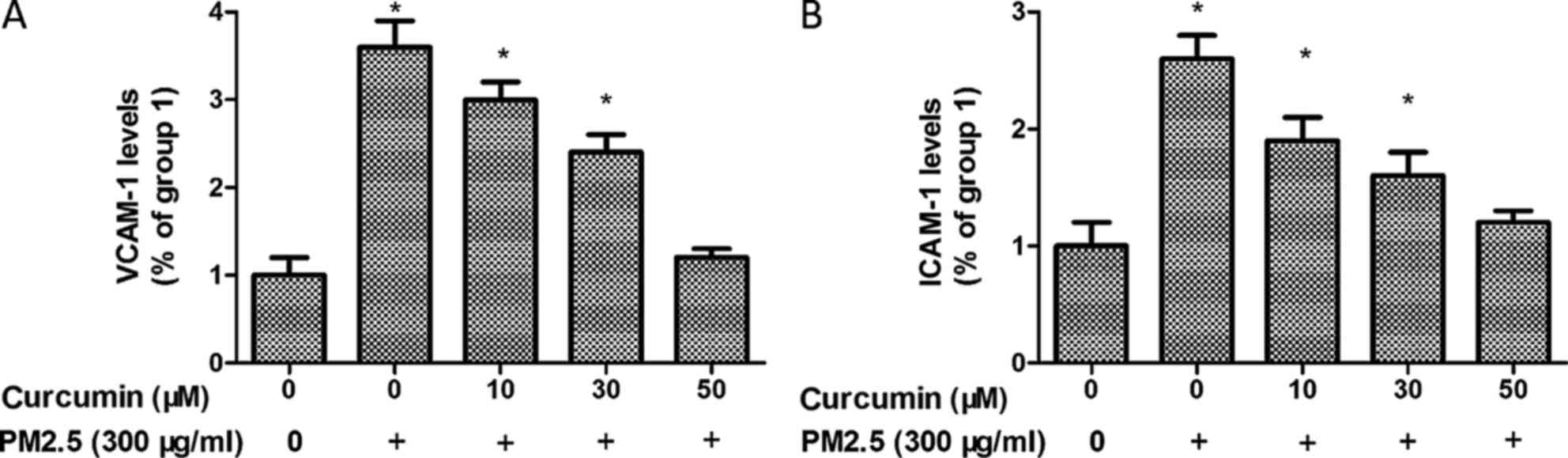
Curcumin attenuates PM2.5 induced
adhesion molecules expression in HMEC-1
To further analyze the potential inflammatory effect
of PM2.5 in HMEC-1 cells, ICAM-1 and VCAM-1 expression were
identified. As demonstrated in Fig.
7, the levels of ICAM-1 and VCAM-1 were significantly
upregulated by PM2.5 treatment.
In addition, pretreated with curcumin induced a
significant reduction of ICAM-1 and VCAM-1 levels in PM2.5 exposed
HMEC-1 (Fig. 7).
Discussion
Vascular endothelial injury leads to vascular
disease in patients. Previous studies have demonstrated that PM2.5
induces oxidative stress (27–30).
Notably, epidemiological and clinical studies have indicated that
air pollution is associated with the development of vascular
disease. However, the exact cellular mechanisms of PM2.5-induced
vascular endothelial injury remain to be elucidated. There are no
studies, to the best of the authors' knowledge, concerning the
potential protective effects of curcumin on PM2.5-induced vascular
endothelial injury. The implication of the present study is that
PM2.5 markedly induced oxidative stress and inflammation in HMEC-1.
Curcumin reversed the effects of PM2.5 by decreasing the expression
of ROS and inhibiting inflammation via the NF-κB pathway.
Increasing evidence has suggested that PM2.5 induces
cells apoptosis (31–33), however the precise molecular
mechanisms of PM2.5-induced apoptosis remain unclear, although it
is known that PM2.5 decreases viability of HUVECs in vitro
(14). The present study
documented that PM2.5 could promote HMEC-1 apoptosis by activity
the caspase 3.
Certain previous studies have suggested that lipid
levels are associated with PM2.5 (34–36),
although this remains to be confirmed. A previous study indicated
that exposure to air pollution aggravated the oxLDL levels in
vivo (21). OxLDL is well
known to trigger the ECs to induce adhesion molecules and
chemotactic cytokines expression, attracting monocytes to the
vascular wall for an inflammatory response. ECs secrete
inflammatory cytokines that promote the proliferation and migration
of smooth-muscle cells, resulting in atherosclerotic lesion
formation. It was identified that PM2.5 exposure induced an
increase in oxLDL levels in the present study and it has been
previously reported that PM2.5 exposure is a potential pathogenic
mediator for atherogenesis in vivo (37). Therefore, oxLDL levels were
associated with PM2.5 exposure. Further studies are required to
explain the effects of PM2.5 exposure on lipid metabolism and its
role.
Activation of IL-8 and TNF-α has been associated
with vascular inflammation. The data from the present study
demonstrated that PM2.5 induced IL-8 and TNF-α release in HMEC-1.
ROS overexpression is a mediator of numerous biological processes,
including cell inflammation. ROS overproduction has additionally
been regarded as a potential mediator of vascular diseases. The
present study suggested that PM2.5 induced vascular inflammation
via ROS activation. It also identified that PM2.5 increased the
expression of ICAM-1 and VCAM-1, which mediate vascular
inflammation. The results of the present study suggested that PM2.5
exhibits a number of effects on vascular inflammation. In addition,
it confirmed that curcumin reversed PM2.5-induced oxidative stress
in HMEC-1 through the inhibition of ROS. Curcumin decreased oxLDL,
IL-8, TNF-α, ICAM-1 and VCAM-1 expression and increased cell
viability in the context of PM2.5 treatment. These results
suggested that curcumin protected HMEC-1 from PM2.5-induced injury
at least in part by decreasing the activation of inflammation.
A previous study reported that the activation of the
NF-κB pathway was involved in the apoptosis process (38). Additional studies have also
demonstrated that the NF-κB pathway participates in PM2.5-induced
oxidative stress (29,39,40).
The present study demonstrated that PM2.5 significantly increased
the NF-κB activity in HMEC-1. Therefore, the downregulation of
NF-κB may be required for the protective effects of curcumin
against PM2.5-induced injury in HMEC-1.
In conclusion, the key results of the present study
suggested that curcumin reduces PM2.5-induced injury in HMEC-1 and
curcumin is able to reverse PM2.5-induced oxidant activity via
decreased ROS, oxLDL, ICAM-1 and VCAM-1 levels in HMEC-1. The
present study demonstrated that curcumin inhibited PM2.5-induced
vascular inflammation in HMEC-1. These results suggested that
curcumin may be able to be used as a potential agent for the
prevention of vascular inflammatory processes. However, further
in vivo investigations are required to confirm the results
of the present study.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (grant nos. 81401870 and
81400927), the Shanghai Municipal Science and Technology Commission
(grant no. 13ZR1459000) and Major Science and Technology Program
for Water Pollution Control and Treatment, China (grant no.
2014ZX07405002D).
References
|
1
|
Lee YJ, Kim NY, Suh YA and Lee C:
Involvement of ROS in curcumin-induced autophagic cell death.
Korean J Physiol Pharmacol. 15:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng A, Li H, Wang X, Feng Z, Xu J, Cao
K, Zhou B, Wu J and Liu J: Anticancer effect of a curcumin
derivative B63: ROS production and mitochondrial dysfunction. Curr
Cancer Drug Targets. 14:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garige M and Walters E: Curcumin inhibits
development and cell adhesion in Dictyostelium discoideum:
Implications for YakA signaling and GST enzyme function. Biochem
Biophys Res Commun. 467:275–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tapia E, Soto V, Ortiz-Vega KM,
Zarco-Márquez G, Molina-Jijón E, Cristóbal-García M, Santamaría J,
García-Niño WR, Correa F, Zazueta C and Pedraza-Chaverri: Curcumin
induces Nrf2 nuclear translocation and prevents glomerular
hypertension, hyperfiltration, oxidant stress, and the decrease in
antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell
Longev. 2012:2690392012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao F, Liu T, Xu Y, Xu D and Feng S:
Curcumin inhibits cell proliferation and promotes apoptosis in
human osteoclastoma cell through MMP-9, NF-κB and JNK signaling
pathways. Int J Clin Exp Pathol. 8:6037–6045. 2015.PubMed/NCBI
|
|
6
|
Katsori AM, Palagani A, Bougarne N,
Hadjipavlou-Litina D, Haegeman G and Vanden Berghe W: Inhibition of
the NF-κB signaling pathway by a novel heterocyclic curcumin
analogue. Molecules. 20:863–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Royt M, Mukherjee S, Sarkar R and Biswas
J: Curcumin sensitizes chemotherapeutic drugs via modulation of
PKC, telomerase, NF-kappaB and HDAC in breast cancer. Ther Deliv.
2:1275–1293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kioumourtzoglou MA, Schwartz JD, Weisskopf
MG, Melly SJ, Wang Y, Dominici F and Zanobetti A: Long-term PM2.5
exposure and neurological hospital admissions in the northeastern
united states. Environ Health Perspect. 124:23–29. 2016.PubMed/NCBI
|
|
9
|
Buczyńska AJ, Krata A, Van Grieken R,
Brown A, Polezer G, De Wael K and Potgieter-Vermaak S: Composition
of PM2.5 and PM1 on high and low pollution event days and its
relation to indoor air quality in a home for the elderly. Sci Total
Environ. 490:134–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohammed MO, Song WW, Ma WL, Li WL, Li YF,
Khan AU, Ibrahim MA, Maarouf OA, Ahmed AA and Ambuchi JJ: Potential
toxicological and cardiopulmonary effects of PM2.5 exposure and
related mortality: Findings of recent studies published during
2003–2013. Biomed Environ Sci. 29:66–79. 2016.PubMed/NCBI
|
|
11
|
Wang Y, Yang W, Han B, Zhang W, Chen M and
Bai Z: Gravimetric analysis for PM2.5 mass concentration based on
year-round monitoring at an urban site in Beijing. J Environ Sci
(China). 40:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang X, Li R, Xu Q, Bottai M, Fang F and
Cao Y: A two-stage method to estimate the contribution of road
traffic to PM2.5 concentrations in Beijing, China. Int J Environ
Res Public Health. 13:pii: E124. 2016. View Article : Google Scholar
|
|
13
|
Han L, Zhou W and Li W: Fine particulate
(PM2.5) dynamics during rapid urbanization in Beijing, 1973–2013.
Sci Rep. 6:236042016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan L, Xiu G, Feng L, Cheng N and Wang C:
The mercury species and their association with carbonaceous
compositions, bromine and iodine in PM2.5 in Shanghai. Chemosphere.
146:263–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao M, Qiao T, Huang Z, Zhu M, Xu W, Xiu
G, Tao J and Lee S: Comparison of ionic and carbonaceous
compositions of PM2.5 in 2009 and 2012 in Shanghai, China. Sci
Total Environ. 536:695–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haikerwal A, Akram M, Del Monaco A, Smith
K, Sim MR, Meyer M, Tonkin AM, Abramson MJ and Dennekamp M: Impact
of fine particulate Matter (PM2.5) exposure during wildfires on
cardiovascular health outcomes. J Am Heart Assoc. 4:pii: e001653.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu J, Liberda EN, Qu S, Guo X, Li X,
Zhang J, Meng J, Yan B, Li N, Zhong M, et al: The role of metal
components in the cardiovascular effects of PM2.5. PLoS One.
8:e837822013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montiel-Dávalos A, Alfaro-Moreno E and
López-Marure R: PM2.5 and PM10 induce the expression of adhesion
molecules and the adhesion of monocytic cells to human umbilical
vein endothelial cells. Inhal Toxicol. 19:(Suppl 1). S91–S98. 2007.
View Article : Google Scholar
|
|
19
|
Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv
JY and Zhang MS: Epigallocatechin-3-gallate protects HUVECs from
PM2.5-induced oxidative stress injury by activating critical
antioxidant pathways. Molecules. 20:6626–6639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soares SR, Carvalho-Oliveira R,
Ramos-Sanchez E, Catanozi S, da Silva LF, Mauad T, Gidlund M, Goto
H and Garcia ML: Air pollution and antibodies against modified
lipoproteins are associated with atherosclerosis and vascular
remodeling in hyperlipemic mice. Atherosclerosis. 207:368–373.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takenaka T, Takahashi K, Kobayashi T,
Oshima E, Iwasaki S and Suzuki H: Oxidized low density lipoprotein
(Ox-LDL) as a marker of atherosclerosis in hemodialysis (HD)
patients. Clin Nephrol. 58:33–37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GF, Shi CG, Sun MZ, Wang L, Wu SX,
Wang HF, Xu ZQ and Chen DM: Tetramethylpyrazine attenuates
atherosclerosis development and protects endothelial cells from
ox-LDL. Cardiovasc Drugs Ther. 27:199–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bo L, Jiang S, Xie Y, Kan H, Song W and
Zhao J: Effect of vitamin e and omega-3 fatty acids on protecting
ambient PM2.5-induced inflammatory response and oxidative stress in
vascular endothelial cells. PLoS One. 11:e01522162016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Kou X, Xie L, Cheng F and Geng H:
Effects of ambient PM2.5 on pathological injury, inflammation,
oxidative stress, metabolic enzyme activity, and expression of
c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int.
22:20167–20176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang HP, Zheng FL, Zhao JH, Guo DX and
Chen XL: Genistein inhibits ox-LDL-induced VCAM-1, ICAM-1 and MCP-1
expression of HUVECs through heme oxygenase-1. Arch Med Res.
44:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng X, Rui W, Zhang F and Ding W: PM2.5
induces Nrf2-mediated defense mechanisms against oxidative stress
by activating PIK3/AKT signaling pathway in human lung alveolar
epithelial A549 cells. Cell Biol Toxicol. 29:143–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su R, Jin X, Zhang W, Li Z, Liu X and Ren
J: Particulate matter exposure induces the autophagy of macrophages
via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere.
167:444–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Chen M, Zhong M, Hu Z, Qiu L,
Rajagopalan S, Fossett NG, Chen LC and Ying Z: Exposure to
concentrated ambient PM2.5 shortens lifespan and induces
inflammation-associated signaling and oxidative stress in
drosophila. Toxicol Sci. 156:199–207. 2017.PubMed/NCBI
|
|
30
|
Hong Z, Guo Z, Zhang R, Xu J, Dong W,
Zhuang G and Deng C: Airborne fine particulate matter induces
oxidative stress and inflammation in human nasal epithelial cells.
Tohoku J Exp Med. 239:117–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Deng Z, Feng Y, Liao F, Zhou F,
Feng S and Wang X: PM2.5 induced apoptosis in endothelial cell
through the activation of the p53-bax-caspase pathway. Chemosphere.
177:135–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng X, Zhang F, Wang L, Rui W, Long F,
Zhao Y, Chen D and Ding W: Airborne fine particulate matter induces
multiple cell death pathways in human lung epithelial cells.
Apoptosis. 19:1099–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soberanes S, Urich D, Baker CM, Burgess Z,
Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM,
et al: Mitochondrial complex III-generated oxidants activate ASK1
and JNK to induce alveolar epithelial cell death following exposure
to particulate matter air pollution. J Biol Chem. 284:2176–2186.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rice MB, Cavallari J, Fang S and
Christiani D: Acute decrease in HDL cholesterol associated with
exposure to welding fumes. J Occup Environ Med. 53:17–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan B, Li J, Guo J, Ma P, Wu Z, Ling Z,
Guo H, Hiroshi Y, Yanagi U, Yang X, et al: The toxic effects of
indoor atmospheric fine particulate matter collected from allergic
and non-allergic families in Wuhan on mouse peritoneal macrophages.
J Appl Toxicol. 36:596–608. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yeatts K, Svendsen E, Creason J, Alexis N,
Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, et al:
Coarse particulate matter (PM2.5–10) affects heart rate
variability, blood lipids, and circulating eosinophils in adults
with asthma. Environ Health Perspect. 115:709–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brucker N, Moro AM, Charão MF, Durgante J,
Freitas F, Baierle M, Nascimento S, Gauer B, Bulcão RP, Bubols GB,
et al: Biomarkers of occupational exposure to air pollution,
inflammation and oxidative damage in taxi drivers. Sci Total
Environ. 463–464:884–493. 2013. View Article : Google Scholar
|
|
38
|
Li Q, Wang Y, Li H, Shen G and Hu S:
Ox-LDL influences peripheral Th17/Treg balance by modulating Treg
apoptosis and Th17 proliferation in atherosclerotic cerebral
infarction. Cell Physiol Biochem. 33:1849–1862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kafoury RM and Madden MC: Diesel exhaust
particles induce the over expression of tumor necrosis factor-alpha
(TNF-alpha) gene in alveolar macrophages and failed to induce
apoptosis through activation of nuclear factor-kappaB (NF-kappaB).
Int J Environ Res Public Health. 2:107–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu LZ, Sun H and Chen JH: Histone
deacetylases 3 deletion restrains PM2.5-induced mice lung injury by
regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed
Pharmacother. 85:756–762. 2017. View Article : Google Scholar : PubMed/NCBI
|